Abstract
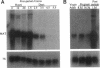
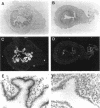
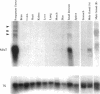
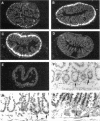
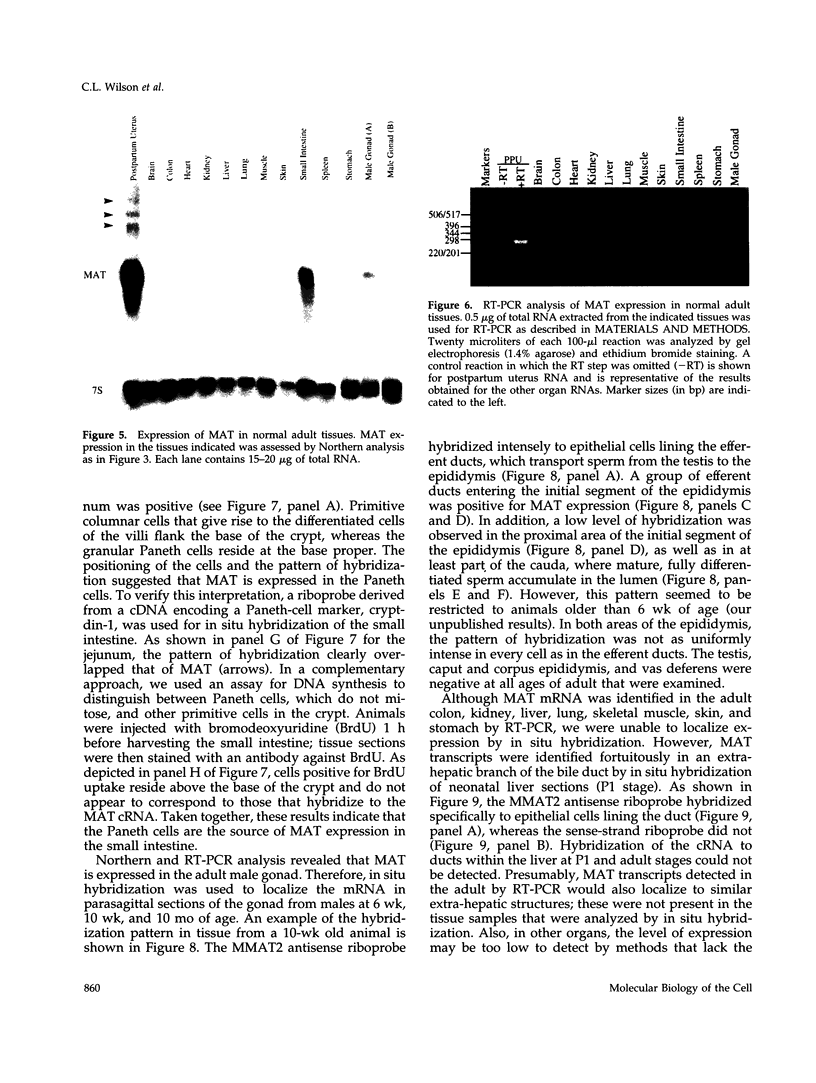
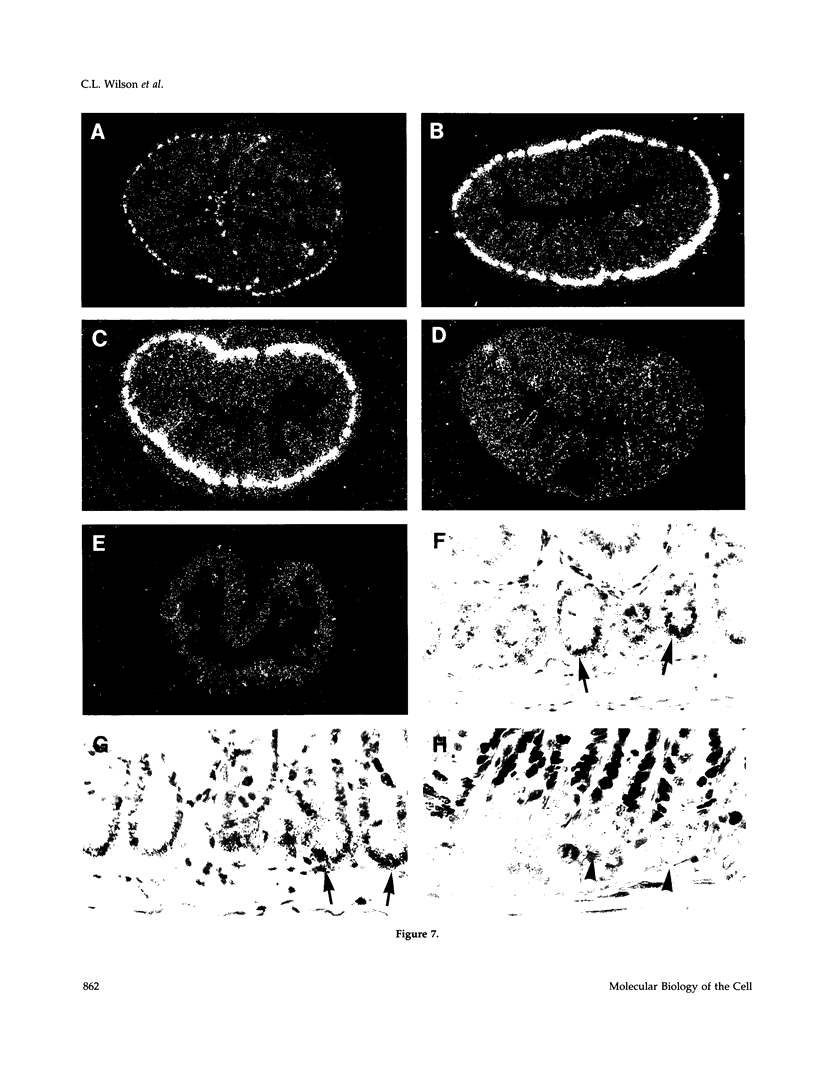
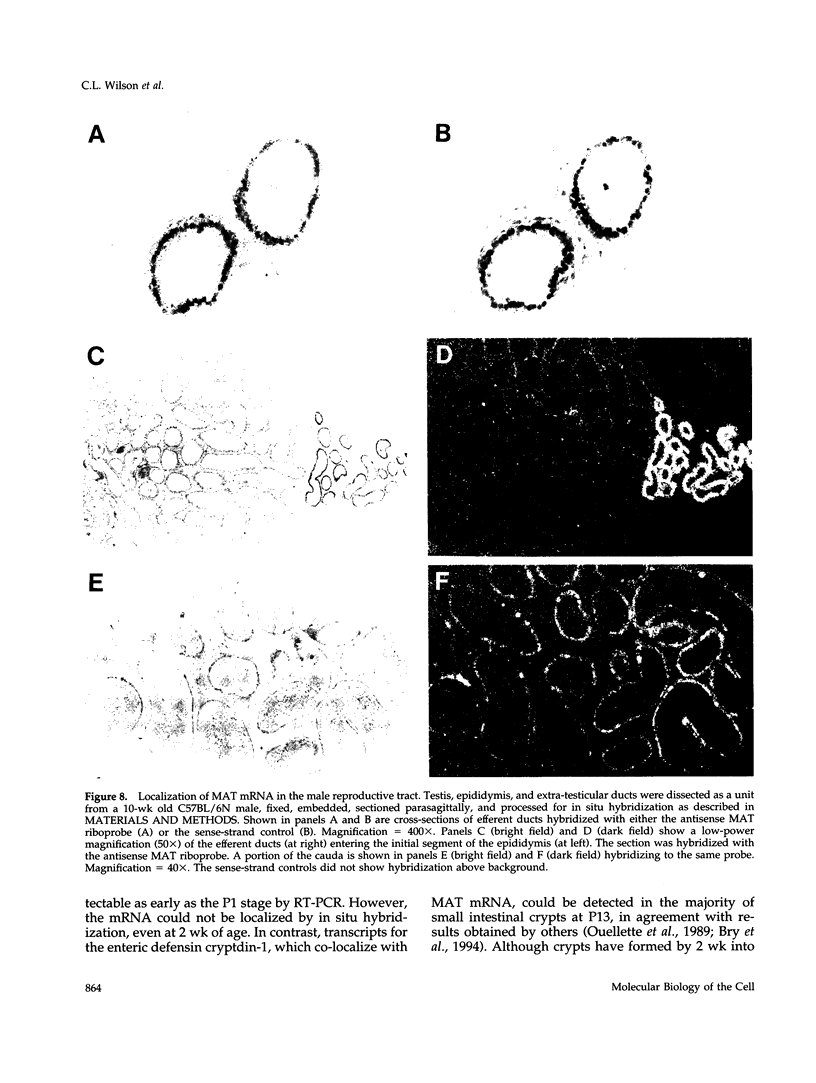
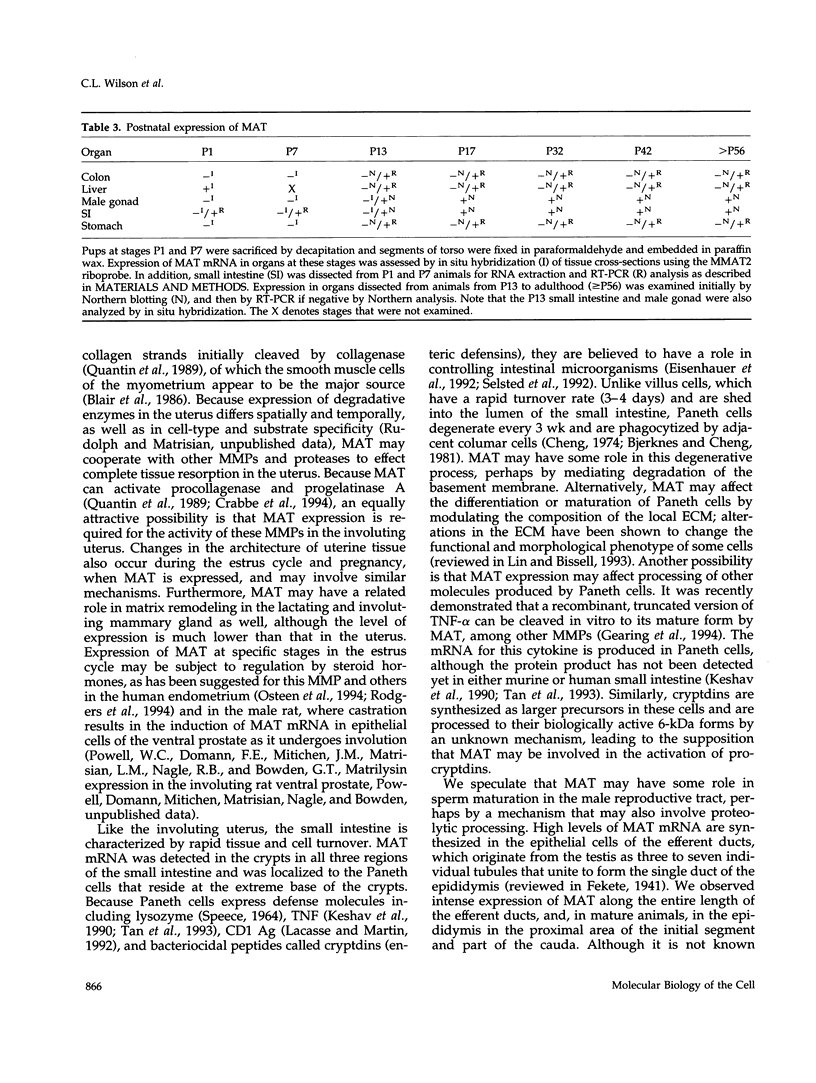
To explore the role of the matrix metalloproteinase matrilysin (MAT) in normal tissue remodeling, we cloned the murine homologue of MAT from postpartum uterus using RACE polymerase chain reaction and examined its pattern of expression in embryonic, neonatal, and adult mice. The murine coding sequence and the corresponding predicted protein sequence were found to be 75% and 70% identical to the human sequences, respectively, and organization of the six exons comprising the gene is similar to the human gene. Northern analysis and in situ hybridization revealed that MAT is expressed in the normal cycling, pregnant, and postpartum uterus, with levels of expression highest in the involuting uterus at early time points (6 h to 1.5 days postpartum). The mRNA was confined to epithelial cells lining the lumen and some glandular structures. High constitutive levels of MAT transcripts were also detected in the small intestine, where expression was localized to the epithelial Paneth cells at the base of the crypts. Similarly, MAT expression was found in epithelial cells of the efferent ducts, in the initial segment and cauda of the epididymis, and in an extra-hepatic branch of the bile duct. MAT transcripts were detectable only by reverse transcription-polymerase chain reaction in the colon, kidney, lung, skeletal muscle, skin, stomach, juvenile uterus, and normal, lactating, and involuting mammary gland, as was expression primarily late in embryogenesis. Analysis of MAT expression during postnatal development indicated that although MAT is expressed in the juvenile small intestine and reproductive organs, the accumulation of significant levels of MAT mRNA appears to correlate with organ maturation. These results show that MAT expression is restricted to specific organs in the mouse, where the mRNA is produced exclusively by epithelial cells, and suggest that in addition to matrix degradation and remodeling, MAT may play an important role in the differentiated function of these organs.
Full text
PDF











Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alexander C. M., Werb Z. Proteinases and extracellular matrix remodeling. Curr Opin Cell Biol. 1989 Oct;1(5):974–982. doi: 10.1016/0955-0674(89)90068-9. [DOI] [PubMed] [Google Scholar]
- Apte S. S., Hayashi K., Seldin M. F., Mattei M. G., Hayashi M., Olsen B. R. Gene encoding a novel murine tissue inhibitor of metalloproteinases (TIMP), TIMP-3, is expressed in developing mouse epithelia, cartilage, and muscle, and is located on mouse chromosome 10. Dev Dyn. 1994 Jul;200(3):177–197. doi: 10.1002/aja.1002000302. [DOI] [PubMed] [Google Scholar]
- Balmain A., Krumlauf R., Vass J. K., Birnie G. D. Cloning and characterisation of the abundant cytoplasmic 7S RNA from mouse cells. Nucleic Acids Res. 1982 Jul 24;10(14):4259–4277. doi: 10.1093/nar/10.14.4259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnett J., Straub K., Nguyen B., Chow J., Suttman R., Thompson K., Tsing S., Benton P., Schatzman R., Chen M. Production, purification, and characterization of human matrilysin (PUMP) from recombinant Chinese hamster ovary cells. Protein Expr Purif. 1994 Feb;5(1):27–36. doi: 10.1006/prep.1994.1004. [DOI] [PubMed] [Google Scholar]
- Basset P., Bellocq J. P., Wolf C., Stoll I., Hutin P., Limacher J. M., Podhajcer O. L., Chenard M. P., Rio M. C., Chambon P. A novel metalloproteinase gene specifically expressed in stromal cells of breast carcinomas. Nature. 1990 Dec 20;348(6303):699–704. doi: 10.1038/348699a0. [DOI] [PubMed] [Google Scholar]
- Birkedal-Hansen H., Moore W. G., Bodden M. K., Windsor L. J., Birkedal-Hansen B., DeCarlo A., Engler J. A. Matrix metalloproteinases: a review. Crit Rev Oral Biol Med. 1993;4(2):197–250. doi: 10.1177/10454411930040020401. [DOI] [PubMed] [Google Scholar]
- Bjerknes M., Cheng H. The stem-cell zone of the small intestinal epithelium. I. Evidence from Paneth cells in the adult mouse. Am J Anat. 1981 Jan;160(1):51–63. doi: 10.1002/aja.1001600105. [DOI] [PubMed] [Google Scholar]
- Blair H. C., Teitelbaum S. L., Ehlich L. S., Jeffrey J. J. Collagenase production by smooth muscle: correlation of immunoreactive with functional enzyme in the myometrium. J Cell Physiol. 1986 Oct;129(1):111–123. doi: 10.1002/jcp.1041290116. [DOI] [PubMed] [Google Scholar]
- Blobel C. P., Myles D. G., Primakoff P., White J. M. Proteolytic processing of a protein involved in sperm-egg fusion correlates with acquisition of fertilization competence. J Cell Biol. 1990 Jul;111(1):69–78. doi: 10.1083/jcb.111.1.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brenner C. A., Adler R. R., Rappolee D. A., Pedersen R. A., Werb Z. Genes for extracellular-matrix-degrading metalloproteinases and their inhibitor, TIMP, are expressed during early mammalian development. Genes Dev. 1989 Jun;3(6):848–859. doi: 10.1101/gad.3.6.848. [DOI] [PubMed] [Google Scholar]
- Bry L., Falk P., Huttner K., Ouellette A., Midtvedt T., Gordon J. I. Paneth cell differentiation in the developing intestine of normal and transgenic mice. Proc Natl Acad Sci U S A. 1994 Oct 25;91(22):10335–10339. doi: 10.1073/pnas.91.22.10335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bucher P. Weight matrix descriptions of four eukaryotic RNA polymerase II promoter elements derived from 502 unrelated promoter sequences. J Mol Biol. 1990 Apr 20;212(4):563–578. doi: 10.1016/0022-2836(90)90223-9. [DOI] [PubMed] [Google Scholar]
- Busiek D. F., Ross F. P., McDonnell S., Murphy G., Matrisian L. M., Welgus H. G. The matrix metalloprotease matrilysin (PUMP) is expressed in developing human mononuclear phagocytes. J Biol Chem. 1992 May 5;267(13):9087–9092. [PubMed] [Google Scholar]
- Cheng H. Origin, differentiation and renewal of the four main epithelial cell types in the mouse small intestine. IV. Paneth cells. Am J Anat. 1974 Dec;141(4):521–535. doi: 10.1002/aja.1001410406. [DOI] [PubMed] [Google Scholar]
- Chomczynski P., Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987 Apr;162(1):156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- Crabbe T., Smith B., O'Connell J., Docherty A. Human progelatinase A can be activated by matrilysin. FEBS Lett. 1994 May 23;345(1):14–16. doi: 10.1016/0014-5793(94)00412-9. [DOI] [PubMed] [Google Scholar]
- Eisenhauer P. B., Harwig S. S., Lehrer R. I. Cryptdins: antimicrobial defensins of the murine small intestine. Infect Immun. 1992 Sep;60(9):3556–3565. doi: 10.1128/iai.60.9.3556-3565.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frohman M. A., Dush M. K., Martin G. R. Rapid production of full-length cDNAs from rare transcripts: amplification using a single gene-specific oligonucleotide primer. Proc Natl Acad Sci U S A. 1988 Dec;85(23):8998–9002. doi: 10.1073/pnas.85.23.8998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaire M., Magbanua Z., McDonnell S., McNeil L., Lovett D. H., Matrisian L. M. Structure and expression of the human gene for the matrix metalloproteinase matrilysin. J Biol Chem. 1994 Jan 21;269(3):2032–2040. [PubMed] [Google Scholar]
- Gearing A. J., Beckett P., Christodoulou M., Churchill M., Clements J., Davidson A. H., Drummond A. H., Galloway W. A., Gilbert R., Gordon J. L. Processing of tumour necrosis factor-alpha precursor by metalloproteinases. Nature. 1994 Aug 18;370(6490):555–557. doi: 10.1038/370555a0. [DOI] [PubMed] [Google Scholar]
- HARKNESS R. D., MORALEE B. E. The time-course and route of loss of collagen from the rat's uterus during post-partum involution. J Physiol. 1956 Jun 28;132(3):502–508. doi: 10.1113/jphysiol.1956.sp005543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hermo L., Oko R., Morales C. R. Secretion and endocytosis in the male reproductive tract: a role in sperm maturation. Int Rev Cytol. 1994;154:106–189. [PubMed] [Google Scholar]
- Hirose T., Patterson C., Pourmotabbed T., Mainardi C. L., Hasty K. A. Structure-function relationship of human neutrophil collagenase: identification of regions responsible for substrate specificity and general proteinase activity. Proc Natl Acad Sci U S A. 1993 Apr 1;90(7):2569–2573. doi: 10.1073/pnas.90.7.2569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karelina T. V., Goldberg G. I., Eisen A. Z. Matrilysin (PUMP) correlates with dermal invasion during appendageal development and cutaneous neoplasia. J Invest Dermatol. 1994 Oct;103(4):482–487. doi: 10.1111/1523-1747.ep12395596. [DOI] [PubMed] [Google Scholar]
- Keshav S., Lawson L., Chung L. P., Stein M., Perry V. H., Gordon S. Tumor necrosis factor mRNA localized to Paneth cells of normal murine intestinal epithelium by in situ hybridization. J Exp Med. 1990 Jan 1;171(1):327–332. doi: 10.1084/jem.171.1.327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozak M. The scanning model for translation: an update. J Cell Biol. 1989 Feb;108(2):229–241. doi: 10.1083/jcb.108.2.229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lacasse J., Martin L. H. Detection of CD1 mRNA in Paneth cells of the mouse intestine by in situ hybridization. J Histochem Cytochem. 1992 Oct;40(10):1527–1534. doi: 10.1177/40.10.1382091. [DOI] [PubMed] [Google Scholar]
- Lin C. Q., Bissell M. J. Multi-faceted regulation of cell differentiation by extracellular matrix. FASEB J. 1993 Jun;7(9):737–743. doi: 10.1096/fasebj.7.9.8330681. [DOI] [PubMed] [Google Scholar]
- Marti H. P., McNeil L., Thomas G., Davies M., Lovett D. H. Molecular characterization of a low-molecular-mass matrix metalloproteinase secreted by glomerular mesangial cells as PUMP-1. Biochem J. 1992 Aug 1;285(Pt 3):899–905. doi: 10.1042/bj2850899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matrisian L. M., Hogan B. L. Growth factor-regulated proteases and extracellular matrix remodeling during mammalian development. Curr Top Dev Biol. 1990;24:219–259. doi: 10.1016/s0070-2153(08)60089-7. [DOI] [PubMed] [Google Scholar]
- Matrisian L. M. Metalloproteinases and their inhibitors in matrix remodeling. Trends Genet. 1990 Apr;6(4):121–125. doi: 10.1016/0168-9525(90)90126-q. [DOI] [PubMed] [Google Scholar]
- Matrisian L. M. The matrix-degrading metalloproteinases. Bioessays. 1992 Jul;14(7):455–463. doi: 10.1002/bies.950140705. [DOI] [PubMed] [Google Scholar]
- McDonnell S., Navre M., Coffey R. J., Jr, Matrisian L. M. Expression and localization of the matrix metalloproteinase pump-1 (MMP-7) in human gastric and colon carcinomas. Mol Carcinog. 1991;4(6):527–533. doi: 10.1002/mc.2940040617. [DOI] [PubMed] [Google Scholar]
- Miyazaki K., Hattori Y., Umenishi F., Yasumitsu H., Umeda M. Purification and characterization of extracellular matrix-degrading metalloproteinase, matrin (pump-1), secreted from human rectal carcinoma cell line. Cancer Res. 1990 Dec 15;50(24):7758–7764. [PubMed] [Google Scholar]
- Muller D., Breathnach R., Engelmann A., Millon R., Bronner G., Flesch H., Dumont P., Eber M., Abecassis J. Expression of collagenase-related metalloproteinase genes in human lung or head and neck tumours. Int J Cancer. 1991 Jun 19;48(4):550–556. doi: 10.1002/ijc.2910480412. [DOI] [PubMed] [Google Scholar]
- Muller D., Quantin B., Gesnel M. C., Millon-Collard R., Abecassis J., Breathnach R. The collagenase gene family in humans consists of at least four members. Biochem J. 1988 Jul 1;253(1):187–192. doi: 10.1042/bj2530187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mullins D. E., Rohrlich S. T. The role of proteinases in cellular invasiveness. Biochim Biophys Acta. 1983 Dec 29;695(3-4):177–214. doi: 10.1016/0304-419x(83)90011-2. [DOI] [PubMed] [Google Scholar]
- Murphy G., Allan J. A., Willenbrock F., Cockett M. I., O'Connell J. P., Docherty A. J. The role of the C-terminal domain in collagenase and stromelysin specificity. J Biol Chem. 1992 May 15;267(14):9612–9618. [PubMed] [Google Scholar]
- Murphy G., Cockett M. I., Ward R. V., Docherty A. J. Matrix metalloproteinase degradation of elastin, type IV collagen and proteoglycan. A quantitative comparison of the activities of 95 kDa and 72 kDa gelatinases, stromelysins-1 and -2 and punctuated metalloproteinase (PUMP). Biochem J. 1991 Jul 1;277(Pt 1):277–279. doi: 10.1042/bj2770277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newell K. J., Witty J. P., Rodgers W. H., Matrisian L. M. Expression and localization of matrix-degrading metalloproteinases during colorectal tumorigenesis. Mol Carcinog. 1994 Aug;10(4):199–206. doi: 10.1002/mc.2940100404. [DOI] [PubMed] [Google Scholar]
- Nomura S., Hogan B. L., Wills A. J., Heath J. K., Edwards D. R. Developmental expression of tissue inhibitor of metalloproteinase (TIMP) RNA. Development. 1989 Mar;105(3):575–583. doi: 10.1242/dev.105.3.575. [DOI] [PubMed] [Google Scholar]
- Osteen K. G., Rodgers W. H., Gaire M., Hargrove J. T., Gorstein F., Matrisian L. M. Stromal-epithelial interaction mediates steroidal regulation of metalloproteinase expression in human endometrium. Proc Natl Acad Sci U S A. 1994 Oct 11;91(21):10129–10133. doi: 10.1073/pnas.91.21.10129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ouellette A. J., Greco R. M., James M., Frederick D., Naftilan J., Fallon J. T. Developmental regulation of cryptdin, a corticostatin/defensin precursor mRNA in mouse small intestinal crypt epithelium. J Cell Biol. 1989 May;108(5):1687–1695. doi: 10.1083/jcb.108.5.1687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pajouh M. S., Nagle R. B., Breathnach R., Finch J. S., Brawer M. K., Bowden G. T. Expression of metalloproteinase genes in human prostate cancer. J Cancer Res Clin Oncol. 1991;117(2):144–150. doi: 10.1007/BF01613138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phelps B. M., Koppel D. E., Primakoff P., Myles D. G. Evidence that proteolysis of the surface is an initial step in the mechanism of formation of sperm cell surface domains. J Cell Biol. 1990 Nov;111(5 Pt 1):1839–1847. doi: 10.1083/jcb.111.5.1839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powell W. C., Knox J. D., Navre M., Grogan T. M., Kittelson J., Nagle R. B., Bowden G. T. Expression of the metalloproteinase matrilysin in DU-145 cells increases their invasive potential in severe combined immunodeficient mice. Cancer Res. 1993 Jan 15;53(2):417–422. [PubMed] [Google Scholar]
- Quantin B., Murphy G., Breathnach R. Pump-1 cDNA codes for a protein with characteristics similar to those of classical collagenase family members. Biochemistry. 1989 Jun 27;28(13):5327–5334. doi: 10.1021/bi00439a004. [DOI] [PubMed] [Google Scholar]
- Reponen P., Sahlberg C., Huhtala P., Hurskainen T., Thesleff I., Tryggvason K. Molecular cloning of murine 72-kDa type IV collagenase and its expression during mouse development. J Biol Chem. 1992 Apr 15;267(11):7856–7862. [PubMed] [Google Scholar]
- Reponen P., Sahlberg C., Munaut C., Thesleff I., Tryggvason K. High expression of 92-kD type IV collagenase (gelatinase B) in the osteoclast lineage during mouse development. J Cell Biol. 1994 Mar;124(6):1091–1102. doi: 10.1083/jcb.124.6.1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodgers W. H., Matrisian L. M., Giudice L. C., Dsupin B., Cannon P., Svitek C., Gorstein F., Osteen K. G. Patterns of matrix metalloproteinase expression in cycling endometrium imply differential functions and regulation by steroid hormones. J Clin Invest. 1994 Sep;94(3):946–953. doi: 10.1172/JCI117461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodgers W. H., Osteen K. G., Matrisian L. M., Navre M., Giudice L. C., Gorstein F. Expression and localization of matrilysin, a matrix metalloproteinase, in human endometrium during the reproductive cycle. Am J Obstet Gynecol. 1993 Jan;168(1 Pt 1):253–260. doi: 10.1016/s0002-9378(12)90922-9. [DOI] [PubMed] [Google Scholar]
- SPEECE A. J. HISTOCHEMICAL DISTRIBUTION OF LYSOZYME ACTIVITY IN ORGANS OF NORMAL MICE AND RADIATION CHIMERAS. J Histochem Cytochem. 1964 May;12:384–391. doi: 10.1177/12.5.384. [DOI] [PubMed] [Google Scholar]
- Sellers A., Woessner J. F., Jr The extraction of a neutral metalloproteinase from the involuting rat uterus, and its action on cartilage proteoglycan. Biochem J. 1980 Sep 1;189(3):521–531. doi: 10.1042/bj1890521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selsted M. E., Miller S. I., Henschen A. H., Ouellette A. J. Enteric defensins: antibiotic peptide components of intestinal host defense. J Cell Biol. 1992 Aug;118(4):929–936. doi: 10.1083/jcb.118.4.929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sires U. I., Griffin G. L., Broekelmann T. J., Mecham R. P., Murphy G., Chung A. E., Welgus H. G., Senior R. M. Degradation of entactin by matrix metalloproteinases. Susceptibility to matrilysin and identification of cleavage sites. J Biol Chem. 1993 Jan 25;268(3):2069–2074. [PubMed] [Google Scholar]
- Tan X., Hsueh W., Gonzalez-Crussi F. Cellular localization of tumor necrosis factor (TNF)-alpha transcripts in normal bowel and in necrotizing enterocolitis. TNF gene expression by Paneth cells, intestinal eosinophils, and macrophages. Am J Pathol. 1993 Jun;142(6):1858–1865. [PMC free article] [PubMed] [Google Scholar]
- Ward J. M., Hagiwara A., Anderson L. M., Lindsey K., Diwan B. A. The chronic hepatic or renal toxicity of di(2-ethylhexyl) phthalate, acetaminophen, sodium barbital, and phenobarbital in male B6C3F1 mice: autoradiographic, immunohistochemical, and biochemical evidence for levels of DNA synthesis not associated with carcinogenesis or tumor promotion. Toxicol Appl Pharmacol. 1988 Dec;96(3):494–506. doi: 10.1016/0041-008x(88)90009-9. [DOI] [PubMed] [Google Scholar]
- Witty J. P., McDonnell S., Newell K. J., Cannon P., Navre M., Tressler R. J., Matrisian L. M. Modulation of matrilysin levels in colon carcinoma cell lines affects tumorigenicity in vivo. Cancer Res. 1994 Sep 1;54(17):4805–4812. [PubMed] [Google Scholar]
- Woessner J. F., Jr Matrix metalloproteinases and their inhibitors in connective tissue remodeling. FASEB J. 1991 May;5(8):2145–2154. [PubMed] [Google Scholar]
- Woessner J. F., Jr, Taplin C. J. Purification and properties of a small latent matrix metalloproteinase of the rat uterus. J Biol Chem. 1988 Nov 15;263(32):16918–16925. [PubMed] [Google Scholar]
- Wolfsberg T. G., Bazan J. F., Blobel C. P., Myles D. G., Primakoff P., White J. M. The precursor region of a protein active in sperm-egg fusion contains a metalloprotease and a disintegrin domain: structural, functional, and evolutionary implications. Proc Natl Acad Sci U S A. 1993 Nov 15;90(22):10783–10787. doi: 10.1073/pnas.90.22.10783. [DOI] [PMC free article] [PubMed] [Google Scholar]