Abstract
Approximately half the human genome is composed of repetitive DNA sequences classified into microsatellites, minisatellites, tandem repeats, and dispersed repeats. These repetitive sequences have coevolved within the genome but little is known about their potential interactions. Trinucleotide repeats (TNRs) are a subclass of microsatellites that are implicated in human disease. Expansion of CAG·CTG TNRs is responsible for Huntington disease, myotonic dystrophy, and a number of spinocerebellar ataxias. In yeast DNA double-strand break (DSB) formation has been proposed to be associated with instability and chromosome fragility at these sites and replication fork reversal (RFR) to be involved either in promoting or in preventing instability. However, the molecular basis for chromosome fragility of repetitive DNA remains poorly understood. Here we show that a CAG·CTG TNR array stimulates instability at a 275-bp tandem repeat located 6.3 kb away on the Escherichia coli chromosome. Remarkably, this stimulation is independent of both DNA double-strand break repair (DSBR) and RFR but is dependent on a functional mismatch repair (MMR) system. Our results provide a demonstration, in a simple model system, that MMR at one type of repetitive DNA has the potential to influence the stability of another. Furthermore, the mechanism of this stimulation places a limit on the universality of DSBR or RFR models of instability and chromosome fragility at CAG·CTG TNR sequences. Instead, our data suggest that explanations of chromosome fragility should encompass the possibility of chromosome gaps formed during MMR.
Keywords: DNA repair, DNA replication, fragile site
Repetitive DNA is a prominent characteristic of many eukaryotic genomes including that of humans (1). This complexity of DNA sequence carries with it a danger of genome rearrangement as repetitive sequences are sites where recombination or replication slippage events can occur. Human microsatellites composed of CAG·CTG trinucleotide repeats (TNRs) have received considerable attention because expanded arrays are associated with neurological diseases such as Huntington disease, myotonic dystrophy, and several spinocerebellar ataxias. Expanded CCG·CGG TNRs have been associated with fragile-X syndrome and expanded GAA·TTC TNRs with Friedrich ataxia. CAG·CTG TNRs can expand and contract in length via replicative and nonreplicative mechanisms that have been studied extensively in Escherichia coli, yeast, cultured mammalian cells, and mice (2, 3). Furthermore, studies in yeast have suggested that these repeated sequences are sites of DNA double-strand breakage and this has been compared with chromosome fragility (4, 5). Recently, interplay between DNA breakage and instability of CAG·CTG TNRs has been suggested with models involving DNA ends generated via replication fork reversal (RFR) (5, 6). It is therefore critical to establish whether CAG·CTG TNRs are sites of DSB formation via chromosome breakage or RFR, using the model systems available.
The fragility of human chromosomes at specific DNA sites is well known (see refs. 7 and 8 for recent reviews). These sites are often associated with regions of DNA that complete replication late especially under conditions where DNA synthesis is partially inhibited. Fragile sites are subdivided into common sites that can be induced by treatment with aphidicolin, bromodeoxyuridine, or 5-azacytidine and rare sites that are either sensitive or not sensitive to folate. Common fragile sites are characterized by extended regions of AT-rich DNA with unusual flexibility that may exclude nucleosomes, may be slow to complete replication, and hence have a propensity to remain single stranded over longer periods than normal. This situation can lead to chromosome breakage and rearrangement. Folate-sensitive rare fragile sites correspond to CCG·CGG TNRs, whereas folate-insensitive rare sites correspond to AT-rich repeats. In Saccharomyces cerevisiae model systems, CAG·CTG and GAA·TTC TNRs have also been shown to be fragile, both directly via the observation of DNA double-strand breaks and via their stimulation of chromosome rearrangements (4, 5, 9).
Mismatch repair (MMR), which repairs nucleotide mismatches and insertion–deletion loops that can arise during DNA replication, has been implicated in TNR stability through a variety of biochemical and genetic approaches. The human MSH2 protein has been shown to bind with increasing affinity to expanding lengths of CAG·CTG TNRs (10) and is proficient at repairing short tandem repeat slip outs of between one and three copies. Interestingly, larger loop-outs or clusters of small loop-outs are refractory to repair by the human MSH2/3 complex, MutSβ (11). Studies in yeast in have shown that MMR can prevent CAG instability at the single triplet level but is unable to repair larger expansions or deletions (12). Similarly, loss of MMR results in the formation of single triplet expansions and contractions in E. coli (13). MMR has also been shown to modulate CTG instability in mice with elevated levels of TNR tract length changes in msh2 null mice compared with wild type (14).
It was recently established that a 246-bp DNA palindrome inserted at the lacZ locus in the E. coli chromosome is cleaved by the hairpin endonuclease SbcCD (the homolog of Rad50/Mre11 in eukaryotes) to generate a double-strand break (DSB) (15). Furthermore, the DSB generated by this cleavage stimulates recombination at a 275-bp direct repeat located 6.3 kb away in the cynX gene (15). This result provided us with a sensitive assay for the detection of DSB formation at the site of expanded CAG·CTG TNR arrays inserted in the place of the palindrome at the lacZ locus.
To our surprise, the results of our assay showed that the primary lesion generated at an expanded CAG·CTG TNR is not a DSB but is in fact a lesion generated by MMR, most probably single-stranded DNA. This result is important for the understanding of the processing of CAG·CTG TNRs and more generally of the potential for repetitive DNA to generate long single-stranded regions in chromosomes. Furthermore, it demonstrates that MMR at one type of repetitive DNA sequence, a TNR, can affect the stability of another, a 275-bp tandem repeat.
Results
Expanded CAG·CTG TNRs Stimulate Tandem Repeat Recombination.
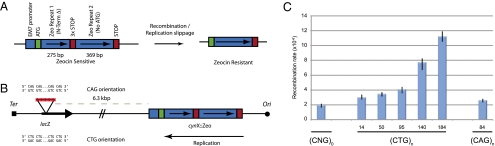
To test whether CAG·CTG repeats are sites of DSB formation, we integrated an array of these TNRs in the lacZ gene of an E. coli chromosome that contained a recombination reporter cassette composed of two direct repeats of parts of the zeocin resistance gene in the cynX gene located 6.3 kb away (Fig. 1 A and B). This tandem repeat cassette was previously used to show that a DSB caused by cleavage of a 246-bp palindrome in lacZ by the hairpin endonuclease SbcCD can result in recombination between the two zeocin repeats to form an intact zeocin resistance gene (15). As shown in Fig. 1C, the presence of the repeated trinucleotide sequence CTG on the leading-strand template (CTG TNRs) stimulated zeocin recombination. The observed rate of recombination increased as a function of the length of the repeat tract. Although CAG TNRs (CAG on the leading-strand template) were also shown to stimulate zeocin recombination slightly, their length dependency could not be tested as the CAG repeats are highly prone to deletions (16), preventing the construction of an array longer than CAG84.
Fig. 1.
CAG·CTG TNRs stimulate recombination at a 275-bp tandem repeat located at a distance of 6.3 kb on the E. coli chromosome. (A) Structure of the 275-bp tandem repeat of zeocin resistance gene sequences. Recombination or replication slippage between the two incomplete copies of the tandem duplication generates an intact zeocin resistance gene, as previously described (15). (B) Experimental system. CAG·CTG TNRs were inserted in the lacZ gene and a 275-bp tandem repeat of zeocin resistance gene sequences was inserted in the cynX gene at a distance of 6.3 kb on the E. coli chromosome (15, 16). (C) Recombination rates determined by fluctuation analysis as a function of the length of the CAG·CTG TNR array at lacZ. Error bars represent 95% confidence limits.
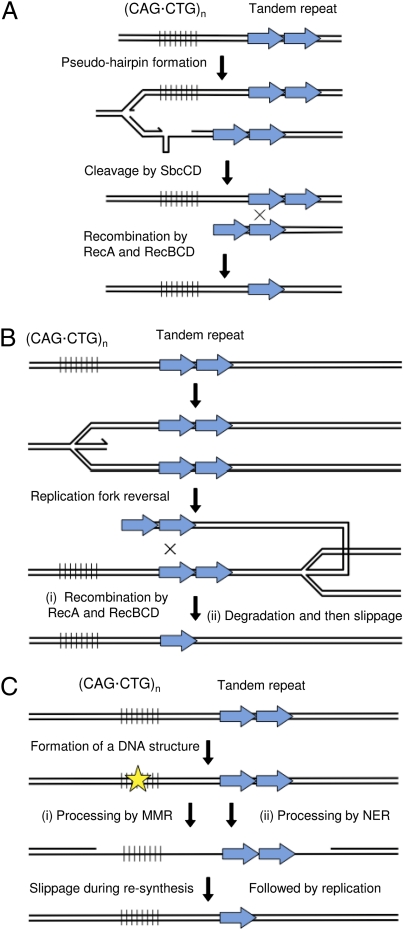
We then set out to distinguish between the potential mechanisms underlying the stimulation of tandem repeat recombination by the expanded TNR arrays. The first hypothesis tested was that a single strand of the TNR array was able to form a hairpin-like structure that was cleaved by SbcCD to generate a DSB, as was previously shown to occur at the site of a DNA palindrome (15). The second hypothesis was that a double-strand end was generated at the site of the TNR array by replication fork reversal. The third was that single-stranded DNA initiated at the TNR array, by the action of MMR or nucleotide excision repair (NER), stimulated recombination at the tandem repeat via a strand-slippage mechanism. These hypotheses are illustrated in Fig. 2.
Fig. 2.
Hypotheses tested. (A) Cleavage of a pseudohairpin structure followed by homologous recombination between sister chromosomes. (B) RFR followed either by (i) homologous recombination between the newly replicated strands and the parental strands or by (ii) degradation followed by resynthesis involving strand slippage at the tandem repeat. (C) Formation of a lesion at the TNR followed by repair either by (i) MMR or by (ii) NER. In either case, resynthesis would involve strand slippage at the tandem repeat.
TNR-Stimulated Tandem Repeat Recombination Is Independent of recA, recB, recF, recO, and sbcCD.
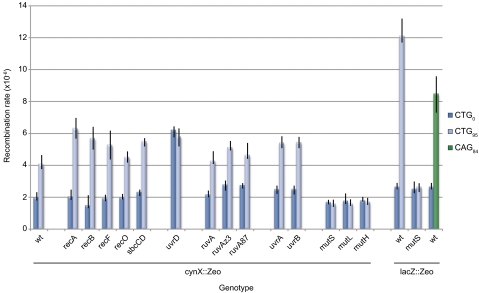
Genetic analysis showed that the observed stimulation of tandem repeat recombination was independent of the presence of the genes recA, recB, recF, and recO, which encode proteins that are implicated in homologous recombination pathways of double-strand break repair (DSBR) and ssDNA gap repair (Fig. 3). Furthermore, tandem repeat recombination was independent of sbcCD (Fig. 3). This result was in marked contrast to the stimulation of tandem repeat recombination initiated by a DSB at a palindrome in lacZ (15). We conclude that CAG·CTG TNRs stimulate tandem repeat instability by a mechanism such as strand slippage that is independent of homologous recombination. Significantly, independence of the mechanism from the RecA–RecB pathway of homologous recombination, which operates exclusively at double-strand ends, provides strong evidence against the formation of a DSB at the site of the TNR array. Furthermore, independence from the pathway of SbcCD-mediated hairpin cleavage provides further support that the stimulation of tandem repeat recombination observed in vivo is not caused by DSBs generated by cleavage of pseudohairpins that can form in vitro (17) and can be cleaved by SbcCD in vitro (18). Consistent with this, extensive attempts to detect a DSB by pulsed-field gel electrophoresis of TNR-containing recB mutants failed to reveal any double-strand ends.
Fig. 3.
Genetic requirements for stimulation of tandem repeat recombination. Recombination rates for the zeocin resistance cassette at cynX were determined by fluctuation analysis as a function of inactivation of recombination, hairpin cleavage, RuvAB-dependent RFR, NER, and MMR. Also shown are recombination rates for the zeocin resistance cassette at lacZ in the presence and the absence of MMR. Error bars represent 95% confidence limits.
TNR-Stimulated Tandem Repeat Recombination Does Not Occur via RFR.
Because RFR has been proposed to play a role in TNR processing, we set out to test whether the inactivation of genes that encode proteins implicated in RFR affects TNR-stimulated tandem repeat recombination. As can be seen in Fig. 3, the uvrD mutation, which inhibits RuvAB-dependent RFR (19), eliminated stimulation by the CTG95 repeat array. However, deletion of ruvA or the mutations ruvAz3 and ruvA87, which also inhibit RuvAB-dependent RFR (20), did not eliminate the stimulation. We therefore conclude that the stimulation is dependent not on RuvAB-dependent RFR but on some other reaction that requires UvrD.
TNR-Stimulated Tandem Repeat Recombination Requires MMR.
UvrD is also required for NER and MMR. We therefore determined the effects of inactivating other genes encoding proteins essential in these pathways. As can be seen in Fig. 3, uvrB and uvrC mutations had no effect, but TNR-stimulated tandem repeat recombination was eliminated in the absence of mutS, mutL, and mutH. These data demonstrate that NER is not involved but that MMR is critical for this reaction. An increase in the baseline stimulation of tandem repeat recombination (in the absence of TNRs) was observed in the uvrD mutant (Fig. 3). This increase was not observed in uvrB, uvrC, mutS, mutL, or mutH mutants, indicating that this result was not caused by inactivating NER or MMR (Fig. 3). Stimulation of tandem repeat instability in a uvrD mutant was previously shown to be caused by an increase in RecF-mediated recombination (21) and this result may relate to the antirecombination role of the UvrD protein (22).
To test whether MMR initiated at the TNR array generates a signal that is propagated along the DNA to the tandem repeat, we tested the prediction that decreasing the distance between the TNRs and the tandem repeat would increase stimulation of recombination. As can be seen in Fig. 3, reducing this distance to 0.5 kb (lacZ::Zeo) increased the stimulation significantly and the increased rate of tandem repeat recombination caused by the presence of the TNRs was eliminated entirely in the absence of mutS. Finally, moving the tandem repeat closer to the TNRs allowed us to clearly demonstrate that the unstable CAG leading-stand orientation of the repeat array was also capable of stimulating recombination.
Elevated TNR Instability in MMR Mutants.
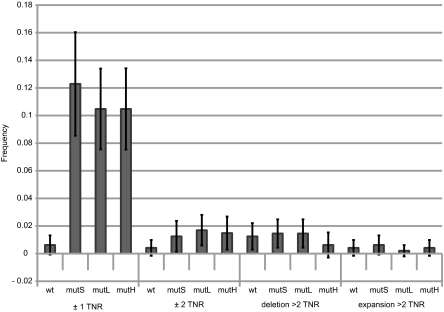
To determine the likely molecular trigger for the MMR reaction that stimulates tandem repeat instability, we analyzed the effect of inactivation of MMR genes mutS, mutL, and mutH on TNR instability. As can be seen in Fig. 4, inactivation of MMR dramatically increased the frequency of single trinucleotide changes (+1 or −1 repeat unit) in the CAG·CTG TNR arrays, as previously observed in a plasmid assay (13). The frequencies of changes greater than one trinucleotide were not affected by inactivation of MMR, consistent with the previous observation that a maximum loop size of 4 bp is required for recognition by the MMR system (23). It is likely that in MMR-proficient cells, the precursors to the single trinucleotide changes trigger recognition by the MMR system, generating single-stranded DNA that is propagated to the tandem duplication where slippage is stimulated during resynthesis (Fig. 2C, i).
Fig. 4.
Frequencies of changes in CAG·CTG TNR arrays (CTG leading strand orientation) as a function of MMR genotypes. The frequencies of length changes in CAG·CTG TNR arrays were determined by GeneMapper analysis following capillary electrophoresis of 480 individual PCR reactions for each strain, as described in Materials and Methods. Error bars represent 95% confidence limits.
Discussion
We asked the question: Does one class of repetitive DNA influence the stability of another and, if so, how does it carry out this action? This work demonstrates that, in an E. coli model system, a CAG·CTG TNR array stimulates instability of a 275-bp tandem repeat located up to 6.3 kb away. This reaction is independent of the hairpin endonuclease SbcCD (Mre11/Rad50), homologous recombination, and RuvAB-dependent RFR, providing no evidence for the formation of a DSB as a consequence of cleavage or RFR. By contrast, our data show that MMR at the TNR array stimulates instability at the 275-bp tandem repeat.
We confirmed, by using TNR instability assays, that MMR mutants display an elevated frequency of +1 and −1 repeat unit changes, implying that CAG·CTG repeats can form loop-outs composed of a single trinucleotide unit on the template or on the newly synthesized strands of replication. We propose that these loop-outs are recognized by the MMR system that removes them by a process that involves the formation of ssDNA by UvrD helicase following a ssDNA nick generated by MutH and that it is the repair of this ssDNA that results in recombination of the tandem zeocin repeats, via strand slippage as shown in Fig. 2C, i.
Whether a similar MMR-dependent interaction between classes of repeated DNA sequences occurs in eukaryotic genomes remains to be determined. In yeast, MMR has been shown to act at a GAA·TTC TNR array to stimulate large deletions, DNA double-strand breaks, and chromosome arm loss (9). Kim et al. (9) proposed that triplex DNA formed in GAA·TTC TNR arrays may trigger double-strand breakage via the action of MMR proteins. This proposal cannot be the explanation of our observations, as CAG·CTG TNRs are unable to adopt a triplex conformation and the tandem repeat recombination we observe is stimulated via a mechanism that does not involve the processing of DNA double-strand breaks.
In human chromosomes, actual chromosome breakage may be a rare event with respect to other properties of fragile sites such as exclusion of nucleosomes and accumulation of persistent single-stranded DNA. The importance of single-stranded DNA in fragility is emphasized by the central role of ATR and not ATM in counteracting the expression of common fragile sites (not associated with TNRs) (7, 20), the ATR-dependent checkpoint response being elicited primarily by single-stranded DNA and the ATM checkpoint response being elicited primarily by DSBs. Our results raise the parallel possibility that single-stranded DNA generated during MMR could play a part in chromosome fragility at repetitive DNA sequences characteristic of rare fragile sites such as TNRs. The long distance over which the stimulation of tandem repeat recombination can be propagated (6.3 kb) argues for the formation during MMR of single-stranded regions considerably longer than Okazaki fragments. If such long single-stranded regions were generated in a human chromosome containing an expanded array of a foldable TNR sequence (e.g., CCG·CGG at the fragile-X locus), the folded structure could pose a serious impediment to DNA synthesis. This folding could result in regions of persistent single-stranded DNA that would be visualized as nucleosome-free regions characteristic of fragile sites.
Methods
Bacterial strains used are listed in Table S1.
Rates of formation of zeocin-resistant recombinants were determined by fluctuation analysis, as previously described (15, 24). Twenty-four cultures derived from independent single colonies were analyzed for the frequencies of zeocin-resistant colonies. For each strain this analysis was repeated three times.
CAG·CTG TNR instability assays were carried out by GeneMapper analysis following capillary electrophoresis as previously described (16). Twelve cultures derived from independent single colonies were plated on LB plates to generate single colonies. Eight single colonies from each of these 12 plates were analyzed for instability. This analysis was repeated five times for each strain to monitor instability in a total of 60 independent cultures (480 instability tests). A fluorescently labeled PCR product 8 bp smaller than the fragment containing the intact TNR array was included in the capillary electrophoresis as an additional size marker to ensure accurate determination of +1 and −1 TNR changes.
Supplementary Material
Acknowledgments
We thank Benedicte Michel (Centre de Génétique Moléculaire, Gif-sur-Yvette, France) for providing ruvA mutants; Adam Jackson for statistical analysis; and Elise Darmon, Robyn Emmins, and Martin White for critical reading of the manuscript. J.K.B. was supported by a Cooperative Award in Science and Engineering (CASE) studentship from the Biotechnology and Biological Sciences Research Council and Transgenomic Ltd. D.R.F.L. is supported by the Medical Research Council.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1012906108/-/DCSupplemental.
References
- 1.Richard GF, Kerrest A, Dujon B. Comparative genomics and molecular dynamics of DNA repeats in eukaryotes. Microbiol Mol Biol Rev. 2008;72:686–727. doi: 10.1128/MMBR.00011-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kovtun IV, McMurray CT. Features of trinucleotide repeat instability in vivo. Cell Res. 2008;18:198–213. doi: 10.1038/cr.2008.5. [DOI] [PubMed] [Google Scholar]
- 3.Kovtun IV, et al. OGG1 initiates age-dependent CAG trinucleotide expansion in somatic cells. Nature. 2007;447:447–452. doi: 10.1038/nature05778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Freudenreich CH, Kantrow SM, Zakian VA. Expansion and length-dependent fragility of CTG repeats in yeast. Science. 1998;279:853–856. doi: 10.1126/science.279.5352.853. [DOI] [PubMed] [Google Scholar]
- 5.Kerrest A, et al. SRS2 and SGS1 prevent chromosomal breaks and stabilize triplet repeats by restraining recombination. Nat Struct Mol Biol. 2009;16:159–167. doi: 10.1038/nsmb.1544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mirkin SM. Expandable DNA repeats and human disease. Nature. 2007;447:932–940. doi: 10.1038/nature05977. [DOI] [PubMed] [Google Scholar]
- 7.Arlt MF, Durkin SG, Ragland RL, Glover TW. Common fragile sites as targets for chromosome rearrangements. DNA Repair (Amst) 2006;5:1126–1135. doi: 10.1016/j.dnarep.2006.05.010. [DOI] [PubMed] [Google Scholar]
- 8.Lukusa T, Fryns JP. Human chromosome fragility. Biochim Biophys Acta. 2008;1779:3–16. doi: 10.1016/j.bbagrm.2007.10.005. [DOI] [PubMed] [Google Scholar]
- 9.Kim HM, et al. Chromosome fragility at GAA tracts in yeast depends on repeat orientation and requires mismatch repair. EMBO J. 2008;27:2896–2906. doi: 10.1038/emboj.2008.205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pearson CE, Ewel A, Acharya S, Fishel RA, Sinden RR. Human MSH2 binds to trinucleotide repeat DNA structures associated with neurodegenerative diseases. Hum Mol Genet. 1997;6:1117–1123. doi: 10.1093/hmg/6.7.1117. [DOI] [PubMed] [Google Scholar]
- 11.Panigrahi GB, Slean MM, Simard JP, Gileadi O, Pearson CE. Isolated short CTG/CAG DNA slip-outs are repaired efficiently by hMutSbeta, but clustered slip-outs are poorly repaired. Proc Natl Acad Sci USA. 2010;107:12593–12598. doi: 10.1073/pnas.0909087107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Schweitzer JK, Livingston DM. Destabilization of CAG trinucleotide repeat tracts by mismatch repair mutations in yeast. Hum Mol Genet. 1997;6:349–355. doi: 10.1093/hmg/6.3.349. [DOI] [PubMed] [Google Scholar]
- 13.Schmidt KH, Abbott CM, Leach DR. Two opposing effects of mismatch repair on CTG repeat instability in Escherichia coli. Mol Microbiol. 2000;35:463–471. doi: 10.1046/j.1365-2958.2000.01727.x. [DOI] [PubMed] [Google Scholar]
- 14.Savouret C, et al. CTG repeat instability and size variation timing in DNA repair-deficient mice. EMBO J. 2003;22:2264–2273. doi: 10.1093/emboj/cdg202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Eykelenboom JK, Blackwood JK, Okely E, Leach DR. SbcCD causes a double-strand break at a DNA palindrome in the Escherichia coli chromosome. Mol Cell. 2008;29:644–651. doi: 10.1016/j.molcel.2007.12.020. [DOI] [PubMed] [Google Scholar]
- 16.Zahra R, Blackwood JK, Sales J, Leach DR. Proofreading and secondary structure processing determine the orientation dependence of CAG × CTG trinucleotide repeat instability in Escherichia coli. Genetics. 2007;176:27–41. doi: 10.1534/genetics.106.069724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gacy AM, Goellner G, Juranić N, Macura S, McMurray CT. Trinucleotide repeats that expand in human disease form hairpin structures in vitro. Cell. 1995;81:533–540. doi: 10.1016/0092-8674(95)90074-8. [DOI] [PubMed] [Google Scholar]
- 18.Connelly JC, de Leau ES, Leach DR. DNA cleavage and degradation by the SbcCD protein complex from Escherichia coli. Nucleic Acids Res. 1999;27:1039–1046. doi: 10.1093/nar/27.4.1039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Flores MJ, Bidnenko V, Michel B. The DNA repair helicase UvrD is essential for replication fork reversal in replication mutants. EMBO Rep. 2004;5:983–988. doi: 10.1038/sj.embor.7400262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Baharoglu Z, Bradley AS, Le Masson M, Tsaneva I, Michel B. ruvA mutants that resolve Holliday junctions but do not reverse replication forks. PLoS Genet. 2008;4:e1000012. doi: 10.1371/journal.pgen.1000012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bierne H, Seigneur M, Ehrlich SD, Michel B. uvrD mutations enhance tandem repeat deletion in the Escherichia coli chromosome via SOS induction of the RecF recombination pathway. Mol Microbiol. 1997;26:557–567. doi: 10.1046/j.1365-2958.1997.6011973.x. [DOI] [PubMed] [Google Scholar]
- 22.Veaute X, et al. UvrD helicase, unlike Rep helicase, dismantles RecA nucleoprotein filaments in Escherichia coli. EMBO J. 2005;24:180–189. doi: 10.1038/sj.emboj.7600485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Modrich P, Lahue R. Mismatch repair in replication fidelity, genetic recombination, and cancer biology. Annu Rev Biochem. 1996;65:101–133. doi: 10.1146/annurev.bi.65.070196.000533. [DOI] [PubMed] [Google Scholar]
- 24.Spell RM, Jinks-Robertson S. Determination of mitotic recombination rates by fluctuation analysis in Saccharomyces cerevisiae. Methods Mol Biol. 2004;262:3–12. doi: 10.1385/1-59259-761-0:003. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.