Abstract
Memory T cells of the effector type (TEM) account for the characteristic rapidity of memory T-cell responses, whereas memory T cells of the central type (TCM) account for long-lasting, vigorously proliferating memory T-cell responses. How antigen-stimulated (primed) T cells develop into different memory T-cell subsets with diverse tissue distributions is largely unknown. Here we show that after respiratory tract infection of mice with influenza virus, viral antigen associated with dendritic cells (DCs) was abundant in lung-draining lymph nodes (DLN) and the spleen for more than a week but was scant and transient in nondraining lymph nodes (NDLN). Correspondingly, activated CD8 T cells proliferated extensively in DLN and the spleen but minimally in NDLN. Strikingly, however, although most persisting CD8 T cells in DLN and spleen exhibited the TEM phenotype, those persisting in NDLN exhibited the TCM phenotype. Reducing antigen exposure by depleting DCs at the peak of primary T-cell responses enhanced the development of TCM, whereas subjecting primed CD8 T cells from NDLN to additional antigen stimulation inhibited TCM development. These findings demonstrate that differences in persistence of antigen-bearing DCs in various tissues regulate the tissue-specific pattern of memory CD8 T-cell development. The findings have significant implications for design of vaccines and immunization strategies.
Memory CD8 T cells generally provide protection against many viruses, including respiratory tract infection by virulent influenza A viruses. Based upon their cell-surface markers, tissue localization, persistence, and responses to restimulation by antigen, memory CD8 T cells often are divided into two major subsets (1, 2). Effector memory T cells (TEM) are CD62Llo CCR7lo, reside primarily in nonlymphoid (parenchymal) tissues, and decline gradually over time because they undergo little homeostatic proliferation. After restimulation by antigen, TEM rapidly exercise effector functions, such as cytolytic activity and IFN-γ secretion, but they hardly proliferate. In contrast, central memory T cells (TCM) are CD62Lhi CCR7hi, reside predominantly in lymphoid tissues, undergo sufficient homeostatic proliferation to maintain steady cell numbers over long times, and proliferate extensively upon antigen restimulation. Because of their persistence and robust proliferation upon antigen restimulation, TCM probably are the principal mediators of long-term protection conferred by T cells against infection by viral pathogens (1, 3).
Since their initial description, many studies have investigated the relationship between TEM and TCM and factors that might regulate their development (4). In particular, the duration of signals initiated by antigen, costimulation, and inflammation following naïve T cells’ initial response to antigen (priming) has been shown to play an important role (5). Short exposure to antigen favors TCM development, whereas prolonged exposure favors development of TEM and short-lived effector cells (6–8). For instance, in the secondary (“memory”) CD8 T-cell response observed after primary intradermal DNA immunization, the cell proliferation response was greater if the duration of antigen expression was shortened (9). In contrast, when T cells were primed by prolonged antigen exposure by DNA immunization, the number of resting memory CD8 T cells was greater, but they showed very limited expansion upon secondary antigen challenge (10). Likewise, in systemic Listeria infection the frequency of persisting antigen-specific memory CD8 T cells was greater in infected mice that received a second dose of bacteria 6 d after primary infection, but these mice later mounted a smaller proliferative recall response upon reinfection (11).
Although the duration of antigen exposure following T-cell priming affects TCM versus TEM development, the underlying mechanisms are largely unknown. In particular, this generalization does not explain differences in the relative abundance of TCM and TEM in various organs or even in the same tissues at various times after a natural infection. One reason for the lack of a more thorough understanding is that most studies have not directly measured antigen levels in different organs during the course of an immune response. In addition, many previous studies introduced antigen in the form of disseminated (systemic) infection by Listeria or lymphocytic choriomeningitis virus (11–13), probably obscuring differences in antigen distribution in various organs. Because of the low frequencies of antigen-specific T cells in immunized or infected hosts, most previous studies also have been unable to assess T-cell responses in certain organs during natural infections. Memory T cells that develop in such sites could contribute significantly to subsequent immune responses and may be underappreciated.
To investigate the mechanism by which antigen regulates tissue-specific patterns of memory T-cell development, we used cohorts of T-cell receptor (TCR)-transgenic CD8 T cells as tools in two ways. One was to analyze antigen-specific responses in tissues that are near or remote from the influenza virus-infected respiratory tract. Second, adoptively transferred naïve CD8 T cells that proliferate specifically in response to a viral antigen were used as reporters to examine the distribution and persistence of that antigen in different tissues. The results show that the distribution of antigen-bearing dendritic cells (DCs) regulates the tissue-variable pattern of memory CD8 T-cell development. They highlight mechanisms at the cellular level by which effector T cells are generated in different organs to control current infections and to develop into TEM and TCM for defenses against future encounters with the same pathogen.
Results
Tissue-Specific Patterns of TCM Versus TEM Development After Influenza Virus Infection.
To surmount the difficulty of following responses of relatively rare endogenous antigen-specific CD8 cells in various host organs, we used a mouse model of influenza virus infection in which cohorts of antigen-specific CD8 T cells, mainly TCR-transgenic T cells, can be monitored at any time and in any organ (14). In this model, the CD8 T cells express the 2C TCR, which specifically recognizes the SIYRYYGL (SIY) peptide bound to the MHC-I Kb molecule (SIY-Kb complexes). Naïve 2C T cells were injected into C57BL/6 (B6) mice, and the mice then were infected intranasally (i.n.) with the WSN-SIY influenza A virus that expresses the SIY peptide in infected cells (Fig. S1A) (14). Although the 2C T cells expressed the same TCR and initially were activated by the same infection in the same mouse, at 30 d postinfection (dpi) the persisting memory 2C cells exhibited characteristic TCM and TEM phenotypes in different tissues (Fig. S1B). In the lungs, the site of viral infection, the persisting memory 2C cells were all TEM-like (CD62Llo), whereas in nondraining lymph nodes (NDLN) they were predominantly TCM-like (CD62Lhi). Both subsets were present in the spleen and lung draining lymph nodes (DLN). To measure their proliferative recall potentials, memory 2C cells were sorted on the basis of CD62L expression and transferred into secondary B6 recipients that then were infected i.n. with the WSN-SIY virus. The number of 2C cells in bronchial alveolar lavage (BAL) fluid of these infected recipients was a measure of the transferred cells’ proliferative recall potential. The CD62Lhi memory 2C cells from DLN, NDLN, and spleen proliferated extensively, whereas the CD62Llo memory 2C cells from lung, DLN, and spleen proliferated poorly (Fig. S1C). Thus, as in other acute viral infections (15), memory 2C cells with features of TCM and TEM develop to different extents in different tissues from the same cohort of activated 2C cells following influenza virus infection in the lungs.
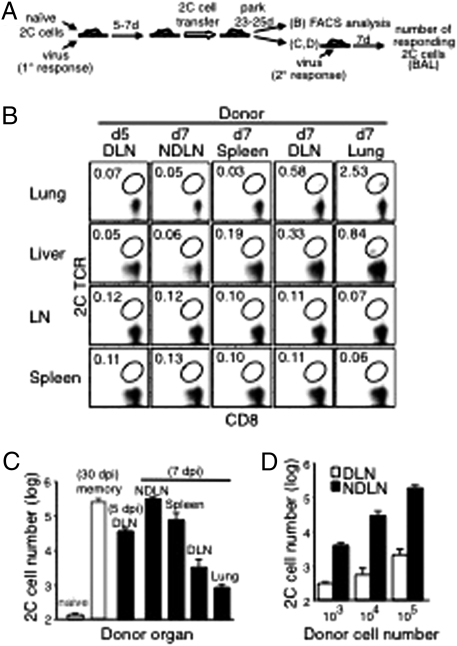
After i.n. influenza virus infection, naïve T cells initially are activated in the lung DLN (Fig. S2A). The activated T cells start to migrate to other tissues 4–5 dpi (14). We examined whether activated 2C cells in various organs at the peak of the T-cell response (7 dpi; Fig. S2B) already were committed to differentiate into different subsets of memory T cells. Equal numbers of activated 2C cells taken at this time from different tissues were transferred into naïve B6 recipients, and their persistence and recall responses were assayed 23 d later (equivalent to 30 dpi) (Fig. 1A). When the transferred 2C cells were from NDLN, the frequency of 2C cells persisting in the recipients’ organs was low (Fig. 1B), but when these recipient mice were challenged with WSN-SIY virus to measure the transferred cells’ recall potential, a large number (∼3 × 105) of responding 2C cells was detected in the BAL fluid (Fig. 1C).
Fig. 1.
Recall potential of 2C cells in different organs after influenza virus infection. (A) Scheme of the experimental procedures to detect memory 2C cells in various tissues (B) and to measure their proliferative recall potentials (C and D). B6 mice were injected i.v. with naive 2C cells (2 × 105) and infected i.n with WSN-SIY virus (primary response). Five or seven days later, activated 2C cells (5 × 105) from the indicated donor organs were injected into naive B6 recipient mice. After 25 d (for donor cells from DLN 5 dpi) or 23 d (for other donor tissues), cells from the recipients’ lung, liver, mesenteric lymph nodes, and spleen were analyzed for 2C TCR and CD8. Plots shown are gated on CD8+ cells, and the percentages of 2C cells among total CD8+ cells are shown (B). (C) Activated 2C cells, taken 5 dpi or 7 dpi from various tissues of virus-infected donors were parked for 23–25 d in recipient mice, which then were infected with the WSN-SIY virus (secondary response), and the responding 2C cells in BAL fluid were enumerated 7 d later (black bars). For reference and controls, the same number (5 × 105) of naïve 2C cells were transferred and parked for 30 d in one group of recipient mice (naïve, white bar), and memory 2C cells from spleens of mice that had been infected with the WSN-SIY virus 30 d earlier were transferred into another group of recipient mice (memory, white bar). The proliferative potentials of 2C cells in all of the recipient mice then were measured by infecting them i.n. with the virus and 7 d later counting 2C cells in BAL fluid (y axis). (D) Graded numbers of activated 2C cells derived from DLN or NDLN (7 dpi, generated as described in A) were injected into recipient mice and parked for 23 d. The recall responses were analyzed in BAL fluid 7 d after virus infection, as in C. Error bars in C and D represent SEM from three recipient mice per group.
In contrast, when the initially transferred 2C cells were from the lung or DLN, the frequency of persisting 2C cells in the recipients' nonlymphoid organs, i.e., in the lung and liver, was much higher (Fig. 1B), but their proliferative recall potential was weak, as indicated by the at least 100-fold fewer 2C cells in BAL fluid of the virus-challenged recipient mice (Fig. 1C). The validity of this in vivo recall assay is indicated by the proportionality between the numbers of responding 2C cells in BAL fluid and the numbers of activated 2C cells transferred initially from DLN and NDLN (Fig. 1D). That the responding 2C cells in recipient mice were descendants of the transferred activated 2C cells was evident from the absence of significant numbers of responding 2C cells in BAL fluid examined 7 d after the virus challenge from control mice that were injected with equal numbers of naïve 2C cells and then challenged with the WSN-SIY virus 30 d later (naïve control in Fig. 1C). Moreover, similar results were obtained when recipient mice were transferred with activated 2C cells that were generated by initially injecting only 500 or 10,000 naïve 2C cells during the primary response to mimic the low frequency of antigen-specific CD8 T cells seen in natural infections (Fig. S3). Taken together, these results indicate that at the peak of the primary immune response (7 dpi) activated 2C cells residing in different tissues already differ in their potential to differentiate into various kinds of memory T cells: The relatively few 2C cells in NDLN were committed to become predominantly TCM-like memory T cells, whereas the much more abundant 2C cells in DLN and especially in the lung were committed to become TEM-like memory T cells.
We also found that activated 2C cells isolated from DLN at various times differed in the ability to develop into the different subsets of memory T cells. Higher percentages of persisting 2C cells were detected in the lung and liver of recipient mice when the transferred 2C cells were taken from infected donors at 7 dpi rather than at 5 dpi (Fig. 1B). However, after virus rechallenge, ∼10 times more responding 2C cells were detected in recipients of activated 2C cells taken at 5 dpi than in recipients of activated cells taken at 7 dpi (Fig. 1C). Thus, the developmental potential of activated 2C cells in DLN changed rapidly with time after infection. Together, these results strongly point to time-dependent and tissue-associated factors that determine memory CD8 T-cell development during the initial phase of the response to an acute viral infection.
Changes in the Distribution of Antigen-Bearing Dendritic Cells over Time and in Different Tissues Following Influenza Virus Infection.
We examined the presence of antigen-bearing DCs in NDLN, DLN, and spleen in CD11c-DTR/EGFP transgenic mice, in which CD11c+ DCs can be depleted transiently by an injection of diphtheria toxin (16) (Fig. S4 A and B). When carboxyfluorescein succinimidyl ester (CFSE)-labeled naïve 2C cells were adoptively transferred into these mice 1 d after virus infection and recovered 1 d later, without injection of diphtheria toxin (no DC depletion), CFSE dilution (i.e., cell proliferation) was pronounced in 2C cells recovered from DLN, spleen, and NDLN (Fig. S4C). In particular, most 2C cells from DLN underwent an average of six divisions, indicating a higher antigen load in DLN than in spleen and NDLN. Depletion of DCs by diphtheria toxin injection at the time of naïve T-cell transfer dramatically reduced the fraction of proliferating 2C cells, indicating that CD11c+ DCs in these lymphoid tissues were presenting the viral antigen (SIY-Kb). When CFSE-labeled 2C cells were injected into virus-infected mice 7 dpi and recovered 1 d later, without DC depletion, CFSE dilution was observed only in 2C cells recovered from DLN and spleen but not in cells recovered from NDLN (Fig. S4D). Again, depletion of DCs by injection of diphtheria toxin markedly reduced both the fraction of 2C cells that proliferated and the average number of divisions. In the absence of DC depletion, a significant fraction of transferred naïve T cells proliferated when recovered from DLN and spleen at 9 dpi but not at 14 dpi (Fig. S5). Thus, after a local influenza virus infection in the lungs, viral antigen persists in DLN and spleen for at least 9 d but does not persist that long in NDLN.
Antigen-Bearing DCs Drive Activated CD8 T Cells in the Same Tissue to Proliferate Continuously.
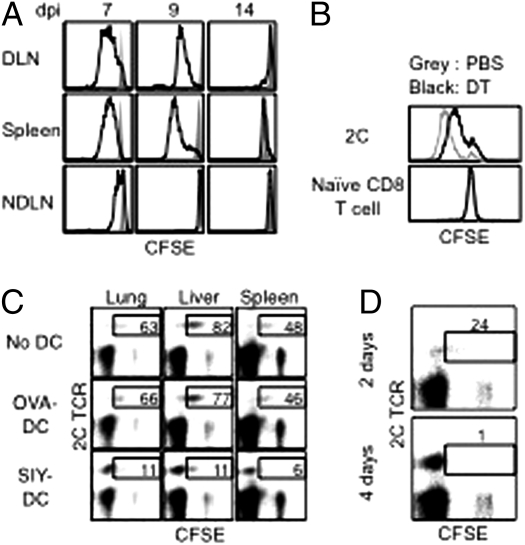
2C cells taken from DLN, spleen, and NDLN 7, 9, and 14 d after influenza virus infection were labeled with CFSE, and their proliferation profiles were analyzed. At 7 dpi the cells from DLN and spleen proliferated extensively, whereas those from NDLN hardly proliferated (Fig. 2A). By 9 dpi, 2C cells from NDLN had completely stopped proliferating, whereas 2C cells from DLN and spleen of the same mouse still proliferated vigorously. Thus, activated 2C cells continued to proliferate in organs where viral antigen persisted.
Fig. 2.
Antigen-bearing DCs promote proliferation of resident-activated CD8 T cells. (A) B6 mice were injected with naive 2C cells and infected with WSN-SIY virus. Cells from the indicated tissues 7, 9, and 14 dpi were labeled with CFSE, and the CFSE intensities of 2C cells (solid line) and of naïve endogenous CD8 T cells (shaded area) after 3-d culture are shown. (B) Naïve 2C cells were injected into CD11c-DTR/EGFP mice followed by i.n. infection with the WSN-SIY virus. Five dpi the mice were injected with diphtheria toxin (DT) or PBS. Two days later (7 dpi), cells were isolated from the spleen, labeled with CFSE, and cultured for 3 d, followed by flow cytometry. CFSE profiles of 2C cells (Upper) and naive endogenous CD8 T cells (Lower) from diphtheria toxin- and PBS-treated mice are shown. (C) B6 mice were injected with naive 2C cells and infected with WSN-SIY virus. Then 2C cells from DLN 5 dpi were labeled with CFSE and transferred into naive mice or mice that were concomitantly injected with BMDCs (1 × 106) loaded with SIY or control (OVA) peptide. 2C cells from the indicated organs 7 d posttransfer were analyzed by flow cytometry. 2C TCR versus CFSE profiles are shown for CD8+ cells. Numbers indicate the percentages of 2C cells still containing CFSE (indicating five or fewer divisions) among total 2C cells. (D) CFSE-labeled 2C cells (from DLN 5 dpi) were transferred into mice that were injected with SIY peptide for 2 or 4 d, starting 1 d before the cell transfer. Cells from the liver were isolated 9 d posttransfer and analyzed as in C. Numbers have the same significance as in C.
To demonstrate a direct role of antigen-bearing DCs in promoting proliferation of activated CD8 T cells, we used CD11c-DTR/EGFP mice. These mice were given naïve 2C cells and at the same time were infected with the WSN-SIY virus. Five days postinfection, the mice were injected with diphtheria toxin to deplete antigen-bearing DCs or with PBS as control. Two days later, cells from the spleens were labeled with CFSE, and their proliferation profiles were analyzed. The 2C cells from diphtheria toxin-treated mice retained more CFSE label than did 2C cells from PBS-treated mice (Fig. 2B), suggesting that antigen-bearing DCs are required for the continuing proliferation of activated CD8 T cells.
Conversely, activated 2C cells from DLN 5 dpi were labeled with CFSE and adoptively transferred into naive recipient mice that either were untreated or were injected at the same time with SIY peptide-loaded bone marrow-derived DCs (BMDCs), or, as controls, with OVA peptide (SIINFEKL)-loaded BMDCs. Seven days later (equivalent to 12 dpi), the percentages of 2C cells that were CFSE+ were much lower in recipient mice that received SIY peptide-loaded BMDCs than in those that received OVA peptide-loaded BMDCs (6–11% vs. 46–77%; Fig. 2C). Injection of the SIY peptide alone had the same effect as SIY-loaded BMDCs in promoting proliferation of activated 2C cells. The transferred activated 2C cells also proliferated more when the recipient mice were treated with SIY peptide for 4 d than when treated for 2 d (Fig. 2D). Together, these results suggest that continuous proliferation of activated CD8 T cells in various locations is driven by local antigen-bearing DCs.
Antigen-Bearing DCs Inhibit Development of TCM-Like Memory Cells.
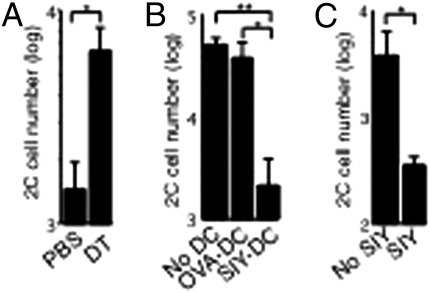
To examine the effect of local antigen-bearing DCs on memory T-cell development, we modulated the level of antigen-bearing DCs using complementary approaches. In one approach, CD11c-DTR/EGFP mice were injected with naïve 2C cells and infected with the WSN-SIY virus. Five days postinfection, the mice were treated with either PBS or diphtheria toxin to deplete DCs. Two days later (7 dpi), equal numbers of activated 2C cells harvested from spleens were transferred into naïve B6 recipients, and 23 d later the recall responses of the persisting 2C cells were measured in BAL fluid of the recipients 7 d after they were infected with the virus. As shown in Fig. 3A, ≈4.3 times more 2C cells were detected in BAL fluid of recipient mice that received activated 2C cells from the diphtheria toxin-treated (DC-depleted) CD11c-DTR/EGFP mice than in BAL fluid from the PBS-treated mice. Conversely, activated 2C cells from DLN 5 dpi were transferred into naive recipient mice that were injected at the same time with BMDCs loaded with SIY or OVA (control) peptide. Recall responses of the persisting 2C cells were measured 25 d later. Approximately 20 times fewer 2C cells were recovered from BAL fluid of recipient mice that received SIY peptide-loaded BMDCs than from BAL fluid of mice that received control OVA peptide-loaded BMDCs (Fig. 3B). Similar results were obtained when activated 2C cells from NDLN 7 dpi were transferred into B6 recipient mice that were injected daily for 4 d with either PBS or SIY peptide and recall potentials were measured 23 d later. We found that daily injection of the peptide for 4 d, which simulated the duration of antigen exposure in DLN and spleen following influenza virus infection (Fig. S5), reduced the subsequent recall responses of the persisting 2C cells by ∼10-fold (Fig. 3C). These complementary findings suggest that an excess of antigen-bearing DCs inhibits the development of memory T cells with strong recall potential (TCM-like memory T cells).
Fig. 3.
Antigen-bearing DCs inhibit TCM development. (A) Naïve 2C cells were injected into CD11c-DTR/EGFP transgenic mice followed by i.n. infection with the WSN-SIY virus. The mice were injected with diphtheria toxin (DT) or PBS 5 dpi. Two days later (7 dpi), 2C cells (2 × 104) from the spleen were transferred into B6 recipient mice and parked for 23 d. Recipient mice then were challenged i.n. with WSN-SIY virus, and 2C cell numbers were counted in BAL fluid 7 d later to measure recall responses (y axis). (B) B6 mice were injected with naive 2C cells and infected with WSN-SIY virus. Activated 2C cells (5 × 105) from DLN 5 dpi were transferred into naive mice or mice that were injected i.v. with BMDCs (1 × 106) loaded with the SIY or control (OVA) peptide upon 2C cell transfer. Twenty-five days posttransfer, recall responses were measured as in A. (C) B6 mice were injected with naive 2C cells and infected with WSN-SIY virus. Activated 2C cells (5 × 103) from NDLN 7 dpi were transferred into mice that also were given SIY peptide or PBS daily for 4 d. The recall response of 2C cells was measured 23 d posttransfer, as in A. Error bars represent the SEM from three recipient mice per group. *P < 0.05; **P < 0.01.
Prolonged Antigen Stimulation of Activated CD8 T Cells Promotes Development of TEM-Like Memory Cells.
To examine whether additional antigen drives virus-activated 2C cells to differentiate into memory T cells with the TEM phenotype, activated 2C cells from DLN 5 dpi were transferred into B6 recipient mice that were left untreated or were injected with SIY peptide daily for 4 d. Twenty-five days later (equivalent to 30 dpi), 2C cells were considerably more abundant in all organs of the recipient mice that had been injected with the SIY peptide (Fig. S6A). The proliferative recall responses, however, were greater in recipient mice that had not been injected with the SIY peptide (Fig. S6B). Similarly, SIY peptide injections significantly diminished the recall response in recipient mice that were adoptively transferred with SIY-specific endogenous CD8 T cells (not 2C cells) from mice infected with WSN-SIY influenza virus taken either from DLN at 5 dpi or from NDLN at 8 dpi (Fig. S6 C and D). These findings show that prolonged antigen stimulation of virus-activated cells induces more persisting memory 2C cells, but their proliferative recall potential is low.
Studies have shown that persistent antigen stimulation can lead to T-cell exhaustion, as indicated by elevated programmed death 1 (PD-1) expression and lack of IFN-γ secretion upon antigen restimulation. To examine whether T-cell exhaustion occurs in the SIY peptide-stimulated persisting memory 2C cells, we analyzed their PD-1 expression and function. In the spleen of virus-infected mice the level of PD-1 expression was lower on CD62Lhi memory 2C cells (i.e., TCM-like memory T cells) than on the CD62Llo memory 2C cells (i.e., TEM-like memory T cells) (SI Fig. 7A). The persisting memory 2C cells in mice that received virus-activated cells and additional SIY peptide injections were CD62Llo and expressed PD-1. As expected, they did not produce a significant proliferative recall response (SI Fig. 7B). However, they readily expressed IFN-γ after restimulation with SIY peptide in vitro (SI Fig. 8A), and they effectively inhibited WSN-SIY virus infection in recipient mice (SI Fig. 8B). These results show that persisting virus-activated memory 2C cells that were exposed to additional SIY peptide during the primary immune response acquired the TEM phenotype but were not functionally exhausted despite expressing high PD-1 levels.
Discussion
In this study, we used 2C TCR-transgenic T cells that recognize a particular viral antigen (SIY-Kb) to probe the role of antigen-bearing DCs in regulating the differentiation of influenza virus-activated CD8 T cells into different memory T-cell subsets. Differences in the distribution and frequency of these subsets in various anatomical sites, some near and other remote from the influenza virus-infected respiratory tract, suggested that T-cell–extrinsic factors regulate memory T-cell development. To determine if DCs that present this antigen are a significant factor, we used naïve 2C cells as reporters to monitor the antigen-bearing DCs at different times and in different tissues following virus infection. By 1–2 dpi, viral antigen already was present not only in DLN but also in the spleen and NDLN (Fig. S4), and, importantly, the antigen at all these sites was associated with CD11c+ DCs. These results are consistent with the finding that lung DCs migrate widely to various lymphoid tissues in a synchronous wave almost immediately following a pulmonary virus infection (17). The wide distribution of antigen-bearing DCs probably maximizes opportunities to prime naïve antigen-specific CD8 T cells in DLN and in other lymphoid tissues as well.
At 7–8 dpi, antigen-bearing DCs still were present in DLN and the spleen, but they were no longer detectable in NDLN. By transiently depleting DCs with diphtheria toxin and, conversely, by enhancing the antigen level by administering the epitope as synthetic peptide (SIY), we found that differences in the level and persistence of antigen-bearing DCs at various sites exerted a profound effect on the development of different memory CD8 T-cell subsets. First, in accord with the transient presence of antigen-bearing DCs in NDLN, the 2C cells that persisted at this site at 30 dpi exhibited TCM-like properties almost exclusively: They expressed high levels of CD62L and proliferated extensively when restimulated by antigen. In contrast, in antigen-rich tissues, such as DLN and lung, the persisting 2C cells exhibited mostly the TEM phenotype (Fig. S1). Second, when activated 2C cells taken at 7 dpi were adoptively transferred into naïve B6 recipient mice, the cells taken from NDLN gave rise predominantly to TCM-like memory T cells (proliferating extensively on antigen restimulation), whereas those from DLN and the lung gave rise to memory T cells that on restimulation proliferated only weakly (TEM-like memory T cells). Third, when equal numbers of virus-activated 2C cells from DLN were transferred into naïve recipients, cells taken at 5 dpi gave rise to memory T cells with a significantly greater proliferative response than those taken at 7 dpi, probably because there had been less exposure to antigen at 5 dpi (Fig. 1). This observation is consistent with previous reports that TCM precursors are present in DLN at 3.5 d but not at 8 d after influenza virus infection of the respiratory tract (8) and that CD8 T cells can be activated to differentiate into memory T cells by a brief contact (<24 h) with antigen (18, 19). Fourth, depletion of DCs from the spleen at 5–7 dpi led to development of memory 2C cells with greater proliferative recall potential. Conversely, further stimulation of virus-activated 2C cells taken 5 dpi from DLN or 7 dpi from NDLN with supplementary injections of SIY peptide or SIY peptide-loaded DCs resulted in memory T cells with diminished proliferative response to antigen restimulation (Fig. 3). Together, this series of complementary results indicates that differences in distribution and persistence of antigen-bearing DCs regulate the development of various memory CD8 T-cell subsets.
All these findings suggest that, after natural infections (or at least after influenza virus infection), differentiation of antigen-primed T cells into various memory subsets does not simply follow a confined linear pathway, as proposed by many models (1, 2, 5, 20). Activated T cells in DLN at 5 dpi have the potential to differentiate into TCM. If, however, these activated T cells experience further stimulation by antigen-bearing DCs in DLN or spleen, they may be inhibited from developing into TCM. In contrast, TCM precursors migrating to NDLN are spared further antigen stimulation, making NDLN, rather than DLN, favorable sites for generating TCM. A high frequency of TCM precursors is obtained in DLN only if the priming process is artificially interrupted, as shown previously (8, 18, 21) and as shown here by adoptive transfer of newly activated (i.e., 5 dpi) CD8 T cells into naïve recipients. Thus, in addition to previously identified factors, such as the density of DCs and the frequency of responding CD8 T cells in the DLN after T-cell priming (22, 23), we show that variations in the levels of antigen-bearing DCs in different tissues play an important role in regulating memory CD8 T-cell phenotype. The role of antigen-bearing DCs may allow the immune system to use different anatomical sites to produce effector T cells to control current infections while simultaneously generating both TEM and TCM precursors for future defenses.
In antigen-rich tissues or after treatment with SIY peptide or SIY peptide-loaded DCs, activated 2C cells continued to proliferate. Correspondingly, the frequency of persisting memory T cells was significantly elevated, especially in nonlymphoid tissues such as infected lungs. Because memory CD8 T-cell development is proportional to the number of effector T cells at the peak of the response (24), continued proliferation of activated CD8 T cells is expected to result in more memory T cells. The qualifying finding here is that continued proliferation of activated T cells leads to the generation of more TEM-like cells at the expense of TCM cells. This result is consistent with a large body of evidence that systemic infections in which antigen stimulation is prolonged generated abundant TEM cells (12). Because different pathogens exhibit different tissue tropisms and growth kinetics, variations in the distribution of antigen-bearing DCs may help account for differences in the development and distribution of TCM and TEM in diverse infections. Elucidation of the molecular mechanisms that govern the extent of exposure to antigen-bearing DCs to regulate memory cell development may lead to improved strategies for CD8 T-cell vaccines.
Materials and Methods
Mice.
2C TCR transgenic mice on the RAG1−/− and B6 background (2C+ RAG−/−) (25) were used as donors. B6, B6-CD11c-DTR/EGFP, B6-Thy1.1, and B6-CD45.1 mice (The Jackson Laboratory) were used as recipients at age 8–12 wk. All studies with animals were conducted in compliance with institutional guidelines.
Flow Cytometry.
SIY peptide was bound noncovalently to H-2Kb:Ig fusion protein (BD Biosciences) to stain SIY-Kb–specific T cells. In B6 recipient mice, 2C cells were identified by costaining with anti-CD8 and 1B2 antibodies specific for the 2C TCR. In B6-Thy1.1 or B6-CD45.1 recipient mice, 2C cells also were identified by staining with anti-CD8 together with anti-Thy1.2 or anti-CD45.2 antibodies. For IFN-γ staining, CD8+ cells from spleen of B6 recipient mice first were enriched with the CD8α+ T-cell isolation kit. Aliquots of cells were cocultured with 1 × 106 splenocytes from naive B6-CD45.1 mice in the presence or absence of SIY peptide (10 μg/mL) for 4 h. Secretion of IFN-γ from 2C cells was detected with the Mouse IFN-γ Secretion Assay Detection Kit (Miltenyi Biotec Inc.). Samples were analyzed on a FACSCalibur flow cytometer (BD Biosciences) with FlowJo software (Tree Star Inc.).
Infection, Cell Preparation, Adoptive Transfer, and Recall Response.
Naive 2C cells were injected i.v. into B6, B6-Thy1.1, or B6-CD45.1 recipient mice. One day later, recipient mice were infected i.n. with 100 pfu of WSN-SIY virus (14). For adoptive transfer of activated 2C cells or endogenous SIY-Kb–specific T cells, CD8+ cells from various organs were enriched at the indicated times with the CD8α+ T-cell isolation kit (Miltenyi Biotec Inc.) followed by flow cytometry to determine the percentage of 2C cells or endogenous SIY-Kb–specific T cells. Total CD8+ cells containing the indicated numbers of 2C cells or endogenous SIY-Kb–specific T cells were injected i.v. into recipient mice. In some experiments, cells were labeled with CFSE (5 μM) at room temperature for 10 min before adoptive transfer. In other experiments, recipient mice were injected with BMDCs (see below) concomitantly or with SIY peptide (5 μg daily) i.p. for 2 or 4 d starting 1 d before 2C cell transfer. For adoptive transfer of sorted memory 2C cells (either Thy1.2+ or CD45.2+ and CD8+), cells were isolated from the recipient mice, sorted by the MoFlow cell sorter (BD Biosciences) based on the expression level of CD62L, and transferred into naïve mice. For recall responses of transferred T cells, the recipient mice were infected i.n. with 100 pfu of the WSN-SIY virus, and 7 d later cells from BAL fluid were analyzed by flow cytometry for either 2C cells or Thy1.1+ SIY-Kb CD8 T cells. The recipients’ own endogenous antigen (SIY/Kb)-specific CD8+ T cells (Thy1.2+) also were measured in the BAL fluid in some experiments. These primary responses by Thy1.2+ T cells served as internal controls that ensured adequacy of the challenge virus infection (Fig. S9). To measure virus titers, BAL fluid was collected at 3 dpi, and plaque assays were performed with Madin–Darby canine kidney cells.
T-Cell Priming and Proliferation in DC-Depleted CD11c-DTR/EGFP Mice.
Depletion of DCs in CD11c-DTR/EGFP mice has been described previously (16). For T-cell priming assays, CD11c-DTR/EGFP mice were infected with WSN-SIY virus, and at the indicated times mice were injected i.p. with either diphtheria toxin (4 ng/g body weight) or an equal volume of PBS. CFSE-labeled naïve 2C cells (2.5 × 106) were transferred into mice 1 dpi and were retrieved 24 h later from different organs for in vitro culture for 3 d in the presence of IL-2 (10 ng/mL). CFSE profiles of 2C cells were analyzed by flow cytometry. For measuring cell proliferation and recall potentials of activated 2C cells, naive 2C cells (1 × 105) were injected into CD11c-DTR/EGFP mice, followed by i.n. infection with WSN-SIY virus. Mice were injected with either diphtheria toxin or PBS 5 dpi, and splenocytes were isolated 7 dpi. For proliferation profile analysis, cells were labeled with CFSE and were cultured for 3 d in vitro, and CFSE profiles were analyzed by flow cytometry as described above. For recall potential analysis, activated 2C cells (2 × 104) from the splenocytes were transferred into B6 recipients and were parked for 23 d, and recall responses were measured as described above (i.n. infection with WSN-SIY virus followed 7 d later by counting 2C cells in BAL fluid).
Preparation of Peptide-Loaded BMDCs.
BMDCs from naive B6 mice were generated as described (26). For activation, BMDCs were treated with LPS (10 μg/mL) at 37 °C for 24 h. After being washed twice with medium, cells were incubated with SIY or OVA peptide (SIINFEKL) (5 μg/mL) at 37 °C for 2 h. BMDCs (1 × 106) then were washed once with medium and injected i.v. into B6 recipient mice.
Statistical Analysis.
Logarithmic transformation (log10) was applied to cell numbers obtained from recall responses, and unpaired one-tailed t tests were performed for statistical analysis.
Supplementary Material
Acknowledgments
We thank members of the J.C. laboratory for discussion and Camille Jusino for technical assistance. This work was supported in part by National Institutes of Health Grant AI69208 (to J.C.), funds from the Singapore-MIT Alliance (to J.C.), and a Cancer Center Core Grant (to T. Jacks).
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1016350108/-/DCSupplemental.
References
- 1.Wherry EJ, et al. Lineage relationship and protective immunity of memory CD8 T cell subsets. Nat Immunol. 2003;4:225–234. doi: 10.1038/ni889. [DOI] [PubMed] [Google Scholar]
- 2.Bouneaud C, Garcia Z, Kourilsky P, Pannetier C. Lineage relationships, homeostasis, and recall capacities of central- and effector-memory CD8 T cells in vivo. J Exp Med. 2005;201:579–590. doi: 10.1084/jem.20040876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Surh CD, Boyman O, Purton JF, Sprent J. Homeostasis of memory T cells. Immunol Rev. 2006;211:154–163. doi: 10.1111/j.0105-2896.2006.00401.x. [DOI] [PubMed] [Google Scholar]
- 4.Joshi NS, Kaech SM. Effector CD8 T cell development: A balancing act between memory cell potential and terminal differentiation. J Immunol. 2008;180:1309–1315. doi: 10.4049/jimmunol.180.3.1309. [DOI] [PubMed] [Google Scholar]
- 5.Ahmed R, Gray D. Immunological memory and protective immunity: Understanding their relation. Science. 1996;272:54–60. doi: 10.1126/science.272.5258.54. [DOI] [PubMed] [Google Scholar]
- 6.Prlic M, Hernandez-Hoyos G, Bevan MJ. Duration of the initial TCR stimulus controls the magnitude but not functionality of the CD8+ T cell response. J Exp Med. 2006;203:2135–2143. doi: 10.1084/jem.20060928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Williams MA, Holmes BJ, Sun JC, Bevan MJ. Developing and maintaining protective CD8+ memory T cells. Immunol Rev. 2006;211:146–153. doi: 10.1111/j.0105-2896.2006.00389.x. [DOI] [PubMed] [Google Scholar]
- 8.Kedzierska K, et al. Location rather than CD62L phenotype is critical in the early establishment of influenza-specific CD8+ T cell memory. Proc Natl Acad Sci USA. 2007;104:9782–9787. doi: 10.1073/pnas.0703699104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hovav AH, et al. Duration of antigen expression in vivo following DNA immunization modifies the magnitude, contraction, and secondary responses of CD8+ T lymphocytes. J Immunol. 2007;179:6725–6733. doi: 10.4049/jimmunol.179.10.6725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Radcliffe JN, Roddick JS, Stevenson FK, Thirdborough SM. Prolonged antigen expression following DNA vaccination impairs effector CD8+ T cell function and memory development. J Immunol. 2007;179:8313–8321. doi: 10.4049/jimmunol.179.12.8313. [DOI] [PubMed] [Google Scholar]
- 11.Busch DH, Kerksiek KM, Pamer EG. Differing roles of inflammation and antigen in T cell proliferation and memory generation. J Immunol. 2000;164:4063–4070. doi: 10.4049/jimmunol.164.8.4063. [DOI] [PubMed] [Google Scholar]
- 12.Voehringer D, et al. Viral infections induce abundant numbers of senescent CD8 T cells. J Immunol. 2001;167:4838–4843. doi: 10.4049/jimmunol.167.9.4838. [DOI] [PubMed] [Google Scholar]
- 13.Williams MA, Bevan MJ. Shortening the infectious period does not alter expansion of CD8 T cells but diminishes their capacity to differentiate into memory cells. J Immunol. 2004;173:6694–6702. doi: 10.4049/jimmunol.173.11.6694. [DOI] [PubMed] [Google Scholar]
- 14.Shen C-H, et al. Loss of IL-7R and IL-15R expression is associated with disappearance of memory T cells in respiratory tract following influenza infection. J Immunol. 2008;180:171–178. doi: 10.4049/jimmunol.180.1.171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sallusto F, Lenig D, Förster R, Lipp M, Lanzavecchia A. Two subsets of memory T lymphocytes with distinct homing potentials and effector functions. Nature. 1999;401:708–712. doi: 10.1038/44385. [DOI] [PubMed] [Google Scholar]
- 16.Jung S, et al. In vivo depletion of CD11c+ dendritic cells abrogates priming of CD8+ T cells by exogenous cell-associated antigens. Immunity. 2002;17:211–220. doi: 10.1016/s1074-7613(02)00365-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Legge KL, Braciale TJ. Accelerated migration of respiratory dendritic cells to the regional lymph nodes is limited to the early phase of pulmonary infection. Immunity. 2003;18:265–277. doi: 10.1016/s1074-7613(03)00023-2. [DOI] [PubMed] [Google Scholar]
- 18.Kaech SM, Ahmed R. Memory CD8+ T cell differentiation: Initial antigen encounter triggers a developmental program in naïve cells. Nat Immunol. 2001;2:415–422. doi: 10.1038/87720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chang JT, et al. Asymmetric T lymphocyte division in the initiation of adaptive immune responses. Science. 2007;315:1687–1691. doi: 10.1126/science.1139393. [DOI] [PubMed] [Google Scholar]
- 20.Manjunath N, et al. Effector differentiation is not prerequisite for generation of memory cytotoxic T lymphocytes. J Clin Invest. 2001;108:871–878. doi: 10.1172/JCI13296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.van Stipdonk MJ, Lemmens EE, Schoenberger SP. Naïve CTLs require a single brief period of antigenic stimulation for clonal expansion and differentiation. Nat Immunol. 2001;2:423–429. doi: 10.1038/87730. [DOI] [PubMed] [Google Scholar]
- 22.Sarkar S, et al. Strength of stimulus and clonal competition impact the rate of memory CD8 T cell differentiation. J Immunol. 2007;179:6704–6714. doi: 10.4049/jimmunol.179.10.6704. [DOI] [PubMed] [Google Scholar]
- 23.Marzo AL, et al. Initial T cell frequency dictates memory CD8+ T cell lineage commitment. Nat Immunol. 2005;6:793–799. doi: 10.1038/ni1227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hou S, Hyland L, Ryan KW, Portner A, Doherty PC. Virus-specific CD8+ T-cell memory determined by clonal burst size. Nature. 1994;369:652–654. doi: 10.1038/369652a0. [DOI] [PubMed] [Google Scholar]
- 25.Chen J, Eisen HN, Kranz DM. A model T-cell receptor system for studying memory T-cell development. Microbes Infect. 2003;5:233–240. doi: 10.1016/s1286-4579(03)00016-9. [DOI] [PubMed] [Google Scholar]
- 26.Inaba K, et al. Generation of large numbers of dendritic cells from mouse bone marrow cultures supplemented with granulocyte/macrophage colony-stimulating factor. J Exp Med. 1992;176:1693–1702. doi: 10.1084/jem.176.6.1693. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.