Abstract
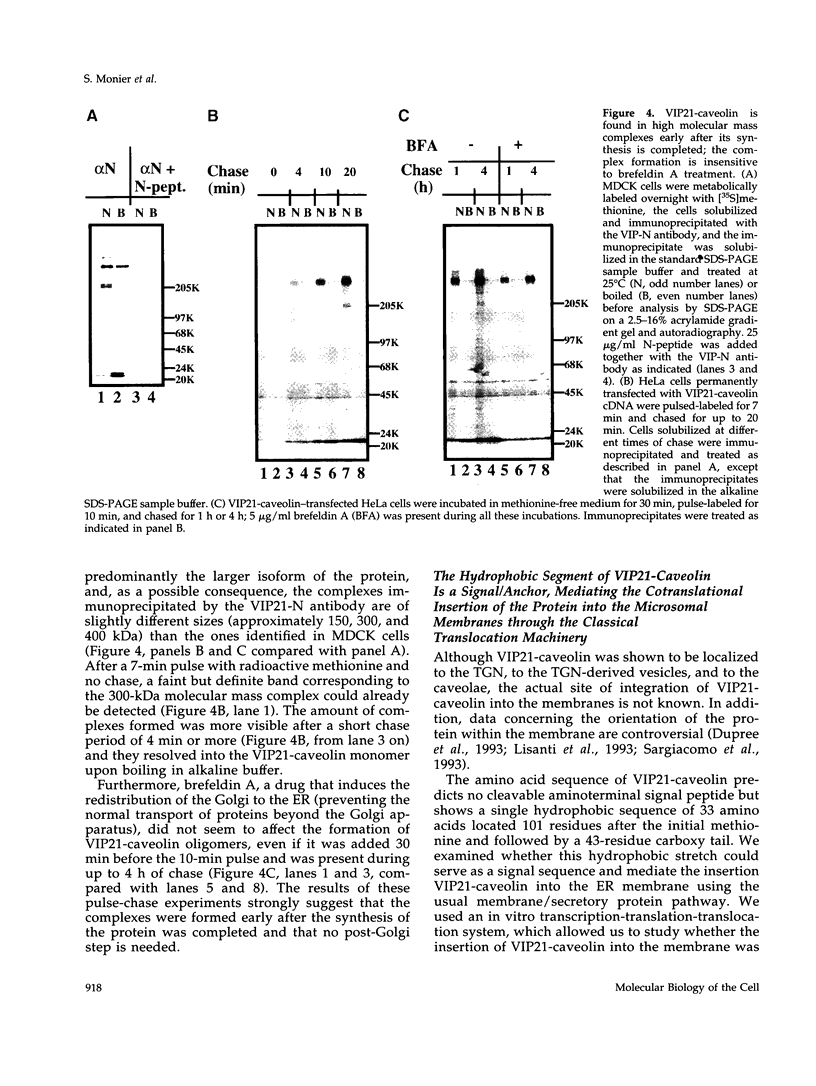
VIP21-caveolin is a membrane protein, proposed to be a component of the striated coat covering the cytoplasmic surface of caveolae. To investigate the biochemical composition of the caveolar coat, we used our previous observation that VIP21-caveolin is present in large complexes and insoluble in the detergents CHAPS or Triton X-114. The mild treatment of these insoluble structures with sodium dodecyl sulfate leads to the detection of high molecular mass complexes of approximately 200, 400, and 600 kDa. The 400-kDa complex purified to homogeneity from dog lung is shown to consist exclusive of the two isoforms of VIP21-caveolin. Pulse-chase experiments indicate that the oligomers form early after the protein is synthesized in the endoplasmic reticulum (ER). VIP21-caveolin does indeed insert into the ER membrane through the classical translocation machinery. Its hydrophobic domain adopts an unusual loop configuration exposing the N- and C-flanking regions to the cytoplasm. Similar high molecular mass complexes can be produced from the in vitro-synthesized VIP21-caveolin. The complex formation occurs only if VIP21-caveolin isoforms are properly inserted into the membrane; formation is cytosol-dependent and does not involve a vesicle fusion step. We propose that high molecular mass oligomers of VIP21-caveolin represent the basic units forming the caveolar coat. They are formed in the ER and later, between the ER and the plasma membrane, these oligomers could associate into larger detergent-insoluble structures.
Full text
PDF















Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson R. G. Caveolae: where incoming and outgoing messengers meet. Proc Natl Acad Sci U S A. 1993 Dec 1;90(23):10909–10913. doi: 10.1073/pnas.90.23.10909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson R. G., Kamen B. A., Rothberg K. G., Lacey S. W. Potocytosis: sequestration and transport of small molecules by caveolae. Science. 1992 Jan 24;255(5043):410–411. doi: 10.1126/science.1310359. [DOI] [PubMed] [Google Scholar]
- Anderson R. G. Plasmalemmal caveolae and GPI-anchored membrane proteins. Curr Opin Cell Biol. 1993 Aug;5(4):647–652. doi: 10.1016/0955-0674(93)90135-d. [DOI] [PubMed] [Google Scholar]
- Arreaza G., Melkonian K. A., LaFevre-Bernt M., Brown D. A. Triton X-100-resistant membrane complexes from cultured kidney epithelial cells contain the Src family protein tyrosine kinase p62yes. J Biol Chem. 1994 Jul 22;269(29):19123–19127. [PubMed] [Google Scholar]
- Beckmann R. P., Mizzen L. E., Welch W. J. Interaction of Hsp 70 with newly synthesized proteins: implications for protein folding and assembly. Science. 1990 May 18;248(4957):850–854. doi: 10.1126/science.2188360. [DOI] [PubMed] [Google Scholar]
- Behlke J., Bommer U. A., Lutsch G., Henske A., Bielka H. Structure of initiation factor eIF-3 from rat liver. Hydrodynamic and electron microscopic investigations. Eur J Biochem. 1986 Jun 16;157(3):523–530. doi: 10.1111/j.1432-1033.1986.tb09698.x. [DOI] [PubMed] [Google Scholar]
- Blobel G. Intracellular protein topogenesis. Proc Natl Acad Sci U S A. 1980 Mar;77(3):1496–1500. doi: 10.1073/pnas.77.3.1496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braakman I., Helenius J., Helenius A. Manipulating disulfide bond formation and protein folding in the endoplasmic reticulum. EMBO J. 1992 May;11(5):1717–1722. doi: 10.1002/j.1460-2075.1992.tb05223.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braks J. A., Martens G. J. 7B2 is a neuroendocrine chaperone that transiently interacts with prohormone convertase PC2 in the secretory pathway. Cell. 1994 Jul 29;78(2):263–273. doi: 10.1016/0092-8674(94)90296-8. [DOI] [PubMed] [Google Scholar]
- Brown D. A., Rose J. K. Sorting of GPI-anchored proteins to glycolipid-enriched membrane subdomains during transport to the apical cell surface. Cell. 1992 Feb 7;68(3):533–544. doi: 10.1016/0092-8674(92)90189-j. [DOI] [PubMed] [Google Scholar]
- Chang W. J., Ying Y. S., Rothberg K. G., Hooper N. M., Turner A. J., Gambliel H. A., De Gunzburg J., Mumby S. M., Gilman A. G., Anderson R. G. Purification and characterization of smooth muscle cell caveolae. J Cell Biol. 1994 Jul;126(1):127–138. doi: 10.1083/jcb.126.1.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Compton T., Ivanov I. E., Gottlieb T., Rindler M., Adesnik M., Sabatini D. D. A sorting signal for the basolateral delivery of the vesicular stomatitis virus (VSV) G protein lies in its luminal domain: analysis of the targeting of VSV G-influenza hemagglutinin chimeras. Proc Natl Acad Sci U S A. 1989 Jun;86(11):4112–4116. doi: 10.1073/pnas.86.11.4112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dupree P., Parton R. G., Raposo G., Kurzchalia T. V., Simons K. Caveolae and sorting in the trans-Golgi network of epithelial cells. EMBO J. 1993 Apr;12(4):1597–1605. doi: 10.1002/j.1460-2075.1993.tb05804.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiedler K., Kobayashi T., Kurzchalia T. V., Simons K. Glycosphingolipid-enriched, detergent-insoluble complexes in protein sorting in epithelial cells. Biochemistry. 1993 Jun 29;32(25):6365–6373. doi: 10.1021/bi00076a009. [DOI] [PubMed] [Google Scholar]
- Fujimoto T. Calcium pump of the plasma membrane is localized in caveolae. J Cell Biol. 1993 Mar;120(5):1147–1157. doi: 10.1083/jcb.120.5.1147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujimoto T., Nakade S., Miyawaki A., Mikoshiba K., Ogawa K. Localization of inositol 1,4,5-trisphosphate receptor-like protein in plasmalemmal caveolae. J Cell Biol. 1992 Dec;119(6):1507–1513. doi: 10.1083/jcb.119.6.1507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gething M. J., Sambrook J. Protein folding in the cell. Nature. 1992 Jan 2;355(6355):33–45. doi: 10.1038/355033a0. [DOI] [PubMed] [Google Scholar]
- Ghitescu L., Fixman A., Simionescu M., Simionescu N. Specific binding sites for albumin restricted to plasmalemmal vesicles of continuous capillary endothelium: receptor-mediated transcytosis. J Cell Biol. 1986 Apr;102(4):1304–1311. doi: 10.1083/jcb.102.4.1304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glenney J. R., Jr, Soppet D. Sequence and expression of caveolin, a protein component of caveolae plasma membrane domains phosphorylated on tyrosine in Rous sarcoma virus-transformed fibroblasts. Proc Natl Acad Sci U S A. 1992 Nov 1;89(21):10517–10521. doi: 10.1073/pnas.89.21.10517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glenney J. R., Jr The sequence of human caveolin reveals identity with VIP21, a component of transport vesicles. FEBS Lett. 1992 Dec 7;314(1):45–48. doi: 10.1016/0014-5793(92)81458-x. [DOI] [PubMed] [Google Scholar]
- Glenney J. R., Jr Tyrosine phosphorylation of a 22-kDa protein is correlated with transformation by Rous sarcoma virus. J Biol Chem. 1989 Dec 5;264(34):20163–20166. [PubMed] [Google Scholar]
- Glenney J. R., Jr, Zokas L. Novel tyrosine kinase substrates from Rous sarcoma virus-transformed cells are present in the membrane skeleton. J Cell Biol. 1989 Jun;108(6):2401–2408. doi: 10.1083/jcb.108.6.2401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Görlich D., Rapoport T. A. Protein translocation into proteoliposomes reconstituted from purified components of the endoplasmic reticulum membrane. Cell. 1993 Nov 19;75(4):615–630. doi: 10.1016/0092-8674(93)90483-7. [DOI] [PubMed] [Google Scholar]
- Hoessli D., Rungger-Brändle E. Association of specific cell-surface glycoproteins with a triton X-100-resistant complex of plasma membrane proteins isolated from T-lymphoma cells (P1798). Exp Cell Res. 1985 Jan;156(1):239–250. doi: 10.1016/0014-4827(85)90278-2. [DOI] [PubMed] [Google Scholar]
- Ivessa N. E., De Lemos-Chiarandini C., Tsao Y. S., Takatsuki A., Adesnik M., Sabatini D. D., Kreibich G. O-glycosylation of intact and truncated ribophorins in brefeldin A-treated cells: newly synthesized intact ribophorins are only transiently accessible to the relocated glycosyltransferases. J Cell Biol. 1992 Jun;117(5):949–958. doi: 10.1083/jcb.117.5.949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim P. S., Bole D., Arvan P. Transient aggregation of nascent thyroglobulin in the endoplasmic reticulum: relationship to the molecular chaperone, BiP. J Cell Biol. 1992 Aug;118(3):541–549. doi: 10.1083/jcb.118.3.541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurzchalia T. V., Dupree P., Parton R. G., Kellner R., Virta H., Lehnert M., Simons K. VIP21, a 21-kD membrane protein is an integral component of trans-Golgi-network-derived transport vesicles. J Cell Biol. 1992 Sep;118(5):1003–1014. doi: 10.1083/jcb.118.5.1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lisanti M. P., Scherer P. E., Tang Z., Sargiacomo M. Caveolae, caveolin and caveolin-rich membrane domains: a signalling hypothesis. Trends Cell Biol. 1994 Jul;4(7):231–235. doi: 10.1016/0962-8924(94)90114-7. [DOI] [PubMed] [Google Scholar]
- Lisanti M. P., Scherer P. E., Vidugiriene J., Tang Z., Hermanowski-Vosatka A., Tu Y. H., Cook R. F., Sargiacomo M. Characterization of caveolin-rich membrane domains isolated from an endothelial-rich source: implications for human disease. J Cell Biol. 1994 Jul;126(1):111–126. doi: 10.1083/jcb.126.1.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lisanti M. P., Tang Z. L., Sargiacomo M. Caveolin forms a hetero-oligomeric protein complex that interacts with an apical GPI-linked protein: implications for the biogenesis of caveolae. J Cell Biol. 1993 Nov;123(3):595–604. doi: 10.1083/jcb.123.3.595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monier S., Van Luc P., Kreibich G., Sabatini D. D., Adesnik M. Signals for the incorporation and orientation of cytochrome P450 in the endoplasmic reticulum membrane. J Cell Biol. 1988 Aug;107(2):457–470. doi: 10.1083/jcb.107.2.457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montesano R., Roth J., Robert A., Orci L. Non-coated membrane invaginations are involved in binding and internalization of cholera and tetanus toxins. Nature. 1982 Apr 15;296(5858):651–653. doi: 10.1038/296651a0. [DOI] [PubMed] [Google Scholar]
- Parton R. G., Joggerst B., Simons K. Regulated internalization of caveolae. J Cell Biol. 1994 Dec;127(5):1199–1215. doi: 10.1083/jcb.127.5.1199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parton R. G. Ultrastructural localization of gangliosides; GM1 is concentrated in caveolae. J Histochem Cytochem. 1994 Feb;42(2):155–166. doi: 10.1177/42.2.8288861. [DOI] [PubMed] [Google Scholar]
- Pelham H. R. Control of protein exit from the endoplasmic reticulum. Annu Rev Cell Biol. 1989;5:1–23. doi: 10.1146/annurev.cb.05.110189.000245. [DOI] [PubMed] [Google Scholar]
- Peters K. R., Carley W. W., Palade G. E. Endothelial plasmalemmal vesicles have a characteristic striped bipolar surface structure. J Cell Biol. 1985 Dec;101(6):2233–2238. doi: 10.1083/jcb.101.6.2233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Picot D., Loll P. J., Garavito R. M. The X-ray crystal structure of the membrane protein prostaglandin H2 synthase-1. Nature. 1994 Jan 20;367(6460):243–249. doi: 10.1038/367243a0. [DOI] [PubMed] [Google Scholar]
- Poruchynsky M. S., Maass D. R., Atkinson P. H. Calcium depletion blocks the maturation of rotavirus by altering the oligomerization of virus-encoded proteins in the ER. J Cell Biol. 1991 Aug;114(4):651–656. doi: 10.1083/jcb.114.4.651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothberg K. G., Heuser J. E., Donzell W. C., Ying Y. S., Glenney J. R., Anderson R. G. Caveolin, a protein component of caveolae membrane coats. Cell. 1992 Feb 21;68(4):673–682. doi: 10.1016/0092-8674(92)90143-z. [DOI] [PubMed] [Google Scholar]
- Rothberg K. G., Ying Y. S., Kolhouse J. F., Kamen B. A., Anderson R. G. The glycophospholipid-linked folate receptor internalizes folate without entering the clathrin-coated pit endocytic pathway. J Cell Biol. 1990 Mar;110(3):637–649. doi: 10.1083/jcb.110.3.637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sargiacomo M., Sudol M., Tang Z., Lisanti M. P. Signal transducing molecules and glycosyl-phosphatidylinositol-linked proteins form a caveolin-rich insoluble complex in MDCK cells. J Cell Biol. 1993 Aug;122(4):789–807. doi: 10.1083/jcb.122.4.789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schweizer A., Rohrer J., Hauri H. P., Kornfeld S. Retention of p63 in an ER-Golgi intermediate compartment depends on the presence of all three of its domains and on its ability to form oligomers. J Cell Biol. 1994 Jul;126(1):25–39. doi: 10.1083/jcb.126.1.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simionescu M., Simionescu N., Palade G. E. Differentiated microdomains on the luminal surface of capillary endothelium: distribution of lectin receptors. J Cell Biol. 1982 Aug;94(2):406–413. doi: 10.1083/jcb.94.2.406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smart E. J., Ying Y. S., Conrad P. A., Anderson R. G. Caveolin moves from caveolae to the Golgi apparatus in response to cholesterol oxidation. J Cell Biol. 1994 Dec;127(5):1185–1197. doi: 10.1083/jcb.127.5.1185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tran D., Carpentier J. L., Sawano F., Gorden P., Orci L. Ligands internalized through coated or noncoated invaginations follow a common intracellular pathway. Proc Natl Acad Sci U S A. 1987 Nov;84(22):7957–7961. doi: 10.1073/pnas.84.22.7957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ungewickell E. The 70-kd mammalian heat shock proteins are structurally and functionally related to the uncoating protein that releases clathrin triskelia from coated vesicles. EMBO J. 1985 Dec 16;4(13A):3385–3391. doi: 10.1002/j.1460-2075.1985.tb04094.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urbani L., Simoni R. D. Cholesterol and vesicular stomatitis virus G protein take separate routes from the endoplasmic reticulum to the plasma membrane. J Biol Chem. 1990 Feb 5;265(4):1919–1923. [PubMed] [Google Scholar]
- Voorberg J., Fontijn R., Calafat J., Janssen H., van Mourik J. A., Pannekoek H. Biogenesis of von Willebrand factor-containing organelles in heterologous transfected CV-1 cells. EMBO J. 1993 Feb;12(2):749–758. doi: 10.1002/j.1460-2075.1993.tb05709.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weisz O. A., Swift A. M., Machamer C. E. Oligomerization of a membrane protein correlates with its retention in the Golgi complex. J Cell Biol. 1993 Sep;122(6):1185–1196. doi: 10.1083/jcb.122.6.1185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Meer G. Transport and sorting of membrane lipids. Curr Opin Cell Biol. 1993 Aug;5(4):661–673. doi: 10.1016/0955-0674(93)90137-f. [DOI] [PubMed] [Google Scholar]