Abstract
The hippocampus is a region of the mammalian brain that shows an impressive capacity for structural reorganization. Preexisting neural circuits undergo modifications in dendritic complexity and synapse number, and entirely novel neural connections are formed through the process of neurogenesis. These types of structural change were once thought to be restricted to development. However, it is now generally accepted that the hippocampus remains structurally plastic throughout life. This article reviews structural plasticity in the hippocampus over the lifespan, including how it is investigated experimentally. The modulation of structural plasticity by various experiential factors as well as the possible role it may have in hippocampal functions such as learning and memory, anxiety, and stress regulation are also considered. Although significant progress has been made in many of these areas, we highlight some of the outstanding issues that remain.
Keywords: adult neurogenesis, anxiety, learning, memory, synapse
INTRODUCTION
Throughout most of the twentieth century, the neuroscience community assumed that the central nervous system of mammals became structurally stable soon after birth. The complex architecture and functions of the mammalian brain argued against the possibility of structural remodeling of neural circuits during adulthood. This assumption was coupled with a lack of compelling evidence in favor of structural plasticity throughout life. Technical advances over the past several decades, however, have forced a dramatic revision of this view. It is now clear that rather than being fixed and immutable, the brain displays persistent plasticity across the lifespan.
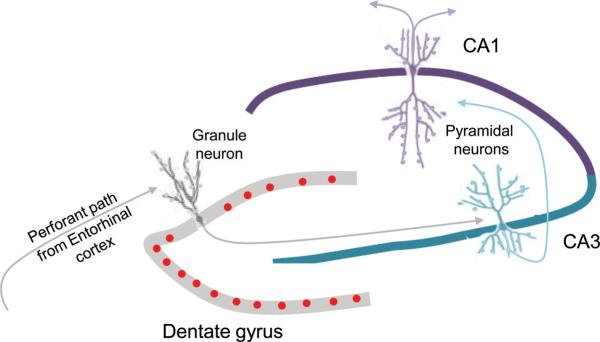
The initial discovery of adult neurogenesis in the 1960s was largely ignored, and two decades later, groundbreaking work documenting evidence of neurogenesis in the adult bird brain was viewed as irrelevant to the mammalian brain. Since that time, however, evidence has been mounting in support of adult neurogenesis in many mammalian species. Consequently, there is now almost universal acceptance that some neurons are continually generated in adulthood and added to established neural circuits (Shors 2008). There remains controversy about the regional extent of adult neurogenesis (reviewed in Gould 2007), but the hippocampus is one brain region where consensus has been reached that the production of new granule neurons is ongoing throughout life (Figure 1). Since the creation of entirely new granule neurons is accompanied by the growth of their axons, dendrites, and synapses, adult neurogenesis increases the plasticity of the hippocampus through multiple processes. In addition, preexisting granule neurons of the dentate gyrus and pyramidal neurons in areas CA3 and CA1 (Figure 1) undergo dynamic modifications in the form of dendritic extension and retraction, as well as synapse formation and elimination. All of these types of structural plasticity are subject to modification by a variety of factors and conditions, suggesting that they may be substrates for experience-dependent change.
Figure 1.
Schematic diagram of the hippocampus showing a mature granule neuron of the dentate gyrus and mature pyramidal neurons of areas CA3 and CA1 as well as their main axonal connections. For each of these cell types, the size and complexity of the dendritic trees as well as the size, shape, and number of dendritic spines can change. In the dentate gyrus, substantial numbers of new neurons (red) are also produced in adulthood.
In this review, we discuss the variety of traditional and emerging methodologies that have facilitated the study of structural plasticity in the adult hippocampus. We also describe evidence for lifelong structural plasticity and summarize how these processes are modulated by environmental experience, including learning and stress. Finally, the possibility that structural plasticity participates in hippocampal functions such as learning and memory, anxiety, and stress regulation will be considered. Even though much of what is known about structural plasticity, its experiential modulation, and its functional relevance comes from work done in rodents, there is evidence for adult neurogenesis and dendritic remodeling in the hippocampus of primates, including humans. Thus, information gathered from animal studies is likely to be applicable to humans and may shed light on the clinical importance of structural plasticity in the adult brain.
STRUCTURAL PLASTICITY ACROSS THE LIFESPAN
The hippocampus consists of a heterogeneous population of neurons distinguished by their age, morphological characteristics, and connectivity (Figure 1. The synapses and dendrites of mature neurons undergo continuous rearrangement, and entirely new neurons are formed throughout life. These various forms of structural change, which are typically associated with development, continue to occur during the postnatal period and beyond, persisting into young adulthood and throughout middle age and senescence. Despite the fact that cellular events traditionally associated with development take place in adulthood, each life stage is characterized by varying degrees of plasticity and different endpoints for structura l change.
Early Postnatal Life
Although pyramidal neurons of the CA3 and CA1 regions are generated exclusively during embryonic development, the granule cell population of the dentate gyrus is produced during an extended period that begins during gestation and continues postnatally (reviewed in Seress 2007). In rodents, the granule cell layer (GCL) forms along four general gradients—caudal to rostral, suprapyramidal to infrapyramidal, tip to crest, and superficial to deep (Figure 2). Thus, superficially located granule cells in the caudal tip of the suprapyramidal blade are the oldest, produced exclusively during the embryonic period. At the time of birth, the gross structure of the GCL is mostly formed except for the rostral infrapyramidal blade, which is virtually nonexistent. During the first postnatal week, granule cell genesis is maximal, and the result is that the remainder of the GCL coalesces (Schlessinger et al. 1975). Also, a considerable amount of cell death occurs among the newly generated granule cell population at this time. The day of maximal granule cell genesis is the same day as maximal granule cell death (Gould et al. 1991a). The massive neurogenesis and concomitant cell death that occur during the early postnatal period complete the process of laying down the foundation of the GCL. New granule cells are then inserted into this foundation throughout the ensuing stages of life.
Figure 2.
Cell birth and cell death in the dentate gyrus across the lifespan. On postnatal day 1, granule neurons that were generated embryonically have begun to form the tip of the suprapyramidal blade of the granule cell layer (GCL). During the first postnatal week, the GCL continues to be formed from progenitor cells located within the hilus along four general gradients—caudal to rostral, suprapyramidal to infrapyramidal, suprapyramidal tip through crest to infrapyramidal tip, and superficial to deep. Thereafter, the production of new granule neurons tapers off but remains substantial in adulthood until animals reach middle age and become aged. Alongside neurogenesis, there is substantial death of granule neurons. Cell death peaks at the end of the first postnatal week as indicated by the presence of pyknotic (i.e., dying) cells. In adulthood, substantial cell death continues, especially of newborn neurons located primarily within the subgranular zone (SGZ) or deep within the GCL.
Granule cell dendrites begin to develop shortly after the production of each new cell—at first dendrites are rudimentary and bare, but in the weeks that follow, they develop numerous branches and become covered with dendritic spines (reviewed in Rahimi & Claiborne 2007). Although the exact events leading to spine formation remain to be fully determined, filopodia have been implicated in synaptogenesis and early spine development (Fiala et al. 1998). Regressive events also sculpt hippocampal circuitry during development—pruning of dendritic branches occurs on the granule cells during this time (Rahimi & Claiborne 2007). Similar events occur throughout the hippocampus—postnatal development of dendritic arbors and the formation of dendritic spines followed by dendritic pruning and synapse elimination are also features of the pyramidal neuron population (Liu et al. 2005).
Juvenile Period and Adolescence
Following the early postnatal period, continued differentiation of neuronal structure occurs in all hippocampal regions. Although levels of neurogenesis are highest perinatally, neurogenesis remains robust during the juvenile period and adolescence (He & Crews 2007, Hodes et al. 2009). In addition, spine densities increase during this time and, on pyramidal neurons, do not reach adult levels until the time of sexual maturity in both rats and monkeys (~45 days in rats and 4 years in rhesus monkeys) (Pokorný & Yamamoto 1981, Seress & Ribak 1995). In the dentate gyrus, granule cells also undergo dendritic pruning during adolescence. This dendritic regression appears to be restricted to the infrapyramidal blade during this life stage (Zehr et al. 2008), perhaps because this part of the GCL is less mature than the suprapyramidal blade.
Young Adulthood
Altman and colleagues were the first to demonstrate that neurogenesis in the dentate gyrus persists into adulthood (Altman 1962, Altman & Das 1965). Although neurogenesis is not limited to the postnatal and juvenile periods, the level of new neuron production undergoes a progressive decline during the transition into adulthood (He & Crews 2007, Hodes et al. 2009). Except for a few species of bats (Amrein et al. 2007), adult neurogenesis in the hippocampus appears to be a common characteristic of all species from rodents to primates, including humans (Cameron et al. 1993, Eriksson et al. 1998, Gould et al. 1999a, Manganas et al. 2007).
Neurogenesis in the adult brain is a complex process characterized by distinct milestones (reviewed in Kempermann et al. 2004). In adulthood, new neurons are generated from a resident population of mitotic cells that are believed to be neural stem cells, although some debate remains as to whether they should be classified as such (Bull & Bartlett 2005, Jessberger et al. 2008a, Walker et al. 2008). These putative neural stem cells have some characteristics of astroglia (Garcia et al. 2004, Seri et al. 2001) and are localized to the subgranular zone (SGZ), where they divide asymmetrically to either self-renew or give rise to progenitor cells (Figure 2). The progenitor cells, while committed to a neuronal phenotype, act as transit-amplifying cells, dividing again to produce additional committed daughter cells that mature and become postmitotic. As the postmitotic progeny differentiate, they migrate a short distance into the GCL, where maturation continues. Over time, newly born cells express proteins that are characteristic of granule neurons (reviewed in Christie & Cameron 2006; Figure 3), elaborate dendritic projections with dendritic spines (reviewed in Ribak & Shapiro 2007; see also Toni et al. 2007, van Praag et al. 2002, Zhao et al. 2006), receive synaptic inputs (Kaplan & Hinds 1977, Markakis & Gage 1999), extend axons into the appropriate targets (Hastings & Gould 1999, Markakis & Gage 1999, Toni et al. 2008), and release glutamate as their main neurotransmitter (Toni et al. 2008). Eventually, adult-generated neurons produce action potentials (Laplagne et al. 2006, van Praag et al. 2002) and show other signs of activation, i.e., they express immediate early genes (IEGs) in response to a variety of stimuli (Kee et al. 2007; Ramirez-Amaya et al. 2006; Snyder et al. 2009a,b; Tashiro et al. 2007), thereby indicating that they become integrated into the existing hippocampal circuitry.
Figure 3.

(A, B) Photomicrographs of newly born neurons (arrows) in the dentate gyrus of an adult rat labeled with BrdU (red) coexpressing (A) NeuN (green), a marker of mature neurons or (B) TuJ1 (green), a marker of immature and mature neurons. Scale bars, 10 μm. Eventually, adult-generated neurons become morphologically indistinguishable from granule neurons generated during development, like those shown in (C) which were labeled with the lipophilic tracer DiI. Scale bar, 25 μm. Parts of this panel have been previously published (Leuner et al. 2004, Stranahan et al. 2007).
Numerous lines of evidence suggest that the maturational processes of adult-generated neurons follow stages characteristic of development even though they are maturing in the context of an adult hippocampus (Espósito et al. 2005; reviewed in Overstreet-Wadiche & Westbrook 2006, Ribak & Shapiro 2007). For example, like the developing brain, the inhibitory neurotransmitter gamma-aminobutyric acid (GABA) initially elicits excitatory responses in newborn cells of the adult brain (Ge et al. 2006, Karten et al. 2006). Once mature, adult-generated granule cells become indistinguishable in many ways from granule cells born during embryonic development (Ge et al. 2007, Laplagne et al. 2006). An exception to this seems to be with the axonal projection patterns of some early generated neurons—these appear to be more widely divergent than those of adult-generated granule cells (Hastings et al. 2002). This widespread divergence of axonal projections most likely occurs when embryonically generated granule cells extend axon collaterals to an immature CA3 region that subsequently grows and tows the collaterals farther apart. Late-generated granule cells do not display this feature, presumably because they are forming connections with a target area that is no longer undergoing major changes in size.
It is important to note that not all neurons produced in adulthood mature and persist. Indeed, a large proportion of newly generated neurons die within a few weeks after mitosis (Dayer et al. 2003; Gould et al. 1999b, 2001). The survival of adult-generated cells is highly sensitive to experience, suggesting that the overproduction of granule cells prepares the dentate gyrus for environmental conditions that might benefit from the incorporation of more new neurons. In the absence of these conditions, such as in laboratory control settings, these neurons are not needed and instead degenerate. The extent to which such a phenomenon reflects naturally occurring plasticity that enables a response to variable environmental demands versus an artifact of living in an abnormal laboratory setting remains unknown.
During adulthood, the morphology of mature hippocampal neurons also displays plasticity under normal conditions. For example, new spines and spine synapses form and regress on CA1 pyramidal neurons across the estrous cycle of the female rat (Woolley et al. 1990a). Similar to early postnatal spinogenesis, the mechanism by which spines are formed in the adult hippocampus likely involves the formation of filopodia and the eventual transition to a mature spine shape. Besides the production of new dendritic spines on hippocampal neurons, existing dendritic spines are also known to be quite motile and exhibit rapid changes in size and shape in adulthood (reviewed in Bonhoeffer & Yuste 2002).
Middle Age to Senescence
The hippocampus maintains the ability to undergo structural reorganization even later in life. In aged rodents and monkeys, the hippocampus produces new neurons, albeit at a much reduced level compared to young adulthood (Cameron & McKay 1999, Kuhn et al. 1996, Leuner et al. 2007a; Figure 2). The decline in new neuron production begins during middle-age, well before the onset of senescence, as a result of a reduction in the rate of proliferation, a loss in the number of progenitors, and a decrease in the proportion of progenitor cells that adopt a neuronal phenotype (Kuhn et al. 1996, Olariu et al. 2007, van Praag et al. 2005). These changes in turn may be a consequence of age-associated changes in extrinsic trophic signals and a reduction in the responsiveness of progenitor cells to these signals (reviewed in Klempin & Kempermann 2007). Although aging substantially reduces proliferation of neural progenitor cells and the differentiation of their progeny, dendritic morphology, including dendritic length, branching, and spine density, of new neurons is similar to those produced in the young brain (Morgenstern et al. 2008, van Praag et al. 2005). Thus, new neurons generated in the aged hippocampus maintain the capacity to achieve a level of complexity that is comparable to that of other dentate granule cells.
In contrast to the substantial reduction in the rate of neurogenesis, aging has subtler and regionally specific effects on dendritic and synaptic structure of mature hippocampal neurons (reviewed in Burke & Barnes 2006). Although the density of synaptic contacts formed onto dentate gyrus granule cells is reduced by aging (Geinisman et al. 1992), dendritic complexity and/or spine density of granule cells, as well as of pyramidal neurons of CA3 and CA1, are unaltered. Similar effects have been observed in aged humans, when patients with dementia are excluded (reviewed in Flood 1993).
METHODOLOGIES USED TO STUDY STRUCTURAL PLASTICITY IN THE ADULT BRAIN
The now widespread acceptance of structural plasticity in the intact adult brain represents a major milestone in the neuroscience community. This change in thinking has been fueled, at least in part, by methodological advances. The application of new techniques to the question of structural plasticity has produced a rapidly expanding literature. Findings in this subfield of neuroscience, however, have often been discrepant, raising issues about the need for a standardization of techniques and the importance of a critical evaluation of the strengths and weaknesses of the available methods.
Detection of Adult Neurogenesis
Tritiated thymidine
The earliest reports of adult neurogenesis in the mammalian brain used 3H-thymidine labeling (Altman 1962, Altman & Das 1965). This method involves injecting animals with radio-labeled thymidine, which is incorporated into DNA of dividing cells. By varying the survival time after labeling, 3H-thymidine can be a marker of proliferating cells (at short survival times) or their progeny (longer survival times). 3H-thymidine-labeled cells are identified using autoradiographic techniques (Altman 1962). Although 3H-thymidine autoradiography can be combined with immunocytochemistry for verifying neuronal phenotype (Cameron et al. 1993), colabeling is difficult to establish with certainty. Because conventional light microscopy only allows for visualizing tissue in two dimensions, it's possible that a putative 3H-thymidine-labeled neuron is actually a 3H-thymidine-labeled nonneuronal cell lying directly on top of a mature neuron. Another problem with this technique is that it underestimates the numbers of labeled cells because 3H only exposes photographic emulsion if it is located in the upper few microns of a tissue section. With the emergence of various new techniques, this method to label newly generated cells is utilized relatively infrequently.
Bromodeoxyuridine
Currently, administration of the thymidine analog bromodeoxyuridine (BrdU) is the most widely used method to study adult neurogenesis. BrdU, like thymidine, is injected into animals, becomes incorporated into the DNA of cells during the synthesis stage (i.e., S-phase) of the cell cycle, and depending on the survival time employed, is a marker of proliferating cells and their progeny. The use of BrdU as a marker of dividing cells has a number of advantages over 3H-thymidine. First, BrdU labeling is a nonisotopic method that can be easily combined with immunohistochemistry, and unlike autoradiography, takes only a few days for incubation and staining (Wojtowicz & Kee 2006). Second, antibodies to BrdU can identify labeled nuclei throughout the thickness of the section, and labeling can be quantitatively assessed with stereological counting techniques. Third, BrdU allows for the use of fluorescent immunohistochemical methods to label newly generated cells that can be combined with multiple markers to identify phenotypes of new cells (Figure 3) as well as to assess the expression of neurotransmitter and growth factor receptors and IEGs. The colocalization of BrdU with these markers can then be confirmed using three-dimensional reconstruction with confocal microscopy.
BrdU labeling is relatively straightforward. However, its extensive use in studies of adult neurogenesis has raised several questions about important variables that alter experimental outcomes. Two issues appear to be critical in designing and interpreting studies involving BrdU labeling. One issue involves differences in the dose, timing, and treatment regimen of BrdU, all of which can affect the number of labeled cells. For instance, Cameron & McKay (2001) have shown that low doses of BrdU (50 mg/kg) underestimate the number of newly born cells. A single 200–300 mg/kg BrdU injection appears to label the maximal number of cells in S-phase. The other issue that can greatly influence the detection of BrdU-labeled cells involves differences in immunohistochemical methods. Antibody selection is an important consideration given that some commonly used BrdU antibodies label substantially fewer cells than others (Leuner et al. 2009; Figure 4). Additional variability in BrdU labeling occurs with different DNA denaturation techniques. DNA denaturation is required because BrdU antibodies recognize the antigen only in single-stranded DNA. Denaturation can be achieved by high or low pH, high temperature, or treatment with solvents such as formamide. These techniques alter the number of detectable BrdU-labeled cells and the intensity of staining for NeuN, a standard marker of neuronal phenotype (Leuner et al. 2009; Figure 4).
Figure 4.
Methods for studies of adult neurogenesis are not equally sensitive. (A) BrdU antibodies do not label the same number of newborn cells in the dentate gyrus. Vector and Novocastra antibodies stain fewer BrdU-labeled cells as compared to BD, Roche, Dako, and Accurate antibodies (two-hour post-BrdU survival time). (B) Additional variability in BrdU labeling occurs with different DNA denaturation pretreatment methods. HCl alone and HCl + formamide pretreatments stain more newborn cells in the dentate gyrus than does steam heating. (C) Pretreatments also differentially affect immunoflurorescent staining for the mature neuronal marker, NeuN; greater staining is observed with HCl-alone pretreatment. *p < 0.05. Adapted from Leuner et al. (2009).
The two points raised above appear to be much more problematic for studies using the BrdU method than are other commonly mentioned potential pitfalls of this method. For example, numerous papers have raised the possibility that BrdU produces false positive results because it labels cells undergoing DNA repair or in the process of dying. This concern appears to be unimportant, since BrdU incorporation is specific to dividing cells even when the level of DNA repair is experimentally increased in adults (Bauer & Patterson 2005). Furthermore, no available evidence suggests that BrdU labels dying cells in the adult brain, unless they are cells that incorporated BrdU during mitosis and ultimately degenerated. Other issues raised in criticism of BrdU labeling are that it is toxic to cells and that it stimulates cell proliferation. Neither of these possibilities appears to be the case for the adult brain. Even at high doses, BrdU does not have cytotoxic effects and does not alter cell proliferation rates (Cameron & McKay 2001, Hancock et al. 2009, Kee et al. 2002). Finally, there is no evidence that BrdU incorporation alters neuronal differentiation—the percentage of new cells that express neuronal markers and the age of those cells at which such expression can be detected is the same as with 3H-thymidine labeling.
Other nucleotide analogs
In addition to BrdU, recent reports have used iododeoxyuridine (IdU) and chlorodeoxyuridine (CldU), thymidine analogs with a structure similar to BrdU, to label newly generated cells in the adult brain (Bauer & Patterson 2005, Burns & Kuan 2005, Dupret et al. 2007, Thomas et al. 2007, Vega & Peterson 2005). These analogs can be detected individually by different antibodies that have been reported to recognize IdU or CldU exclusively. Thus, IdU and CldU have an advantage over BrdU labeling alone; both can be injected into the same animal so cells produced at different time points can be simultaneously assessed. For this technique to produce interpretable results, antibodies for one analog must not crossreact with the other. Previous work identified antibodies that recognize IdU or CldU exclusively (Burns & Kuan 2005, Vega & Peterson 2005), although these studies did not use IdU and CldU at molarities comparable to the dose of BrdU that labels the maximal number of cells (i.e., 200–300 mg/kg; Cameron & McKay 2001). Even at higher molarities, IdU and CldU do not label as many cells as BrdU, and furthermore, antibody selectivity is lost (Leuner et al. 2009). As a result, these analogs may not provide an accurate estimate of the number of newly born cells.
Most recently, a new thymidine analog, ethynyldeoxyuridine (EdU), has been developed that reportedly has a number of benefits over the other thymidine analogs currently in use. As mentioned above, BrdU, IdU, and CldU require DNA denaturation procedures so that antigen/antibody binding can occur. This typically involves harsh pretreatments, such as the application of strong acids and/or high heat (Wojtowicz & Kee 2006), that affect histological quality as well as the detection of newborn cells (Leuner et al. 2009). In contrast, EdU does not require denaturation but instead can be detected by a fluorescent azide through a reaction known as “click chemistry” (Salic & Mitchinson 2008). This reaction is quick—it can be done in minutes as compared to hours or days—and compatible with multiple probes for fluorescent immunochemistry (Chehrehasa et al. 2009). Like BrdU, IdU, and CldU, EdU can be administered peripherally and shows strong labeling of proliferating cells that is localized within nuclei. But because this is a new technique, additional quantitative studies are needed before definitive conclusions can be made about its usefulness for studies of adult neurogenesis.
Endogenous markers
Another strategy for identifying dividing cells in the adult brain involves the detection of endogenous proteins that are only expressed in mitotically active cells (reviewed in Eisch & Mandyam 2007). Although endogenous markers, such as Ki-67, are limited in that they can only be used to examine cell proliferation, they are useful when it is not feasible to deliver thymidine analogs, such as in the study of natural populations of animals (Amrein et al. 2004, Epp et al. 2009) or postmortem human tissue (Boldrini et al. 2009, Jin et al. 2006, Reif et al. 2006). Also, endogenous markers can be used to confirm findings obtained with thymidine analogs, especially when experimental conditions may alter the availability or uptake of exogenous nucleotides. Indeed, the expression of endogenous markers mimics that of BrdU labeling, though typically more cells are stained with endogenous markers since BrdU labels only those cells that are in S-phase for at most two hours after the BrdU injection (Cameron & McKay 2001, Kee et al. 2002).
Viral vectors
An increasing number of studies use retroviral vectors that drive the expression of fluorescent proteins, most often green fluorescent protein (GFP), to label dividing cells and their progeny. Unlike the thymidine analog methods that show label dilution with cell division, viral-mediated labeling is permanent because the retroviruses incorporate into dividing cells by integrating into the genome. This technique is advantageous because it can be used to identify adult-generated neurons in living-slice preparations and characterize their electrophysiological properties (Ge et al. 2007, Laplagne et al. 2006, van Praag et al. 2002). Since the fluorescent protein is distributed throughout the entire infected neuron, retroviral-mediated labeling also enables the morphological development of newborn neurons to be examined (Espósito et al. 2005; Toni et al. 2007, 2008; Zhao et al. 2006). In addition, the regulation of adult neurogenesis by specific genes can be studied by engineering viral vectors to induce specific types of genetic manipulations (Ge et al. 2006, Jessberger et al. 2008b, Tashiro et al. 2006). However, as with the other techniques used to study adult neurogenesis, there are limitations to the application of virus-mediated cell labeling. Most notably, the retroviral method is inefficient and variable across animals, making it unsuitable for quantitative analyses. Even with improved retroviruses, the numbers of labeled cells are lower and the proportion of cells expressing neuronal markers is decreased compared to BrdU incorporation (van Praag et al. 2002, Zhao et al. 2006). Another concern is that stereotaxic surgery is necessary to deliver the retrovirus to the region of interest. This delivery method is not only more labor intensive but also is potentially problematic because damage is inevitable, and injury-induced neurogenesis has been reported (Gould & Tanapat 1997). Also, the immune-mediated side effects resulting from the virus and/or surgery may cause the proliferation of microglia that incorporate the retrovirus to subsequently fuse with existing postmitotic neurons, thereby creating a false demonstration of neurogenesis, although this has only been reported for the neocortex (Ackman et al. 2006). In addition to retroviral vectors, lentiviral vectors have been employed in studies of adult neurogenesis. However, these are not specific for newborn cells and label a broader range of cell types (van Hooijdonk et al. 2009).
Reporter Mice
Transgenic mouse lines expressing genetically encoded fluorescent reporters such as GFP have become an important tool for studying adult neurogenesis (Overstreet et al. 2004, Yamaguchi et al. 2000). These lines allow for selective identification of cells at discrete developmental stages including neural stem cells, progenitor cells, and postmitotic, immature neurons (Couillard-Despres et al. 2006, Encinas et al. 2006, Overstreet et al. 2004, Yamaguchi et al. 2000). Like retroviral labeling, the full morphology of labeled cells can be visualized and electrophysiological analysis performed. Transgenic reporter lines in which the fluorescent protein is directed to the nucleus have the added benefit of quantitative analysis (Encinas et al. 2006). Moreover, the effect of specific genes or mutations on adult neurogenesis can be examined by crossing reporter mice with other genetically modified mice. Although transgenic labeling is noninvasive and thus not confounded by issues related to inflammation or injury, expression of the reporter in these mice is often transient, making it impossible to follow the same population of cells across maturation (Overstreet et al. 2004, Yamaguchi et al. 2000). In order to overcome this issue, inducible transgenic mouse lines have been developed that permit labeling and tracking of progenitor cells and their progeny in the dentate gyrus (Imayoshi et al. 2008, Lagace et al. 2008). The inducible transgenic systems have also been useful for gene deletion and progenitor ablation studies that investigate adult neurogenesis from a mechanistic and functional perspective.
Imaging
Currently, all methods to directly study adult neurogenesis involve assessment of postmortem tissue. Investigating this process in vivo has been delayed because of a lack of noninvasive methods to detect new neurons over time. However, efforts are underway to address this gap (reviewed in Schroeder 2008). One approach has relied on the relationship between neurogenesis and angiogenesis. Since angiogenesis has been coupled to cerebral blood volume, which can be measured with magnetic resonance imaging, this method has been suggested to provide an in vivo correlate of neurogenesis (Pereira et al. 2007). Although this approach does not specifically measure neurogenesis, other work has reported the discovery of a biomarker for neural progenitor cells in humans that is detectable using magnetic resonance spectroscopy (Manganas et al. 2007). However, questions about the validity of the detection techniques and data analysis methods of this latter approach have been raised. Even so, the ability to monitor neural stem/progenitor cells in the hippocampus of live animals and humans represents a major technological breakthrough, one that would have important implications for understanding the functional consequences of adult neurogenesis as well as its role in psychiatric and neurological disorders.
Analysis of Dendritic Structure and Dendritic Spines
Golgi technique
The vast majority of studies until recently have used light microscopic analysis of brain sections stained with Golgi techniques to examine dendritic structure and dendritic spines. Several versions of this technique are currently in use, but all are based on the same principles of metallic impregnation first developed and used more than 100 years ago by Golgi and Ramon y Cajal. The Golgi technique is useful because it randomly labels a small number of cells in their entirety so that detailed information regarding dendritic branching, length, and spine density can be measured. Since more than 90% of excitatory synapses are formed on dendritic spines (reviewed in Nimchinsky et al. 2002), dendritic spine numbers obtained from Golgi-stained tissue provide an indirect measure of excitatory synaptic inputs. However, quantification of the actual number of synapses can only be done using electron microscopic procedures. Also, due to limits in resolution with light microscopy, Golgi staining doesn't reveal subtler changes in spine morphology and other aspects of spine ultrastructure.
Fluorescent labeling
More recent work has employed fluorescent labeling of neurons followed by confocal microscopy to assess dendritic and synaptic structure (Dailey et al. 1999, Kozorovitskiy et al. 2005). In this regard, intra-cellular microinjection of fluorescent dyes such as Lucifer yellow has been used in hippocampal slices. Cell filling has also been achieved with lipophilic fluorescent dyes such as DiI, which diffuse readily across neuronal membranes of fixed tissue (O'Brien & Lummis 2006). Crystals of the dye can be applied directly to slices, delivered ballistically with a gene gun, or inserted into small tissue blocks where it serves as a retrograde label. Not only do these approaches produce spine images of a higher resolution when viewed with a confocal microscope, allowing for the assessment of spine morphology, but when used in slices, synaptic activity can be manipulated and its effects on dendritic morphology determined.
Imaging
Two-photon laser-scanning microscopy in combination with fluorescent molecular tools has allowed for high-resolution, time-lapse imaging of spines in living slices (reviewed in Nimchinsky et al. 2002). It is now even possible to study dendritic spines in vivo using transgenic mouse lines that sparsely express genetically encoded fluorescent proteins in specific neuronal types. A “window” is created in the skulls of these mice, and when secured under a two-photon laser-scanning microscope, the same dendritic segments can be imaged over extended periods of time as well as before and after exposure to stimuli. This form of imaging has been primarily used to visualize neurons in the most superficial layers of the cortex, although preparations for in vivo imaging of hippocampal dendrites and spines have been reported (Mizrahi et al. 2004). Dynamic changes in the individual shapes of spines can be detected with this technique, but resolution is not sufficient to count numbers or identify exactly where synapses occur. There are also data to suggest that the cranial window itself can cause substantial glial activation and influence spine dynamics (Xu et al. 2007). An alternative approach involves viewing neurons through a thinned skull preparation. Results from this method have shown naturally occurring changes in dendritic spine number and shape but of a less extensive nature than that observed with a cranial window. However, examining deep structures such as the hippocampus with this method is difficult. Another limitation of current imaging techniques is that because anesthesia is required, examination of structural plasticity in an animal as it actively engages in specific behaviors cannot yet be done. With the development of miniaturized fiber optic microscopy (Flusberg et al. 2008), cellular-level imaging of the hippocampus may soon be possible in behaving animals.
EXPERIENCE MODULATES STRUCTURAL PLASTICITY
Structural plasticity in the hippocampus is sensitive to a wide range of experiences, many of which appear to have substantial effects throughout the lifespan of the animal. Within the context of adult neurogenesis, the discrete stages that characterize the progression from progenitor cell to mature granule neuron are each subject to experiential modification. In addition, hippocampal neurons produced during development are responsive to many of the same experiential factors and exhibit alterations in dendritic architecture and dendritic spines. This prolonged sensitivity of the hippocampus to experience may have positive and negative consequences. On the one hand, the extended restructuring of the hippocampus according to experiential cues may confer important adaptive plasticity. On the other hand, the perpetual capacity for structural change might render the hippocampus particularly sensitive to environmental perturbations that may have adverse consequences on hippocampal function.
Negative Versus Positive Stress
Stressors are typically defined in terms of their ability to activate the hypothalamic-pituitary-adrenal (HPA) axis and ultimately increase glucocorticoid levels (reviewed in Ulrich-Lai & Herman 2009). Most experiences known to cause HPA axis activation are aversive. Exposure to aversive stressors adversely influences numerous aspects of hippocampal structure.
With few exceptions (Bain et al. 2004, Snyder et al. 2009a, Thomas et al. 2007), new cell production in the dentate gyrus is inhibited by a variety of acute and chronic aversive experiences, including both physical and psychosocial stressors (reviewed in Mirescu & Gould 2006). Stress-induced suppression of cell proliferation has been demonstrated in various species (mouse, rat, tree shrew, monkey) and occurs throughout life, with similar results reported for the early postnatal period, young adulthood, and aging (Coe et al. 2003; Gould et al. 1997, 1998; Simon et al. 2005; Tanapat et al. 1998, 2001; Veenema et al. 2007). When stressor exposure occurs during development, the effects are enduring and can persist into adulthood (Lemaire et al. 2000, Lucassen et al. 2009, Mirescu et al. 2004). However, it is unclear whether stress experienced in adulthood has a long-lasting influence on hippocampal neurogenesis. Prolonged effects of stress on new neuron production (Heine et al. 2004, Malberg & Duman 2003, Pham et al. 2003) and survival (Koo & Duman 2008, Thomas et al. 2007, Westenbroek et al. 2004) have been observed. Yet, others have shown that the influence of stress on adult neurogenesis is temporary, decreasing cell proliferation and immature neuron production without altering the number of new neurons that survive to maturity (Mirescu et al. 2004, Snyder et al. 2009a, Tanapat et al. 2001). The reason for these discrepancies is unknown but may be related to the duration or intensity of the stressor or timing of BrdU labeling or sacrifice relative to the stressful experience.
In addition to suppressing neurogenesis, aversive stressful experiences alter dendritic architecture in the hippocampus. For example, repeated stress in adulthood induces retraction of CA3 pyramidal neuron dendrites as well as a loss of synapses in adult male rats and tree shrews (Magariños et al. 1996, McKittrick et al. 2000, Stewart et al. 2005). Within hours of stressor onset, dendritic spine density in the CA3 region is also reduced (Chen et al. 2008). The effects of stress on dendritic architecture in other hippocampal regions have been less well-studied. Chronic stress causes dendritic regression and spine synapse loss in the dentate gyrus and CA1 (Hajszan et al. 2009, Sousa et al. 2000). Acute stress also alters dendritic spine density on CA1 pyramidal cells of adult rats, but the direction of the effect is dependent on the sex of the animal, increasing the number of dendritic spines in males but decreasing the number of dendritic spines in females (Shors et al. 2001a).
Some evidence demonstrates that glucocorticoids regulate structural plasticity in the hippocampus and are the primary mediator underlying the detrimental effects of aversive stress on hippocampal structure. First, an inhibition of neurogenesis occurs in response to natural changes in glucocorticoids across the lifespan. Neurogenesis in the dentate gyrus is maximal during the early postnatal period, when levels of circulating glucocorticoids are low (Gould et al. 1991b) but diminished during life stages when glucocorticoids are elevated, including aging and the postpartum period (Cameron & McKay 1999; Kuhn et al. 1996; Leuner et al. 2007a,b). Second, glucocorticoid administration during the early postnatal period and in adulthood suppresses neurogenesis (Cameron & Gould 1994, Gould et al. 1991b). Conversely, removal of circulating glucocorticoids by bilateral adrenalectomy increases neurogenesis in adult and aged rats (Cameron & Gould 1994, Cameron & McKay 1999) and prevents the stress-induced reduction in neurogenesis (Mirescu et al. 2004, Tanapat et al. 2001). Third, blocking glucocorticoid receptors can reverse the reduction in neurogenesis after glucocorticoid treatment (Mayer et al. 2006) or stressor exposure (Oomen et al. 2007). Like neurogenesis, the stress-induced atrophy of CA3 pyramidal neurons is prevented by pharmacological blockade of the glucocorticoid stress response and can be mimicked by exogenous glucocorticoid administration (Magariños & McEwen 1995, Woolley et al. 1990b).
It is becoming increasingly clear, however, that glucocorticoids are not the sole factor mediating the suppressive action of stress on hippocampal structure (Gould et al. 1997, Koo & Duman 2008, Van der Borght et al. 2005a) and that the effects of glucocorticoids on structural plasticity are complex. Notably, conditions associated with elevated glucocorticoids do not necessarily have detrimental effects on structural plasticity and in some cases, those conditions can be beneficial. Physical activity is an example of this paradox—despite substantial elevations in circulating glucocorticoids, running enhances adult neurogenesis (Stranahan et al. 2006, van Praag et al. 1999, Zhao et al. 2006) and dendritic architecture (Eadie et al. 2005, Stranahan et al. 2007) in the hippocampus. Although a variety of factors are likely to contribute to stress outcomes, including stressor controllability (Shors et al. 2007), social context (Stranahan et al. 2006), and coping strategy (Veenema et al. 2007), emotional valence of the stressor seems to be a critical variable. Since the systematic descriptions of stress by Selye (1976), a distinction has been made between negative and positive stress. Although both types of experience activate stress hormone systems and are generally arousing, positive stress is, by definition, rewarding. For example, rodents will develop a conditioned place preference to contexts in which they previously had access to a running wheel and will show signs of distress if they are denied access after chronic exposure (reviewed in Brené et al. 2007). The beneficial effects of running suggest that a rewarding stress can exert the opposite action on hippocampal structure as an aversive stress. However, since running is naturally rewarding to rodents, the possibility that these findings may not apply to other animals without a strong motivation to run must be considered. Nonetheless, the ability of this stimulus to override the negative actions of glucocorticoids is interesting and raises questions about the underlying mechanisms that enable running-induced neurogenesis despite activation of the HPA axis.
Environmental Enrichment
The first evidence that environmental complexity influences hippocampal neurogenesis was provided by Barnea & Nottebohm (1994), who showed that compared to black-capped chickadees living in captivity, those living in the wild maintained a higher number of new neurons. Similar findings have been reported in rodents using “environmental enrichment” protocols—housing conditions that enhance opportunities for social, cognitive, sensory, and motor stimulation. Compared to animals living in standard laboratory cages, hippocampal neurogenesis is enhanced in juvenile, adult, and aged rodents living in enriched environments (Kempermann et al. 1997, 1998). In adulthood, environmental enrichment is able to influence new neurons during a narrow temporal window shortly after they are produced, suggesting that there is a sensitive period during which newborn neurons are susceptible to experiential modulation (Tashiro et al. 2007).
Enriched environments have long been known to have a beneficial effect on other aspects of brain structure (reviewed in van Praag et al. 2000). Rosenzweig and colleagues (Globus et al. 1973) were the first to demonstrate that exposure to enriched settings either during development or in adulthood enhance multiple measures of neuronal structure. Rodents and monkeys living in an enriched environment have more dendritic spines and synapses as well as increased dendritic branching in the hippocampus compared to laboratory controls (Kozorovitskiy et al. 2005, Moser et al. 1994).
There is currently no consensus on the specific environmental feature that contributes to the beneficial consequences of enrichment on hippocampal structure (van Praag et al. 2000). Since environmental enrichment protocols typically include access to a running wheel, some have suggested physical activity may play an important role. For instance, exercise and living in enriched environments similarly enhance dendritic spine density and neurogenesis in the hippocampus (Eadie et al. 2005, Stranahan et al. 2007). Environmental complexity also provides a larger number of learning opportunities than are in standard housing conditions, which, as discussed below, can have a beneficial effect on neurogenesis and dendritic architecture. Regardless of the specific environmental feature that may be responsible for these effects, it is important to keep in mind that standard laboratory housing conditions are impoverished and that under these conditions, hippocampal structure may atrophy from disuse. Thus, experimental enrichment may be a reversal of the impoverishment generally found in the laboratory setting rather than enrichment over a natural setting. Indeed, like birds, some species of wild-living rodents have a greater amount of adult neurogenesis compared to those living in the laboratory (Amrein et al. 2004, Epp et al. 2009).
Learning
A variety of learning tasks that depend on the hippocampus have been shown to alter the number of new neurons in the dentate gyrus of adult rats. For example, the number of newborn cells is increased following the associative learning task of trace eyeblink conditioning (Gould et al. 1999b). This effect persists until the new neurons are at least two months old (Leuner et al. 2004). Other hippocampus-dependent tasks, such as long-delay eyeblink conditioning, as well as spatial learning in the Morris water maze and conditioned food preference, also increase the number of newborn cells (Ambrogini et al. 2000, Döbrössy et al. 2003, Dupret et al. 2007, Gould et al. 1999b, Hairston et al. 2005, Lemaire et al. 2000, Leuner et al. 2006a, Olariu et al. 2005). In contrast, learning tasks that do not require the hippocampus (i.e., short-delay eyeblink conditioning, cued water-maze training, active shock avoidance) do not change the number of newborn neurons (Gould et al. 1999b, Van der Borght et al. 2005a). However, classifying a task as hippocampal dependent or independent cannot completely account for the effects of learning on adult neurogenesis (reviewed in Shors 2008). Rather, more recent data suggest that for learning to have a stimulatory effect on neurogenesis, the task must be sufficiently difficult (Leuner et al. 2006a, Waddell & Shors 2008) and it must be learned well (Dalla et al. 2007, 2009; Leuner et al. 2004). Even so, there are several reported contradictions. Notably, spatial learning in the water-maze task has been linked to no effect or to a decrease in the number of newborn neurons in the dentate gyrus (Ambrogini et al. 2004, Döbrössy et al. 2003, Dupret et al. 2007, Ehninger & Kempermann 2006, Epp et al. 2007, Mohapel et al. 2006, Van der Borght et al. 2005b). In addition to differences in BrdU injection protocols and training paradigms employed, the maturity of the labeled adult-born cells at the time of learning may determine whether and how learning alters them. Both spatial learning and trace eyeblink conditioning encourage the survival of new cells born about one week prior to training, when these cells are immature but already differentiated into neurons (Ambrogini et al. 2000, Dupret et al. 2007, Epp et al. 2007, Gould et al. 1999b, Hairston et al. 2005). In contrast, some studies suggest that spatial learning induces the death of cells that are less mature (i.e., ≤4 days of age; Dupret et al. 2007, Mohapel et al. 2006), whereas others show that the death of older and perhaps more mature cells occurs (i.e., ≥10 days of age; Ambrogini et al. 2004). Therefore, learning may have a differential capacity to influence neurogenesis depending on the age of the cell. This is consistent with observations showing that experience-induced modulation of adult neurogenesis occurs at a critical period during an immature stage (Tashiro et al. 2007).
Further adding to the complex effects of learning on adult neurogenesis are studies showing that besides affecting cell survival and cell death, learning also influences cell proliferation, depending on the specific phase of the learning process assessed (Döbrössy et al. 2003, Dupret et al. 2007). That learning can affect cell proliferation, cell survival, and cell death has been proposed to reflect an activity-dependent selective stabilization process analogous to what occurs during development (Dupret et al. 2007). Accordingly, neurogenesis may be promoted by synaptic plasticity during learning, an idea that is consistent with electrophysiological data showing that enhanced synaptic activity associated with long-term potentiation (LTP) increases cell proliferation and cell survival (Bruel-Jungerman et al. 2006).
The purpose of a learning-induced enhancement of neurogenesis remains to be fully determined. One possibility is that these newborn neurons can contribute to learning and memory by creating a neural representation of previous experience (Aimone et al. 2009). Work showing that neurons made to survive by exposure to an enriched environment are preferentially activated at a later time to the same, but not a different, experience lends some support to the possibility that such a process takes place (Tashiro et al. 2007).
Learning has also been reported to induce dendritic spine alterations of mature hippocampal neurons. The effects of learning include changes in spine number and morphology (Knafo et al. 2004; Leuner et al. 2003; Moser et al. 1994; O'Malley et al. 1998, 2000) and changes in the number and distribution of synapses along dendrites (Andersen & Soleng 1998, Eyre et al. 2003, Geinisman et al. 2001, Miranda et al. 2006, Rusakov et al. 1997). Although a variety of learning tasks (i.e., trace eyeblink conditioning, water-maze training, olfactory discrimination, avoidance conditioning) have been associated with these alterations, there are some differences in the magnitude, time course, and location of the effects across studies. Similar to the effects of learning on adult neurogenesis, it appears that changes in spine formation are activity driven. Indeed, studies using live imaging with two-photon microscopy have shown that LTP is associated with the growth of new spines and synapses as well as changes in spine morphology (reviewed in De Roo et al. 2008, Lamprecht & LeDoux 2004).
STRUCTURAL PLASTICITY AND HIPPOCAMPAL FUNCTION
Although it is generally assumed that dendritic and synaptic rearrangement of mature neurons underlie at least some aspects of hippocampal function, the significance of adult neurogenesis remains elusive. One possibility is that new neurons are continuously added to the existing hippocampal circuitry to replace older cells that die. Because a substantial amount of cell death occurring in this region involves the adult-generated populations, adult neurogenesis as a replacement mechanism seems unlikely (Dayer et al. 2003). Instead, neurogenesis may be required for the modulation and refinement of existing neuronal circuits in the dentate gyrus (Imayoshi et al. 2008) and may serve one or several aspects of hippocampal function that cannot be accomplished exclusively by existing mature neurons. Even though the level of neurogenesis in the adult hippocampus is substantial, with an estimated 9000 new neurons produced daily in the young adult rat, this still represents only a relatively small proportion (~0.5%) of the total population of mature granule neurons (Cameron & McKay 2001). How can so few neurons have functional significance? The answer to this question may be related to the unique properties of adult-generated neurons. One distinct feature of newborn neurons in the adult hippocampus is that they are structurally plastic during their immature stages (Hastings & Gould 1999, Toni et al. 2007, Zhao et al. 2006). Adult-generated neurons also possess, at least transiently, several electrophysiological characteristics that differ from those of mature neurons. For example, immature neurons exhibit depolarization by GABA, enhanced excitability, low LTP-induction threshold, and more robust LTP that unlike mature granule cells is insensitive to GABA inhibition (Ge et al. 2006, 2007; Karten et al. 2006; Schmidt-Hieber et al. 2004; Snyder et al. 2001; Wang et al. 2000). Collectively, these findings support the view that a somewhat small population of immature neurons might exert a substantial influence on hippocampal function.
Experimental Approaches to Study the Role of Structural Plasticity in Hippocampal Function
Several strategies have been used to link structural change to hippocampal function. One is correlative and involves evaluating whether there is a positive relationship between structural plasticity and hippocampal function. Another is more direct and involves blocking structural changes and examining whether hippocampal functions are altered. With respect to adult neurogenesis, the depletion of newborn cells has been achieved pharmacologically by systemic administration of the antimitotic agent methylazoxymethanol (MAM; Bruel-Jungerman et al. 2005, Shors et al. 2001b) or the DNA-alkylating agent temozolomide (TMZ; Garthe et al. 2009) as well as by central infusion of the mitotic blocker cytosine arabinoside (AraC; Mak et al. 2007). However, none of these agents diminish the number of newborn neurons exclusively within the dentate gyrus. Irradiation is another approach to block hippocampal neurogenesis and when applied focally, spares neurogenesis in the rest of the brain (Clelland et al. 2009, Santarelli et al. 2003, Saxe et al. 2006, Winocur et al. 2006). One downside common to all of these methods is their nonspecific side effects that may complicate the interpretation of results (Dupret et al. 2005, Monje et al. 2003). Genetic ablation of dividing progenitors may be less susceptible to this criticism, but again assessing behavioral consequences is difficult because inhibition of the dividing precursors is not restricted to the dentate gyrus (Garcia et al. 2004, Saxe et al. 2006). For this reason, virus-based strategies have been developed to prevent neurogenesis exclusively in the dentate gyrus at a specific time in adulthood (Clelland et al. 2009, Jessberger et al. 2009). However, the possibility that postmitotic neurons are affected by the virus and may contribute to behavioral alterations cannot be ruled out. Most recently, inducible genetic approaches to ablate specific populations of adult-generated neurons in a temporally and spatially precise manner have been used (Imayoshi et al. 2008, Revest et al. 2009, Zhang et al. 2008), although these too are not without practical drawbacks.
Immunohistochemical procedures to quantify whether newly born cells labeled with BrdU express IEGs is a complementary noninvasive way in which the role of adult-generated neurons in hippocampal functions has been examined and allows for a comparison of mature versus new populations of neurons (Kee et al. 2007, Ramirez-Amaya et al. 2006, Snyder et al. 2009b, Tashiro et al. 2007). Although examining the expression of IEGs in adult-generated neurons provides an indication of whether recently born neurons participate in functionally relevant hippocampal networks, it does not reveal whether new neurons are necessary for those functions. An additional downside to this approach seems to be the very small number of new neurons that stain for IEGs, even under conditions of environmental stimulation. Because of the shortcomings inherent to each strategy, it is critical that multiple, independent methods be used when examining the role of adult neurogenesis in a specific behavior.
A Possible Role in Learning and Memory
The role of the hippocampus in learning and memory has long been recognized. However, the hippocampus has been associated with a range of learning tasks (e.g., trace conditioning, contextual fear conditioning, social transmission of food preference, spatial navigation, and object recognition, to name a few) that do not readily fall into a single category, making a unifying theory of hippocampal function difficult to pin down. One reason for this may be related to findings from recent studies incorporating a subregional analysis of the hippocampus that suggests a heterogeneous distribution of function within its different subfields (reviewed in Rolls & Kesner 2006) as well as along the septotemporal axis (reviewed in Bannerman et al. 2004, Moser & Moser 1998). Despite this complexity, it has been proposed that learning and memory might require structural changes in the hippocampus (reviewed in Lamprecht & LeDoux 2004, Nottebohm 2002). In support of this, numerous positive correlations between learning and structural plasticity have been demonstrated.
In rodents, a variety of conditions that decrease adult neurogenesis in the dentate gyrus are associated with learning impairments. These include stress, increased levels of circulating glucocorticoids, and aging (Drapeau et al. 2003, Montaron et al. 2006). Similarly, adverse prenatal or early-life experiences produce persistent reductions in neurogenesis and reduced learning abilities in adulthood (Lemaire et al. 2000). Conversely, conditions that increase neurogenesis, such as environmental enrichment and physical exercise, also tend to enhance performance on hippocampal-dependent learning tasks (Kempermann et al. 1997; van Praag et al. 1999, 2005). There are also a number of studies that have found no correlation or even a negative correlation between neurogenesis and learning (reviewed in Leuner et al. 2006b). However, it is important to keep in mind that a positive correlation between the number of new neurons and learning performance implies a relationship between neurogenesis and learning, although not necessarily a causal one. Another issue to consider is that the time course for alterations in cell production may not correspond to changes in learning abilities. For example, it seems unlikely that the production of new cells would have an immediate effect on processes involved in learning because the cells probably require a certain level of differentiation to have an impact on behavior. Perhaps an even more important consideration, and one that is impossible to discount, is the fact that many of the factors known to affect neurogenesis alter other aspects of brain structure, such as dendritic architecture and synapse number. Since these types of changes are also likely to be involved in hippocampal-dependent learning, it is difficult to interpret correlations between new neurons and learning.
Because of the caveats associated with correlative studies, other work has attempted to demonstrate a casual relationship between learning and neurogenesis, but these too have yielded mixed findings (Leuner et al. 2006b). Reducing or blocking hippocampal neurogenesis disrupts various hippocampal-dependent forms of learning and memory (Clelland et al. 2009; Dupret et al. 2008; Garthe et al. 2009; Hernandez-Rabaza et al. 2009; Jessberger et al. 2009; Imayoshi et al. 2008; Madsen et al. 2003; Raber et al. 2004; Rola et al. 2004; Saxe et al. 2006; Shors et al. 2001b, 2002; Snyder et al. 2005; Winocur et al. 2006; Zhang et al. 2008). Recent findings further suggest that the correct differentiation and integration of new neurons may be necessary for acquisition of new information and the recall of memories consolidated in tasks previously performed (Farioli-Vecchioli et al. 2008). However, a large number of studies have failed to demonstrate an involvement of newly generated cells in hippocampal-dependent learning (Hernandez-Rabaza et al. 2009, Jessberger et al. 2009, Shors et al. 2002, Snyder et al. 2005, Zhang et al. 2008), and there is at least one demonstration of enhanced learning following the suppression of neurogenesis (Saxe et al. 2007). These discrepancies can be attributed to numerous factors, including the animal species and strain tested and the method of ablation, as well as the specifics of the design, analysis, and interpretation of the learning paradigm employed (Garthe et al. 2009). Another possibility is that the age of the newborn neurons may be critical in determining their specific involvement in cognitive function. On the one hand, new immature neurons (because of their unique electrophysiological properties) may participate in learning for only a discrete period after their production, thus requiring a specific timing and length of neuron depletion in order to detect a learning deficit. A depletion paradigm that is insufficient in length, uses an inappropriate interval between depletion and training, or is too long, allowing compensatory mechanisms to come into play, could lead to contradictory results. On the other hand, new neurons may be critical for learning only when they are mature and integrated into similar neural networks as preexisting neurons. There is some disagreement regarding the exact age at which this occurs as well as whether the distinct functional properties of adult-born neurons continue to exist upon maturation. Some studies suggest that young mature neurons do not retain their unique electrophysiological properties, eventually becoming part of the same functional population as old mature neurons (Ge et al. 2007, Laplagne et al. 2006). In contrast, results from other work indicate that mature newborn cells show enhanced plasticity—adult-born neurons are more likely to be activated than are older cells during spatial exploration and hippocampal-dependent spatial learning (Ramirez-Amaya et al. 2006, Snyder et al. 2009b) and are also preferentially reactivated during recall of the spatial memory (Kee et al. 2007). A third possibility, and most probable, is that neurons of different ages—young adult, mature adult, and preexisting—may be optimally suited for different functions (Aimone et al. 2009). Indeed, it has been proposed that neurogenesis serves to complement the synaptic plasticity and memory function of older neurons. In this context, new neurons would provide more synapses for learning and added processing abilities (Schinder & Gage 2004).
Numerous other theories about the functions of newborn cells in the hippocampus have been proposed, including the possibility that a rapidly changing population of adult-generated neurons may provide a temporary substrate for memory storage (Gould et al. 1999b). Accordingly, one might predict that the longevity of a new neuron would correspond to the duration of the memory that it supports. However, this is not necessarily the case since learning increases the survival of new neurons in the hippocampus where they remain for at least two months after training (Leuner et al. 2004), well beyond the time when the hippocampus is required for the retention of those memories. These findings do not exclude the possibility that new neurons participate transiently in memory storage or encoding. In fact, computational (Aimone et al. 2009) and experimental (Clelland et al. 2009) work suggests that immature neurons may be critical for creating associations between memories learned close in time (Aimone et al. 2009) and may aid in distinguishing memories closely related in space (Clelland et al. 2009). How these memories are then transferred to the prefrontal cortex, the site of long-term storage and retrieval of memories (reviewed in Frankland & Bontempi 2005), is an open question that highlights the need to investigate the consequences of structural changes in the hippocampus on related brain regions. One possibility is that the learning-induced enhancement of neurogenesis, as well as dendritic spines, alters the excitability or activity of the circuitry between the hippocampus and prefrontal cortex, which in turn alters the structure of the prefrontal cortex in a way that supports memory storage. Regardless, even if new neurons are somehow involved in a time-limited way in memory storage, they must eventually outlive their usefulness, perhaps becoming important for some other, still unknown, function.
A Possible Role in Anxiety and Depression
The involvement of the hippocampus in mood disorders is suggested by magnetic resonance imaging studies demonstrating a small reduction in hippocampal volume in depressed patients (reviewed in Campbell & MacQueen 2004). It has been proposed that reduced neurogenesis contributes to these volumetric changes, as well as the symptoms associated with depression and anxiety, and that the efficacy of antidepressant treatments may depend on their ability to restore neurogenesis to normal levels. These ideas, collectively referred to as the neurogenic hypothesis of depression, have been fueled by the observation in rodents and monkeys that stress, which pre-disposes some individuals to develop anxiety and depression, inhibits hippocampal neurogenesis (see discussion above), and that antidepressants, which are effective in alleviating behavioral symptoms of these disorders, stimulate neurogenesis, at least in adults (Encinas et al. 2006, Hodes et al. 2009, Malberg et al. 2000, Perera et al. 2007). Additional support for a link between adult neurogenesis and affective disorders comes from work examining the effects of focal irradiation on the behavioral effects of antidepressants. Prevention of the antidepressant-induced increase in neurogenesis blocks the ability of these treatments to reduce the behavioral signs of anxiety (Santarelli et al. 2003) or depression (Airan et al. 2007). However, the potential role for adult hippocampal neurogenesis as a mechanism underlying the etiology and treatment of mood disorders remains a matter of intense discussion and investigation (reviewed in Sahay & Hen 2007, Sapolsky 2004). This is in part because some studies have failed to demonstrate that neurogenesis is required for the behavioral effects of antidepressants (Holick et al. 2008, Surget et al. 2008), but also because stressful procedures that reduce neurogenesis do not necessarily cause a depressive phentotype in animals models (Vollmayr et al. 2003). Furthermore, it has not been shown that a disruption of hippocampal neurogenesis, either by using irradiation or antimitotic drugs, enhances susceptibility to stress (Surget et al. 2008) or induces depression-like or anxiety-related behaviors (Airan et al. 2007, Santarelli et al. 2003, Saxe et al. 2006, Shors et al. 2002). One exception to this is a recent study showing that arresting neurogenesis leads to increased anxiety (Revest et al. 2009). Because specific depletion of newborn neurons was accomplished with an inducible transgenic strategy, it is possible that the less-specific ablation methods used in earlier work may have produced confounding behavioral perturbations. Nevertheless, the “neurogenic hypothesis of depression” continues to be controversial. Currently, there is no evidence for differences in the number of proliferating cells in the hippocampus of individuals with depression (Boldrini et al. 2009, Reif et al. 2006), although the possibility remains that other stages of neurogenesis such as survival, differentiation, or integration of new neurons into the existing hippocampal circuitry are affected. Moreover, only one study has demonstrated increased progenitor cell proliferation in depressed patients after antidepressant treatment (Boldrini et al. 2009). Further studies are necessary to determine whether this effect is associated with an improvement of depressive symptoms, a critical issue as most of the patients in this study died from suicide.
Like the role of adult neurogenesis in learning and memory, the specific involvement new neurons might have in the pathogenesis of and recovery from depression is not yet clear. However, given the complexity of mood disorders, it is important to consider that multiple mechanisms are likely to be involved (reviewed in Sahay & Hen 2007). That the anxiolytic effects of some manipulations, such as drugs that directly target the HPA axis, can be achieved even after suppression of hippocampal neurogenesis is at least one indication that neurogenesis-independent mechanisms exist (Surget et al. 2008). These may include other types of structural change (reviewed in McEwen 2005), a possibility that is supported by the demonstration that hippocampal spine synapse loss occurs in animal models of depression (Hajszan et al. 2009). Analogous effects have been observed in the hippocampus of depressed patients (Law et al. 2004).
One additional point worth mentioning is that although the hippocampus has been linked to anxiety-related behavior, lesions of the hippocampus lead to decreased, not increased, levels of anxiety (Bannerman et al. 2004). Thus, under normal conditions, the hippocampus is anxiogenic. How the addition of new neurons that are known to be excitatory to an anxiogenic brain region would lead to less anxiety is a paradox that is not addressed by the neurogenic hypothesis of depression.
A Possible Role in Stress Regulation
A less well known function of the hippocampus is its role as a negative feedback regulator of the HPA axis. The high concentration of adrenal steroid receptors in the hippocampus (reviewed in de Kloet et al. 1998) and the hippocampal projections to the hypothalamus (reviewed in Ulrich-Lai & Herman 2009) provide an indirect link between the hippocampus and regulation of the stress response. Direct evidence comes from numerous studies showing that destruction of the hippocampus prevents the efficient shut-off of the HPA axis and the return of glucocorticoid levels to their basal state after stressor exposure, whereas stimulation of the hippocampus inhibits stress-induced HPA activation (reviewed in Jacobson & Sapolsky 1991, Ulrich-Lai & Herman 2009).
Adult-generated neurons may play a role in regulating the HPA axis as suggested by work examining the effects of adverse early-life experiences on the development of the HPA axis and adult neurogenesis. In both rodents and monkeys, exposure to prenatal stress results in a persistent dampening in the production of new neurons in adulthood (Coe et al. 2003, Lemaire et al. 2000). Likewise, adult rats subjected to maternal separation in early life exhibit reduced neurogenesis (Mirescu et al. 2004). Prenatal and early postnatal stressors have additionally been associated with impaired negative feedback of the HPA axis response to stressful situations during adulthood. Taken together, these studies raise the possibility that adult-generated neurons are involved in hippocampal neuroendocrine function. This idea has been recently tested using transgenic mice in which adult neurogenesis was conditionally suppressed. Compared to wild-type controls, mice lacking adult neurogenesis exhibited greater stress-induced activation of the HPA axis (Schloesser et al. 2009), indicating that newly born neurons may in fact contribute to the ability of the hippocampus to mediate proper inhibitory control over the HPA axis. Again, adult neurogenesis is likely not the sole mechanism involved in HPA axis regulation. Alterations in CA3 dendritic complexity caused by chronic stress have been proposed to make the hippocampus less effective in regulating the HPA axis, which in turn leads to hypersecretion of glucocorticoids, but currently there is only correlative evidence to support this view (reviewed in Conrad 2006).
Other Possible Roles
In addition to the functions already discussed, accumulating evidence suggests that hippocampal neurogenesis may be linked to social behaviors, a function first suggested by work in birds (reviewed in Gheusi et al. 2009; see also Kozorovitskiy & Gould 2004, Mak et al. 2007). Structural plasticity in the hippocampus has been proposed to play a role in a number of neuropsychiatric and neurological disorders. Although a discussion of these is beyond the scope of this review, adult neurogenesis and other forms of structural plasticity have been implicated in addiction, schizophrenia, Alzheimer's, and epilepsy (reviewed in Burke & Barnes 2006, Eisch et al. 2008, Manji et al. 2001, Russo et al. 2009, Swann et al. 2000).
OVERALL CONCLUSIONS AND DIRECTIONS
A number of outstanding issues remain before definitive conclusions about the role of structural plasticity in hippocampal function can be reached. First and foremost, given the various types of structural change that occur within the hippocampus, it is currently impossible to attribute functional alterations to one specific type. Thus, a critical direction for future research will be the development of new techniques or approaches that selectively alter one form of plasticity while sparing the others. If different forms of structural plasticity are linked mechanistically, this might be difficult to achieve. Advances in imaging technologies allowing for the observation of structural changes in behaving animals combined with molecular approaches enabling the manipulation of structural plasticity in vivo will be critical to this endeavor and will help to elucidate the role of specific types of structural change in functions mediated by the hippocampus.
Within the context of adult neurogenesis, most studies to date have focused on quantitative changes. However, it is possible that the qualitative properties of new neurons could be altered instead of, or in addition to, absolute numbers. By analyzing the response properties of young cells to different experiences and by comparing these to mature cells, information can be obtained regarding the contribution of these different populations of neurons to hippocampal functions. Along similar lines, although much has been elucidated about the maturation of young cells, it will be critical to examine whether different aspects of their development are subject to experiential modification. For example, antidepressants have been shown to not only increase neurogenesis but also to impact the maturation and synaptic plasticity of adult-born hippocampal granule cells (Wang et al. 2008). Extending this type of analysis to other factors and experiences that modulate neurogenesis will undoubtedly provide novel insights into how adult-generated cells contribute to hippocampal functions.
It is also becoming increasingly appreciated that the hippocampus is not a homogenous structure and that it exhibits differences in connectivity and function, as well as gene expression, along the longitudinal or septotemporal axis (Bannerman et al. 2004, Leonardo et al. 2006). Whereas the dorsal hippocampus participates in working and spatial memory, the ventral hippocampus is primarily involved in emotional memory and anxiety, and unlike the dorsal region, has connections to amygdala, hypothalamus, and prefrontal cortex structures associated with the HPA axis and emotion (Moser & Moser 1998, Swanson & Cowan 1977). Thus, additional consideration of the distinctions between the dorsal and ventral hippocampus is necessary and may be key to understanding the specific contributions that structural change makes to learning and memory, anxiety, and stress regulation. For instance, some newer work suggests that spatial and associative learning may have a more pronounced effect on the survival and subsequent activation of new cells in the ventral hippocampus (Dalla et al. 2009, Snyder et al. 2009b).
Finally, various forms of structural plasticity have been tied to numerous psychiatric and neurological conditions. Although no definitive link has been established because of obstacles in studying structural plasticity in the human brain, technological advances are on the horizon and should be valuable in this regard. Given that there is now widespread acceptance that the adult brain is capable of undergoing dramatic structural reorganization, it is likely only a matter of time before definitive conclusions are obtained about the precise role of structural plasticity in hippocampal functions under normal and pathological conditions.
ACKNOWLEDGMENTS
The work was supported by a Young Investigator Award from the National Alliance for Research on Schizophrenia and Depression to B.L. and grants from the National Institute of Mental Health to B.L. (K99MH084148) and E.G. (MH54970).
Glossary
- GCL
granule cell layer
- Dendritic spines
protrusions covering the surface of dendrites that are major sites of excitatory synaptic input
- Filopodia
long, thin, dynamic protrusions that are the precursors of dendritic spines
- Neural stem cells
cells that self-renew and are multipotent, giving rise to neurons and glia
- SGZ
subgranular zone
- Progenitor cells
proliferative progeny of stem cells that generate differentiated cells but that have a limited capacity for self-renewal
- Immediate early genes (IEGs)
genes that are rapidly and transiently expressed in response to various cellular stimuli that are often used as indirect markers for neuronal activity
- Gamma-aminobutyric acid (GABA)
major inhibitory neurotransmitter in the adult brain that is excitatory to immature neurons during development and to neural progenitor cells during adult neurogenesis
- BrdU
bromodeoxyuridine
- NeuN
neuronal nuclei
- GFP
green fluorescent protein
- Angiogenesis
the growth of new blood vessels
- HPA
hypothalamic-pituitary-adrenal
- Glucocorticoid
steroid hormone released by the adrenal cortex in response to stress
Footnotes
DISCLOSURE STATEMENT The authors are not aware of any biases that might be perceived as affecting the objectivity of this review.
LITERATURE CITED
- Ackman JB, Siddiqi F, Walikonis RS, LoTurco JJ. Fusion of microglia with pyramidal neurons after retroviral infection. J. Neurosci. 2006;26:11413–22. doi: 10.1523/JNEUROSCI.3340-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aimone JB, Wiles J, Gage FH. Computational influence of adult neurogenesis on memory encoding. Neuron. 2009;61:187–202. doi: 10.1016/j.neuron.2008.11.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Airan RD, Meltzer LA, Roy M, Gong Y, Chen H, Deisseroth K. High-speed imaging reveals neuro-physiological links to behavior in an animal model of depression. Science. 2007;317:819–23. doi: 10.1126/science.1144400. [DOI] [PubMed] [Google Scholar]
- Altman J. Are new neurons formed in the brains of adult mammals? Science. 1962;135:1127–28. doi: 10.1126/science.135.3509.1127. [DOI] [PubMed] [Google Scholar]
- Altman J, Das GD. Autoradiographic and histological evidence of postnatal hippocampal neurogenesis in rats. J. Comp. Neurol. 1965;124:319–35. doi: 10.1002/cne.901240303. [DOI] [PubMed] [Google Scholar]
- Ambrogini P, Cuppini R, Cuppini C, Ciaroni S, Cecchini T, et al. Spatial learning affects immature granule cell survival in the adult dentate gyrus. Neurosci. Lett. 2000;286:21–24. doi: 10.1016/s0304-3940(00)01074-0. [DOI] [PubMed] [Google Scholar]
- Ambrogini P, Orsini L, Mancini C, Ferri P, Ciaroni S, Cuppini R. Learning may reduce neurogenesis in the adult rat dentate gyrus. Neurosci. Lett. 2004;359:13–16. doi: 10.1016/j.neulet.2003.12.123. [DOI] [PubMed] [Google Scholar]
- Amrein I, Dechmann DK, Winter Y, Lipp HP. Absent or low rate of adult neurogenesis in the hippocampus of bats (Chiroptera) PLoS One. 2007;2:e455. doi: 10.1371/journal.pone.0000455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amrein I, Slomianka L, Lipp HP. Granule cell number, cell death and cell proliferation in the dentate gyrus of wild-living rodents. Eur. J. Neurosci. 2004;20:3342–50. doi: 10.1111/j.1460-9568.2004.03795.x. [DOI] [PubMed] [Google Scholar]
- Andersen P, Soleng AF. Long-term potentiation and spatial training are both associated with the generation of new excitatory synapses. Brain Res. Brain Res. Rev. 1998;26:353–59. doi: 10.1016/s0165-0173(97)00042-8. [DOI] [PubMed] [Google Scholar]
- Bain MJ, Dwyer SM, Rusak B. Restraint stress affects hippocampal cell proliferation differently in rat and mice. Neurosci. Lett. 2004;368:7–10. doi: 10.1016/j.neulet.2004.04.096. [DOI] [PubMed] [Google Scholar]
- Bannerman DM, Rawlins JN, McHugh SB, Deacon RM, Yee BK, et al. Regional dissociations within the hippocampus—memory and anxiety. Neurosci. Biobehav. Rev. 2004;28:273–83. doi: 10.1016/j.neubiorev.2004.03.004. [DOI] [PubMed] [Google Scholar]
- Barnea A, Nottebohm F. Seasonal recruitment of hippocampal neurons in adult free-ranging black-capped chickadees. Proc. Natl. Acad. Sci. USA. 1994;91:11217–21. doi: 10.1073/pnas.91.23.11217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauer S, Patterson PH. The cell cycle-apoptosis connection revisited in the adult brain. J. Cell Biol. 2005;171:641–50. doi: 10.1083/jcb.200505072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boldrini M, Underwood MD, Hen R, Rosoklija GB, Dwork AJ, et al. Antidepressants increase neural progenitor cells in the human hippocampus. Neuropsychopharmacology. 2009;34:2376–89. doi: 10.1038/npp.2009.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonhoeffer T, Yuste R. Spine motility. Phenomenology, mechanisms, and function. Neuron. 2002;35:1019–27. doi: 10.1016/s0896-6273(02)00906-6. [DOI] [PubMed] [Google Scholar]
- Brené S, Bjørnebekk A, Aberg E, Mathé AA, Olson L, Werme M. Running is rewarding and antidepressive. Physiol. Behav. 2007;92:136–40. doi: 10.1016/j.physbeh.2007.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruel-Jungerman E, Davis S, Rampon C, Laroche S. Long-term potentiation enhances neurogenesis in the adult dentate gyrus. J. Neurosci. 2006;26:5888–93. doi: 10.1523/JNEUROSCI.0782-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruel-Jungerman E, Laroche S, Rampon C. New neurons in the dentate gyrus are involved in the expression of enhanced long-term memory following environmental enrichment. Eur. J. Neurosci. 2005;21:513–21. doi: 10.1111/j.1460-9568.2005.03875.x. [DOI] [PubMed] [Google Scholar]
- Bull ND, Bartlett PF. The adult mouse hippocampal progenitor is neurogenic but not a stem cell. J. Neurosci. 2005;25:10815–21. doi: 10.1523/JNEUROSCI.3249-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burke SN, Barnes CA. Neural plasticity in the ageing brain. Nat. Rev. Neurosci. 2006;7:30–40. doi: 10.1038/nrn1809. [DOI] [PubMed] [Google Scholar]
- Burns KA, Kuan CY. Low doses of bromo- and iododeoxyuridine produce near-saturation labeling of adult proliferative populations in the dentate gyrus. Eur. J. Neurosci. 2005;21:803–7. doi: 10.1111/j.1460-9568.2005.03907.x. [DOI] [PubMed] [Google Scholar]
- Cameron HA, Gould E. Adult neurogenesis is regulated by adrenal steroids in the dentate gyrus. Neuroscience. 1994;61:203–9. doi: 10.1016/0306-4522(94)90224-0. [DOI] [PubMed] [Google Scholar]
- Cameron HA, McKay RD. Restoring production of hippocampal neurons in old age. Nat. Neurosci. 1999;2:894–97. doi: 10.1038/13197. [DOI] [PubMed] [Google Scholar]
- Cameron HA, McKay RD. Adult neurogenesis produces a large pool of new granule cells in the dentate gyrus. J. Comp. Neurol. 2001;435:406–17. doi: 10.1002/cne.1040. [DOI] [PubMed] [Google Scholar]
- Cameron HA, Woolley CS, McEwen BS, Gould E. Differentiation of newly born neurons and glia in the dentate gyrus of the adult rat. Neuroscience. 1993;56:337–44. doi: 10.1016/0306-4522(93)90335-d. [DOI] [PubMed] [Google Scholar]
- Campbell S, MacQueen G. The role of the hippocampus in the pathophysiology of major depression. J. Psychiatry Neurosci. 2004;29:417–26. [PMC free article] [PubMed] [Google Scholar]
- Chehrehasa F, Meedeniya ACB, Dwyer P, Abrahamsen G, Mackay-Sim A. EdU, a new thymidine analogue for labeling proliferating cells in the nervous system. J. Neurosci. Methods. 2009;177:122–30. doi: 10.1016/j.jneumeth.2008.10.006. [DOI] [PubMed] [Google Scholar]
- Chen Y, Dubé CM, Rice CJ, Baram TZ. Rapid loss of dendritic spines after stress involves derangement of spine dynamics by corticotropin-releasing hormone. J. Neurosci. 2008;28:2903–11. doi: 10.1523/JNEUROSCI.0225-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christie BR, Cameron HA. Neurogenesis in the adult hippocampus. Hippocampus. 2006;16:199–207. doi: 10.1002/hipo.20151. [DOI] [PubMed] [Google Scholar]
- Clelland CD, Choi M, Romberg C, Clemenson GD, Jr, Fragniere A, et al. A functional role for adult hippocampal neurogenesis in spatial pattern separation. Science. 2009;325:210–13. doi: 10.1126/science.1173215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coe CL, Kramer M, Cźeh B, Gould E, Reeves AJ, et al. Prenatal stress diminishes neurogenesis in the dentate gyrus of juvenile rhesus monkeys. Biol. Psychiatry. 2003;54:1025–34. doi: 10.1016/s0006-3223(03)00698-x. [DOI] [PubMed] [Google Scholar]
- Conrad CD. What is the functional significance of chronic stress-induced CA3 dendritic retraction within the hippocampus? Behav. Cogn. Neurosci. Rev. 2006;5:41–60. doi: 10.1177/1534582306289043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Couillard-Despres S, Winner B, Karl C, Lindemann G, Schmid P, et al. Targeted transgene expression in neuronal precursors: watching young neurons in the old brain. Eur. J. Neurosci. 2006;24:1535–45. doi: 10.1111/j.1460-9568.2006.05039.x. [DOI] [PubMed] [Google Scholar]
- Dailey M, Marrs G, Satz J, Waite M. Concepts in imaging and microscopy. Exploring biological structure and function with confocal microscopy. Biol. Bull. 1999;197:115–22. doi: 10.2307/1542608. [DOI] [PubMed] [Google Scholar]
- Dalla C, Bangasser DA, Edgecomb C, Shors TJ. Neurogenesis and learning: acquisition and asymptotic performance predict how many new cells survive in the hippocampus. Neurobiol. Learn. Mem. 2007;88:143–48. doi: 10.1016/j.nlm.2007.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalla C, Papachristos EB, Whetstone AS, Shors TJ. Female rats learn trace memories better than male rats and consequently retain a greater proportion of new neurons in their hippocampi. Proc. Natl. Acad. Sci. USA. 2009;106:2927–32. doi: 10.1073/pnas.0809650106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dayer AG, Ford AA, Cleaver KM, Yassaee M, Cameron HA. Short-term and long-term survival of new neurons in the rat dentate gyrus. J. Comp. Neurol. 2003;460:563–72. doi: 10.1002/cne.10675. [DOI] [PubMed] [Google Scholar]
- de Kloet ER, Vreugdenhil E, Oitzl MS, Joëls M. Brain corticosteroid receptor balance in health and disease. Endocr. Rev. 1998;19:269–301. doi: 10.1210/edrv.19.3.0331. [DOI] [PubMed] [Google Scholar]
- De Roo M, Klauser P, Garcia PM, Poglia L, Muller D. Spine dynamics and synapse remodeling during LTP and memory processes. Prog. Brain Res. 2008;169:199–207. doi: 10.1016/S0079-6123(07)00011-8. [DOI] [PubMed] [Google Scholar]
- Döbrössy MD, Drapeau E, Aurousseau C, Le Moal M, Piazza PV, Abrous DN. Differential effects of learning on neurogenesis: learning increases or decreases the number of newly born cells depending on their birth date. Mol. Psychiatry. 2003;8:974–82. doi: 10.1038/sj.mp.4001419. [DOI] [PubMed] [Google Scholar]
- Drapeau E, Mayo W, Aurousseau C, Le Moal M, Piazza PV, Abrous DN. Spatial memory performances of aged rats in the water maze predict levels of hippocampal neurogenesis. Proc. Natl. Acad. Sci. USA. 2003;100:14385–90. doi: 10.1073/pnas.2334169100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dupret D, Fabre A, Döbrössy MD, Panatier A, Rodríguez JJ, et al. Spatial learning depends on both the addition and removal of new hippocampal neurons. PLoS Biol. 2007;5:e214. doi: 10.1371/journal.pbio.0050214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dupret D, Montaron MF, Drapeau E, Aurousseau C, Le Moal M, et al. Methylazoxymethanol acetate does not fully block cell genesis in the young and aged dentate gyrus. Eur. J. Neurosci. 2005;22:778–83. doi: 10.1111/j.1460-9568.2005.04262.x. [DOI] [PubMed] [Google Scholar]
- Dupret D, Revest JM, Koehl M, Ichas F, De Giorgi F, et al. Spatial relational memory requires hippocampal adult neurogenesis. PLoS ONE. 2008;3:e1959. doi: 10.1371/journal.pone.0001959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eadie BD, Redila VA, Christie BR. Voluntary exercise alters the cytoarchitecture of the adult dentate gyrus by increasing cellular proliferation, dendritic complexity, and spine density. J. Comp. Neurol. 2005;486:39–47. doi: 10.1002/cne.20493. [DOI] [PubMed] [Google Scholar]
- Ehninger D, Kempermann G. Paradoxical effects of learning the Morris water maze on adult hippocampal neurogenesis in mice may be explained by a combination of stress and physical activity. Genes Brain Behav. 2006;5:29–39. doi: 10.1111/j.1601-183X.2005.00129.x. [DOI] [PubMed] [Google Scholar]
- Eisch AJ, Cameron HA, Encinas JM, Meltzer L, Ming GL, Overstreet-Wadiche LS. Adult neurogenesis, mental health, and mental illness: hope or hype? J. Neurosci. 2008;28:11785–91. doi: 10.1523/JNEUROSCI.3798-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisch AJ, Mandyam CD. Adult neurogenesis: Can analysis of cell cycle proteins move us “Beyond BrdU”? Curr. Pharm. Biotechnol. 2007;8:147–65. doi: 10.2174/138920107780906540. [DOI] [PubMed] [Google Scholar]
- Encinas JM, Vaahtokari A, Enikolopov G. Fluoxetine targets early progenitor cells in the adult brain. Proc. Natl. Acad. Sci. USA. 2006;103:8233–38. doi: 10.1073/pnas.0601992103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epp JR, Barker JM, Galea LA. Running wild: neurogenesis in the hippocampus across the lifespan in wild and laboratory-bred Norway rats. Hippocampus. 2009 doi: 10.1002/hipo.20546. In press. [DOI] [PubMed] [Google Scholar]
- Epp JR, Spritzer MD, Galea LA. Hippocampus-dependent learning promotes survival of new neurons in the dentate gyrus at a specific time during cell maturation. Neuroscience. 2007;149:273–85. doi: 10.1016/j.neuroscience.2007.07.046. [DOI] [PubMed] [Google Scholar]
- Eriksson PS, Perfilieva E, Björk-Eriksson T, Alborn AM, Nordborg C, et al. Neurogenesis in the adult human hippocampus. Nat. Med. 1998;4:1313–17. doi: 10.1038/3305. [DOI] [PubMed] [Google Scholar]
- Espósito MS, Piatti VC, Laplagne DA, Morgenstern NA, Ferrari CC, et al. Neuronal differentiation in the adult hippocampus recapitulates embryonic development. J. Neurosci. 2005;25:10074–86. doi: 10.1523/JNEUROSCI.3114-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eyre MD, Richter-Levin G, Avital A, Stewart MG. Morphological changes in hippocampal dentate gyrus synapses following spatial learning in rats are transient. Eur. J. Neurosci. 2003;17:1973–80. doi: 10.1046/j.1460-9568.2003.02624.x. [DOI] [PubMed] [Google Scholar]
- Farioli-Vecchioli S, Saraulli D, Costanzi M, Pacioni S, Cinà I, et al. The timing of differentiation of adult hippocampal neurons is crucial for spatial memory. PLoS Biol. 2008;6:e246. doi: 10.1371/journal.pbio.0060246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiala JC, Feinberg M, Popov V, Harris KM. Synaptogenesis via dendritic filopodia in developing hippocampal area CA1. J. Neurosci. 1998;18:8900–11. doi: 10.1523/JNEUROSCI.18-21-08900.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flood DG. Critical issues in the analysis of dendritic extent in aging humans, primates, and rodents. Neurobiol. Aging. 1993;14:649–54. doi: 10.1016/0197-4580(93)90058-j. [DOI] [PubMed] [Google Scholar]
- Flusberg BA, Nimmerjahn A, Cocker ED, Mukamel EA, Barretto RP. High-speed, miniaturized fluorescence microscopy in freely moving mice. Nat. Methods. 2008;5:935–38. doi: 10.1038/nmeth.1256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frankland PW, Bontempi B. The organization of recent and remote memories. Nat. Rev. Neurosci. 2005;6:119–30. doi: 10.1038/nrn1607. [DOI] [PubMed] [Google Scholar]
- Garcia AD, Doan NB, Imura T, Bush TG, Sofroniew MV. GFAP-expressing progenitors are the principal source of constitutive neurogenesis in adult mouse forebrain. Nat. Neurosci. 2004;7:1233–41. doi: 10.1038/nn1340. [DOI] [PubMed] [Google Scholar]
- Garthe A, Behr J, Kempermann G. Adult-generated hippocampal neurons allow the flexible use of spatially precise learning strategies. PLoS One. 2009;5:e5464. doi: 10.1371/journal.pone.0005464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ge S, Goh EL, Sailor KA, Kitabatake Y, Ming GL, Song H. GABA regulates synaptic integration of newly generated neurons in the adult brain. Nature. 2006;439:589–93. doi: 10.1038/nature04404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ge S, Yang CH, Hsu KS, Ming GL, Song H. A critical period for enhanced synaptic plasticity in newly generated neurons of the adult brain. Neuron. 2007;54:559–66. doi: 10.1016/j.neuron.2007.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geinisman Y, Berry RW, Disterhoft JF, Power JM, Van Der Zee EA. Associative learning elicits the formation of multiple-synapse boutons. J. Neurosci. 2001;21:5568–73. doi: 10.1523/JNEUROSCI.21-15-05568.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geinisman Y, deToledo-Morrell L, Morrell F, Persina IS, Rossi M. Age-related loss of axospinous synapses formed by two afferent systems in the rat dentate gyrus as revealed by the unbiased stereological dissector technique. Hippocampus. 1992;2:437–44. doi: 10.1002/hipo.450020411. [DOI] [PubMed] [Google Scholar]
- Gheusi G, Ortega-Perez I, Murray K, Lledo PM. A niche for adult neurogenesis in social behavior. Behav. Brain Res. 2009;200:315–22. doi: 10.1016/j.bbr.2009.02.006. [DOI] [PubMed] [Google Scholar]
- Globus A, Rosenzweig MR, Bennett EL, Diamond MC. Effects of differential experience on dendritic spine counts in rat cerebral cortex. J. Comp. Physiol. Psychol. 1973;82:175–81. doi: 10.1037/h0033910. [DOI] [PubMed] [Google Scholar]
- Gould E. How widespread is adult neurogenesis in mammals? Nat. Rev. Neurosci. 2007;8:481–88. doi: 10.1038/nrn2147. [DOI] [PubMed] [Google Scholar]
- Gould E, Beylin A, Tanapat P, Reeves AJ, Shors TJ. Learning enhances adult neurogenesis in the hippocampal formation. Nat. Neurosci. 1999b;2:260–65. doi: 10.1038/6365. [DOI] [PubMed] [Google Scholar]
- Gould E, McEwen BS, Tanapat P, Galea LA, Fuchs E. Neurogenesis in the dentate gyrus of the adult tree shrew is regulated by psychosocial stress and NMDA receptor activation. J. Neurosci. 1997;17:2492–98. doi: 10.1523/JNEUROSCI.17-07-02492.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gould E, Reeves AJ, Fallah M, Tanapat P, Gross CG, Fuchs E. Hippocampal neurogenesis in adult Old World primates. Proc. Natl. Acad. Sci. USA. 1999a;96:5263–67. doi: 10.1073/pnas.96.9.5263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gould E, Tanapat P. Lesion-induced proliferation of neuronal progenitors in the dentate gyrus of the adult rat. Neuroscience. 1997;80:427–36. doi: 10.1016/s0306-4522(97)00127-9. [DOI] [PubMed] [Google Scholar]
- Gould E, Tanapat P, McEwen BS, Flügge G, Fuchs E. Proliferation of granule cell precursors in the dentate gyrus of adult monkeys is diminished by stress. Proc. Natl. Acad. Sci. USA. 1998;95:3168–71. doi: 10.1073/pnas.95.6.3168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gould E, Vail N, Wagers M, Gross CG. Adult-generated hippocampal and neocortical neurons in macaques have a transient existence. Proc. Natl. Acad. Sci. USA. 2001;98:10910–17. doi: 10.1073/pnas.181354698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gould E, Woolley CS, Cameron HA, Daniels DC, McEwen BS. Adrenal steroids regulate postnatal development of the rat dentate gyrus: II. Effects of glucocorticoids and mineralocorticoids on cell birth. J. Comp. Neurol. 1991b;313:486–93. doi: 10.1002/cne.903130309. [DOI] [PubMed] [Google Scholar]
- Gould E, Woolley CS, McEwen BS. Naturally occurring cell death in the developing dentate gyrus of the rat. J. Comp. Neurol. 1991a;304:408–18. doi: 10.1002/cne.903040306. [DOI] [PubMed] [Google Scholar]
- Hairston IS, Little MT, Scanlon MD, Barakat MT, Palmer TD, et al. Sleep restriction suppresses neurogenesis induced by hippocampus-dependent learning. J. Neurophysiol. 2005;94:4224–33. doi: 10.1152/jn.00218.2005. [DOI] [PubMed] [Google Scholar]
- Hajszan T, Dow A, Warner-Schmidt JL, Szigeti-Buck K, Sallam NL, et al. Remodeling of hippocampal spine synapses in the rat learned helplessness model of depression. Biol. Psychiatry. 2009;65:392–400. doi: 10.1016/j.biopsych.2008.09.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hancock A, Priester C, Kidder E, Keith JR. Does 5-bromo-2′-deoxyuridine (BrdU) disrupt cell proliferation and neuronal maturation in the adult rat hippocampus in vivo? Behav. Brain Res. 2009;199:218–21. doi: 10.1016/j.bbr.2008.11.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hastings N, Gould E. Rapid extension of axons into the CA3 region by adult-generated granule cells. J. Comp. Neurol. 1999;413:146–54. doi: 10.1002/(sici)1096-9861(19991011)413:1<146::aid-cne10>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- Hastings NB, Seth MI, Tanapat P, Rydel TA, Gould E. Granule neurons generated during development extend divergent axon collaterals to hippocampal area CA3. J. Comp. Neurol. 2002;452:324–33. doi: 10.1002/cne.10386. [DOI] [PubMed] [Google Scholar]
- He J, Crews FT. Neurogenesis decreases during brain maturation from adolescence to adulthood. Pharmacol. Biochem. Behav. 2007;86:327–33. doi: 10.1016/j.pbb.2006.11.003. [DOI] [PubMed] [Google Scholar]
- Heine V, Maslam S, Zareno J, Joëls M, Lucassen PJ. Suppressed proliferation and apoptotic changes in the rat dentate gyrus after acute and chronic stress are reversible. Eur. J. Neurosci. 2004;19:131–44. doi: 10.1046/j.1460-9568.2003.03100.x. [DOI] [PubMed] [Google Scholar]
- Hernández-Rabaza V, Llorens-Martín M, Velázquez-Sánchez C, Ferragud A, Arcusa A, et al. Inhibition of adult hippocampal neurogenesis disrupts contextual learning but spares spatial working memory, long-term conditional rule retention and spatial reversal. Neuroscience. 2009;159:59–68. doi: 10.1016/j.neuroscience.2008.11.054. [DOI] [PubMed] [Google Scholar]
- Hodes GE, Yang L, VanKooy J, Santollo J, Shors TJ. Prozac during puberty: distinctive effects on neurogenesis as a function of age and sex. Neuroscience. 2009;163:609–17. doi: 10.1016/j.neuroscience.2009.06.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holick KA, Lee DC, Hen R, Dulawa SC. Behavioral effects of chronic fluoxetine in BALB/cJ mice do not require adult hippocampal neurogenesis or the serotonin 1A receptor. Neuropsychopharmacology. 2008;33:406–17. doi: 10.1038/sj.npp.1301399. [DOI] [PubMed] [Google Scholar]
- Imayoshi I, Sakamoto M, Ohtsuka T, Takao K, Miyakawa T, et al. Roles of continuous neurogenesis in the structural and functional integrity of the adult forebrain. Nat. Neurosci. 2008;11:1153–61. doi: 10.1038/nn.2185. [DOI] [PubMed] [Google Scholar]
- Jacobson L, Sapolsky R. The role of the hippocampus in feedback regulation of the hypothalamic-pituitary-adrenocortical axis. Endocr. Rev. 1991;12:118–34. doi: 10.1210/edrv-12-2-118. [DOI] [PubMed] [Google Scholar]
- Jessberger S, Aigner S, Clemenson GD, Jr, Toni N, Lie DC, et al. Cdk5 regulates accurate maturation of newborn granule cells in the adult hippocampus. PLoS Biol. 2008b;6:e272. doi: 10.1371/journal.pbio.0060272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jessberger S, Clark RE, Broadbent NJ, Clemson GD, Jr, Consiglio A, et al. Dentate gyrus-specific knockdown of adult neurogenesis impairs spatial and object recognition memory in adult rats. Learn. Mem. 2009;16:147–54. doi: 10.1101/lm.1172609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jessberger S, Toni N, Clemenson GD, Jr, Ray J, Gage FH. Directed differentiation of hippocampal stem/progenitor cells in the adult brain. Nat. Neurosci. 2008a;11:888–93. doi: 10.1038/nn.2148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin K, Wang X, Xie L, Mao XO, Zhu W, et al. Evidence for stroke-induced neurogenesis in the human brain. Proc. Natl. Acad. Sci. USA. 2006;103:13198–202. doi: 10.1073/pnas.0603512103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplan MS, Hinds JW. Neurogenesis in the adult rat: electron microscopic analysis of light radioautographs. Science. 1977;197:1092–94. doi: 10.1126/science.887941. [DOI] [PubMed] [Google Scholar]
- Karten YJ, Jones MA, Jeurling SI, Cameron HA. GABAergic signaling in young granule cells in the adult rat and mouse dentate gyrus. Hippocampus. 2006;16:312–20. doi: 10.1002/hipo.20165. [DOI] [PubMed] [Google Scholar]
- Kee N, Sivalingam S, Boonstra R, Wojtowicz JM. The utility of Ki-67 and BrdU as proliferative markers of adult neurogenesis. J. Neurosci. Methods. 2002;115:97–105. doi: 10.1016/s0165-0270(02)00007-9. [DOI] [PubMed] [Google Scholar]
- Kee N, Teixeira CM, Wang AH, Frankland PW. Preferential incorporation of adult-generated granule cells into spatial memory networks in the dentate gyrus. Nat. Neurosci. 2007;10:355–62. doi: 10.1038/nn1847. [DOI] [PubMed] [Google Scholar]
- Kempermann G, Jessberger S, Steiner B, Kronenberg G. Milestones of neuronal development in the adult hippocampus. Trends Neuroci. 2004;27:447–52. doi: 10.1016/j.tins.2004.05.013. [DOI] [PubMed] [Google Scholar]
- Kempermann G, Kuhn HG, Gage FH. More hippocampal neurons in adult mice living in an enriched environment. Nature. 1997;386:493–95. doi: 10.1038/386493a0. [DOI] [PubMed] [Google Scholar]
- Kempermann G, Kuhn HG, Gage FH. Experience-induced neurogenesis in the senescent dentate gyrus. J. Neurosci. 1998;18:3206–12. doi: 10.1523/JNEUROSCI.18-09-03206.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klempin F, Kempermann G. Adult hippocampal neurogenesis and aging. Eur. Arch. Psychiatry Clin. Neurosci. 2007;257:271–80. doi: 10.1007/s00406-007-0731-5. [DOI] [PubMed] [Google Scholar]
- Knafo S, Ariav G, Barkai E, Libersat F. Olfactory learning-induced increase in spine density along the apical dendrites of CA1 hippocampal neurons. Hippocampus. 2004;14:819–25. doi: 10.1002/hipo.10219. [DOI] [PubMed] [Google Scholar]
- Koo JW, Duman RS. IL-1beta is an essential mediator of the antineurogenic and anhedonic effects of stress. Proc. Natl. Acad. Sci. USA. 2008;105:751–56. doi: 10.1073/pnas.0708092105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozorovitskiy Y, Gould E. Dominance hierarchy influences adult neurogenesis in the dentate gyrus. J. Neurosci. 2004;24:6755–59. doi: 10.1523/JNEUROSCI.0345-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozorovitskiy Y, Gross CG, Kopil C, Battaglia L, McBreen M, et al. Experience induces structural and biochemical changes in the adult primate brain. Proc. Natl. Acad. Sci. USA. 2005;102:17478–82. doi: 10.1073/pnas.0508817102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuhn HG, Dickinson-Anson H, Gage FH. Neurogenesis in the dentate gyrus of the adult rat: age-related decrease of neuronal progenitor proliferation. J. Neurosci. 1996;16:2027–33. doi: 10.1523/JNEUROSCI.16-06-02027.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lagace DC, Benavides DR, Kansy JW, Mapelli M, Greengard P, et al. Cdk5 is essential for adult hippocampal neurogenesis. Proc. Natl. Acad. Sci. USA. 2008;105:18567–71. doi: 10.1073/pnas.0810137105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamprecht R, LeDoux J. Structural plasticity and memory. Nat. Rev. Neurosci. 2004;5:45–54. doi: 10.1038/nrn1301. [DOI] [PubMed] [Google Scholar]
- Laplagne DA, Espósito MS, Piatti VC, Morgenstern NA, Zhao C, et al. Functional convergence of neurons generated in the developing and adult hippocampus. PLoS Biol. 2006;4:e409. doi: 10.1371/journal.pbio.0040409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Law AJ, Weickert CS, Hyde TM, Kleinman JE, Harrison PJ. Reduced spinophilin but not microtubule-associated protein 2 expression in the hippocampal formation in schizophrenia and mood disorders: molecular evidence for a pathology of dendritic spines. Am. J. Psychiatry. 2004;161:1848–55. doi: 10.1176/ajp.161.10.1848. [DOI] [PubMed] [Google Scholar]
- Lemaire V, Koehl M, LeMoal M, Abrous DN. Prenatal stress produces learning deficits associated with an inhibition of neurogenesis in the hippocampus. Proc. Natl. Acad. Sci. USA. 2000;97:11032–37. doi: 10.1073/pnas.97.20.11032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leonardo ED, Richardson-Jones JW, Sibille E, Kottman A, Hen R. Molecular heterogeneity along the dorsal-ventral axis of the murine hippocampal CA1 field: a microarray analysis of gene expression. Neuroscience. 2006;137:177–86. doi: 10.1016/j.neuroscience.2005.08.082. [DOI] [PubMed] [Google Scholar]
- Leuner B, Falduto J, Shors TJ. Associative memory formation increases the observation of dendritic spines in the hippocampus. J. Neurosci. 2003;23:659–65. doi: 10.1523/JNEUROSCI.23-02-00659.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leuner B, Glasper ER, Gould E. Thymidine analog methods for studies of adult neurogenesis are not equally sensitive. J. Comp. Neurol. 2009;517:123–33. doi: 10.1002/cne.22107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leuner B, Gould E, Shors TJ. Is there a link between adult neurogenesis and learning? Hippocampus. 2006b;16:216–24. doi: 10.1002/hipo.20153. [DOI] [PubMed] [Google Scholar]
- Leuner B, Kozorovitskiy Y, Gross CG, Gould E. Diminished adult neurogenesis in the marmoset brain precedes old age. Proc. Natl. Acad. Sci. USA. 2007a;104:17169–73. doi: 10.1073/pnas.0708228104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leuner B, Mendolia-Loffredo S, Kozorovitskiy Y, Samburg D, Gould E, Shors TJ. Learning enhances the survival of new neurons beyond the time when the hippocampus is required for memory. J. Neurosci. 2004;24:7477–81. doi: 10.1523/JNEUROSCI.0204-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leuner B, Mirescu C, Noiman L, Gould E. Maternal experience inhibits the production of immature neurons in the hippocampus during the postpartum period through elevations in adrenal steroids. Hippocampus. 2007b;17:434–42. doi: 10.1002/hipo.20278. [DOI] [PubMed] [Google Scholar]
- Leuner B, Waddell J, Gould E, Shors TJ. Temporal discontiguity is neither necessary nor sufficient for learning-induced effects on adult neurogenesis. J. Neurosci. 2006a;26:13437–42. doi: 10.1523/JNEUROSCI.2781-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu XB, Low LK, Jones EG, Cheng HJ. Stereotyped axon pruning via plexin signaling is associated with synaptic complex elimination in the hippocampus. J. Neurosci. 2005;25:9124–34. doi: 10.1523/JNEUROSCI.2648-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucassen PJ, Bosch OJ, Jousma E, Krömer SA, Andrew R, et al. Prenatal stress reduces postnatal neurogenesis in rats selectively bred for high, but not low, anxiety: possible key role of placental 11bet-ahydroxysteroid dehydrogenase type 2. Eur. J. Neurosci. 2009;29:97–103. doi: 10.1111/j.1460-9568.2008.06543.x. [DOI] [PubMed] [Google Scholar]
- Madsen TM, Kristjansen PEG, Bolwig TG, Wortwein G. Arrested neuronal proliferation and impaired hippocampal function following fractionated brain irradiation in the adult rat. Neuroscience. 2003;119:635–42. doi: 10.1016/s0306-4522(03)00199-4. [DOI] [PubMed] [Google Scholar]
- Magariños AM, McEwen BS. Stress-induced atrophy of apical dendrites of hippocampal CA3c neurons: involvement of glucocorticoid secretion and excitatory amino acid receptors. Neuroscience. 1995;69:89–98. doi: 10.1016/0306-4522(95)00259-l. [DOI] [PubMed] [Google Scholar]
- Magariños AM, McEwen BS, Flügge G, Fuchs E. Chronic psychosocial stress causes apical dendritic atrophy of hippocampal CA3 pyramidal neurons in subordinate tree shrews. J. Neurosci. 1996;16:3534–40. doi: 10.1523/JNEUROSCI.16-10-03534.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mak G, Enwere EK, Gregg C, Pakarainen T, Poutanen M, et al. Male-pheromone-stimulated neurogenesis in the adult female brain: possible role in mating behavior. Nat. Neurosci. 2007;10:1003–11. doi: 10.1038/nn1928. [DOI] [PubMed] [Google Scholar]
- Malberg JE, Duman RS. Cell proliferation in adult hippocampus is decreased by inescapable stress: reversal by fluoxetine treatment. Neuropsychopharmacology. 2003;28:1562–71. doi: 10.1038/sj.npp.1300234. [DOI] [PubMed] [Google Scholar]
- Malberg JE, Eisch AJ, Nestler EJ, Duman RS. Chronic antidepressant treatment increases neurogenesis in adult rat hippocampus. J. Neurosci. 2000;20:9104–10. doi: 10.1523/JNEUROSCI.20-24-09104.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manganas LN, Zhang X, Li Y, Hazel RD, Smith SD, et al. Magnetic resonance spectroscopy identifies neural progenitor cells in the live human brain. Science. 2007;318:980–85. doi: 10.1126/science.1147851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manji HK, Drevets WC, Charney DS. The cellular neurobiology of depression. Nat. Med. 2001;7:541–47. doi: 10.1038/87865. [DOI] [PubMed] [Google Scholar]
- Markakis E, Gage F. Adult-generated neurons in the dentate gyrus send axonal projections to field CA3 and are surrounded by synaptic vesicles. J. Comp. Neurol. 1999;406:449–60. [PubMed] [Google Scholar]
- Mayer JL, Klumpers L, Maslam S, de Kloet ER, Joëls M, Lucassen PJ. Brief treatment with the glucocorticoid receptor antagonist mifepristone normalises the corticosterone-induced reduction of adult hippocampal neurogenesis. J. Neuroendocrinol. 2006;18:629–31. doi: 10.1111/j.1365-2826.2006.01455.x. [DOI] [PubMed] [Google Scholar]
- McEwen BS. Glucocorticoids, depression, and mood disorders: structural remodeling in the brain. Metabolism. 2005;54:20–23. doi: 10.1016/j.metabol.2005.01.008. [DOI] [PubMed] [Google Scholar]
- McKittrick CR, Magariños AM, Blanchard DC, Blanchard RJ, McEwen BS, Sakai RR. Chronic social stress reduces dendritic arbors in CA3 of hippocampus and decreases binding to serotonin transporter sites. Synapse. 2000;36:85–94. doi: 10.1002/(SICI)1098-2396(200005)36:2<85::AID-SYN1>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- Miranda R, Blanco E, Begega A, Santín LJ, Arias JL. Reversible changes in hippocampal CA1 synapses associated with water maze training in rats. Synapse. 2006;59:177–81. doi: 10.1002/syn.20229. [DOI] [PubMed] [Google Scholar]
- Mirescu C, Gould E. Stress and adult neurogenesis. Hippocampus. 2006;16:233–38. doi: 10.1002/hipo.20155. [DOI] [PubMed] [Google Scholar]
- Mirescu C, Peters JD, Gould E. Early life experience alters response of adult neurogenesis to stress. Nat. Neurosci. 2004;7:841–46. doi: 10.1038/nn1290. [DOI] [PubMed] [Google Scholar]
- Mizrahi A, Crowley JC, Shtoyerman E, Katz LC. High-resolution in vivo imaging of hippocampal dendrites and spines. J. Neurosci. 2004;24:3147–51. doi: 10.1523/JNEUROSCI.5218-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohapel P, Mundt-Petersen K, Brundin P, Frielingsdorf H. Working memory training decreases hippocampal neurogenesis. Neuroscience. 2006;142:609–13. doi: 10.1016/j.neuroscience.2006.07.033. [DOI] [PubMed] [Google Scholar]
- Monje ML, Toda H, Palmer TD. Inflammatory blockade restores adult hippocampal neurogenesis. Science. 2003;302:1760–65. doi: 10.1126/science.1088417. [DOI] [PubMed] [Google Scholar]
- Montaron MF, Drapeau E, Dupret D, Kitchener P, Aurousseau C, et al. Lifelong corticosterone level determines age-related decline in neurogenesis and memory. Neurobiol. Aging. 2006;27:645–54. doi: 10.1016/j.neurobiolaging.2005.02.014. [DOI] [PubMed] [Google Scholar]
- Morgenstern NA, Lombardi G, Schinder AF. Newborn granule cells in the ageing dentate gyrus. J. Physiol. 2008;586:3751–57. doi: 10.1113/jphysiol.2008.154807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moser MB, Moser EI. Functional differentiation in the hippocampus. Hippocampus. 1998;6:608–19. doi: 10.1002/(SICI)1098-1063(1998)8:6<608::AID-HIPO3>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- Moser MB, Trommald M, Andersen P. An increase in dendritic spine density on hippocampal CA1 pyramidal cells following spatial learning in adult rats suggests the formation of new synapses. Proc. Natl. Acad. Sci. USA. 1994;91:12673–75. doi: 10.1073/pnas.91.26.12673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nimchinsky EA, Sabatini BL, Svoboda K. Structure and function of dendritic spines. Annu. Rev. Physiol. 2002;64:313–53. doi: 10.1146/annurev.physiol.64.081501.160008. [DOI] [PubMed] [Google Scholar]
- Nottebohm F. Why are some neurons replaced in adult brain? J. Neurosci. 2002;22:624–28. doi: 10.1523/JNEUROSCI.22-03-00624.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Brien JA, Lummis SC. Diolistic labeling of neuronal cultures and intact tissue using a hand-held gene gun. Nat. Protoc. 2006;1:1517–21. doi: 10.1038/nprot.2006.258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olariu A, Cleaver KM, Cameron HA. Decreased neurogenesis in aged rats results from loss of granule cell precursors without lengthening of the cell cycle. J. Comp. Neurol. 2007;501:659–67. doi: 10.1002/cne.21268. [DOI] [PubMed] [Google Scholar]
- Olariu A, Cleaver KM, Shore LE, Brewer MD, Cameron HA. A natural form of learning can increase and decrease the survival of new neurons in the dentate gyrus. Hippocampus. 2005;15:750–62. doi: 10.1002/hipo.20097. [DOI] [PubMed] [Google Scholar]
- O'Malley A, O'Connell C, Murphy KJ, Regan CM. Transient spine density increases in the mid-molecular layer of hippocampal dentate gyrus accompany consolidation of a spatial learning task in the rodent. Neuroscience. 2000;99:229–32. doi: 10.1016/s0306-4522(00)00182-2. [DOI] [PubMed] [Google Scholar]
- O'Malley A, O'Connell C, Regan CM. Ultrastructural analysis reveals avoidance conditioning to induce a transient increase in hippocampal dentate spine density in the 6 hour post-training period of consolidation. Neuroscience. 1998;87:607–13. doi: 10.1016/s0306-4522(98)00178-x. [DOI] [PubMed] [Google Scholar]
- Oomen CA, Mayer JL, de Kloet ER, Joëls M, Lucassen PJ. Brief treatment with the glucocorticoid receptor antagonist mifepristone normalizes the reduction in neurogenesis after chronic stress. Eur. J. Neurosci. 2007;2612:3395–401. doi: 10.1111/j.1460-9568.2007.05972.x. [DOI] [PubMed] [Google Scholar]
- Overstreet LS, Hentges ST, Bumaschny VF, de Souza FS, Smart JL, et al. A transgenic marker for newly born granule cells in dentate gyrus. J. Neurosci. 2004;24:3251–59. doi: 10.1523/JNEUROSCI.5173-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Overstreet-Wadiche LS, Westbrook GL. Functional maturation of adult-generated granule cells. Hippocampus. 2006;16:208–15. doi: 10.1002/hipo.20152. [DOI] [PubMed] [Google Scholar]
- Pereira AC, Huddleston DE, Brickman AM, Sosunov AA, Hen R, et al. An in vivo correlate of exercise-induced neurogenesis in the adult dentate gyrus. Proc. Natl. Acad. Sci. USA. 2007;104:5638–43. doi: 10.1073/pnas.0611721104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perera TD, Coplan JD, Lisanby SH, Lipira CM, Arif M, et al. Antidepressant-induced neurogenesis in the hippocampus of adult nonhuman primates. J. Neurosci. 2007;27:4894–901. doi: 10.1523/JNEUROSCI.0237-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pham K, Nacher J, Hof PR, McEwen BS. Repeated restraint stress suppresses neurogenesis and induces biphasic PSA-NCAM expression in the adult rat dentate gyrus. Eur. J. Neurosci. 2003;7:879–86. doi: 10.1046/j.1460-9568.2003.02513.x. [DOI] [PubMed] [Google Scholar]
- Pokorný J, Yamamoto Postnatal ontogenesis of hippocampal CA1 area in rats. II. Development of ultrastructure in stratum lacunosum and moleculare. Brain Res. Bull. 1981;7:121–30. doi: 10.1016/0361-9230(81)90076-9. [DOI] [PubMed] [Google Scholar]
- Raber J, Rola R, LeFevour A, Morhardt D, Curley J, et al. Radiation induced cognitive impairments are associated with changes in indicators of hippocampal neurogenesis. Radiat. Res. 2004;162:39–47. doi: 10.1667/rr3206. [DOI] [PubMed] [Google Scholar]
- Rahimi O, Claiborne BJ. Morphological development and maturation of granule neuron dendrites in the rat dentate gyrus. Prog. Brain Res. 2007;163:167–81. doi: 10.1016/S0079-6123(07)63010-6. [DOI] [PubMed] [Google Scholar]
- Ramirez-Amaya V, Marrone DF, Gage FH, Worley PF, Barnes CA. Integration of new neurons into functional neural networks. J. Neurosci. 2006;26:12237–41. doi: 10.1523/JNEUROSCI.2195-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reif A, Fritzen S, Finger M, Strobel A, Lauer M, et al. Neural stem cell proliferation is decreased in schizophrenia, but not in depression. Mol. Psychiatry. 2006;11:514–22. doi: 10.1038/sj.mp.4001791. [DOI] [PubMed] [Google Scholar]
- Revest JM, Dupret D, Koehl M, Funk-Reiter C, Grosjean N, et al. Adult hippocampal neurogenesis is involved in anxiety-related behaviors. Mol. Psychiatry. 2009 doi: 10.1038/mp.2009.15. In press. [DOI] [PubMed] [Google Scholar]
- Ribak CE, Shapiro LA. Dendritic development of newly generated neurons in the adult brain. Brain. Res Rev. 2007;55:390–94. doi: 10.1016/j.brainresrev.2006.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rola R, Raber J, Rizk A, Otsuka S, VandenBerg SR, et al. Radiation-induced impairment of hippocampal neurogenesis is associated with cognitive deficits in young mice. Exp. Neurol. 2004;188:316–30. doi: 10.1016/j.expneurol.2004.05.005. [DOI] [PubMed] [Google Scholar]
- Rolls ET, Kesner RP. A computational theory of hippocampal function, and empirical tests of the theory. Prog. Neurobiol. 2006;79:1–48. doi: 10.1016/j.pneurobio.2006.04.005. [DOI] [PubMed] [Google Scholar]
- Rusakov DA, Davies HA, Harrison E, Diana G, Richter-Levin G, et al. Ultrastructural synaptic correlates of spatial learning in rat hippocampus. Neuroscience. 1997;80:69–77. doi: 10.1016/s0306-4522(97)00125-5. [DOI] [PubMed] [Google Scholar]
- Russo SJ, Mazei-Robison MS, Ables JL, Nestler EJ. Neurotrophic factors and structural plasticity in addiction. Neuropharmacology. 2009;56:73–82. doi: 10.1016/j.neuropharm.2008.06.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sahay A, Hen R. Adult hippocampal neurogenesis in depression. Nat. Neurosci. 2007;10:1110–15. doi: 10.1038/nn1969. [DOI] [PubMed] [Google Scholar]
- Salic A, Mitchinson TJ. A chemical method for fast and sensitive detection of DNA synthesis in vivo. proc. Natl. Acad. Sci. USA. 2008;105:2415–20. doi: 10.1073/pnas.0712168105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santarelli L, Saxe M, Gross C, Surget A, Battaglia F, et al. Requirement of hippocampal neurogenesis for the behavioral effects of antidepressants. Science. 2003;301:805–9. doi: 10.1126/science.1083328. [DOI] [PubMed] [Google Scholar]
- Sapolsky RM. Is impaired neurogenesis relevant to the affective symptoms of depression? Biol. Psychiatry. 2004;56:137–39. doi: 10.1016/j.biopsych.2004.04.012. [DOI] [PubMed] [Google Scholar]
- Saxe MD, Battaglia F, Wang JW, Malleret G, David DJ, et al. Ablation of hippocampal neurogenesis impairs contextual fear conditioning and synaptic plasticity in the dentate gyrus. Proc. Natl. Acad. Sci. USA. 2006;103:17501–6. doi: 10.1073/pnas.0607207103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saxe MD, Malleret G, Vronskaya S, Mendez I, Garcia AD, et al. Paradoxical influence of hippocampal neurogenesis on working memory. Proc. Natl. Acad. Sci. USA. 2007;104:4642–46. doi: 10.1073/pnas.0611718104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schinder AF, Gage FH. A hypothesis about the role of adult neurogenesis in hippocampal function. Physiology (Bethesda) 2004;19:253–61. doi: 10.1152/physiol.00012.2004. [DOI] [PubMed] [Google Scholar]
- Schlessinger AR, Cowan WM, Gottlieb DI. An autoradiographic study of the time of origin and the pattern of granule cell migration in the dentate gyrus of the rat. J. Comp. Neurol. 1975;159:149–75. doi: 10.1002/cne.901590202. [DOI] [PubMed] [Google Scholar]
- Schloesser RJ, Manji HK, Martinowich K. Suppression of adult neurogenesis leads to increased hypothalamo-pituitary-adrenal axis response. Neuroreport. 2009;20:553–57. doi: 10.1097/WNR.0b013e3283293e59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt-Hieber C, Jonas P, Bischofberger J. Enhanced synaptic plasticity in newly generated granule cells of the adult hippocampus. Nature. 2004;429:184–87. doi: 10.1038/nature02553. [DOI] [PubMed] [Google Scholar]
- Schroeder T. Imaging stem-cell-driven regeneration in mammals. Nature. 2008;453:345–51. doi: 10.1038/nature07043. [DOI] [PubMed] [Google Scholar]
- Seress L. Comparative anatomy of the hippocampal dentate gyrus in adult and developing rodents, nonhuman primates and humans. Prog. Brain Res. 2007;163:23–41. doi: 10.1016/S0079-6123(07)63002-7. [DOI] [PubMed] [Google Scholar]
- Seress L, Ribak CE. Postnatal development of CA3 pyramidal neurons and their afferents in the Ammon's horn of rhesus monkeys. Hippocampus. 1995;5:217–31. doi: 10.1002/hipo.450050308. [DOI] [PubMed] [Google Scholar]
- Seri B, García-Verdugo JM, McEwen BS, Alvarez-Buylla A. Astrocytes give rise to new neurons in the adult mammalian hippocampus. J. Neurosci. 2001;21:7153–60. doi: 10.1523/JNEUROSCI.21-18-07153.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selye H. The Stress of Life. McGraw-Hill; New York: 1976. [Google Scholar]
- Shors TJ. From stem cells to grandmother cells: how neurogenesis relates to learning and memory. Cell Stem Cell. 2008;3:253–58. doi: 10.1016/j.stem.2008.08.010. [DOI] [PubMed] [Google Scholar]
- Shors TJ, Chua C, Falduto J. Sex differences and opposite effects of stress on dendritic spine density in the male versus female hippocampus. J. Neurosci. 2001a;21:6292–97. doi: 10.1523/JNEUROSCI.21-16-06292.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shors TJ, Mathew J, Sisti HM, Edgecomb C, Beckoff S, Dalla C. Neurogenesis and helplessness are mediated by controllability in males but not in females. Biol. Psychiatry. 2007;62:487–95. doi: 10.1016/j.biopsych.2006.10.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shors TJ, Miesagaes G, Beylin A, Zhao M, Rydel T, Gould E. Neurogenesis in the adult rat is involved in the formation of trace memories. Nature. 2001b;410:372–76. doi: 10.1038/35066584. [DOI] [PubMed] [Google Scholar]
- Shors TJ, Townsend DA, Zhao M, Kozorovitskiy Y, Gould E. Neurogenesis may relate to some but not all types of hippocampal-dependent learning. Hippocampus. 2002;12:578–84. doi: 10.1002/hipo.10103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon M, Czéh B, Fuchs E. Age-dependent susceptibility of adult hippocampal cell proliferation to chronic psychosocial stress. Brain Res. 2005;1049:244–48. doi: 10.1016/j.brainres.2005.05.006. [DOI] [PubMed] [Google Scholar]
- Snyder JS, Kee N, Wojtowicz JM. Effects of adult neurogenesis on synaptic plasticity in the rat dentate gyrus. J. Neurophysiol. 2001;85:2423–31. doi: 10.1152/jn.2001.85.6.2423. [DOI] [PubMed] [Google Scholar]
- Snyder JS, Glover LR, Sanzone KM, Kamhi JF, Cameron HA. The effects of exercise and stress on the survival and maturation of adult-generated granule cells. Hippocampus. 2009a doi: 10.1002/hipo.20552. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snyder JS, Hong NS, McDonald RJ, Wojtowicz JM. A role for adult neurogenesis in spatial long-term memory. Neuroscience. 2005;130:843–52. doi: 10.1016/j.neuroscience.2004.10.009. [DOI] [PubMed] [Google Scholar]
- Snyder JS, Radik R, Wojtowicz JM, Cameron HA. Anatomical gradients of adult neurogenesis and activity: Young neurons in the ventral dentate gyrus are activated by water maze training. Hippocampus. 2009b;19:360–70. doi: 10.1002/hipo.20525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sousa N, Lukoyanov NV, Madeira MD, Almeida OF, Paula-Barbosa MM. Reorganization of the morphology of hippocampal neurites and synapses after stress-induced damage correlates with behavioral improvement. Neuroscience. 2000;97:253–66. doi: 10.1016/s0306-4522(00)00050-6. [DOI] [PubMed] [Google Scholar]
- Stewart MG, Davies HA, Sandi C, Kraev IV, Rogachevsky VV, et al. Stress suppresses and learning induces plasticity in CA3 of rat hippocampus: a three-dimensional ultrastructural study of thorny excrescences and their postsynaptic densities. Neuroscience. 2005;131:43–54. doi: 10.1016/j.neuroscience.2004.10.031. [DOI] [PubMed] [Google Scholar]
- Stranahan A, Kahlil D, Gould E. Social isolation delays the positive effects of running on adult neurogenesis. Nat. Neurosci. 2006;9:526–33. doi: 10.1038/nn1668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stranahan A, Kahlil D, Gould E. Running induces widespread structural alterations in the hippocampus and entorhinal cortex. Hippocampus. 2007;17:1017–22. doi: 10.1002/hipo.20348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Surget A, Saxe M, Leman S, Ibarguen-Vargas Y, Chalon S, et al. Drug-dependent requirement of hippocampal neurogenesis in a model of depression and of antidepressant reversal. Biol. Psychiatry. 2008;64:293–301. doi: 10.1016/j.biopsych.2008.02.022. [DOI] [PubMed] [Google Scholar]
- Swann JW, Al-Noori S, Jiang M, Lee CL. Spine loss and other dendritic abnormalities in epilepsy. Hippocampus. 2000;10:617–25. doi: 10.1002/1098-1063(2000)10:5<617::AID-HIPO13>3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- Swanson LW, Cowan WM. An autoradiographic study of the organization of the efferent connections of the hippocampal formation in the rat. J. Comp. Neurol. 1977;172:49–84. doi: 10.1002/cne.901720104. [DOI] [PubMed] [Google Scholar]
- Tanapat P, Galea LA, Gould L. Stress inhibits the proliferation of granule cell precursors in the developing dentate gyrus. Int. J. Dev. Neurosci. 1998;16:235–39. doi: 10.1016/s0736-5748(98)00029-x. [DOI] [PubMed] [Google Scholar]
- Tanapat P, Hastings NB, Rydel TA, Galea LA, Gould E. Exposure to fox odor inhibits cell proliferation in the hippocampus of adult rats via an adrenal hormone-dependent mechanism. J. Comp. Neurol. 2001;437:496–504. doi: 10.1002/cne.1297. [DOI] [PubMed] [Google Scholar]
- Tashiro A, Makino H, Gage FH. Experience-specific functional modification of the dentate gyrus through adult neurogenesis: a critical period during an immature stage. J. Neurosci. 2007;27:3252–59. doi: 10.1523/JNEUROSCI.4941-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tashiro A, Zhao C, Gage FH. Retrovirus-mediated single-cell gene knockout technique in adult newborn neurons in vivo. Nat. Protoc. 2006;1:3049–55. doi: 10.1038/nprot.2006.473. [DOI] [PubMed] [Google Scholar]
- Thomas RM, Hotsenpiller G, Peterson DA. Acute psychosocial stress reduces cell survival in adult hippocampal neurogenesis without altering proliferation. J. Neurosci. 2007;27:2734–43. doi: 10.1523/JNEUROSCI.3849-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toni N, Laplagne DA, Zhao C, Lombardi G, Ribak CE, et al. Neurons born in the adult dentate gyrus form functional synapses with target cells. Nat. Neurosci. 2008;11:901–7. doi: 10.1038/nn.2156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toni N, Teng EM, Bushong EA, Aimone JB, Zhao C, et al. Synapse formation on neurons born in the adult hippocampus. Nat. Neurosci. 2007;10:727–34. doi: 10.1038/nn1908. [DOI] [PubMed] [Google Scholar]
- Ulrich-Lai YM, Herman JP. Neural regulation of endocrine and autonomic stress responses. Nat. Rev. Neurosci. 2009;10:397–409. doi: 10.1038/nrn2647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Der Borght K, Meerlo P, Luiten PG, Eggen BJ, Van Der Zee EA. Effects of active shock avoidance learning on hippocampal neurogenesis and plasma levels of corticosterone. Behav. Brain Res. 2005a;157:23–30. doi: 10.1016/j.bbr.2004.06.004. [DOI] [PubMed] [Google Scholar]
- Van Der Borght K, Wallinga AE, Luiten PG, Eggen BJ, Van Der Zee EA. Morris water maze learning in two rat strains increases the expression of the polysialylated form of the neural cell adhesion molecule in the dentate gyrus but has no effect on hippocampal neurogenesis. Behav. Neurosci. 2005b;119:926–32. doi: 10.1037/0735-7044.119.4.926. [DOI] [PubMed] [Google Scholar]
- van Hooijdonk LW, Ichwan M, Dijkmans TF, Schouten TG, de Backer MW, et al. Lentivirus-mediated transgene delivery to the hippocampus reveals subfield specific differences in expression. BMC Neurosci. 2009;10:2. doi: 10.1186/1471-2202-10-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Praag H, Kempermann G, Gage FH. Running increases cell proliferation and neurogenesis in the adult mouse dentate gyrus. Nat. Neurosci. 1999;2:266–70. doi: 10.1038/6368. [DOI] [PubMed] [Google Scholar]
- van Praag H, Kempermann G, Gage FH. Neural consequences of environmental enrichment. Nat. Rev. Neurosci. 2000;1:191–98. doi: 10.1038/35044558. [DOI] [PubMed] [Google Scholar]
- van Praag H, Schinder AF, Christie BR, Toni N, Palmer TD, Gage FH. Functional neurogenesis in the adult hippocampus. Nature. 2002;415:1030–34. doi: 10.1038/4151030a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Praag H, Shubert T, Zhao C, Gage FH. Exercise enhances learning and hippocampal neurogenesis in aged mice. J. Neurosci. 2005;25:8680–85. doi: 10.1523/JNEUROSCI.1731-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veenema AH, de Kloet ER, de Wilde MC, Roelofs AJ, Kawata M, et al. Differential effects of stress on adult hippocampal cell proliferation in low and high aggressive mice. J. Neuroendocrinol. 2007;19:489–98. doi: 10.1111/j.1365-2826.2007.01555.x. [DOI] [PubMed] [Google Scholar]
- Vega CJ, Peterson DA. Stem cell proliferative history in tissue revealed by temporal halogenated thymidine analog discrimination. Nat. Methods. 2005;2:167–69. doi: 10.1038/nmeth741. [DOI] [PubMed] [Google Scholar]
- Vollmayr B, Simonis C, Weber S, Gass P, Henn F. Reduced cell proliferation in the dentate gyrus is not correlated with the development of learned helplessness. Biol. Psychiatry. 2003;54:1035–40. doi: 10.1016/s0006-3223(03)00527-4. [DOI] [PubMed] [Google Scholar]
- Waddell J, Shors TJ. Neurogenesis, learning and associative strength. Eur. J. Neurosci. 2008;27:3020–28. doi: 10.1111/j.1460-9568.2008.06222.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker TL, White A, Black DM, Wallace RH, Sah P, Bartlett PF. Latent stem and progenitor cells in the hippocampus are activated by neural excitation. J. Neurosci. 2008;28:5240–47. doi: 10.1523/JNEUROSCI.0344-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang JW, David DJ, Monckton JE, Battaglia F, Hen R. Chronic fluoxetine stimulates maturation and synaptic plasticity of adult-born hippocampal granule cells. J. Neurosci. 2008;28:1374–84. doi: 10.1523/JNEUROSCI.3632-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang S, Scott BW, Wojtowicz JM. Heterogeneous properties of dentate granule neurons in the adult rat. J. Neurobiol. 2000;42:248–57. [PubMed] [Google Scholar]
- Westenbroek C, Den Boer JA, Veenhuis M, Ter Horst GJ. Chronic stress and social housing differentially affect neurogenesis in male and female rats. Brain Res. Bull. 2004;64:303–8. doi: 10.1016/j.brainresbull.2004.08.006. [DOI] [PubMed] [Google Scholar]
- Winocur G, Wojtowicz JM, Sekeres M, Snyder JS, Wang S. Inhibition of neurogenesis interferes with hippocampus-dependent memory function. Hippocampus. 2006;16:296–304. doi: 10.1002/hipo.20163. [DOI] [PubMed] [Google Scholar]
- Wojtowicz JM, Kee N. BrdU assay for neurogenesis in rodents. Nat. Protoc. 2006;1:1399–405. doi: 10.1038/nprot.2006.224. [DOI] [PubMed] [Google Scholar]
- Woolley CS, Gould E, Frankfurt M, McEwen BS. Naturally occurring fluctuation in dendritic spine density on adult hippocampal pyramidal neurons. J. Neurosci. 1990a;10:4035–39. doi: 10.1523/JNEUROSCI.10-12-04035.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woolley CS, Gould E, McEwen BS. Exposure to excess glucocorticoids alters dendritic morphology of adult hippocampal pyramidal neurons. Brain Res. 1990b;531:225–31. doi: 10.1016/0006-8993(90)90778-a. [DOI] [PubMed] [Google Scholar]
- Xu HT, Pan F, Yang G, Gan WB. Choice of cranial window type for in vivo imaging affects dendritic spine turnover in the cortex. Nat. Neurosci. 2007;10:549–51. doi: 10.1038/nn1883. [DOI] [PubMed] [Google Scholar]
- Yamaguchi M, Saito H, Suzuki M, Mori K. Visualization of neurogenesis in the central nervous system using nestin promoter-GFP transgenic mice. Neuroreport. 2000;11:1991–96. doi: 10.1097/00001756-200006260-00037. [DOI] [PubMed] [Google Scholar]
- Zehr JL, Nichols LR, Schulz KM, Sisk CL. Adolescent development of neuron structure in dentate gyrus granule cells of male Syrian hamsters. Dev. Neurobiol. 2008;68:1517–26. doi: 10.1002/dneu.20675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang CL, Zou Y, He W, Gage FH, Evans RM. A role for adult TLX-positive neural stem cells in learning and behavior. Nature. 2008;451:1004–7. doi: 10.1038/nature06562. [DOI] [PubMed] [Google Scholar]
- Zhao C, Teng EM, Summers RG, Jr, Ming GL, Gage FH. Distinct morphological stages of dentate granule neuron maturation in the adult mouse hippocampus. J. Neurosci. 2006;26:3–11. doi: 10.1523/JNEUROSCI.3648-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]