Abstract
Puberty onset is initiated by activation of neurons that secrete gonadotropin-releasing hormone (GnRH). The timing and progression of puberty may depend upon temporal coordination of two opposing central mechanisms—a restraint of GnRH secretion before puberty onset, followed by enhanced stimulation of GnRH release to complete reproductive maturation during puberty. Neuronal estrogen receptor α (ERα) has been implicated in both controls; however, the underlying neural circuits are not well understood. Here we test whether these mechanisms are mediated by neurons that express kisspeptin, a neuropeptide that modulates GnRH neurosecretion. Strikingly, conditional ablation of ERα in kisspeptin neurons results in a dramatic advancement of puberty onset in female mice. Furthermore, subsequent pubertal maturation is arrested in these animals, as they fail to acquire normal ovulatory cyclicity. We show that the temporal coordination of juvenile restraint and subsequent pubertal activation is likely mediated by ERα in two separate kisspeptin neuronal populations in the hypothalamus.
Keywords: estradiol-17β, conditional gene targeting, kiss1, arcuate nucleus, anteroventral periventricular nucleus
Puberty is the transition to adulthood that culminates in the production of mature gametes and the initiation of reproductive activity. The process begins within the central nervous system, where gonadotropin-releasing hormone (GnRH) neurons are activated to release high-frequency pulses of the neurohormone, stimulating pituitary gonadotropic hormone secretions that in turn direct gonadal steroid hormone production and maturation of gametes (1–3). An ill-defined developmental clock, as well as permissive somatic and environmental signals, govern the onset, progression, and completion of the pubertal acceleration of GnRH release. However, the cellular signaling pathways that mediate the pubertal stimulation and/or disinhibition of GnRH neurons have remained obscure.
In rodents, sheep, and other animals, a “gonadostat” hypothesis has held that the pace of puberty is a function of changing sensitivity of the GnRH neurosecretory system to the inhibitory feedback actions of gonadal steroids. In females, the hypothesis stipulates that the sensitivity of the central GnRH pulse generator to inhibition by circulating gonadal steroids, principally estradiol-17β (E2), is reduced in the late juvenile stage of development (4–6). The concept has been strongly supported in female mice, where classic analyses revealed that a 10-fold increase in the dose of E2 is required to fully suppress LH secretion in adult vs. juvenile animals (7). This mechanistic model has been revised to apply largely to the later stages of puberty in monkeys (8). A second steroid-dependent mechanism has also been proposed, in which E2’s positive neurotropic effects contribute to the maturation of the GnRH pulse generator during puberty (9). We sought to determine the specific neuronal populations in which estrogen receptors may mediate either the juvenile feedback suppression of GnRH release or the pubertal stimulation of the GnRH releasing system, or both.
The identification of kisspeptins, neuropeptides expressed by the Kiss1 gene, and their receptor, GPR54, has provided an opportunity for understanding how the timing and progression of puberty are controlled. Inactivating mutations of the human GPR54 gene are associated with hypogonadotropic hypogonadism and an absence of puberty (10, 11). Ablation of the GPR54 (12, 13) or the Kiss1 (13, 14) gene renders mice of either sex hypogonadotropic and infertile. Kisspeptin is a powerful GnRH secretagogue (15, 16), and kisspeptin administration advances puberty onset (17, 18), whereas GPR54 antagonists delay puberty onset (19). These data provide compelling support for the idea that activation of kisspeptin neurons precipitates the pubertal stimulation of GnRH neurosecretion. Estrogens are key regulators of kisspeptin expression in pre- and postpubertal female mice (20, 21) and rats (22), as they potently exert inhibitory effects on hypothalamic arcuate nucleus (ARC) neurons and stimulatory actions in anteroventral periventricular (AVPe) neurons. Furthermore, the majority of kisspeptin neurons in both hypothalamic regions coexpress ERα (23), raising the possibility that these receptors in kisspeptin neurons directly modulate the progression of puberty. To test this hypothesis, we generated mice with a conditional knockout of the ERα (Esr1) gene in kisspeptin neurons, and assessed the tempo and completeness of their reproductive maturation.
Results and Discussion
Generation of Kisspeptin Cell-Specific ERα Knockout Mice.
We first used gene targeting in embryonic stem cells to express Cre recombinase under the control of the mouse kisspeptin promoter. We inserted an internal ribosome entry site (IRES) sequence followed by a Cre recombinase cDNA downstream of the Kiss1 coding region (Fig. 1A). Transcription of the recombinant Kiss1 allele yields a bicistronic messenger RNA, from which kisspeptin and Cre recombinase are independently translated.
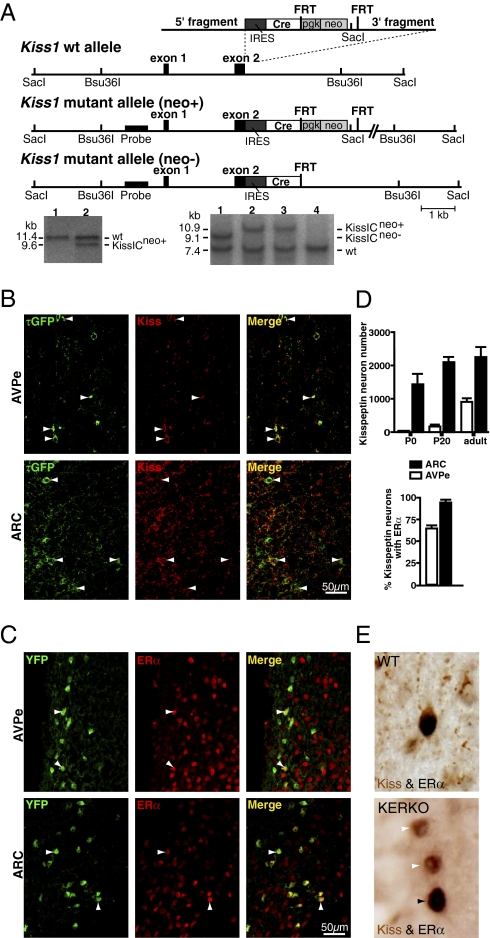
Fig. 1.
Conditional ERα knockout in kisspeptin cells. (A) Targeting strategy to express Cre recombinase under control of the Kiss1 promoter. The targeting vector, the WT alleles, and the targeted allele before (neo+) and after (neo−) removal of the neomycin selection cassette are shown from top to bottom. Restriction sites used for Southern blot analysis, as well as the location of the probes, are indicated. Black boxes represent exons. The inserted cassette is composed of an internal ribosomal entry site (IRES) followed by the coding sequence for Cre recombinase (Cre), and a phosphoglycerate kinase promoter-driven neomycin resistance cassette flanked by Flp recombinase recognition sites (FRT). Southern blot analysis of ES-cell DNA after digestion with SacI. The expected fragment sizes detected by the probe are indicated (WT, 11.4 kb; mutant, 9.6 kb). Clone 2 carries the mutant KissI allele (KissICneo+). Southern blot analysis of DNA digested with Bsu36I from WT, and heterozygous mutant mice before and after removal of the neomycin selection cassette. The expected fragment sizes detected by the probe are indicated (WT, 7.4 kb; mutant allele I KissICneo+, 9.1 kb; mutant allele II KissICneo−, 10.9 kb). Mice 2 and 3 carry mutant allele I (KissICneo+), mouse 1 carries mutant allele II after Flp recombinase mediated excision of the neomycin cassette (KissICneo−). (B) Cre-mediated recombination is restricted to kisspeptin neurons. τGFP+ neurons were found in the ARC and AVPe of female KissIC/eR26-τGPF (green) mice. Immunofluorescence analysis of ARC and AVPe sections using antibodies against kisspeptin shows that >95% of kisspeptin containing cells (red) display τGFP fluorescence (green), demonstrating faithful activation of the ROSA26-τGFP reporter gene in kisspeptin neurons. (Scale bar, 50 μm.) (C) ERα expression in kisspeptin neurons. Immunofluorescence analysis of ARC and AVPe sections from female KissIC/R26-YFP mice using antibodies against ERα shows that most ARC and AVPe kisspeptin neurons (green) express ERα (red). (Scale bar, 50 μm.) (D) Differential onset of kisspeptin expression in the AVPe and ARC. Number of kisspeptin neurons (YFP+) in the AVPe and ARC of female KissIC/R26-YFP mice on the day of birth (P0) and before (P20) and after (adult) puberty. Percentage of kisspeptin neurons expressing ERα in the AVPe and ARC of adult female KissIC/R26-YFP mice. Data are presented as mean ± SEM. (E) Conditional ERα knockout in kisspeptin neurons confirmed by dual labeling of ERα (black nuclear staining) and kisspeptin (brown cytoplasmic staining). (Upper) Double-labeled ERα-kisspeptin neuron in the AVPe of a WT mouse. (Lower) Two kisspeptin neurons lacking ERα immunoreactivity and one nonkisspeptin immunoreactive neuron expressing ERα in the AVPe of a KERKO mouse.
To verify kisspeptin cell-specific Cre expression, kisspeptin-IRES-Cre (KissIC) mice were bred to mice carrying a Cre-activated fluorescent reporter. Cre-mediated recombination was evident in restricted cell populations within the ARC and the AVPe of the hypothalamus (Fig. 1B), consistent with the reported distribution of kisspeptin neurons in mice (20). Immunofluorescence analysis on hypothalamic sections prepared from these animals using anti-kisspeptin antibodies showed that >95% of kisspeptin neurons are fluorescent (97 ± 0.4%; n = 3), demonstrating faithful activation of the reporter gene in kisspeptin neurons. Immunofluorescence analysis using antibodies against ERα revealed that ∼90% of the ARC (93.6 ± 2.4%; n = 3) and ∼65% of AVPe kisspeptin neurons express ERα (64.2 ± 2.0%; n = 3; Fig. 1 C and D).
To specifically ablate ERα in KissIC mice, we used a mouse strain in which exon 3 of the Esr1 gene, which encodes the ERα DNA-binding domain, is flanked by loxP sites (ERαlox/lox). Deletion of exon 3 using this model results in a lack of ERα protein (24). First we crossed KissIC with ERαlox/lox mice and then crossed the resultant KissIC+/ERαlox/wt animals with ERαlox/lox mice. The F2 progeny included KissIC+/ERαlox/lox (KERKO) mice and KissIC−/ERαlox/lox mice. The latter were used as WT in all studies, except for qPCR analyses, where heterozygous ERαlox/wt mice were also included as controls. To confirm the lack of ERα in kisspeptin neurons in KERKO mice, we performed double-label immunohistochemical analysis of brain sections containing the AVPe. In ovary-intact adult WT mice (n = 2), kisspeptin staining was robust, and a high proportion of kisspeptin cell bodies (92/222 and 163/363) were colabeled with the anti-ERα antiserum (Fig. 1E). By contrast, kisspeptin immunoreactivity in the AVPe of KERKO mice (n = 2) was dramatically reduced, and among the few identifiable kisspeptin neurons, very few appeared to coexpress ERα (1/28 and 2/12; Fig. 1E). ERα immunoreactivity otherwise remained robust throughout preoptic and mediobasal hypothalamic tissues of KERKO mice.
Pubertal Onset Is Dramatically Advanced in KERKO Mice.
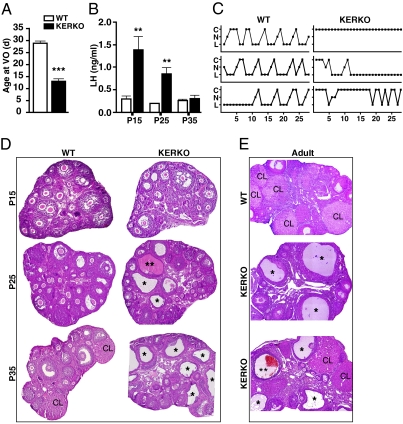
We then assessed pubertal maturation in groups of WT and KERKO mice at postnatal day (P) 15, P25, and P35. Vaginal opening, an external marker of puberty onset, occurred on average at a normal age (28.9 ± 0.9 d; n = 18) in WT mice, but was strikingly advanced in KERKO mice (13.0 ± 1.1 d; n = 7; Fig. 2A). Serum LH levels in WT mice remained low until P35, when they rose to normal basal levels for this age (Fig. 2B). In KERKO mice, however, the profile of serum LH concentrations was profoundly different; these were elevated six- to sevenfold in P15 KERKO mice (1.4 ± 0.3 ng/mL; n = 7) compared with values in corresponding WT mice. The LH levels in KERKO mice remained elevated at P25 (0.9 ± 0.1 ng/mL; n = 10) and declined to values similar to WT by P35 (0.3 ± 0.1 ng/mL; n = 8; Fig. 2B). Estradiol-17β levels in serum samples remained low or undetectable (≤ 2 pg/mL) in P15 (WT: 2.7 ± 0.6 pg/mL; n = 5, and KERKO: 2.0 ± 0.0 pg/mL; n = 4) and P25 (WT: 2.4 ± 0.3 pg/mL; n = 9, and KERKO: 2.8 ± 0.1 pg/mL; n = 3) mice. These data suggest that the absence of ERα in kisspeptin neurons precociously activates GnRH pulse neurosecretion, which leads to premature LH secretion and hence, profoundly advanced puberty onset. Thus, ERα signaling in kisspeptin neurons normally maintains a prepubertal restraint of GnRH release. This restraint mechanism is likely mediated by the ARC kisspeptin neurons, because the absence of ERα in these cells is associated with enhanced kisspeptin expression (see below).
Fig. 2.
KERKO mice exhibit premature activation of the reproductive axis but do not attain normal estrous cyclicity. (A) Vaginal opening is advanced to the early juvenile period in KERKO mice. (B) Serum LH levels are abnormally elevated in juvenile (P15) and peripubertal (P25) KERKO mice compared with WT. Data are presented as mean ± SEM. **P < 0.001; ***P < 0.0001. (C) KERKO animals do not exhibit normal estrous cyclicity, but instead enter a prolonged period of persistent vaginal cornification (Top Right), often followed by a persistent diestrus state (Middle Right). In some animals, isolated cycles occurred followed by additional prolonged periods of acyclicity (Bottom Right). C, cornified (estrus); N, nucleated (proestrus); L, leukocytic (metestrus and diestrus). (D) Corpora lutea (CL) are present in ovaries from P35 WT mice (example is from a KissIC+/ERαlox/wt mouse) and absent in those of P35 KERKO mice. Numerous follicular cysts (*), only rarely hemorrhagic (**), are evident in P25 and P35 KERKO, but not in WT ovaries. (E) Ovarian histology reveals an abundance of corpora lutea in WT mice (Top), which are either absent (Middle) or present in low numbers (Bottom) in KERKO mice. Large follicular cysts (*) are also evident in the ovaries of adult KERKO mice.
KERKO Mice Fail to Develop Normal Estrous Cyclicity.
Though pubertal onset is gauged by vaginal opening in female rodents, the complete process of puberty is defined by vaginal opening followed by first ovulation and the attainment of ovulatory cyclicity. Thus, following pubertal onset, subsequent stages of normal female pubertal maturation are characterized by further enhancement of pulsatile GnRH release and initiation of estrous cyclicity (9). Daily inspection of vaginal cytology in KERKO females, however, revealed an absence of normal estrous cyclicity (Fig. 2C and Fig. S1A). KERKO mice exhibited a prolonged period of persistent vaginal cornification, lasting weeks or even months, often followed by a persistent diestrus state (Fig. 2C). In some animals, isolated “breakthrough” cycles occurred followed by additional prolonged periods of acyclicity. In contrast to P35 WT mouse ovaries, where corpora lutea are present, histological inspection of P35 KERKO mouse ovaries revealed a complete absence of corpora lutea (Fig. 2D), confirming anovulation associated with the observed estrous acyclicity. Ovaries from P25 and P35 KERKO mice also contained numerous follicular cysts (Fig. 2D). Similarly, adult KERKO ovaries contained numerous large follicular cysts, as well as few or no corpora lutea (Fig. 2E and Fig. S1 B and C). These findings reveal that KERKO mice do not acquire the capacity to sustain normal ovulatory cyclicity, and hence fail to fully progress through puberty.
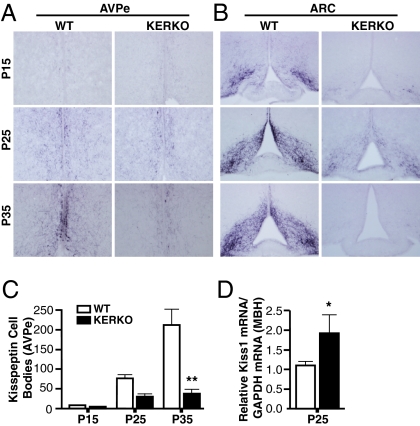
Kisspeptin Expression in KERKO Mice Is Diminished in the AVPe and Increased in ARC Neurons.
The initiation of ovulatory cyclicity during the latter stages of puberty thus depends upon activation of ERα in kisspeptin cells. We next tested whether ERα may normally mediate this process by enhancing kisspeptin expression (20), and thereby prompt further activation of GnRH neurosecretion (20, 23). Kisspeptin immunohistochemical analysis revealed that expression of the peptide in WT AVPe neurons was virtually undetectable at P15 (8.3 ± 3.9 immunostained kisspeptin neurons; n = 4), moderate at P25 (76.2 ± 9.3; n = 5), and robust at P35 (211 ± 39.9; n = 4; Fig. 3 A and C). In contrast, the number of immunoreactive AVPe kisspeptin neurons in KERKO mice at P25 (30.2 ± 6.0; n = 5) and P35 (45.2 ± 10.9; n = 5) was markedly reduced (Fig. 3 A and C). As previously reported (20), analysis of kisspeptin immunoreactivity in the ARC was limited to the dense fiber network that largely precludes the accurate quantitation of cell bodies in this nucleus. Immunoreactivity in these fibers was robust in WT mice by P15 and remained so thereafter. A pronounced diminishment of ARC kisspeptin expression was observed in KERKO mice at all three ages (Fig. 3B). Because the bulk of these fibers likely originate outside of the ARC, it was not possible to assess changes in kisspeptin cellular expression in ARC neurons of KERKO mice using this method. Indeed, our findings in WT and KERKO animals in this regard are remarkably similar to those obtained in WT vs. aromatase knockout (ArKO) mice (20, 25). We therefore analyzed Kiss1 gene expression in the mediobasal hypothalamic (MBH) tissues of separate sets of P25 mice by qPCR. In contrast to the diminishment of kisspeptin immunoreactivity in the KERKO AVPe, Kiss1 mRNA levels were increased in the MBH of KERKO mice vs. tissues obtained from controls (Fig. 3D). Taken together, our analyses reveal that ERα plays opposite roles in kisspeptin neurons in the two nuclei—that is, it mediates an inhibition of kisspeptin expression in ARC neurons and a stimulation of kisspeptin expression in AVPe neurons.
Fig. 3.
Kisspeptin expression fails to develop normally in AVPe of KERKO mice. (A and B) Representative examples of immunohistochemical labeling of kisspeptin cells in the AVPe (A) and ARC (B) of WT and KERKO mice at P15, P25, and P35. (C) Statistical analysis of kisspeptin immunoreactive (kisspeptin-IR) cell numbers reveals the progression of kisspeptin expression from undetectable at P15, through intermediate numbers at P25, and highest numbers at P35 in WT mice; by contrast, kisspeptin-IR cells appear significantly lower in numbers in P35 KERKO mice. (D) Kiss1 gene expression is increased in MBH tissues of P25 KERKO mice, compared with tissues obtained from a corresponding group of WT/ERαlox/wt mice. Data are presented as mean ± SEM. *P < 0.01; **P < 0.001.
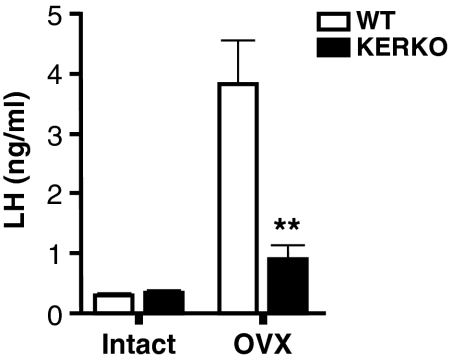
GnRH/LH Secretions in KERKO Mice Do Not Reach Normal Postpubertal Levels.
The enhanced kisspeptin expression in the AVPe during pubertal maturation is thought to increase GnRH/LH release during later stages of puberty (20). We therefore reasoned that diminished kisspeptin expression in KERKO mice would also lead to decreased GnRH secretion in postpubertal animals. In adult females, ovarian hormones exert homeostatic feedback actions in the reproductive axis that include direct suppression of LH from pituitary gonadotropes, and these effects may thus confound the use of serum LH levels as a proxy measure of GnRH release. Therefore, we tested our hypothesis by comparing LH levels in WT vs. KERKO animals both in the presence and absence of ovarian feedback. LH secretion in the absence of ovarian restraint was greatly reduced in KERKO (1.0 ± 0.2 ng/mL; n = 8) vs. WT (3.8 ± 0.7 ng/mL; n = 17) mice (Fig. 4). Thus, activation of ERα in kisspeptin neurons during pubertal development is required for GnRH neurosecretion to reach its maximal capacity in adult animals. This is a specific requirement for female maturation, as male KERKO mice did not show a similar impairment in the LH response to castration (Fig. S2A). Serum LH concentrations in P35 male KERKO mice, as well as testes weights in adult KERKO mice, were also not different from values recorded in their WT counterparts (Fig. S2 B and C), indicating that ERα activation in kisspeptin cells is not integral to male pubertal development as it is in the female.
Fig. 4.
Adult female KERKO mice exhibit diminished LH secretory responses to ovariectomy. Basal LH levels were similar in adult ovary-intact (Intact) mice, WT, and KERKO mice, but the LH response to ovariectomy (OVX) was significantly attenuated in the KERKO mice. Data are presented as mean ± SEM. **P < 0.001.
Conclusions
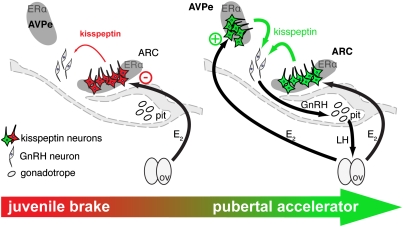
These studies reveal that two ERα-dependent mechanisms—one a brake and the other an accelerator— are sequentially operated in two different populations of kisspeptin neurons during pubertal development of female mice to gate, and then to activate, GnRH release (Fig. 5). A juvenile restraint of GnRH release appears to be mediated by ERα activation in ARC kisspeptin neurons, whereas the subsequent pubertal stimulation of GnRH release depends upon ERα signaling in AVPe kisspeptin neurons. This appears to ensure coordinated progression of neuroendocrine events culminating in increased basal reproductive hormone secretions and the capacity to sustain ovulatory cyclicity, hallmarks of the fully mature adult reproductive state.
Fig. 5.
Schematic representation of the hypothetical mechanisms of the regulation of pubertal onset and progression by ERα signaling in kisspeptin neurons. During the prepubertal period, ERα signaling mediates inhibition of kisspeptin expression in ARC and hence restraint of GnRH release. Pubertal onset is associated with decreased sensitivity to this suppressive mechanism in the ARC, as well as increased activation of ERα in AVPe kisspeptin neurons; the latter process stimulates kisspeptin expression in AVPe cells and increases GnRH release rates throughout the completion of puberty.
It remains to be determined in other species whether ERα in kisspeptin neurons similarly mediates prepubertal restraint and pubertal activation of GnRH release. In contrast to findings in rodents and sheep, the pubertal increase in GnRH and LH secretions in rhesus monkeys can occur independently of the presence or absence of the ovaries (26, 27), indicating that central restraint of the female GnRH pulse generator during the juvenile period in this species is predominantly steroid independent. A role for ERα in ARC kisspeptin neurons in puberty onset in this species may therefore figure less importantly, although it is possible that central ERs are activated in a ligand-independent manner (28) by peripheral and central signals in prepubertal monkeys, thereby constituting an estrogen-independent, yet estrogen receptor-dependent, mechanism for the pubertal restraint. Estrogen treatments suppress gonadotropin secretion more effectively in prepubertal girls than in adult women (29), allowing for the possibility that some steroid-dependent mechanism may contribute to the timing of puberty onset in human females.
We consider it likely, however, that the activation of ERα in rostral kisspeptin neurons is also integral to pubertal maturation in other species. Kisspeptin expression increases throughout puberty in mice, rat, sheep, and monkeys, and previous studies have demonstrated that this is an estrogen-dependent phenomenon (20, 22, 30, 31). Estrogen therapy has been used successfully to jumpstart sexual maturation in girls with constitutive delay of puberty (32), suggesting ER-dependent neurotropic mechanisms governing maturation of central neuronal circuitries that control GnRH release. Our findings implicate ERα in kisspeptin neurons as the targets and mediators of these estrogen effects. The progression and completion of puberty thus appears to be governed by a positive feedback loop, in which rising levels of circulating estrogens in early puberty stimulate kisspeptin expression, which further elevates GnRH release and hence, prompts additional gonadotropin and steroid production. This cycle of stimulatory signals ultimately raises kisspeptin and GnRH release rates into the range that is required to support ovulatory cyclicity (33, 34), and hence completes the process of puberty. The initial events that propel this cycle may occur early in development, and in a sexually differentiated manner; our finding of diminished AVPe kisspeptin neuronal development in the KERKO mice is reminiscent of those obtained in female hypogonadal (hpg) mice (35) and ArKO mice (20, 25), as well as in male mice, in which the AVPe kisspeptin neuronal population is also relatively small (36, 37). Taken together, these findings implicate both E2 and ERα in kisspeptin neurons in the developmental feminization of the AVPe kisspeptin neuronal population, a process that commences as early as P15 (20). This process may also be preceded by active masculinization (diminishment) of the AVPe nucleus in the male subsequent to perinatal androgen exposure (37) and a default organization (enlargement) of the AVPe kisspeptin neuronal population in the absence of androgens in the female. The temporal sequence of the developmental events that lead to maturation of the female AVPe kisspeptin cell group, and hence the attainment of ovulatory cyclicity and full reproductive maturation, remain to be clarified.
Materials and Methods
Animals.
Mice were housed in the University of Hamburg or Northwestern University animal facilities under a 12-h light/12-h dark cycle and had access to water and rodent chow ad libitum. All experimental procedures adhered to guidelines provided in the National Institutes of Health Guide for the Care and Use of Laboratory Animals and were approved by the Institutional Animal Care and Use Committee at Northwestern University and the University of Hamburg.
Generation of Kisspeptin-IRES-Cre (KissIC) Mice.
A total of 4.8 kb of genomic DNA containing exon 3 of the Kiss1 gene (Fig. 1A) was PCR amplified from BAC RP23-240P23 DNA (Wellcome Trust Sanger Institute, Cambridge) and subcloned into the pKO-V901 plasmid (Lexicon Genetics) containing a phosphoglycerate kinase (pgk) promoter-driven diphtheria toxin A cassette. Accuracy of PCR products was confirmed by sequencing. A unique AscI site was created 3′ to the stop codon of the Kiss1 gene to insert an IRES-Cre-FRT-NEO-FRT (38) cassette containing a pgk promoter-driven neomycin resistance cassette flanked by FRT sites. The targeting construct was electroporated into IDG3.2 mouse embryonic stem cells (kindly provided by Ralf Kühn, GSF, Munich), and resistant clones were analyzed by Southern blotting using SacI and an external 629-bp probe (Fig. 1A). Correctly targeted ES cells were injected into C57BL/6J blastocysts to generate chimeras that were backcrossed to C57BL/6J animals to give KissICneo+ mice. For genotyping, genomic DNA from tail biopsies was prepared by proteinase K (Roche) digestion and subsequent purification with DNA Isolation Reagent for genomic DNA (AppliChem). Mice were genotyped by Southern blotting as described. To remove the FRT-flanked neomycin resistance cassette, KissICneo+ animals were bred to Flp recombinase deleter mice (39) to create KissICneo- (KissIC) mice. Flp-mediated excision of the selection cassette was verified by Southern blot analysis using Bsu36I and the same probe as before (Fig. 1A). KissIC mice were backcrossed to C57BL/6J for six generations. ROSA26-YFP (R26-YFP) mice were kindly provided by S. Srinivas (University of Oxford, Oxford, United Kingdom) (40). ROSA26-CAGS-τGFP (eR26-τGFP) mice express a fusion of the microtubule-associated protein tau with GFP (τGFP) (41) under control of the β-actin promoter/CMV enhancer (CAGS) in the ROSA26 locus after Cre-mediated excision of a stop sequence, and were used to visualize kisspeptin fibers.
Immunofluorescence.
KissIC/R26-YFP or KissIC/eR26-τGFP mice were perfused transcardially with 4% paraformaldehyde (fixative) under ketamine/xylazine (Bayer) anesthesia. Tissues were removed and soaked in fixative for 2 h, followed by 30% sucrose for 48 h, and then frozen in tissue freezing medium OCT (Leica) and cut into 14-μm sections with a cryostat (42). Coronal brain sections were blocked in 1× PBS, 0.025% TX-100, 5% horse serum, and then treated either with rabbit anti-kisspeptin (1:500; Millipore) or with rabbit anti-estrogen receptor α (ERα) (1:1,000; Chemicon) antiserum overnight at 4 °C, followed by Cy3-donkey anti-rabbit IgG (1:500; Jackson Labs) for 1 h at room temperature. Sections were then incubated with Hoechst solution for nuclear staining and coverslipped with Fluoromount-G (SouthernBiotech).
Generation of KissIC+/ERαlox/lox (KERKO) Mice.
To specifically ablate ERα in KissIC mice, a mouse strain was used in which exon 3 of the ERα (Esr1) gene, which encodes the ERα DNA-binding domain, is flanked by loxP sites (ERαlox/lox). Deletion of exon 3 using this model results in a lack of ERα protein (24). These mice were previously backcrossed onto the pure C57BL/6J background strain. KissIC mice were crossed with ERαlox/lox mice, and then the resultant KissIC+/ERαlox/wt animals were crossed with ERαlox/lox mice. The F2 progeny included KissIC+/ERαlox/lox (KERKO) mice and KissIC−/ERαlox/lox mice. The latter were used as WT controls.
Immunohistochemistry.
Animals were anesthetized with 80 mg/kg, i.p. ketamine (Fort Dodge Laboratories) and 32 mg/kg, i.p. xylazine (Burns Veterinary Supply, Inc.) and perfused intracardially with 0.1 M PBS followed by 4% paraformaldehyde in 0.1 M PBS. Brains were removed and postfixed in 4% paraformaldehyde for 24 h at 4 °C followed by immersion in 30% sucrose cryoprotectant. Brains were cut into three sets of 30-μm-thick coronal sections, and two of these sets were used for both single- and double-immunocytochemistry staining as previously described (36, 43). Further details appear in SI Materials and Methods.
Vaginal Opening and Estrous Cyclicity.
Beginning at 10 d of age, females were examined for the onset of vaginal opening. To examine the possible effects of genotype on estrous cyclicity, vaginal lavages from female mice (3 wk to 3 mo old) were obtained and viewed under a microscope daily (900–1,000 h) for at least 31 consecutive days. A normal estrous cycle was defined as exhibiting vaginal cytology that was leukocytic (L) for 2 d followed by 1 d of nucleated (N) and 1–2 d of cornified (C) vaginal cytology.
Hormonal Assays.
Animals were anesthetized with ketamine/xylazine i.p., and blood was withdrawn by cardiac puncture (0900–1,100 h). Serum was assayed for LH using RIA reagents obtained from the National Institute of Diabetes and Digestive and Kidney Diseases, including the LH reference (RP-3) and S-11 antibody. The assay had a lower limit of detection of 0.2 ng/mL. The intraassay and interassay coefficients of variance (CVs) were 4.1% and 7.9%, respectively.
Ovarian Histology.
Ovaries were dissected, immersed in 4% paraformaldehyde overnight at 4 °C, and then washed with ethanol, processed, and embedded in paraffin wax. Paraffin-embedded ovaries were mounted on a microtome, cut into 5-μm-thick slices, and then stained with H&E.
Quantitative Real-Time PCR.
Total RNA was extracted from MBH tissue using TRIzol reagent (Invitrogen) according to the manufacturer's protocol. RNA was reverse transcribed using the iScript cDNA synthesis kit (BioRad). PCR reactions were performed with IQ SYBR Green Supermix (BioRad), and fluorescence was measured using the MyiQ quantitative real-time thermocycler (BioRad). The following primer sets were used: Kiss1 (sense 5′-AGCTGCTGCTTCTCCTCTGT-3′ and antisense 5′-GCATACCGCGATTCCTTTT-3′) (44). TaqMan was used to measure GAPDH reference gene levels, and were measured on an IQ5 thermocylcer (BioRad); sense 5′-GGGCATCTTGGGCTACACT-3′, antisense 5′-GGCATCGAAGGTGGAAGAGT-3′, probe 5′-Cal fluoro red-610-AGGACCAGGTTGTCTCCTGCGA-BHQ2 3′. Each reaction was run in triplicate, and for each assay a standard curve was created using 10-fold serial dilutions of cDNA. All experiments had efficiencies between 95% and 105%, and Kiss1 measures displayed normal melt curves. Fold changes in relative gene expression were calculated by 2−Δ(ΔCt), where ΔCt = Ct (Kiss1) − Ct(GAPDH) and Δ(ΔCt) = ΔCt (KERKO) − mean ΔCt (WT). Results are expressed as fold differences in relative gene expression with respect to WT.
Statistical Analysis.
Data are presented as the mean ± SEM. Two-tailed, unpaired t tests with Welch's correction were used to determine statistical significance for age at vaginal opening and Kiss1 expression in the MBH. Two-way ANOVA followed by Bonferroni post hoc tests were used to determine statistical significance in the remaining experiments. Differences were considered significant when P < 0.05.
Supplementary Material
Acknowledgments
We gratefully acknowledge the technical assistance of Brigitte Mann. Support for this work was provided by Deutsche Forschungsgemeinschaft Grant DFG BO1743/2 and National Institutes of Health Grants K99 HD55446, P01 HD21921, T32 HD07068, and P50 HD44405.
Footnotes
The authors declare no conflict of interest.
*This Direct Submission article had a prearranged editor.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1012406108/-/DCSupplemental.
References
- 1.Watanabe G, Terasawa E. In vivo release of luteinizing hormone releasing hormone increases with puberty in the female rhesus monkey. Endocrinology. 1989;125:92–99. doi: 10.1210/endo-125-1-92. [DOI] [PubMed] [Google Scholar]
- 2.Sisk CL, Richardson HN, Chappell PE, Levine JE. In vivo gonadotropin-releasing hormone secretion in female rats during peripubertal development and on proestrus. Endocrinology. 2001;142:2929–2936. doi: 10.1210/endo.142.7.8239. [DOI] [PubMed] [Google Scholar]
- 3.Harris GC, Levine JE. Pubertal acceleration of pulsatile gonadotropin-releasing hormone release in male rats as revealed by microdialysis. Endocrinology. 2003;144:163–171. doi: 10.1210/en.2002-220767. [DOI] [PubMed] [Google Scholar]
- 4.Kulin HE, Grumbach MM, Kaplan SL. Changing sensitivity of the pubertal gonadal hypothalamic feedback mechanism in man. Science. 1969;166:1012–1013. doi: 10.1126/science.166.3908.1012. [DOI] [PubMed] [Google Scholar]
- 5.Steele RE, Weisz J. Changes in sensitivity of the estradiol-LH feedback system with puberty in the female rat. Endocrinology. 1974;95:513–520. doi: 10.1210/endo-95-2-513. [DOI] [PubMed] [Google Scholar]
- 6.Foster DL, Ryan KD. Endocrine mechanisms governing transition into adulthood: A marked decrease in inhibitory feedback action of estradiol on tonic secretion of luteinizing hormone in the lamb during puberty. Endocrinology. 1979;105:896–904. doi: 10.1210/endo-105-4-896. [DOI] [PubMed] [Google Scholar]
- 7.Bronson FH. The regulation of luteinizing hormone secretion by estrogen: Relationships among negative feedback, surge potential, and male stimulation in juvenile, peripubertal, and adult female mice. Endocrinology. 1981;108:506–516. doi: 10.1210/endo-108-2-506. [DOI] [PubMed] [Google Scholar]
- 8.Rapisarda JJ, Bergman KS, Steiner RA, Foster DL. Response to estradiol inhibition of tonic luteinizing hormone secretion decreases during the final stage of puberty in the rhesus monkey. Endocrinology. 1983;112:1172–1179. doi: 10.1210/endo-112-4-1172. [DOI] [PubMed] [Google Scholar]
- 9.Ojeda SR, Skinner MK. Puberty in the rat. In: Neill JD, editor. Physiology of Reproduction. 3rd Ed. St. Louis: Elsevier; 2006. pp. 2061–2126. [Google Scholar]
- 10.Seminara SB, et al. The GPR54 gene as a regulator of puberty. N Engl J Med. 2003;349:1614–1627. doi: 10.1056/NEJMoa035322. [DOI] [PubMed] [Google Scholar]
- 11.de Roux N, et al. Hypogonadotropic hypogonadism due to loss of function of the KiSS1-derived peptide receptor GPR54. Proc Natl Acad Sci USA. 2003;100:10972–10976. doi: 10.1073/pnas.1834399100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Funes S, et al. The KiSS-1 receptor GPR54 is essential for the development of the murine reproductive system. Biochem Biophys Res Commun. 2003;312:1357–1363. doi: 10.1016/j.bbrc.2003.11.066. [DOI] [PubMed] [Google Scholar]
- 13.Lapatto R, et al. Kiss1−/− mice exhibit more variable hypogonadism than Gpr54−/− mice. Endocrinology. 2007;148:4927–4936. doi: 10.1210/en.2007-0078. [DOI] [PubMed] [Google Scholar]
- 14.d'Anglemont de Tassigny X, et al. Hypogonadotropic hypogonadism in mice lacking a functional Kiss1 gene. Proc Natl Acad Sci USA. 2007;104:10714–10719. doi: 10.1073/pnas.0704114104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Irwig MS, et al. Kisspeptin activation of gonadotropin releasing hormone neurons and regulation of KiSS-1 mRNA in the male rat. Neuroendocrinology. 2004;80:264–272. doi: 10.1159/000083140. [DOI] [PubMed] [Google Scholar]
- 16.Messager S, et al. Kisspeptin directly stimulates gonadotropin-releasing hormone release via G protein-coupled receptor 54. Proc Natl Acad Sci USA. 2005;102:1761–1766. doi: 10.1073/pnas.0409330102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Navarro VM, et al. Advanced vaginal opening and precocious activation of the reproductive axis by KiSS-1 peptide, the endogenous ligand of GPR54. J Physiol. 2004;561:379–386. doi: 10.1113/jphysiol.2004.072298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Matsui H, Takatsu Y, Kumano S, Matsumoto H, Ohtaki T. Peripheral administration of metastin induces marked gonadotropin release and ovulation in the rat. Biochem Biophys Res Commun. 2004;320:383–388. doi: 10.1016/j.bbrc.2004.05.185. [DOI] [PubMed] [Google Scholar]
- 19.Pineda R, et al. Critical roles of kisspeptins in female puberty and preovulatory gonadotropin surges as revealed by a novel antagonist. Endocrinology. 2010;151:722–730. doi: 10.1210/en.2009-0803. [DOI] [PubMed] [Google Scholar]
- 20.Clarkson J, Boon WC, Simpson ER, Herbison AE. Postnatal development of an estradiol-kisspeptin positive feedback mechanism implicated in puberty onset. Endocrinology. 2009;150:3214–3220. doi: 10.1210/en.2008-1733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gottsch ML, et al. Regulation of Kiss1 and dynorphin gene expression in the murine brain by classical and nonclassical estrogen receptor pathways. J Neurosci. 2009;29:9390–9395. doi: 10.1523/JNEUROSCI.0763-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Takase K, et al. Possible role of oestrogen in pubertal increase of Kiss1/kisspeptin expression in discrete hypothalamic areas of female rats. J Neuroendocrinol. 2009;21:527–537. doi: 10.1111/j.1365-2826.2009.01868.x. [DOI] [PubMed] [Google Scholar]
- 23.Smith JT, Popa SM, Clifton DK, Hoffman GE, Steiner RA. Kiss1 neurons in the forebrain as central processors for generating the preovulatory luteinizing hormone surge. J Neurosci. 2006;26:6687–6694. doi: 10.1523/JNEUROSCI.1618-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Singh SP, et al. Impaired estrogen feedback and infertility in female mice with pituitary-specific deletion of estrogen receptor alpha (ESR1) Biol Reprod. 2009;81:488–496. doi: 10.1095/biolreprod.108.075259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bakker J, Pierman S, González-Martínez D. Effects of aromatase mutation (ArKO) on the sexual differentiation of kisspeptin neuronal numbers and their activation by same versus opposite sex urinary pheromones. Horm Behav. 2010;57:390–395. doi: 10.1016/j.yhbeh.2009.11.005. [DOI] [PubMed] [Google Scholar]
- 26.Chongthammakun S, Claypool LE, Terasawa E. Ovariectomy increases in vivo luteinizing hormone-releasing hormone release in pubertal, but not prepubertal, female rhesus monkeys. J Neuroendocrinol. 1993;5:41–50. doi: 10.1111/j.1365-2826.1993.tb00362.x. [DOI] [PubMed] [Google Scholar]
- 27.Chongthammakun S, Terasawa E. Negative feedback effects of estrogen on luteinizing hormone-releasing hormone release occur in pubertal, but not prepubertal, ovariectomized female rhesus monkeys. Endocrinology. 1993;132:735–743. doi: 10.1210/endo.132.2.8425492. [DOI] [PubMed] [Google Scholar]
- 28.Olesen KM, Jessen HM, Auger CJ, Auger AP. Dopaminergic activation of estrogen receptors in neonatal brain alters progestin receptor expression and juvenile social play behavior. Endocrinology. 2005;146:3705–3712. doi: 10.1210/en.2005-0498. [DOI] [PubMed] [Google Scholar]
- 29.Kelch RP, Kaplan SL, Ghumbach MM. Suppression of urinary and plasma follicle-stimulating hormone by exogenous estrogens in prepubertal and pubertal children. J Clin Invest. 1973;52:1122–1128. doi: 10.1172/JCI107278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Smith JT, Li Q, Pereira A, Clarke IJ. Kisspeptin neurons in the ovine arcuate nucleus and preoptic area are involved in the preovulatory luteinizing hormone surge. Endocrinology. 2009;150:5530–5538. doi: 10.1210/en.2009-0712. [DOI] [PubMed] [Google Scholar]
- 31.Terasawa E, et al. Recent discoveries on the control of gonadotrophin-releasing hormone neurones in nonhuman primates. J Neuroendocrinol. 2010;22:630–638. doi: 10.1111/j.1365-2826.2010.02027.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kulin HE. Delayed puberty. J Clin Endocrinol Metab. 1996;81:3460–3464. doi: 10.1210/jcem.81.10.8855785. [DOI] [PubMed] [Google Scholar]
- 33.Terasawa E. Developmental changes in the positive feedback effect of estrogen on luteinizing hormone release in ovariectomized female rhesus monkeys. Endocrinology. 1985;117:2490–2497. doi: 10.1210/endo-117-6-2490. [DOI] [PubMed] [Google Scholar]
- 34.Foster DL, Karsch FJ. Development of the mechanism regulating the preovulatory surge of luteinizing hormone in sheep. Endocrinology. 1975;97:1205–1209. doi: 10.1210/endo-97-5-1205. [DOI] [PubMed] [Google Scholar]
- 35.Gill JC, et al. Reproductive hormone-dependent and -independent contributions to developmental changes in kisspeptin in GnRH-deficient hypogonadal mice. PLoS ONE. 2010;5:e11911. doi: 10.1371/journal.pone.0011911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Clarkson J, Herbison AE. Postnatal development of kisspeptin neurons in mouse hypothalamus; sexual dimorphism and projections to gonadotropin-releasing hormone neurons. Endocrinology. 2006;147:5817–5825. doi: 10.1210/en.2006-0787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kauffman AS, et al. Sexual differentiation of Kiss1 gene expression in the brain of the rat. Endocrinology. 2007;148:1774–1783. doi: 10.1210/en.2006-1540. [DOI] [PubMed] [Google Scholar]
- 38.Eggan K, et al. Mice cloned from olfactory sensory neurons. Nature. 2004;428:44–49. doi: 10.1038/nature02375. [DOI] [PubMed] [Google Scholar]
- 39.Rodríguez CI, et al. High-efficiency deleter mice show that FLPe is an alternative to Cre-loxP. Nat Genet. 2000;25:139–140. doi: 10.1038/75973. [DOI] [PubMed] [Google Scholar]
- 40.Srinivas S, et al. Cre reporter strains produced by targeted insertion of EYFP and ECFP into the ROSA26 locus. BMC Dev Biol. 2001;1:4. doi: 10.1186/1471-213X-1-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rodriguez I, Feinstein P, Mombaerts P. Variable patterns of axonal projections of sensory neurons in the mouse vomeronasal system. Cell. 1999;1:199–208. doi: 10.1016/s0092-8674(00)80730-8. [DOI] [PubMed] [Google Scholar]
- 42.Boehm U, Zou Z, Buck LB. Feedback loops link odor and pheromone signaling with reproduction. Cell. 2005;123:683–695. doi: 10.1016/j.cell.2005.09.027. [DOI] [PubMed] [Google Scholar]
- 43.Clarkson J, d'Anglemont de Tassigny X, Moreno AS, Colledge WH, Herbison AE. Kisspeptin-GPR54 signaling is essential for preovulatory gonadotropin-releasing hormone neuron activation and the luteinizing hormone surge. J Neurosci. 2008;28:8691–8697. doi: 10.1523/JNEUROSCI.1775-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Luque RM, Kineman RD, Tena-Sempere M. Regulation of hypothalamic expression of KiSS-1 and GPR54 genes by metabolic factors: Analyses using mouse models and a cell line. Endocrinology. 2007;148:4601–4611. doi: 10.1210/en.2007-0500. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.