Abstract
Cilia function as cell sensors in many organs, and their disorders are referred to as “ciliopathies.” Although ciliary components and transport machinery have been well studied, regulatory mechanisms of ciliary formation and maintenance are poorly understood. Here we show that male germ cell-associated kinase (Mak) regulates retinal photoreceptor ciliary length and subcompartmentalization. Mak was localized both in the connecting cilia and outer-segment axonemes of photoreceptor cells. In the Mak-null retina, photoreceptors exhibit elongated cilia and progressive degeneration. We observed accumulation of intraflagellar transport 88 (IFT88) and IFT57, expansion of kinesin family member 3A (Kif3a), and acetylated α-tubulin signals in the Mak-null photoreceptor cilia. We found abnormal rhodopsin accumulation in the Mak-null photoreceptor cell bodies at postnatal day 14. In addition, overexpression of retinitis pigmentosa 1 (RP1), a microtubule-associated protein localized in outer-segment axonemes, induced ciliary elongation, and Mak coexpression rescued excessive ciliary elongation by RP1. The RP1 N-terminal portion induces ciliary elongation and increased intensity of acetylated α-tubulin labeling in the cells and is phosphorylated by Mak. These results suggest that Mak is essential for the regulation of ciliary length and is required for the long-term survival of photoreceptors.
Cilia are evolutionally conserved microtubule-based organelles that extend from basal bodies and form on the apical surface of cells. In humans, ciliary dysfunction is associated with various diseases that can be broadly classified as “ciliopathies.” As exemplified by Bardet-Biedl syndrome (BBS), diseases linked with a defect in the primary cilia usually are associated with a broad spectrum of pathologies, including polydactyly, craniofacial abnormalities, brain malformation, situs inversus, obesity, diabetes, polycystic kidney, and retinal degeneration (1, 2). In vertebrates, many types of cells in the G1 phase develop cilia, but ciliary length varies in each cell type of different tissues (3). Retinal photoreceptor cells develop a light-sensory structure containing photopigments and light-transducing machinery, the outer segment. Outer segments are formed initially from the primary cilia in photoreceptor precursors (4, 5). The photoreceptor cilium is divided structurally into at least two subcompartments: the connecting cilia, distal to the basal body, and the axoneme in the outer segment, distal to the connecting cilia. The connecting cilium is analogous to the transitional zone of the motile cilia (6, 7). Connecting cilium connects the inner and outer segments of photoreceptors and is essential for protein transport between the inner and outer segments. Defects of the photoreceptor ciliary transport machinery (called “intraflagellar transport,” IFT) cause photoreceptor degeneration in model animals (8–10). The retinitis pigmentosa 1 (RP1) protein is localized specifically in the outer-segment axonemes in photoreceptors, which stabilizes cytosolic microtubules (11). A mutation in human RP1 generating a deletion of the RP1 C-terminal portion causes dominant retinitis pigmentosa (12).
Mechanisms of ciliogenesis have been well studied in the green algae Chlamydomonas reinhardtii. The Chlamydomonas LF4 mutant shows a long-flagella phenotype. LF4 encodes a protein highly similar to mammalian male germ cell-associated kinase (Mak) and intestinal cell kinase (ICK) (13). Loss of function of the LF4 homologs Caenorhabditis elegans dye-filling defective 5 (Dyf-5) and Leishmania Mexicana LmxMPK9 also causes slightly elongated cilia or flagella (14, 15). However, molecular regulatory mechanisms controlling ciliary length remain unknown. Mak was first identified as a gene highly expressed in testicular germ cells (16). Spermatogenesis of the Mak-KO mouse is intact (17). In addition to expression in the testis, Mak is also expressed in the retina (18, 19). However, the molecular function of Mak in the retina has not been reported yet.
Results
Mak Is Expressed in Photoreceptors in the Retina.
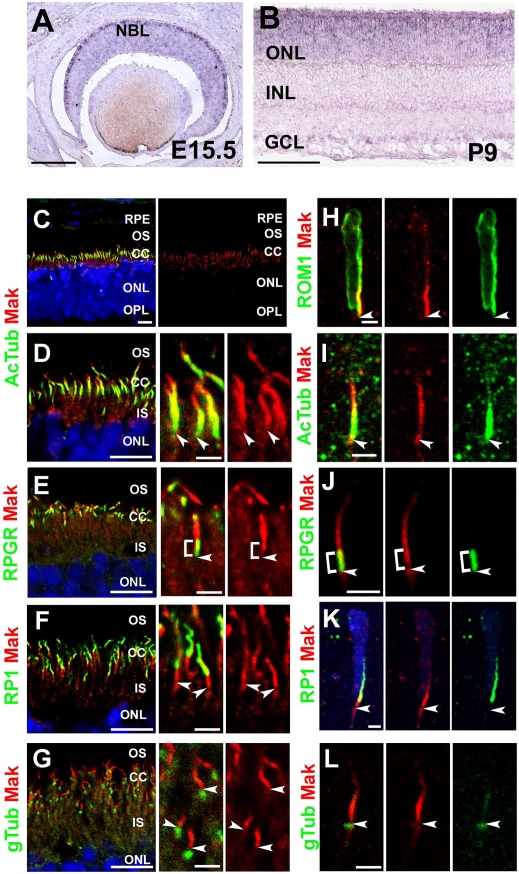
In the course of a microarray screening for genes specifically expressed in photoreceptors (20), we found that the Mak transcript is markedly reduced in the orthodenticle homeobox 2 (Otx2) conditional knockout (CKO) retina in which most of the photoreceptors are converted to amacrine-like cells (21). We confirmed by quantitative PCR analysis that Mak expression is markedly decreased in the Otx2 CKO retina at postnatal day 12 (P12) (Fig. S1A). From a retinal cDNA library we cloned an alternatively spliced form of Mak full-length cDNA containing a 75-bp in-frame insertion to the reported Mak cDNA (19) (Fig. S2). RT-PCR analysis revealed that this form is likely to be the major alternatively spliced form of the Mak transcript in the retina (four of six clones analyzed). To investigate Mak expression in the developing retina, we performed in situ hybridization analysis using a Mak probe (Fig. 1 A and B and Fig. S1 B–D). Mak expression was detected first at embryonic day 15.5 (E15.5) in the outer part of the neuroblastic layer (NBL), corresponding to photoreceptor precursors (Fig. 1A). Mak expression was restricted to the photoreceptor layer after birth (Fig. 1B and Fig. S1 C and D). These results indicate that Mak is expressed predominantly in photoreceptor cells in the retina.
Fig. 1.
Expression and subcellular localization of Mak in the retina. (A and B) In situ hybridization analysis of retinal sections at E15.5 (A) and postnatal day 9 (P9) (B). Mak mRNA is expressed in both photoreceptor precursors and developing photoreceptors in the retina. (C–L) Subcellular localization of Mak in photoreceptors. Retinal sections at P14 (E) and 1 mo (C, D, F, and G) and dissociated photoreceptor cells at P14 (H–L) were stained with anti-Mak (red in C–L) and anti-acetylated α-tubulin (a marker for cilia; green in C, D, and I), anti-RPGR (a marker for connecting cilia; green in E and J), anti-RP1 (a marker for outer-segment axonemes; green in F and K), anti-ROM1 (a marker for outer-segment disks, green in H), or anti–γ-tubulin (a marker for basal bodies; green in G and L) antibodies. [Scale bars: 100 μm (A and B), 2 μm (H–L and D–G Center and Right), and 10 μm (C and D–G Left).] Arrowheads (D–L) indicate basal body-connecting cilium junctions. Brackets (E and J) indicate connecting cilia. CC, connecting cilia; GCL, ganglion cell layer; INL, inner nuclear layer; IS, inner segments; NBL, neuroblastic layer; ONL, outer nuclear layer; OPL, outer plexiform layer OS, outer segments; RPE, retinal pigment epithelium.
Mak Is Localized in the Photoreceptor Connecting Cilia and Outer-Segment Axonemes.
To investigate the subcellular localization of Mak in photoreceptor cells, we immunostained retinal sections using an anti-Mak antibody. To eliminate cross-reaction with other kinases, we raised an antibody which recognizes the C-terminal portion of Mak. We confirmed that the anti-Mak antibody recognizes the major retinal variant of Mak with the 25-amino acid insertion (Fig. S1E). By immunostaining, we observed a layer of Mak signals between the retinal pigment epithelia and outer nuclear layer (ONL) corresponding to the photoreceptor cilia (Fig. 1C).
To identify the precise localization of Mak in the photoreceptor cilia, we immunostained the retina using the Mak antibody along with other ciliary markers including acetylated α-tubulin (a ciliary marker), retinitis pigmentosa GTPase regulator (RPGR, a connecting cilium marker) (7), RP1 (a marker for the outer-segment axonemes) (11), and rod outer-segment membrane protein 1 (ROM1, a marker for the outer-segment disks) (Fig. 1 C–L) (22). The Mak signal was observed broadly in the photoreceptor cilia overlapping with ROM1, RPGR, RP1 and acetylated α-tubulin signals (Fig. 1 C–F and H–K; for summary, see Fig. S9). We observed a slight signal of Mak overlapping with the γ-tubulin signal, a marker for basal bodies (Fig. 1 G and L). These results indicate that Mak is localized in both the connecting cilia and the outer-segment axonemes. Interestingly, the intensity of the Mak signal was not uniform in the photoreceptor cilia. The decreased Mak signal was observed in the proximal portion of the connecting cilia (Fig. 1 E and J) and the distal portion of the outer-segment axonemes (Fig. 1 F and K).
Photoreceptors Degenerate Progressively in the Mak-Deficient Retina.
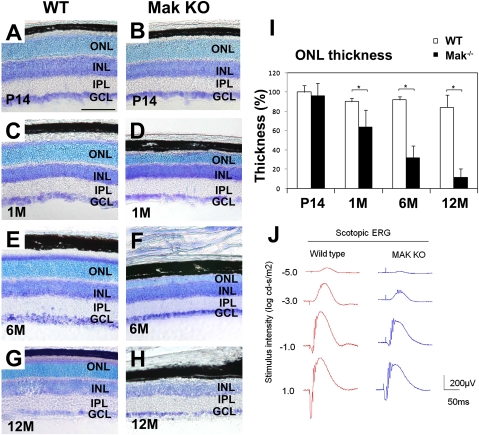
Mak-null mice were established previously, and it was reported that Mak is not essential for spermatogenesis, although Mak is highly expressed in the testis (17). To investigate in vivo Mak function in the retina, we analyzed this KO mouse. We confirmed that none of the normal Mak transcripts, including the major retinal variant of Mak with a 75-bp insertion, were expressed in the Mak-KO retina (Fig. S1F). Until retinogenesis was complete in the normal retina at postnatal day 14 (P14), the Mak-KO retina exhibited normal layering and cell composition, indicating that loss of Mak does not affect cell fates (Fig. 2 A and B and Fig. S3 A–F). We also analyzed the retinas at age 1 mo, 6 mo, and 12 mo. Notably, we found progressive degeneration of the ONL (a photoreceptor layer) in the Mak-KO retina after 1 mo (Fig. 2 C–I). This progressive ONL loss often is observed in animal models of retinitis pigmentosa and Leber's congenital amaurosis (23–27). We observed no obvious structural differences between the heterozygous and wild-type retinas at 6 mo (Fig. S4 A–C). The thickness of the other layers did not differ in the Mak-KO and wild-type retinas (Fig. S5A).
Fig. 2.
Loss of Mak leads to photoreceptor degeneration (A–H). Retinal sections from wild-type and Mak-KO mice at age P14 (A and B), 1 mo (C and D), 6 mo (E and F), and 12 mo (G and H) were stained with toluidine blue. Progressive degeneration of the ONL (a photoreceptor layer) occurs in the Mak-KO retina. (Scale bar: 100 μm.) IPL, inner plexiform layer. (I) Thickness of retinal layers was measured at age P14, 1 mo, 6 mo, and 12 mo. ONL thickness decreased progressively in the Mak-KO retina. Average of layer thickness in the wild-type retina at P14 was set to 100%. Error bars show SD. *P < 0.03. (J) ERGs recorded from Mak-KO mice. Scotopic ERGs elicited by four different stimulus intensities (−5.0 to 1.0 log cd-s/m2).
To examine if loss of Mak in the retina affects photoreceptor function, we recorded electroretinograms (ERG) from adult Mak-KO mice at age 3 mo. Both scotopic and photopic ERG amplitudes of Mak-KO mice were significantly smaller than those of the control mice (Fig. 2J and Fig. S5 B–D). These results show that the loss of Mak impairs the function of both rods and cones.
Cilia Are Elongated in Mak-KO Photoreceptors.
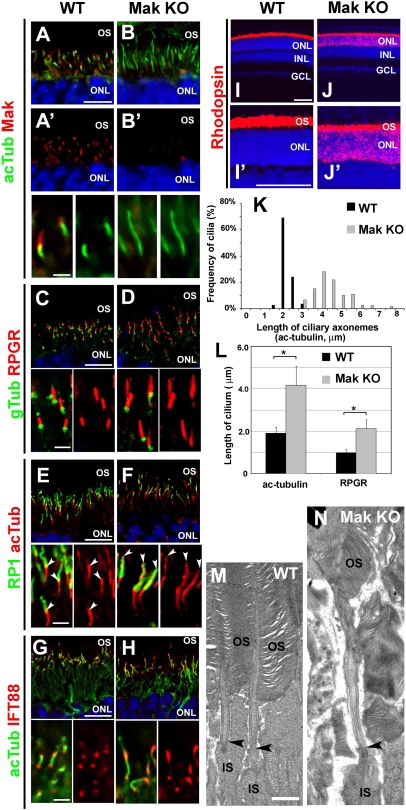
How does progressive photoreceptor death occur in the Mak-KO retina? To explore this question, we examined photoreceptors in the Mak-deficient retina by immunostaining using antibodies against photoreceptor ciliary markers. We first confirmed the loss of Mak in the photoreceptor cilia of the Mak-KO retina at P14 (Fig. 3 A, A′, B, and B′). Notably, we found that the cilia stained with the anti-acetylated α-tubulin antibody were markedly elongated in Mak-deficient rod photoreceptors (Fig. 3B). We measured ciliary length of rod photoreceptors and found that the acetylated α-tubulin–positive cilia in Mak-KO photoreceptors were approximately twice the length of wild-type cilia (Fig. 3 K and L). We also found that cone photoreceptor cilia were elongated in the Mak-null retina (Fig. S6 A–D). To investigate whether ciliary subcompartments are affected in Mak-KO photoreceptors, we immunostained for RPGR (a marker of the connecting cilia) in the Mak-KO retina. We observed an approximately twofold elongation of the RPGR-positive connecting cilia in the Mak-KO retina (Fig. 3 C, D, and L and Fig. S6E). In contrast, γ-tubulin staining (a basal body marker) showed no significant difference between Mak-KO and wild-type photoreceptors (Fig. 3 C and D). Next, we stained for RP1, a marker of the outer-segment axonemes. Unexpectedly, we observed excessively long acetylated α-tubulin labeling in the outer-segment axonemes (Fig. 3 E and F). In most of the wild-type photoreceptors, an acetylated α-tubulin signal is observed in less than half of the proximal portion of the RP1-positive outer-segment axoneme, whereas in the Mak-KO photoreceptors often almost all the outer-segment axoneme is acetylated α-tubulin signal-positive. The percentage of excessively long acetylated α-tubulin labeling of the outer-segment axonemes (wherein more than half of the distal portion of the outer-segment axoneme is acetylated α-tubulin signal-positive) is 5.1% (n = 59) in the wild-type photoreceptors, whereas in Mak-KO photoreceptors 83.3% (n = 66) of the axonemes have excessively long acetylated α-tubulin labeling. The distance from the top of the outer segment to the top of the outer-segment axonemes stained with acetylated α-tubulin decreased in Mak-KO photoreceptors (Fig. S6 F and G). These results demonstrate that loss of Mak affects both subcompartments of the cilia, the connecting cilia and outer-segment axonemes.
Fig. 3.
Ciliary defect in Mak-null photoreceptors. (A–H) Immunohistochemical analysis of the Mak-null photoreceptor cilia. Retinal sections from wild-type mice (A, A′, C, E, and G) and Mak-KO mice (B, B′, D, F, and H) at age P14 (A, A′, B, and B′) and 1 mo (C–H) were stained with anti-Mak (red in A, A′, B, and B′), anti-acetylated α-tubulin (a ciliary marker; green in A, B, G, and H; red in E and F), anti-RPGR (a connecting cilium marker; red in C and D), anti-RP1 (a marker for the outer-segment axonemes; green in E and F), anti-IFT88 (a component of IFT complex; red in G and H) or anti–γ-tubulin (a marker for the basal bodies; green in C and D) antibodies. Arrowheads in E and F indicate the distal tips of acetylated microtubules in the outer-segment axonemes. (I and J) Rhodopsin is mislocalized in the Mak-KO retina. Retinal sections from wild-type mice (I and I′) and Mak-KO mice (J and J′) at age P14 were stained with an anti-rhodopsin antibody. [Scale bars: 10 μm (A, C, E, and G, Upper), 100 μm (I and I′), and 2 μm (A′, C, E, and G, Lower).] (K and L) Length of the ciliary axonemes stained with the anti-acetylated α-tubulin antibody (K and L) and connecting cilia stained with the anti-RPGR (L) antibody in the wild-type photoreceptors (black bars) and Mak-KO photoreceptors (gray bars) were measured. Error bars show SE. *P < 0.03. (M and N) Longitudinal profiles of the connecting cilia in 1-mo-old wild-type photoreceptors (M) and Mak-KO photoreceptors (N) observed by electron microscopy. Arrowheads indicate the basal body-connecting cilium junctions in the photoreceptors. (Scale bar in M: 1 μm.)
To examine whether loss of Mak affects the anterograde IFT and kinesin motors, we stained photoreceptor cilia with anti-IFT88, anti-IFT57, and anti-Kif3a antibodies. We observed that both IFT88 and IFT57 were concentrated on two portions, the tip of the connecting cilia at the outer-segment base and the basal part of the connecting cilia, as previously reported (28). Notably, in Mak-KO photoreceptors, we found that IFT88 and IFT57 were accumulated in outer-segment axonemes (Fig. 3 G and H and Fig. S6 H and I). In wild-type photoreceptors, the Kif3a staining overlaps with the acetylated α-tubulin staining and is concentrated on the basal part of the cilia (Fig. S6 J and K). In Mak-KO photoreceptors, the Kif3a staining extends along the elongated acetylated α-tubulin–positive cilia (Fig. S6 J and K). Interestingly, we also observed an accumulation of rhodopsin in the Mak-KO photoreceptor cell bodies at P14 (Fig. 3 I, I′, J, and J′).
Mak is expressed in epithelia of the nasal cavity and the testis (16, 19). Respiratory epithelia of the nasal cavity have multiple motile cilia which display the 9+2 microtubule structure. We observed Mak localizing in the cilia of the respiratory epithelia (Fig. S6L). The Mak signal is reduced in the Mak-KO nasal cavity (Fig. S6M). However, the ciliary length of respiratory epithelia did not differ in wild-type and Mak-KO mice (Fig. S6 L–N). Similarly, we also found that the acetylated-α-tubulin–positive flagellar length of epididymal sperm does not differ in wild-type and Mak-KO mice (Fig. S6 O–Q).
Aberrant Outer-Segment Disk Formation in Mak-Deficient Photoreceptors.
The 9+0 axonemes of the photoreceptor cilia are assembled by nine peripheral doublet microtubules without a central microtubule (3). To test if loss of Mak affects ultrastructural microtubule organization in the photoreceptor cilia, we performed an electron microscopic analysis. We observed no significant change in ciliary ultrastructures, including the array of the 9+0 microtubule doublets in transverse sections of the connecting cilium in Mak-KO photoreceptors (Fig. S7 A and B). Consistent with the results of the immunofluorescent analysis, we found elongated connecting cilium in the Mak-KO photoreceptors at age 1 mo in a longitudinal section of the connecting cilia (100 ± 2% in wild type, n = 12; 233 ± 22% in Mak-KO, n = 9; P < 0.03) (Fig. 3 M and N).
In both vertebrate photoreceptors and nematode amphid channel cilia, the proximal microtubules of the axonemes are doublets, whereas distal microtubules are singlets (29, 30). In the distal segment of the amphid channel cilia in dyf-5 animals, singlet microtubules were observed (14). We observed singlet microtubules in outer-segment axonemes, which are positioned near the disk clefts, in both wild-type and Mak-null retinas (Fig. S7 C–F).
We observed severely disorganized Mak-KO outer segments at age 1 mo compared with the wild type (Fig. 3 M and N). In contrast, disk rim formation seems to be intact in the Mak-null outer segments at age 1 mo (Fig. S7 G and H). To examine whether the outer-segment disorganization observed in Mak-deficient retinas is caused by a developmental defect or degeneration after normal development, we observed photoreceptor outer segments at P14 by electron microscopy. At this stage, the outer segments are still developing (31). In wild-type photoreceptors, the stack of disk membranes in the outer segments is oriented perpendicular to the long axis of the outer segments (Fig. S7 I and I′). In the Mak-KO retina, however, we observed that the disk membranes were frequently oriented obliquely or parallel to the long axis of the outer segments (Fig. S7 J and J′). In addition, the disk diameters are approximately two to four times larger in Mak-null than in wild-type outer segments (Fig. S7 I′ and J′). Enlarged disks oriented obliquely or parallel to the long axis of photoreceptors were reported in mutant mice including RP1-mutant and RPGRIP1-null mice (24, 25). These results suggest the disorganization of the outer segment observed in the Mak-KO retinas is, at least partially, the result of a developmental defect in outer-segment formation.
Mak Overexpression Reduces Ciliary Elongation in Cultured Cells.
To investigate the mechanisms by which Mak regulates ciliary length, we established a cultured cell system in which NIH 3T3 fibroblast cells develop cilia at a high frequency within 24 h after serum starvation (Fig. S8). We prepared FLAG-tagged constructs expressing a full-length wild-type Mak (Mak-WT), a kinase-dead mutant Mak (Mak-KD), and a deletion-mutant Mak lacking the C-terminal nonkinase domain (Mak-N) (Fig. S8F). The Mak-KD construct was generated by replacing a lysine residue (K33) located in the ATP-binding pocket of the Mak kinase domain with an arginine residue (32).
We transfected these constructs into NIH 3T3 cells and measured ciliary length. We found that the cells transfected with the wild-type Mak construct had shorter cilia than cells transfected with the control constructs (Fig. S8 A, B, and G). On the other hand, cells transfected with the Mak-KD or Mak-N construct showed no significant change in ciliary length (Fig. S8 D, E, and G), showing that kinase activity and/or the C-terminal region of Mak is essential for the regulation of ciliary length.
We then investigated the subcellular localization of Mak in transfected cells using an anti-FLAG antibody. We observed that Mak-WT was localized mainly in the nuclei as previously reported (32). As expected from the ciliary localization of Mak in photoreceptors, we observed that Mak-WT is also localized in the cilia of transfected cells. Mak localization was restricted to the tip of the shortened cilia (Fig. S8B). Although we rarely observed elongated cilia in Mak-WT–transfected cells, ciliary tip localization of Mak-WT was observed in those cells (Fig. S8C). We found that Mak-KD is also localized at the ciliary tip, suggesting that kinase activity is not required for the ciliary localization of Mak. In contrast, Mak-N was not localized in the cilia, showing that the C-terminal portion of Mak is essential for the ciliary localization of Mak.
RP1 Induces Ciliary Elongation and Reduces the Effect of Mak Overexpression.
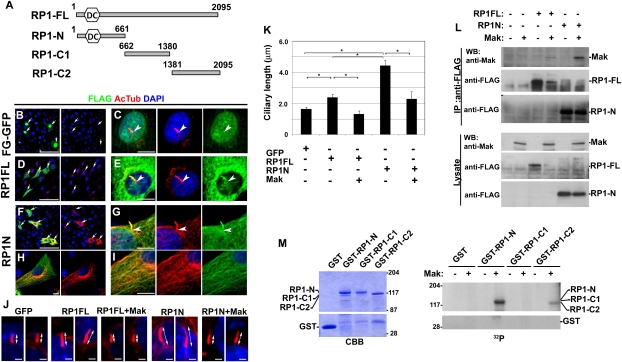
It was previously reported that knock-in mice with a partial deletion of the RP1 gene exhibited shorten cilia (24). As we described above, we found colocalization of Mak with RP1 in the ciliary axoneme of wild-type photoreceptors. Mak-KO photoreceptors exhibited excessively long acetylated α-tubulin labeling. These observations prompted us to investigate whether RP1 is involved in the mechanisms by which Mak regulates ciliary length. To do so, we prepared constructs expressing RP1 and transfected them with or without the Mak-expressing constructs (Fig. 4A). We observed increased ciliary length in the cells transfected with full-length RP1 (RP1-FL) (Fig. 4 B–E, J, and K). In humans, the mutations in the RP1 gene generating deletion of the C-terminal portion of RP1 cause dominant retinitis pigmentosa (12). Interestingly, the intensity of acetylated α-tubulin labeling significantly increased in cells expressing the N-terminal RP1 (RP1-N) construct containing the doublecortin domain, indicating that the cytoplasmic microtubules are more stable in these cells (Fig. 4 F–I). Coimmunostaining of FLAG-tag with acetylated α-tubulin showed that RP1-FL and RP1-N were localized in a large portion of the distal cilia but not in the basal cilia, a putative transition zone (Fig. 4 E and G). These results suggest that RP1 is a positive regulator of ciliary length. Notably, cotransfection of Mak with RP1-FL or RP1-N constructs rescued the excessive elongation of the cilia (Fig. 4 J and K). This result suggests that a functional balance between Mak and RP1 is essential for the regulation of ciliary length and proper formation of the ciliary subcompartments. To test whether Mak rescues increased acetylated α-tubulin signals in cells expressing RP1-N, we cotransfected Mak with an RP1-N construct and observed the acetylated α-tubulin signal levels in the cells. We found that expression of Mak significantly decreased the intensity of acetylated α-tubulin labeling in the cells expressing RP1-N (Fig. S8 H–J).
Fig. 4.
RP1 controls ciliary length and is phosphorylated by Mak. (A–I) Overexpression of RP1 induces ciliary elongation. (A) Schematic diagrams of the RP1-FL, -N, -C1 and -C2 constructs. DC, doublecortin domain. (B–I) FLAG-tagged constructs expressing GFP (B and C), RP1-FL (D and E) or RP1 lacking the C-terminal portion (RP1-N) (F–I) were transfected into NIH 3T3 cells. Localization of FLAG-tagged proteins was observed using anti-FLAG (green) and anti-acetylated α-tubulin (red) antibodies and DAPI (blue). Arrows indicate transfected cells. Arrowheads indicate basal part of cilia. (J and K) RP1 and Mak antagonistically regulate ciliary length. FLAG-tagged constructs expressing GFP, RP1-FL, or RP1-N were transfected with or without a Mak expression plasmid into NIH 3T3 cells. (J) Cilia were observed using the anti-acetylated α-tubulin (red) antibody. (K) The length of the cilia stained with the anti-acetylated α-tubulin antibody (n > 30 for each construct). Error bars show SE. *P < 0.03. (L) Mak interacts with RP1. A Mak expression plasmid was transfected with or without FLAG-tagged RP1 expression plasmids (RP1-FL or RP1-N) into HEK293 cells. RP1 proteins were immunoprecipitated with the anti-FLAG antibody. Immunoprecipitated Mak was detected by Western blotting analysis using the anti-Mak antibody. (M) Mak phosphorylates RP1 in vitro. GST-RP1-N (residues 1–661), GST-RP1-C1 (residues 662–1,380), and GST-RP1-C2 (residues 1,381–2,095) were purified from bacterial extracts and stained with Coomassie brilliant blue (CBB) (Left). GST-RP1 deletion proteins were applied for the in vitro kinase assay using purified GST-Mak (Right). [Scale bars: 100 μm (B, D, and F), 10 μm (C, E, G, H, and I), and 2 μm (J).]
To assess whether Mak physically interacts with RP1, we performed an immunoprecipitation assay. We expressed Mak and FLAG-tagged full-length RP1 or RP1-N in HEK293 cells and performed an immunoprecipitation with an anti-FLAG antibody. We found specific interactions of Mak with both RP1-FL and RP1-N (Fig. 4L).
Then, to examine the possibility that Mak directly phosphorylates RP1, we performed a kinase assay using purified GST-Mak. Interestingly, we found that GST-RP1-N was markedly phosphorylated by Mak, whereas no obvious phosphorylation of the GST-RP1 C-terminal (GST-RP1-C1) construct or GST alone was detected (Fig. 4M). We observed weak phosphorylation of GST-RP1-C2 by Mak. To characterize the kinase activity of Mak with the RP1-N substrate, we performed a kinetic analysis. We found that the Km value for ATP was 19 μM (Fig. S8K). In addition, we confirmed that Mak-phosphorylated RP1-N was dephosphorylated by λ-phosphatase (Fig. S8L). These results support the idea that RP1 is a phosphorylation target of Mak.
Discussion
In the current study, we show that Mak is essential for preventing excessive elongation of the cilia and for maintenance of photoreceptor cells. Our observations in the Mak-KO retina suggest that a negative regulatory mechanism of ciliary length is essential for long-term photoreceptor survival, suggesting that this mechanism is involved in the pathogenesis of human photoreceptor degenerative diseases such as retinitis pigmentosa, Leber's congenital amaurosis, and BBS. In addition, similar negative regulatory mechanisms of ciliary length might be involved in the pathogenesis of other ciliopathies including polydactyly, craniofacial abnormalities, brain malformation, situs inversus, obesity, diabetes, and polycystic kidney (1, 2).
How does the aberrant ciliary elongation in Mak-KO photoreceptors induce progressive photoreceptor death? One possible explanation is that the abnormally elongated cilia affect protein transport from the inner segments to the outer segments in photoreceptors, resulting in photoreceptor degeneration. Several lines of evidence support this idea. We observed an accumulation of rhodopsin in the Mak-KO photoreceptor cell bodies in the retina at P14. The transport efficiency of rhodopsin from the inner to the outer segments through the connecting cilia would be reduced in Mak-KO photoreceptors. Mutations in rhodopsin or protein transport machinery of the cilia (e.g., Kif3a or IFT mutants) cause accumulation of rhodopsin in the photoreceptor cell body and result in photoreceptor cell death. Absence of Dyf-5, a nematode homolog of Mak, affects the motility of kinesin motors and IFT particles in the cilia (14). Similarly, we identified aberrant accumulations of IFT and kinesin in the Mak-KO photoreceptor cilia. Mak may regulate ciliary transport by directly phosphorylating the components of ciliary transport machinery including kinesin and dynein motors, IFT particles, and/or BBSomes (1, 3, 33).
We demonstrate that overexpression of wild-type RP1 induces ciliary elongation. In addition, expression of the N-terminal portion of RP1 induces an increased intensity of acetylated α-tubulin labeling in cultured cells. These results suggest that excess activation of the microtubule-associated protein RP1 can induce excess ciliary elongation. The evidence shown here supports the idea that Mak regulates ciliary elongation through RP1 phosphorylation. First, we observed that coexpression of Mak with the RP1 constructs rescued the excess ciliary elongation. Second, we identified that Mak phosphorylates the N-terminal portion of RP1, which contains the doublecortin domain. This domain was originally identified in Doublecortin, whose mutations cause X-linked lissencephaly and double cortex syndrome in humans (34). Interestingly, phosphorylation of Doublecortin by several kinases, including JNK, protein kinase A, and cyclin-dependent kinase 5, was shown to regulate affinity to microtubules and migration of neurons (35, 36). Similarly, it is possible that phosphorylation of RP1 by Mak regulates microtubule stability and controls ciliary length (Fig. S9). Retinitis pigmentosa 1-like 1 (RP1L1), a putative microtubule-associated protein, is another candidate for phosphorylation by Mak (37). In contrast to the restricted localization of RP1 in the axonemes of the outer segments, RP1L1 is localized both in the connecting cilium and the outer-segment axoneme, suggesting its involvement in the mechanisms regulating the length of connecting cilium in photoreceptors. How does Mak affect the intensity of acetylated α-tubulin labeling in the cilia? The first possibility is that the change of microtubule-binding status of microtubule-associated proteins by phosphorylation leads to the activation of enzymes involved in microtubule acetylation or deacetylation, because several microtubule-associated proteins were reported to induce microtubule acetylation (38). The second possibility is that Mak directly regulates enzymes involved in microtubule acetylation and/or deacetylation. It was reported that Aurora A regulates ciliary disassembly before cell-cycle entry through phosphorylation of tubulin deacetylase, histone deacetylase 6 (HDAC6). Phosphorylated by Aurora A, HDAC6 deacetylates microtubules of the cilia and facilitates disassembly of the cilia (39). Furthermore, microtubule acetylation causes the recruitment of the molecular motors dynein and kinesin to microtubules (40). In the developing photoreceptor cilia, regulatory balance of acetylation and deacetylation of ciliary microtubules seems to be important for keeping proper ciliary length and/or ciliary transport machinery. Mak may regulate this balance by phosphorylation of these molecules.
Materials and Methods
Animals.
We used Mak-KO mice with a deletion of exons 5–8 in the Mak genomic locus which encodes the catalytic kinase domain and the proline and glutamine-rich domain as previously reported (17). The Mak-KO mouse strain was provided by RIKEN BioResource Center through the National Bio-Resource Project of the Japanese Government Ministry of Education, Culture, Sports, Science and Technology. Reagents and procedures are described in detail in SI Materials and Methods.
Supplementary Material
Acknowledgments
We thank Drs. Y. Shinkai (Kyoto University), T. Li (National Institutes of Health), E. A. Pierce (University of Pennsylvania School of Medicine), J. Zuo (St. Jude Children's Research Hospital, Utah), T. Yamashita (St. Jude Children's Research Hospital), G. J. Pazour (University of Massachusetts Medical School), J. C. Besharse (Medical College of Wisconsin), and H. J. Kung (University of California, Davis) for reagents and technical advice. We thank M. Kadowaki, M. Joukan, A. Tani, T. Tsujii, A. Ishimaru, Y. Saioka, K. Sone, and S. Kennedy for technical assistance. This work was supported by Core Research for Evolutional Science and Technology and Precursory Research for Embryonic Science and Technology from the Japan Science and Technology Agency, a grant from Molecular Brain Science, Grants-in-Aid for Scientific Research on Priority Areas and a Grant-in-Aid for Scientific Research (B), Young Scientists (B), the Takeda Science Foundation, the Uehara Memorial Foundation, Novartis Foundation, Senri Life Science Foundation, Kato Memorial Bioscience Foundation, the Naito Foundation, Mochida Memorial Foundation for Medical and Pharmaceutical Research, and the Japan National Society for the Prevention of Blindness.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1009437108/-/DCSupplemental.
References
- 1.Gerdes JM, Davis EE, Katsanis N. The vertebrate primary cilium in development, homeostasis, and disease. Cell. 2009;137:32–45. doi: 10.1016/j.cell.2009.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nigg EA, Raff JW. Centrioles, centrosomes, and cilia in health and disease. Cell. 2009;139:663–678. doi: 10.1016/j.cell.2009.10.036. [DOI] [PubMed] [Google Scholar]
- 3.Fliegauf M, Benzing T, Omran H. When cilia go bad: Cilia defects and ciliopathies. Nat Rev Mol Cell Biol. 2007;8:880–893. doi: 10.1038/nrm2278. [DOI] [PubMed] [Google Scholar]
- 4.Tokuyasu K, Yamada E. The fine structure of the retina studied with the electron microscope. IV. Morphogenesis of outer segments of retinal rods. J Biophys Biochem Cytol. 1959;6:225–230. doi: 10.1083/jcb.6.2.225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rosenbaum JL, Witman GB. Intraflagellar transport. Nat Rev Mol Cell Biol. 2002;3:813–825. doi: 10.1038/nrm952. [DOI] [PubMed] [Google Scholar]
- 6.Röhlich P. The sensory cilium of retinal rods is analogous to the transitional zone of motile cilia. Cell Tissue Res. 1975;161:421–430. doi: 10.1007/BF00220009. [DOI] [PubMed] [Google Scholar]
- 7.Hong DH, et al. RPGR isoforms in photoreceptor connecting cilia and the transitional zone of motile cilia. Invest Ophthalmol Vis Sci. 2003;44:2413–2421. doi: 10.1167/iovs.02-1206. [DOI] [PubMed] [Google Scholar]
- 8.Marszalek JR, et al. Genetic evidence for selective transport of opsin and arrestin by kinesin-II in mammalian photoreceptors. Cell. 2000;102:175–187. doi: 10.1016/s0092-8674(00)00023-4. [DOI] [PubMed] [Google Scholar]
- 9.Pazour GJ, et al. The intraflagellar transport protein, IFT88, is essential for vertebrate photoreceptor assembly and maintenance. J Cell Biol. 2002;157:103–113. doi: 10.1083/jcb.200107108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Omori Y, et al. Elipsa is an early determinant of ciliogenesis that links the IFT particle to membrane-associated small GTPase Rab8. Nat Cell Biol. 2008;10:437–444. doi: 10.1038/ncb1706. [DOI] [PubMed] [Google Scholar]
- 11.Liu Q, Zuo J, Pierce EA. The retinitis pigmentosa 1 protein is a photoreceptor microtubule-associated protein. J Neurosci. 2004;24:6427–6436. doi: 10.1523/JNEUROSCI.1335-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pierce EA, et al. Mutations in a gene encoding a new oxygen-regulated photoreceptor protein cause dominant retinitis pigmentosa. Nat Genet. 1999;22:248–254. doi: 10.1038/10305. [DOI] [PubMed] [Google Scholar]
- 13.Berman SA, Wilson NF, Haas NA, Lefebvre PA. A novel MAP kinase regulates flagellar length in Chlamydomonas. Curr Biol. 2003;13:1145–1149. doi: 10.1016/s0960-9822(03)00415-9. [DOI] [PubMed] [Google Scholar]
- 14.Burghoorn J, et al. Mutation of the MAP kinase DYF-5 affects docking and undocking of kinesin-2 motors and reduces their speed in the cilia of Caenorhabditis elegans. Proc Natl Acad Sci USA. 2007;104:7157–7162. doi: 10.1073/pnas.0606974104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bengs F, Scholz A, Kuhn D, Wiese M. LmxMPK9, a mitogen-activated protein kinase homologue affects flagellar length in Leishmania mexicana. Mol Microbiol. 2005;55:1606–1615. doi: 10.1111/j.1365-2958.2005.04498.x. [DOI] [PubMed] [Google Scholar]
- 16.Matsushime H, Jinno A, Takagi N, Shibuya M. A novel mammalian protein kinase gene (mak) is highly expressed in testicular germ cells at and after meiosis. Mol Cell Biol. 1990;10:2261–2268. doi: 10.1128/mcb.10.5.2261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shinkai Y, et al. A testicular germ cell-associated serine-threonine kinase, MAK, is dispensable for sperm formation. Mol Cell Biol. 2002;22:3276–3280. doi: 10.1128/MCB.22.10.3276-3280.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Blackshaw S, et al. Genomic analysis of mouse retinal development. PLoS Biol. 2004;2:E247. doi: 10.1371/journal.pbio.0020247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bladt F, Birchmeier C. Characterization and expression analysis of the murine rck gene: A protein kinase with a potential function in sensory cells. Differentiation. 1993;53:115–122. doi: 10.1111/j.1432-0436.1993.tb00651.x. [DOI] [PubMed] [Google Scholar]
- 20.Sato S, et al. Pikachurin, a dystroglycan ligand, is essential for photoreceptor ribbon synapse formation. Nat Neurosci. 2008;11:923–931. doi: 10.1038/nn.2160. [DOI] [PubMed] [Google Scholar]
- 21.Nishida A, et al. Otx2 homeobox gene controls retinal photoreceptor cell fate and pineal gland development. Nat Neurosci. 2003;6:1255–1263. doi: 10.1038/nn1155. [DOI] [PubMed] [Google Scholar]
- 22.Bascom RA, et al. Cloning of the cDNA for a novel photoreceptor membrane protein (rom-1) identifies a disk rim protein family implicated in human retinopathies. Neuron. 1992;8:1171–1184. doi: 10.1016/0896-6273(92)90137-3. [DOI] [PubMed] [Google Scholar]
- 23.Hong DH, et al. A retinitis pigmentosa GTPase regulator (RPGR)-deficient mouse model for X-linked retinitis pigmentosa (RP3) Proc Natl Acad Sci USA. 2000;97:3649–3654. doi: 10.1073/pnas.060037497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Liu Q, Lyubarsky A, Skalet JH, Pugh EN., Jr, Pierce EA. (2003) RP1 is required for the correct stacking of outer segment discs. Invest Ophthalmol Vis Sci. 44:4171–4183. doi: 10.1167/iovs.03-0410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhao Y, et al. The retinitis pigmentosa GTPase regulator (RPGR)- interacting protein: Subserving RPGR function and participating in disk morphogenesis. Proc Natl Acad Sci USA. 2003;100:3965–3970. doi: 10.1073/pnas.0637349100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mykytyn K, et al. Bardet-Biedl syndrome type 4 (BBS4)-null mice implicate Bbs4 in flagella formation but not global cilia assembly. Proc Natl Acad Sci USA. 2004;101:8664–8669. doi: 10.1073/pnas.0402354101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ross AJ, et al. Disruption of Bardet-Biedl syndrome ciliary proteins perturbs planar cell polarity in vertebrates. Nat Genet. 2005;37:1135–1140. doi: 10.1038/ng1644. [DOI] [PubMed] [Google Scholar]
- 28.Sedmak T, Wolfrum U. Intraflagellar transport molecules in ciliary and nonciliary cells of the retina. J Cell Biol. 2010;189:171–186. doi: 10.1083/jcb.200911095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Insinna C, Besharse JC. Intraflagellar transport and the sensory outer segment of vertebrate photoreceptors. Dev Dyn. 2008;237:1982–1992. doi: 10.1002/dvdy.21554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Insinna C, Pathak N, Perkins B, Drummond I, Besharse JC. The homodimeric kinesin, Kif17, is essential for vertebrate photoreceptor sensory outer segment development. Dev Biol. 2008;316:160–170. doi: 10.1016/j.ydbio.2008.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.LaVail MM. Kinetics of rod outer segment renewal in the developing mouse retina. J Cell Biol. 1973;58:650–661. doi: 10.1083/jcb.58.3.650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Xia L, et al. Identification of human male germ cell-associated kinase, a kinase transcriptionally activated by androgen in prostate cancer cells. J Biol Chem. 2002;277:35422–35433. doi: 10.1074/jbc.M203940200. [DOI] [PubMed] [Google Scholar]
- 33.Nachury MV, et al. A core complex of BBS proteins cooperates with the GTPase Rab8 to promote ciliary membrane biogenesis. Cell. 2007;129:1201–1213. doi: 10.1016/j.cell.2007.03.053. [DOI] [PubMed] [Google Scholar]
- 34.des Portes V, et al. A novel CNS gene required for neuronal migration and involved in X-linked subcortical laminar heterotopia and lissencephaly syndrome. Cell. 1998;92:51–61. doi: 10.1016/s0092-8674(00)80898-3. [DOI] [PubMed] [Google Scholar]
- 35.Schaar BT, Kinoshita K, McConnell SK. Doublecortin microtubule affinity is regulated by a balance of kinase and phosphatase activity at the leading edge of migrating neurons. Neuron. 2004;41:203–213. doi: 10.1016/s0896-6273(03)00843-2. [DOI] [PubMed] [Google Scholar]
- 36.Tanaka T, et al. Cdk5 phosphorylation of doublecortin ser297 regulates its effect on neuronal migration. Neuron. 2004;41:215–227. doi: 10.1016/s0896-6273(03)00852-3. [DOI] [PubMed] [Google Scholar]
- 37.Yamashita T, et al. Essential and synergistic roles of RP1 and RP1L1 in rod photoreceptor axoneme and retinitis pigmentosa. J Neurosci. 2009;29:9748–9760. doi: 10.1523/JNEUROSCI.5854-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Takemura R, et al. Increased microtubule stability and alpha tubulin acetylation in cells transfected with microtubule-associated proteins MAP1B, MAP2 or tau. J Cell Sci. 1992;103:953–964. doi: 10.1242/jcs.103.4.953. [DOI] [PubMed] [Google Scholar]
- 39.Pugacheva EN, Jablonski SA, Hartman TR, Henske EP, Golemis EA. HEF1-dependent Aurora A activation induces disassembly of the primary cilium. Cell. 2007;129:1351–1363. doi: 10.1016/j.cell.2007.04.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Dompierre JP, et al. Histone deacetylase 6 inhibition compensates for the transport deficit in Huntington's disease by increasing tubulin acetylation. J Neurosci. 2007;27:3571–3583. doi: 10.1523/JNEUROSCI.0037-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.