Abstract
Cognitive dysfunction and memory loss are common features of Alzheimer's disease (AD). Abnormalities in the expression profile of immediate early genes that play a critical role in memory formation, such as the cAMP-response element binding protein (CREB), have been reported in the brains of AD patients. Here we show that amyloid-β (Aβ) accumulation, which plays a primary role in the cognitive deficits of AD, interferes with CREB activity. We further show that restoring CREB function via brain viral delivery of the CREB-binding protein (CBP) improves learning and memory deficits in an animal model of AD. Notably, such improvements occur without changes in Aβ and tau pathology, and instead are linked to an increased level of brain-derived neurotrophic factor. The resulting data suggest that Aβ-induced learning and memory deficits are mediated by alterations in CREB function, based on the finding that restoring CREB activity by directly modulating CBP levels in the brains of adult mice is sufficient to ameliorate learning and memory. Therefore, increasing CBP expression in adult brains may be a valid therapeutic approach not only for AD, but also for various brain disorders characterized by alterations in immediate early genes, further supporting the concept that viral vector delivery may be a viable therapeutic approach in neurodegenerative diseases.
Keywords: tangles, presenilin
Cognitive dysfunction associated with profound memory loss is a major clinical manifestation of Alzheimer's disease (AD). Neuropathologically, AD is characterized by the accumulation of amyloid plaques, formed mainly of the amyloid-β peptide (Aβ), and neurofibrillary tangles, formed of the microtubule-binding protein tau (1). Growing evidence points to soluble Aβ oligomers as the predominant neurotoxic species for neurons and clearly shows that the buildup of Aβ oligomers underlies memory loss in transgenic mice (2). The molecular pathways linking Aβ accumulation to cognitive decline remain elusive. However, soluble Aβ oligomers have been shown to alter signal transduction pathways that play a key role in learning and memory, suggesting that alterations in such pathways might underlie the onset of cognitive decline in AD. NMDA receptors are fundamental for synaptic plasticity and long-term potentiation, and in this context, Aβ accumulation has been shown to reduce glutamatergic transmission and to inhibit synaptic plasticity by interfering with NMDA receptor endocytosis, thereby reducing the receptors’ availability at synapses (3, 4). These results are consistent with the important role of reduced expression of proteins, such as NR2B and GluR1, in synaptic plasticity in transgenic mice (5). Aβ accumulation also has been shown to alter other transduction pathways involved in learning and memory (3, 6–9).
The critical role of immediate early genes (IEGs) in memory formation is widely accepted (10). The expression of some of these IEGs is reduced in AD, as has been shown by in vitro and in vivo experiments (5, 7–9, 11, 12). Most recently, it was reported that gene transcription, mediated by the cAMP-response element binding protein (CREB)-regulated transcription coactivator CRTC1, is impaired in a mouse model of AD (13), further suggesting that Aβ-induced memory deficits may be due to alterations in signaling transduction pathways. CREB is a key immediate early gene involved in learning and memory. The CREB-binding protein (CBP) is a transcriptional coactivator whose function is critical for CREB activity and learning and memory (14). Structurally, CBP has several protein-binding regions and a histone acetyltransferase (HAT) domain; functionally, CBP acts as a transcriptional coactivator by facilitating the recruitment of required components of the transcriptional machinery and as a HAT by altering chromatin structure (15). The role of CBP in AD is unclear, and contradicting reports have been published (16–18). Furthermore, although evidence indicates decreased CREB levels in animal models of AD, more work is needed to gain insight into the cause and consequences of such CREB deficits and how Aβ interferes with CREB activity.
Results
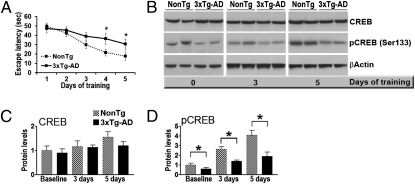
CREB phosphorylation and activity increase following neuronal stimulation; such activity-dependent increases are thought to facilitate the transcription of proteins required for learning and memory (19). Although studies from various laboratories have reported decreased CREB expression in mouse models of AD, whether, and if so, how CREB responds to neuronal stimulation in the presence of Aβ remain unclear. To address these questions, we used 3xTg-AD mice, a widely used animal model that develops Aβ and tau accumulation associated with cognitive dysfunction (20, 21). We trained 6-mo-old 3xTg-AD and NonTg mice (n = 16/genotype) for either 3 or 5 d in the spatial version of the Morris water maze (MWM). At age 6 mo, 3xTg-AD mice show early synaptic and learning and memory dysfunction, associated with the buildup of soluble Aβ levels (20). Eight 3xTg-AD and eight NonTg mice were killed within 30 min of their last learning trial on day 3, and their hippocampi were removed and frozen in dry ice. The remaining mice in each group received 2 additional days of training before being killed, and their hippocampi also were removed and frozen within 30 min of their last learning trial. Two-way ANOVA indicated significant genotype (P < 0.0001) and day (P < 0.0001) effects, as well as a significant genotype–day interaction (P = 0.0049) (Fig. 1A). A Bonferroni post hoc analysis indicated that although no statistically significant differences between the two genotypes were detected after 3 d of training, there was a strong trend supporting better performance by the NonTg mice (P = 0.07) compared with the 3xTg-AD mice (Fig. 1A). After 4 and 5 d of training, however, the average escape latency differed significantly in the NonTg and 3xTg-AD mice (P < 0.01 and < 0.05, respectively; Fig. 1A). At the end of the learning trials, the CREB levels were compared in these mice and in mice that had been killed directly from their housing cages (n = 8/genotype). Total CREB levels were not statistically different in the 3xTg-AD and NonTg mice at any of the time points analyzed (Fig. 1 B and C). In contrast, the steady-state levels of CREB phosphorylated at Ser133 (referred to as pCREB) in the hippocampi of the 3xTg-AD mice were decreased by ∼40% compared with age- and gender-matched NonTg mice at baseline (i.e., before any MWM training) (Fig. 1 B and D). Furthermore, pCREB levels in the NonTg mice increased as a function of training (Fig. 1 B and D). In contrast, although pCREB levels in the 3xTg-AD mice also tended to increase with learning, these changes were not statistically significant (Fig. 1 B and D). Analyzing the data as percentage change within each genotype revealed a 423.8% ± 134.2% increase in the NonTg mice over 5 d of training and a smaller, but not significantly different, increase (305.6% ± 142.9%) in the 3xTg-AD mice. Nevertheless, at all of the time points analyzed, pCREB levels were significantly lower in the 3xTg-AD mice. Furthermore, whereas baseline pCREB levels were ∼40% lower in the hippocampi of the 3xTg-AD mice compared with NonTg mice, after 3 and 5 d of MWM training, the pCREB levels were twice as high in the hippocampi of the NonTg mice compared with the 3xTg-AD mice (Fig. 1D). The hippocampi of 6-mo-old 3xTg-AD mice are characterized by the accumulation of Aβ and early tau mislocalization (20), suggesting that the alterations in CREB phosphorylation might be due to Aβ accumulation and/or tau mislocalization.
Fig. 1.
Activity-dependent CREB activation is impaired in the 3xTg-AD mice. (A) Learning abilities of 6-mo-old 3xTg-AD and NonTg mice (n = 16/genotype) were evaluated in the spatial reference version of the MWM. At the end of the third day, eight mice per genotype were killed, and the remaining mice were trained for 2 additional days. On days 4 and 5 of training, the NonTg mice performed significantly better than the 3xTg-AD mice, as demonstrated by a shorter time to find the hidden platform. (B) Representative Western blots (probed with the indicated antibodies) of proteins extracted from the hippocampi of mice killed directly from their home cages or trained in an MWM for 3 d or 5 d. (C and D) Quantitative analyses of the blots show similar total CREB levels in the NonTg and 3xTg-AD mice at baseline and after neuronal stimulation. In contrast, at baseline, the pCREB levels were significantly reduced in the brains of 3xTg-AD mice compared with NonTg mice. Although the percentage increase in pCREB level did not differ significantly between the NonTg mice and the 3xTg-AD mice, the absolute pCREB levels were significantly lower in the 3xTg-AD mice at all of the time points analyzed. Protein levels are expressed as fold changes over sham-injected NonTg mice and represent mean ± SEM. *P < 0.05; **P < 0.01.
We next sought to determine whether the changes in CREB phosphorylation in the hippocampi of the 3xTg-AD mice are mediated by Aβ accumulation. Toward this end, we first used an immunologic approach to clear Aβ deposits from the brains of the 3xTg-AD mice. We have shown that intrahippocampal injection of anti-Aβ antibodies is sufficient to clear Aβ deposits from the brains of the 3xTg-AD mice (22). Here, the 6E10 antibody (2 μg) was stereotaxically injected into the left hippocampi of 6-mo-old 3xTg-AD mice. The contralateral uninjected hippocampi were used as internal controls. At 3 d postinjection, the hippocampi were removed and analyzed. Sandwich ELISA revealed significantly reduced levels of soluble Aβ40 and Aβ42 in the ipsilateral hippocampi (receiving 6E10) compared with the contralateral uninjected hippocampi (Fig. S1A). Notably, the reduced Aβ levels led to significant increases in pCREB without affecting the total CREB levels (Fig. S1 B and C). To further explore the relationship between Aβ and the CREB phosphorylation, we used a genetic approach to prevent Aβ accumulation in the brains of the 3xTg-AD mice. Toward this end, we made use of the fact that replacing the mutant PS1 gene with its WT counterpart in the 3xTg-AD mice was shown to be sufficient to prevent Aβ accumulation (20). Indeed, these mice (known as APP/tau) exhibited no Aβ immunoreactivity in the brain (Fig. S1D), despite the fact the APP and tau transgene levels remained unchanged (20). Western blot analysis indicated that APP/tau mice, which lack Aβ pathology, had significantly higher pCREB levels compared with age- and gender-matched 3xTg-AD mice (Fig. S1 E and F).
To directly test for a causal relationship between Aβ and CREB deficits, we used Chinese hamster ovary (CHO) cells stably transfected with a cDNA encoding APP751 containing the Val717Phe familial AD mutation (23). These cells, known as 7PA2, are widely used because they secrete high levels of Aβ oligomers, which cause cognitive dysfunction (24). To determine whether Aβ oligomers affect CREB phosphorylation function in vivo, 7PA2 conditioned medium (CM) was concentrated by 50-fold using Amicon Ultra centrifugal filters and stereotaxically injected into the left hippocampi of 2-mo-old NonTg mice (n = 6). The right contralateral hippocampi were used as an internal control. Additional control groups were represented by mice injected with CM prepared from control CHO cells (n = 6) and by mice injected with 7PA2 CM immunodepleted with 6E10 (n = 6). At 3 d postinjection, the mice were killed and their hippocampi used for biochemical assessments. We found significantly lower levels of pCREB (but not of total CREB) in the ipsilateral injected hippocampi compared with the contralateral uninjected hippocampi (Fig. S1 G and H). Notably, the injection of CM from CHO cells or of 7PA2 CM that was depleted of Aβ by immunoprecipitation with 6E10 had no effect on CREB phosphorylation (Fig. S1 G and H). Taken together, these results demonstrate that in 3xTg-AD mice, the alterations in CREB phosphorylation are due to Aβ accumulation.
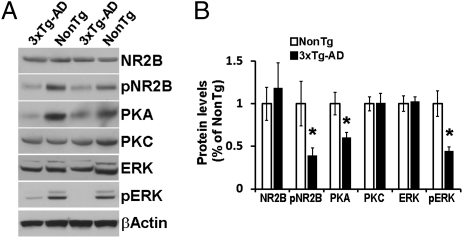
Evidence indicates that Aβ accumulation reduces glutamatergic transmission (4). Notably, during memory formation, CREB is phosphorylated at Ser133 via activation of NMDA receptors (19), suggesting that an alteration in NMDA signaling might account for the decreased pCREB levels in the 3xTg-AD mice. To test this hypothesis, we measured the steady-state levels of NMDA receptor subunit NR2B by Western blot analysis. We found no significant differences between the 3xTg-AD and NonTg mice (Fig. 2 A and B); however, we did note significantly decreased levels of NR2B phosphorylated at Tyr1472 in the hippocampi of the 3xTg-AD mice (Fig. 2 A and B). This finding is noteworthy, given that reduced phosphorylation of NR2B at Thr1472 correlates with receptor endocytosis, which leads to reduced NMDA receptor signaling (3).
Fig. 2.
NMDA signaling is impaired in the 3xTg-AD mice. (A) Representative Western blots of proteins extracted from the hippocampi of 6-mo-old 3xTg-AD and NonTg mice (n = 8/genotype) and probed with the indicated antibodies. (B) Quantitative analysis of the blots shows that the levels of the NMDA receptor subunit NR2B phosphorylated at Tyr1472, PKA, and pERK were significantly decreased in the 3xTg-AD mice compared with the NonTg mice. Protein levels are expressed as fold changes over NonTg mice and represent mean ± SEM. *P < 0.05.
Protein kinase A (PKA), protein kinase C (PKC), and extracellular signal-regulated kinase (ERK) are three proteins downstream of NMDA signaling known to phosphorylate and activate CREB (19). We thus measured the steady-state levels of these proteins in the hippocampi of 6-mo-old mice and found no difference in PKC levels between the 3xTg-AD and NonTg mice, but significantly lower PKA levels in the 3xTg-AD mice (Fig. 2 A and B). We also found significantly lower levels of ERK phosphorylated at Thr202/204 (pERK) in the 3xTg-AD mice (Fig. 2 A and B). Taken together, these findings indicate that two signaling pathways downstream of NMDA are deregulated in the hippocampi of 6-mo-old 3xTg-AD mice and thus might account for the reduced CREB phosphorylation.
Considering the role of CREB in learning and memory, these data suggest that one way in which Aβ accumulation might induce cognitive deficits is by altering CREB phosphorylation and activity. CBP plays a critical role in stimulus-induced activation of CREB (15); thus, we sought to facilitate CREB function in the brains of the 3xTg-AD mice by overexpressing CBP using a lentiviral delivery system. We generated a lentivirus expressing HA-tagged CBP under the control of the neuronal-specific promoter, EF1a (Fig. S2A). The CBO lentivirus was stereotaxically injected into the left dorsal lateral ventricle of 6-mo-old 3xTg-AD mice (n = 18) and NonTg mice (n = 17). In addition, 3xTg-AD mice (n = 17) and NonTg mice (n = 16) received sham injections. The injection site was chosen based on previous reports showing that a viral vector injected into the lateral ventricle diffuses through the hippocampus (25). To determine the extent of the viral diffusion, we stained sections from the sham- and CBP-injected mice with an anti-HA antibody. We observed only background staining in the hippocampi of the sham-injected mice (Fig. S2B), but high HA immunoreactivity in the dentate gyrus and CA1 regions of the hippocampus in the CBP-injected mice (Fig. S2 C and D), with very low HA immunoreactivity in the cortex (Fig. S2D). Double-staining of sections from the CBP-injected mice confirmed that the virus was expressed mainly in neurons and not in astrocytes (Fig. S2E). Notably, the virus also was expressed in the contralateral hippocampus (Fig. S2F).
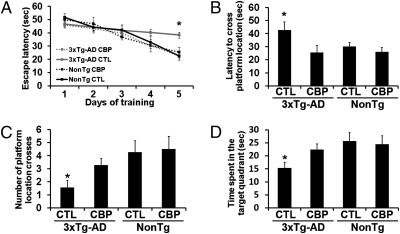
At 7 d after the CBP delivery, all mice were tested using the MWM. Some of the mice were killed at 3 d and 5 d after training (n = 4/group/time point), and their hippocampi were extracted and processed for biochemical measurements (see below). The remaining mice were used to conduct the probe trials. To analyze the learning data, we used a mixed-model repeated-measures ANOVA, with treatment and genotype as categorically fixed effects, days as a numeric covariate, animals as the random effect, and escape latency as the dependent variable. We found a significant effect for days (F = 86.2541; P < 0.0001), indicating that the slope of escape latency across the acquisition sessions was significantly different from 0 (Fig. 3A). We also found a significant group–day interaction (F = 4.5334; P = 0.004091), indicating that one or more of the groups differed from the others. To find the group(s) most responsible for the differences, we performed a post hoc test with Bonferroni correction and compared each of the individual interaction levels (i.e., slopes) to those in the sham-injected NonTg mice. We found that only the sham-injected 3xTg-AD mice had a significantly shallower slope than the sham-injected NonTg mice used as the baseline group, indicating slower improvement in escape latency across the acquisition sessions (P = 0.0049; Fig. 3A). The sham-injected 3xTg-AD mice performed significantly worse than the sham-injected NonTg mice, but the overexpression of CBP rescued the learning deficits of the 3xTg-AD mice (as evidenced by lack of significant differences in the slopes of the CBP-injected 3xTg-AD mice and the sham-injected NonTg mice used as the baseline); indeed, the CBP-injected 3xTg-AD mice performed as well as the sham-injected NonTg mice (P = 0.389; Fig. 3A). The slope of the escape latency was not significantly different between the CBP-injected NonTg mice and the sham-injected mice, indicating that CBP injections have no effect of the escape latency of the NonTg mice (Fig. 3A).
Fig. 3.
CBP gene transfer rescues learning and memory deficits in 3xTg-AD mice. CBP-expressing lentiviruses were injected into the dorsolateral ventricles of 3xTg-AD mice (n = 18) and NonTg mice (n = 17). In addition, 17 3xTg-AD mice and 16 NonTg mice received sham injections. Six mice per group were sacrificed after 3 d and 5 d of training. (A) All mice were evaluated in the spatial reference version of the MWM. The escape latency was significantly lower in the CBP-injected 3xTg-AD mice than in the sham-injected 3xTg-AD mice (P = 0.008). (B–D) Reference memory was significantly improved in the CBP-injected 3xTg-AD mice compared with the sham-injected 3xTg-AD mice in all probe trial measurements conducted. Data are presented as mean ± SEM. *P < 0.05.
To measure spatial memory, we conducted probe trials at 24 h after the final training trial. One-way ANOVA indicated significant changes in the time that the mice took to cross the platform location (P = 0.04; Fig. 3B), in the number of platform location crosses during the 60-s trials (P = 0.03; Fig. 3C), and in the time mice spent in the target quadrant (P = 0.04; Fig. 3D). A post hoc test with Bonferroni correction showed that the CBP-injected 3xTg-AD mice performed significantly worse than the sham-injected CBP mice in all three measurements (P < 0.05). As in the learning trials, here CBP gene delivery did not alter spatial memory in the NonTg mice (Fig. 3 B and D). Notably, the swimming speed was similar in all of the mice tested (Fig. S3). Taken together, these findings clearly indicate that CBP gene delivery rescued learning and memory deficits in 6-mo-old 3xTg-AD mice.
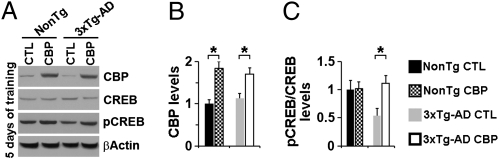
To elucidate the molecular basis underlying the CBP-mediated improvements in learning and memory, we examined the consequences of CBP gene delivery on CREB function. We compared CBP levels between the hippocampi of CBP- and sham-injected mice at baseline (7 d postinjection but without MWM training) and then after 3 d and 5 d of training. CBP levels were significantly higher in the hippocampi of the CBP-injected mice at all three time points, independent of the genotype (Fig. 4 A and B and Fig. S4 A, B, and D). Notably, at baseline, CBP levels were similar in sham-injected NonTg and 3xTg-AD mice (Fig. S4 A and B), suggesting that CBP levels were not altered in the 3xTg-AD mice. Furthermore, CREB phosphorylation was not changed in the NonTg mice at any of the time points analyzed (Fig. 4 A–C and Fig. S4 A, C, and E). In contrast, CBP overexpression restored CREB phosphorylation in the 3xTg-AD mice at baseline and after 3 d and 5 d of training (Fig. 4 A and C and Fig. S4 A, C, and E). These findings are consistent with the improved learning and memory seen in the 3xTg-AD mice during a hippocampal-dependent task.
Fig. 4.
CBP gene delivery restores pCREB levels after 5 d of training. (A) Representative Western blots of proteins extracted from the hippocampi of CBP- and sham-injected 3xTg-AD and NonTg mice (n = 6/group) and probed with the indicated antibodies. (B and C) Quantitative analysis of the blots shows significantly increased CBP levels in both the 3xTg-AD and NonTg mice receiving the virus. In contrast, pCREB levels were significantly increased in the CBP-injected compared with sham-injected 3xTg-AD mice, but not the NonTg mice. Data are presented as fold changes over sham-injected NonTg mice and represent mean ± SEM. *P < 0.05.
The onset of cognitive decline in the 3xTg-AD mice is due to Aβ accumulation, which, as we show here, is also responsible for the altered CREB phosphorylation (Fig. S1). As the 3xTg-AD mice age, cognitive decline becomes more severe and is also mediated by tau pathology (20). Notably, the lentivirus was seen to drive the expression of CBP in Aβ42- and tau-bearing neurons (Fig. S5A). Thus, we next asked whether the CBP-mediated improvement in behavior might be due to a decrease in Aβ and tau pathology. Sandwich ELISA measurements from protein extracted from the hippocampi of 3xTg-AD mice revealed similar levels of soluble Aβ40 and Aβ42 in the CBP-injected and sham-injected 3xTg-AD mice (Fig. S5B). Moreover, immunohistochemical analysis showed no changes in Aβ or tau immunoreactivity in these two groups of mice (Fig. S5 C and D).
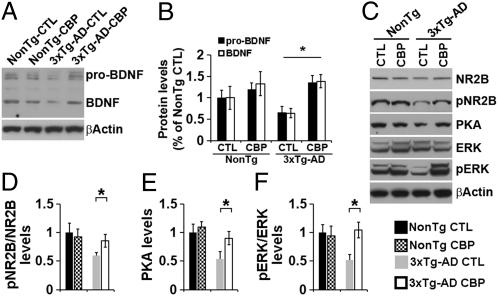
Taken together, the results presented so far indicate that restoration of CREB phosphorylation is sufficient to rescue learning and memory deficits without altering Aβ or tau levels. The role of CREB in cognition is well established; it is thought that, once activated, CREB facilitates the transcription of key proteins necessary for activity-dependent plasticity (19). One of these proteins is brain-derived neurotrophic factor (BDNF), which is known to facilitate synaptic plasticity and memory formation (26) and has been proposed to play a role in the pathogenesis of AD, with reduced BDNF levels detected in AD brains (27, 28). Thus, we next sought to determine whether the improvement in learning and memory following CBP gene transfer might be linked to an increased BDNF level in the hippocampus. Toward this end, we first measured the BDNF levels in the hippocampi of 6-mo-old 3xTg-AD and NonTg mice (n = 9/genotype), and found that BDNF levels were significantly decreased in the 3xTg-AD mice (Fig. S6). These results are consistent with previous reports of reduced BDNF levels in AD brains and other animal models (27–29). We next assessed how BDNF protein levels change in response to CBP gene transfer. Using Western blot analyses, we found higher BDNF levels (but not statistically significant in the CBP-injected NonTg mice compared with the sham-injected NonTg mice (Fig. 5 A and B). In contrast, we found significantly higher BDNF levels in the CBP-injected 3xTg-AD mice compared with sham-injected 3xTg-AD mice; notably, in the 3xTg-AD mice, CBP gene delivery restored BDNF levels to NonTg levels (Fig. 5 A and B).
Fig. 5.
CBP gene delivery rescues BDNF levels. (A) Representative Western blots of proteins extracted from the hippocampi of CBP- and sham-injected 3xTg-AD and NonTg mice and probed with an anti-BDNF antibody. (B) Quantitative analysis of the blots shows that in the hippocampi of the 3xTg-AD mice, CBP gene delivery restored the levels of pro-BDNF and BDNF to NonTg levels. (C) Representative Western blots of proteins extracted from the hippocampi of 6-mo-old sham- and CBP-injected 3xTg-AD and NonTg mice (n = 6/genotype) after 5 d of training and probed with the indicated antibodies. (D–F) Quantitative analysis of the blots shows that after 5 d of training, the pNR2B, PKA, and pERK levels were significantly increased in the CBP-injected 3xTg-AD mice compared with sham-injected 3xTg-AD mice. In contrast, no differences were found between the sham- and CBP-injected NonTg mice. Data are presented as fold changes over sham-injected NonTg mice and represent mean ± SEM. *P < 0.05.
The data presented in Fig. 2 indicate that NMDA signaling is decreased in 3xTg-AD mice; notably, BDNF facilitates NMDA signaling and increases phosphorylation of NR2B (30). Thus, to determine whether the CBP-mediated increase in BDNF facilitates NMDA signaling, we measured pNR2B levels at baseline and after 3 d and 5 d of training. We found significantly higher pNR2B levels at all three time points in the CBP-injected 3xTg-AD mice compared with the sham-injected 3xTg-AD mice (Fig. 5 C and D and Fig. S7 A, B, and E). An increase in pNR2B level has been correlated with increased NMDA signaling (3). Thus, we measured PKA and ERK levels, and found significantly higher PKA levels in the CBP-injected 3xTg-AD mice compared with the sham-injected 3xTg-AD mice at all three time points (Fig. 5 C and E and Fig. S7 A, C, and F). In contrast, we found significantly higher pERK levels in the CBP-injected 3xTg-AD mice after 3 d and 5 d of training, but not at baseline (Fig. 5 C and F and Fig. S7 A–D and G). Finally, we found that CBP injections did not alter NMDA signaling in NonTg mice (Fig. 5 and Fig. S7). Considering the primary role of BDNF and NMDA signaling in activity-dependent synaptic plasticity, the data presented here suggest that some of the beneficial effects of CBP gene transfer on learning and memory may be linked to a BDNF-mediated increase in NMDA signaling.
Discussion
On neuronal stimulation, CREB is normally phosphorylated at Ser133 and activated; this activation is necessary for memory formation and consolidation (19). Consistent with this, impaired CREB activation has been shown to have detrimental effects on various forms of learning and memory, including spatial reference memory, a hippocampal-dependent form of memory that is highly impaired in people with AD (19). Here we report a ∼40% decrease in pCREB levels at baseline in the hippocampi of 3xTg-AD mice, which is consistent with previous reports (7). Most notably, we further show that the difference in pCREB levels between the 3xTg-AD and NonTg mice was greater after neuronal stimulation; indeed, after 5 d of training, pCREB levels were ∼200% lower in the 3xTg-AD mice compared with the NonTg mice. PKA and ERK are two kinases involved in CREB phosphorylation on neuronal stimulation (19). Here we provide compelling in vivo evidence showing that in the hippocampi of the 3xTg-AD mice, Aβ accumulation is responsible for reduced pCREB levels. Considering the role of CREB during the formation of new memories, these data suggest that some of the learning and memory deficits observed in the 3xTg-AD mice might be mediated by deficits in CREB phosphorylation. These results are not meant to imply that this is the only signaling pathway linking Aβ accumulation to cognitive decline; however, it is likely that alterations in other synaptic signaling pathways could contribute to the learning and memory deficits observed. During neuronal stimulation, activation of NMDA receptors leads to PKA- and ERK-mediated activation and CREB phosphorylation, which are necessary for memory formation (19). Dysfunction in NMDA receptor signaling and trafficking has been reported in several animal models of AD (3, 4, 31). Here we report that the phosphorylation of tyrosine 1472 of the NR2B subunit, which increases the activity of NMDA receptors (3), was significantly decreased in the hippocampi of the 3xTg-AD mice at baseline. The Aβ-induced alterations in NMDA/PKA/ERK signaling seen in the 3xTg-AD mice might account for the attenuated response in CREB phosphorylation on exposure to new learning stimuli.
Once phosphorylated at Ser133, CREB binds to the transcriptional coactivator CBP, which facilitates transcription by recruiting other components of the transcriptional machinery and through its intrinsic HAT activity (15). Thus, CBP has been shown to enhance CREB-dependent transcription by directly acetylating CREB (15). Notably, several memory-related signal transduction pathways known to be altered by Aβ accumulation are directly linked to CREB/CBP. Saura et al. (18) reported that cognitive deficits in brain-specific presenilin (PS) 1 and PS2 double-knockout mice are associated with a decrease in CBP levels and CREB/CBP-mediated transcription. In vitro data consistently show that WT PS1, but not familial AD (FAD) mutations, facilitate CREB/CBP-mediated transcription (16). These data contrast with a report of FAD mutations in the PS1 gene increasing CREB/CBP-mediated transcription (17). Our present findings support the hypothesis that loss of function of presenilin and Aβ accumulation can synergistically cause cognitive impairments by interfering with CREB/CBP-mediated transcription, as proposed by Saura et al. (18). Here we show that Aβ accumulation decreased CREB phosphorylation and, more importantly, that increasing CBP expression in the hippocampi of adult 3xTg-AD mice was sufficient to rescue learning and memory deficits without affecting Aβ or tau pathology. Although the 3xTg-AD mice harbor a FAD mutation in the PS1 gene, the CREB deficits appear to be mediated by the accumulation of Aβ, and not directly by the mutant PS1 protein. Indeed, we show that reducing Aβ levels via intrahippocampal injections of 6E10 rescued pCREB levels, and that injections of soluble Aβ were sufficient to induce pCREB deficits in NonTg mice.
Our results indicate that the CBP-mediated improvements in learning and memory were linked to an increase in BDNF levels in the hippocampi. In turn, such an increase potentiates NMDA signaling, which may further facilitate CREB phosphorylation, creating a positive feed-forward loop. BDNF is a CREB target gene that plays a critical role in learning and memory, and there is a large body of literature showing BDNF dysfunction in AD. Indeed, Aβ accumulation is known to decrease BDNF levels in vitro and in animal models of AD (32), and, more importantly, BDNF levels are reduced in AD brains (27, 28). Indeed, several therapeutic strategies designed to ameliorate AD pathology are linked to increased BDNF levels; for example, behavioral enrichment and physical exercise have been found to increase BDNF levels and to ameliorate learning and memory deficits in animal models of AD (33, 34). Recent pioneering work reported by Blurton-Jones et al. (35) has shown that neuronal stem cells improve learning and memory deficits in 3xTg-AD mice by increasing BDNF levels without affecting Aβ and tau pathology. Consistent with these findings, another group has shown that BDNF administration improves learning and memory deficits in several animal models of AD, including in a nonhuman primate, without affecting Aβ pathology (36). These reports and our present findings are consistent with the view that BDNF deficits in AD are downstream of Aβ accumulation and that the Aβ-induced dysfunction in CREB-mediated transcription might account for the BDNF deficits. Although CBP levels were also increased in the hippocampi of NonTg mice, BDNF levels were not significantly higher in the CBP-injected compared with the sham-injected NonTg mice, suggesting that CREB-mediated BDNF transcription is tightly regulated under physiological conditions. Consistent with our hypothesis that NMDA/PKA/ERK signaling is restored by an increase in BDNF level, we found no changes in NMDA signaling in the CBP-injected NonTg mice.
In summary, our data indicate that cognitive dysfunction in AD can be restored without affecting Aβ or tau pathology, and lend support to the use of gene transfer into adult brains as a potential therapeutic approach for AD and other related neurodegenerative disorders. Toward this end, it should be noted that CBP dysfunction has been reported in other neurodegenerative disorders, including Huntington disease and Rubinstein–Taybi syndrome (37), suggesting that CBP gene delivery may have beneficial effects beyond AD.
Materials and Methods
Virus and Surgical Procedures.
Mice were anesthetized with avertin (1.3% tribromoethanol and 0.8% amyl alcohol, at a dose of 0.6 mL/25 g body weight) and placed in a stereotactic apparatus. The mice received either sham injections of PBS or 5 μL of the CBP-expressing lentivirus (∼1.2 × 106 transducing particles/mL; purchased from BioGenova), which was injected into the dorsolateral ventricle through a 33-gauge injector attached to a 5-μL Hamilton syringe. The coordinates with respect to the bregma were −0.34 mm posterior, −2.2 mm lateral, and −1 ventral to the skull. Injections were performed over 5 min, after which the cannula was left in place for an additional 5 min to allow for diffusion. The mice were kept on a warming pad until they had fully recovered from anesthesia.
See SI Materials and Methods for a detailed description of tissue collection, behavioral testing, immunohistochemistry, Western blot analysis, ELISA, and statistical analysis.
Supplementary Material
Acknowledgments
We thank Dr. Edward Koo (University of California, San Diego,CA) for the 7PA2 cells, Dr. Marcelo Wood (University of California, Irvine, CA) for the CBP plasmid, and Andrea Magri and David X. Medina for superb technical assistance. This work was supported by National Institute on Aging Grant R00-AG29729-4 (to S.O.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1012851108/-/DCSupplemental.
References
- 1.Querfurth HW, LaFerla FM. Alzheimer's disease. N Engl J Med. 2010;362:329–344. doi: 10.1056/NEJMra0909142. [DOI] [PubMed] [Google Scholar]
- 2.Crews L, Masliah E. Molecular mechanisms of neurodegeneration in Alzheimer's disease. Hum Mol Genet. 2010;19(R1):R12–R20. doi: 10.1093/hmg/ddq160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Snyder EM, et al. Regulation of NMDA receptor trafficking by amyloid-beta. Nat Neurosci. 2005;8:1051–1058. doi: 10.1038/nn1503. [DOI] [PubMed] [Google Scholar]
- 4.Palop JJ, Mucke L. Amyloid-beta–induced neuronal dysfunction in Alzheimer's disease: From synapses toward neural networks. Nat Neurosci. 2010;13:812–818. doi: 10.1038/nn.2583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dickey CA, et al. Selectively reduced expression of synaptic plasticity-related genes in amyloid precursor protein + presenilin-1 transgenic mice. J Neurosci. 2003;23:5219–5226. doi: 10.1523/JNEUROSCI.23-12-05219.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Caccamo A, Majumder S, Richardson A, Strong R, Oddo S. Molecular interplay between mammalian target of rapamycin (mTOR), amyloid-beta, and Tau: Effects on cognitive impairments. J Biol Chem. 2010;285:13107–13120. doi: 10.1074/jbc.M110.100420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ma QL, et al. Evidence of Abeta- and transgene-dependent defects in ERK-CREB signaling in Alzheimer's models. J Neurochem. 2007;103:1594–1607. doi: 10.1111/j.1471-4159.2007.04869.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Palop JJ, et al. Vulnerability of dentate granule cells to disruption of arc expression in human amyloid precursor protein transgenic mice. J Neurosci. 2005;25:9686–9693. doi: 10.1523/JNEUROSCI.2829-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Palop JJ, et al. Neuronal depletion of calcium-dependent proteins in the dentate gyrus is tightly linked to Alzheimer's disease–related cognitive deficits. Proc Natl Acad Sci USA. 2003;100:9572–9577. doi: 10.1073/pnas.1133381100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lanahan A, Worley P. Immediate-early genes and synaptic function. Neurobiol Learn Mem. 1998;70:37–43. doi: 10.1006/nlme.1998.3836. [DOI] [PubMed] [Google Scholar]
- 11.Vitolo OV, et al. Amyloid beta-peptide inhibition of the PKA/CREB pathway and long-term potentiation: Reversibility by drugs that enhance cAMP signaling. Proc Natl Acad Sci USA. 2002;99:13217–13221. doi: 10.1073/pnas.172504199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tong L, Thornton PL, Balazs R, Cotman CW. Beta -amyloid(1–42) impairs activity-dependent cAMP-response element–binding protein signaling in neurons at concentrations in which cell survival Is not compromised. J Biol Chem. 2001;276:17301–17306. doi: 10.1074/jbc.M010450200. [DOI] [PubMed] [Google Scholar]
- 13.España J, et al. beta-Amyloid disrupts activity-dependent gene transcription required for memory through the CREB coactivator CRTC1. J Neurosci. 2010;30:9402–9410. doi: 10.1523/JNEUROSCI.2154-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Goodman RH, Smolik S. CBP/p300 in cell growth, transformation, and development. Genes Dev. 2000;14:1553–1577. [PubMed] [Google Scholar]
- 15.Vo N, Goodman RH. CREB-binding protein and p300 in transcriptional regulation. J Biol Chem. 2001;276:13505–13508. doi: 10.1074/jbc.R000025200. [DOI] [PubMed] [Google Scholar]
- 16.Francis YI, Stephanou A, Latchman DS. CREB-binding protein activation by presenilin 1 but not by its M146L mutant. Neuroreport. 2006;17:917–921. doi: 10.1097/01.wnr.0000220137.06542.a0. [DOI] [PubMed] [Google Scholar]
- 17.Marambaud P, et al. A CBP-binding transcriptional repressor produced by the PS1/epsilon-cleavage of N-cadherin is inhibited by PS1 FAD mutations. Cell. 2003;114:635–645. doi: 10.1016/j.cell.2003.08.008. [DOI] [PubMed] [Google Scholar]
- 18.Saura CA, et al. Loss of presenilin functon causes impairments of memory and synaptic plasticity followed by age-dependent neurodegeneration. Neuron. 2004;42:23–36. doi: 10.1016/s0896-6273(04)00182-5. [DOI] [PubMed] [Google Scholar]
- 19.Lee YS, Silva AJ. The molecular and cellular biology of enhanced cognition. Nat Rev Neurosci. 2009;10:126–140. doi: 10.1038/nrn2572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Oddo S, et al. Blocking Abeta42 accumulation delays the onset and progression of tau pathology via the C terminus of heat shock protein 70–interacting protein: A mechanistic link between Abeta and tau pathology. J Neurosci. 2008;28:12163–12175. doi: 10.1523/JNEUROSCI.2464-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Oddo S, et al. Triple-transgenic model of Alzheimer's disease with plaques and tangles: Intracellular Abeta and synaptic dysfunction. Neuron. 2003;39:409–421. doi: 10.1016/s0896-6273(03)00434-3. [DOI] [PubMed] [Google Scholar]
- 22.Oddo S, Billings L, Kesslak JP, Cribbs DH, LaFerla FM. Abeta immunotherapy leads to clearance of early, but not late, hyperphosphorylated tau aggregates via the proteasome. Neuron. 2004;43:321–332. doi: 10.1016/j.neuron.2004.07.003. [DOI] [PubMed] [Google Scholar]
- 23.Koo EH, Squazzo SL. Evidence that production and release of amyloid beta-protein involves the endocytic pathway. J Biol Chem. 1994;269:17386–17389. [PubMed] [Google Scholar]
- 24.Cleary JP, et al. Natural oligomers of the amyloid-beta protein specifically disrupt cognitive function. Nat Neurosci. 2005;8:79–84. doi: 10.1038/nn1372. [DOI] [PubMed] [Google Scholar]
- 25.Wang X, et al. The carboxy-terminal fragment of inhibitor-2 of protein phosphatase-2A induces Alzheimer disease pathology and cognitive impairment. FASEB J. 2010;24:4420–4432. doi: 10.1096/fj.10-158477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cowansage KK, LeDoux JE, Monfils MH. Brain-derived neurotrophic factor: a dynamic gatekeeper of neural plasticity. Curr Mol Pharmacol. 2010;3:12–29. doi: 10.2174/1874467211003010012. [DOI] [PubMed] [Google Scholar]
- 27.Hock C, Heese K, Hulette C, Rosenberg C, Otten U. Region-specific neurotrophin imbalances in Alzheimer disease: Decreased levels of brain-derived neurotrophic factor and increased levels of nerve growth factor in hippocampus and cortical areas. Arch Neurol. 2000;57:846–851. doi: 10.1001/archneur.57.6.846. [DOI] [PubMed] [Google Scholar]
- 28.Connor B, et al. Brain-derived neurotrophic factor is reduced in Alzheimer's disease. Brain Res Mol Brain Res. 1997;49:71–81. doi: 10.1016/s0169-328x(97)00125-3. [DOI] [PubMed] [Google Scholar]
- 29.Peng S, et al. Decreased brain-derived neurotrophic factor depends on amyloid aggregation state in transgenic mouse models of Alzheimer's disease. J Neurosci. 2009;29:9321–9329. doi: 10.1523/JNEUROSCI.4736-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Xu F, et al. Brain-derived neurotrophic factor rapidly increases NMDA receptor channel activity through Fyn-mediated phosphorylation. Brain Res. 2006;1121:22–34. doi: 10.1016/j.brainres.2006.08.129. [DOI] [PubMed] [Google Scholar]
- 31.Li S, et al. Soluble oligomers of amyloid Beta protein facilitate hippocampal long-term depression by disrupting neuronal glutamate uptake. Neuron. 2009;62:788–801. doi: 10.1016/j.neuron.2009.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Garzon DJ, Fahnestock M. Oligomeric amyloid decreases basal levels of brain-derived neurotrophic factor (BDNF) mRNA via specific down-regulation of BDNF transcripts IV and V in differentiated human neuroblastoma cells. J Neurosci. 2007;27:2628–2635. doi: 10.1523/JNEUROSCI.5053-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fahnestock M, et al. BDNF increases with behavioral enrichment and an antioxidant diet in the aged dog. Neurobiol Aging. 2010 doi: 10.1016/j.neurobiolaging.2010.03.019. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Neeper SA, Gómez-Pinilla F, Choi J, Cotman CW. Physical activity increases mRNA for brain-derived neurotrophic factor and nerve growth factor in rat brain. Brain Res. 1996;726:49–56. [PubMed] [Google Scholar]
- 35.Blurton-Jones M, et al. Neural stem cells improve cognition via BDNF in a transgenic model of Alzheimer disease. Proc Natl Acad Sci USA. 2009;106:13594–13599. doi: 10.1073/pnas.0901402106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nagahara AH, et al. Neuroprotective effects of brain-derived neurotrophic factor in rodent and primate models of Alzheimer's disease. Nat Med. 2009;15:331–337. doi: 10.1038/nm.1912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rouaux C, Loeffler JP, Boutillier AL. Targeting CREB-binding protein (CBP) loss of function as a therapeutic strategy in neurological disorders. Biochem Pharmacol. 2004;68:1157–1164. doi: 10.1016/j.bcp.2004.05.035. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.