Abstract
Gene assembly of the variable domain of antigen receptors is initiated by DNA cleavage by the RAG1–RAG2 protein complex at sites flanking V, D, and J gene segments. Double-strand breaks are produced via a single-strand nick that is converted to a hairpin end on coding DNA and a blunt end on the neighboring recombination signal sequence. We demonstrate that the C-terminal regions of purified murine RAG1 (aa 1009–1040) and RAG2 (aa 388–520, including a plant homeodomain [PHD domain]) collaborate to inhibit the hairpinning stage of DNA cleavage. The C-terminal region of RAG2 stabilizes the RAG1/2 heterotetramer but destabilizes the RAG–DNA precleavage complex. This destabilization is reversed by binding of the PHD domain to a histone H3 peptide trimethylated on lysine 4 (H3K4me3). The addition of H3K4me3 likewise alleviates the RAG1/RAG2 C-terminus-mediated inhibition of hairpinning and the PHD-mediated inhibition of transposition activity. Thus a negative regulatory function of the noncore regions of RAG1/2 limits the RAG endonuclease activity in the absence of an activating methylated histone tail bound to the complex.
Keywords: diversification, immunoglobulin gene, regulation
V(D)J recombination is the programmed rearrangement of variable (V), diversity (D), and joining (J) gene segments to produce the antigen receptor proteins of lymphocytes. A large number V-J and V-D-J combinations can be assembled from the arrays of V, (D), and J segments for the immunoglobulin (Ig) heavy and light chains and T-cell receptor α and β, γ, and δ chains to create a diverse repertoire of antigen receptors. Each segment of coding DNA is flanked by one of two types of recombination signal sequences (12RSS and 23RSS) differing by the length of spacer (preferably 12 or 23 bp) between a heptamer whose consensus sequence is CACAGTG and a nonamer motif (consensus ACAAAAACC). Preferential synapsis between a 12RSS and a 23RSS by a (RAG1)2–(RAG2)2 heterotetramer ensures correct recombination between the segments. After cutting by the RAG1/2 complex at the recombination signal sequence (RSS)-coding segment boundaries, the coding segments (and separately the RSSs) are then joined using the nonhomologous end joining (NHEJ) pathway (1).
The biochemistry of the cleavage reaction was initially revealed using truncated versions of RAG1 (aa 384–1008, referred to as coreR1) and RAG2 (aa 1–387, coreR2) that improved expression and solubility while maintaining the recombination activity in cellular assays (2). Cleavage with the purified RAG proteins occurs in 2 steps: A nick is produced 5′ of the heptamer; then transesterification using the exposed 3′ hydroxyl group produces a hairpin on the coding end and a blunt ended RSS. The noncore regions of RAG1 and RAG2 (Fig. 1A) are crucial to the regulation of V(D)J recombination, ensuring that broken intermediates feed into the correct NHEJ repair pathway (3), and preventing unwanted reactions (e.g., transposition and hybrid joints). Knock-in mice containing coreR1 and/or coreR2 have decreased numbers of lymphocytes, with higher frequencies of aberrant, unordered recombination events (4–6).
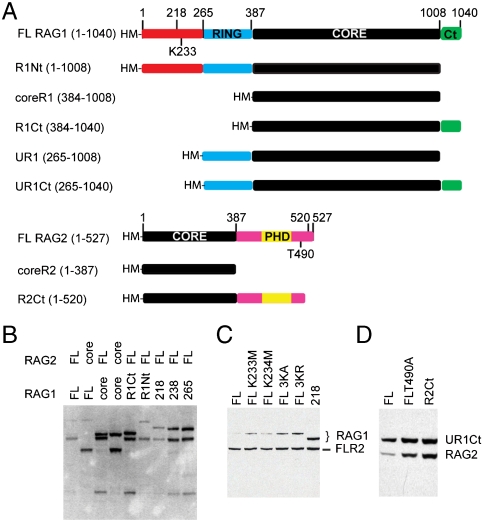
Fig. 1.
Modulation of expression by RAG1 and RAG2 noncore regions. (A) Diagram of RAG1 and RAG2 constructs used in this study. His-tagged MBP fusions (HM) of various truncations were made for transient expression in HEK293 cells. (B) A Western blot (anti-MBP) of lysate from coexpressed samples shows that the N-terminal noncore region of RAG1 reduced expression of RAG1 and full length RAG2 as seen in lanes containing FLR1 and R1Nt (1–1008). Deletion of aa 1–238 of RAG1 (238) or aa 1–265 (265) relieved suppression of expression whereas deletion of aa 1–218 (218) remained inhibitory. (C) The effects on expression of mutating K233 and neighboring lysines (K234 and K236) to nonbasic (alanine) or basic (arginine) residues were analyzed by Western blot. (D) Western blot of various RAG2 constructs containing the majority of the noncore C-terminus.
Potentially damaging double-strand breaks in cells are prevented by regulating the timing of expression of RAG1 and RAG2 at transcriptional and posttranslational levels [reviewed by (7), (8)]. Additionally, V(D)J recombination in lymphoid cells is strongly regulated to insure that it occurs only in appropriate loci and cell types. This epigenetic regulation has been associated with multiple modifications of core histone tails, some of which can stimulate recombination (e.g., H3K4me3) whereas others suppress recombination (e.g., H3K9me3) (9).
Recent attention has been focused on H3K4me3, which is enriched at recombining loci. Correspondingly, cell-wide depletion of K4 methylation causes a decrease in recombination (10, 11). Direct binding of the RAG2 plant homeodomain (PHD domain) to H3K4me3 peptides has been demonstrated both in solution and in a crystal structure (10, 12), providing a plausible explanation of the role of H3K4 trimethylation by tethering of the RAG complex to the activated loci (13). Colocalization of RAG1/2 to H3K4me3 at antigen receptor loci was recently demonstrated in vivo (14). In addition to tethering, a direct effect of an H3K4me3 peptide on the nicking and hairpinning activity of RAG1/2 in vitro has been shown, raising the possibility that the cellular effect of H3K4 trimethylation is dual, both on RAG recruitment and activity (13). The mechanism by which the histone peptide stimulates RAG activity is not clear.
In this work we explored the effects of the noncore regions of RAG1 and RAG2 on expression and activity of the RAG1/2 complex (Fig. 1A). We were able to extend the core proteins by adding the RING domain of RAG1 (aa 265–383), and the C-terminal regions of both RAG1 (aa 1009–1040) and RAG2 (aa 388–520) without greatly disturbing expression or purification. The addition of both C-terminal domains diminished the DNA cleavage activity of RAG1/2, but this inhibition was alleviated by an H3K4me3 peptide. We suggest PHD domain-dependent inhibition as a mechanism for minimizing RAG activity in the absence of the correct epigenetic signal.
Results
Coexpression of RAG1 and RAG2 Containing Noncore Domains.
Expression of full length RAG1/2 has been problematic in either insect or mammalian cell cultures. To assess which regions could be added to the core proteins while retaining expression, we fused full length and various truncated versions of RAG1 and RAG2 to an MBP tag and transiently coexpressed them in HEK293 cells (Fig. 1A). As expected, expression of either full length RAG1 or RAG2 was markedly reduced compared to the core proteins. Removal of the N-terminal noncore region of RAG1 (aa 1–383) rescued the expression of RAG1 and increased that of full length RAG2, whereas removing the C-terminal piece of RAG1 (aa 1009–1040) had no effect on the low expression level of the otherwise full length protein. Addition of the RING finger (aa 265–383) to coreR1 did not reduce its expression, suggesting that in this context the E3 ligase activity of the RING finger was not responsible for the reducing RAG1/2 levels (Fig. 1B).
Because the RING domain has been shown to auto-ubiquitinate RAG1 on K233 in vitro (15), we assessed the expression of truncations and mutations around this basic region. RAG1 (218–1040) was expressed at a lower level than RAG1 (238–1040), suggesting a negative effect of aa 218–238. Mutation of K233 to methionine resulted in slightly elevated RAG1 levels in a full length background. This effect was not observed with the neighboring K234M mutation (Fig. 1C), leading us to speculate that the down-regulation of RAG1 expression was partly dependent on ubiquitination. We therefore mutated all three lysine residues in this region (K233, K234, K236) to alanine (3KA) or arginine (3KR), to ensure the absence of any potential ubiquitination sites. Expression was again slightly elevated, but not fully rescued, with both triple mutants, leading us to conclude that posttranslational modification of K233 is only partially responsible for the reduced expression.
Degradation products and free MBP were particularly evident in cell lysates containing full length RAG2. Phosphorylation of T490 in the noncore region of RAG2 signals for the degradation of RAG2 at the G1/S transition of the cell cycle via a ubiquitin-proteasome pathway (8). We tested a T490A mutant, and a truncation of 7 residues (RAG2 1–520) that has been reported to improve stability (16). Both changes improved RAG2 levels and the 1–520 truncation produced the most reliable expression (Fig. 1D).
Four constructs containing noncore regions, UR1 (RAG1 including the RING, Ubiquitin E3 ligase, aa 265–1008); R1Ct (including the C-terminal region, 384–1040); UR1Ct (aa 265–1040); and R2Ct (aa 1–520), were selected for further examination. All eight combinations, including the core constructs (coreR1 and coreR2) and these RAG1 and RAG2 constructs, were coexpressed and purified on amylose resin.
RAG2 C-Terminus Stabilizes the RAG1/RAG2 Heterotetramer.
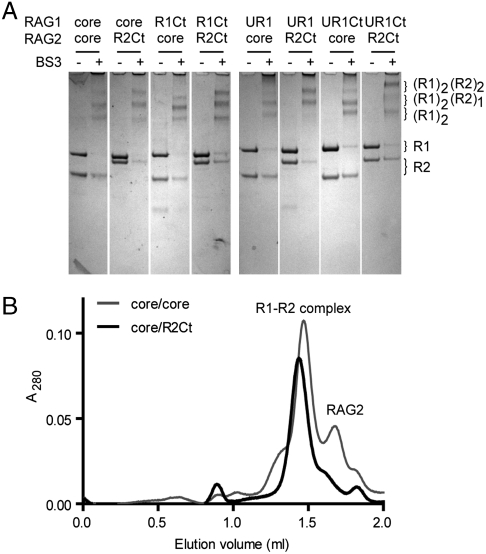
The active RAG1/2 in solution is heterotetrameric, (RAG1)2–(RAG2)2, although species of other stoichiometries also exist. We used chemical cross-linking to semiquantitatively assess the effects of the noncore domains on RAG1/RAG2 tetramer formation in the absence of DNA. Cross-linking with Bis-(sulfosuccinimidyl) suberate (BS3) demonstrated that combinations containing R2Ct formed proportionally more (RAG1)2–(RAG2)2 than those with coreR2 (Fig. 2A). This was true for all RAG1 combinations, including R1Ct, UR1, and UR1Ct. The C-terminal region and RING domain of RAG1 did not appear to separately alter the proportions of complex, but the greatest amounts of heterotetramer were formed between UR1Ct and R2Ct with all noncore domains present, suggesting additional protein–protein interactions beyond coreR1 and coreR2.
Fig. 2.
RAG1/2 heterotetramer formation by noncore domains. (A) BS3 chemical cross-linking of core and noncore constructs. Three cross-linked species were produced with BS3 treatment of RAG1 dimer, (RAG1)2(RAG2) and the complete heterotetramer as indicated. Reactions containing R2Ct produced more heterotetramer than with coreR2. The left and right halves of the figure are from two gels run in parallel; within each half, irrelevant lanes have been removed as indicated. (B) Gel filtration of coreR1/coreR2 and coreR1/R2Ct. The shoulder on the major peak was identified as noncomplexed RAG2.
The complexes formed by coreR1/coreR2 and coreR1/R2Ct preparations were also assessed by gel-filtration (Fig. 2B). The column was unable to separate all of the RAG1/2 species, but the major peak in each case had a shoulder of lower molecular weight, which was identified by SDS-PAGE to be RAG2 alone. The shoulder was particularly apparent in combinations containing coreR2, whereas R2Ct appeared to form the RAG1/2 complexes more efficiently and stably.
The C-Termini of RAG1 and RAG2 Alter DNA Binding.
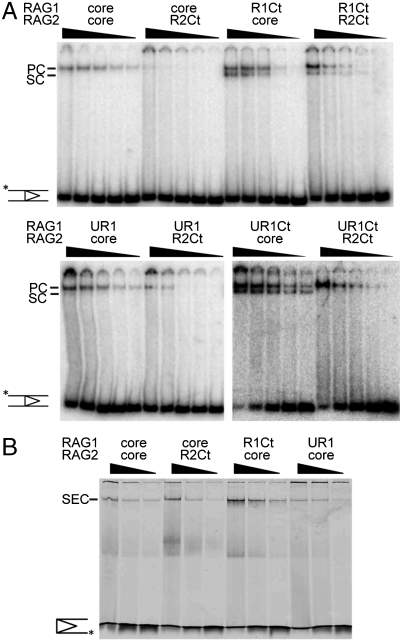
We examined the DNA binding properties of the extended proteins by EMSA, using 12RSS and 23RSS substrates in the absence of HMGB1. Assembly of the paired complex was carried out in the presence of Ca2+, which supports binding but not cleavage. Under these conditions the core constructs formed the paired complex (PC) containing 12/23RSS. In combination with coreR1 (or UR1), R2Ct (containing the C-terminal region) appeared to form less PC than coreR2 despite the greater stability of heterotetramers (Fig. 3A).
Fig. 3.
Complex formation is inhibited by PHD domain. (A) EMSA of proteins containing noncore regions with 5 nM radiolabeled 12RSS and 5 nM 23RSS. The concentrations of RAG proteins were varied (40, 20, 10, 5, 2.5 nM of each, indicated left to right). The 12/23RSS PC and the single substrate complex (SC) are indicated. These reactions contained no HMGB1. (B) EMSA of constructs containing noncore regions with 10 nM precleaved substrates (12SE-cy5 and 23SE). The concentrations of RAG1/RAG2 were each 50, 25, and 12.5 nM estimated as a heterotetramer.
The RAG1 C-terminus increased the total amount of RAG–DNA complex formed. However, a greater proportion of a faster migrating species was also detected, presumed to be a complex with a single substrate (SC), which has been characterized in previous studies (17). The combination of R1Ct/R2Ct produced appreciable amounts of complex with DNA showing that R1Ct could overcome the poorer DNA binding of R2Ct. It was also noted that addition of RAG1 RING (UR1) improved the proportion of PC to SC when R1Ct was present.
Complex formation by coreR1/R2Ct was not as strongly depressed when precleaved substrates (i.e., without the “coding” flank) were supplied in the binding reaction; the levels of signal-end complex were similar to those reached with the core proteins (Fig. 3B). It is possible that the RAG2 C-terminal region destabilizes interactions with the coding flank. As with intact 12/23RSS, the RAG1 C-terminus enhanced complex formation with precleaved 12/23RSS.
The C-Terminal Regions of RAG1 and RAG2 Inhibit Hairpin Formation.
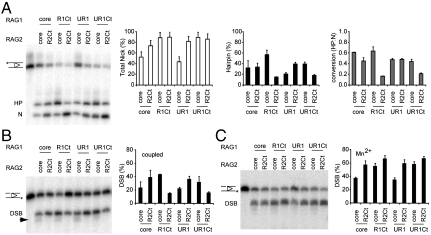
The proteins were tested for DNA cleavage activity in assays containing 12RSS and 23RSS substrates, with Mg2+ (coupled cleavage). The nicked intermediate (N) and hairpin product (HP) were quantified on a denaturing polyacrylamide gel. The differences between the core and extended constructs were more marked when the HMGB1 cofactor was omitted from the reactions, as in the assays shown in Fig. 4, and for most of this study. The RING domain had little effect on overall nicking and hairpinning (comparing the first half of the gel to the second), but addition of the RAG1 C-terminal region had a positive effect on overall activity (increases in both N and HP). Despite the poor formation of paired complex by coreR1/R2Ct, the levels of cleavage appeared normal or slightly elevated. However, in combination with R1Ct constructs, R2Ct led to a notable accumulation of nicked product. The accumulation of the nicked intermediate suggested that hairpin formation was inhibited. When expressed as a conversion factor (i.e., hairpin formed per total nicked DNA), conversion of nick to hairpin was significantly lower with combinations containing both the RAG1 and RAG2 C-termini than with other pairs (p < 0.002).
Fig. 4.
Inhibition of hairpinning by RAG1 and RAG2 C-terminal regions. (A) Equal amounts of core RAG1/2 and noncore combinations were included in coupled cleavage reactions (12RSS/23RSS and Mg2+). The N and HP cleavage products from coupled cleavage reactions were analyzed by TBE-urea gel. (Right) Bar chart displays HP, N (percentage of total substrate + SD) and HP conversion factors (HP formed/total N produced + SD) from 3 independent experiments. (B) TBE-urea gel of hairpinning by various forms of RAG1/2 proteins activity on prenicked substrates, under coupled cleavage conditions. The arrowhead marks the position of shorter, inaccurate cleavage products resulting from alternative hairpinning sites opposite to the nick. Bar chart display the yield of double-strand breaks (DSB) produced from the total substrate as a percentage. The average was obtained from 3 experiments (+SD). (C) TBE-urea gel of hairpinning of prenicked substrates using the less stringent Mn2+ catalytic ion. Average percent DSB formed from total substrate are displayed in the bar chart (+SD).
Prenicked 12/23 RSS substrates were used to test the effects of the C-terminal regions on the hairpinning stage (Fig. 4B). As with intact substrates, we saw that individually the R1Ct and R2Ct stimulated hairpin formation, but together these noncore domains significantly inhibited hairpin production (p < 0.002, when comparing reactions containing R1Ct, with and without R2Ct). This confirms that the C-terminal regions of RAG1 and RAG2 collaborate to inhibit the hairpinning step in coupled cleavage. The gel in Fig. 4B also showed the existence of faster migrating products in the lanes containing coreR2. These bands are indicative of hairpinning at alternative sites within the heptamer instead of at its boundary. The addition of R2Ct to the constructs reduced the appearance of aberrant cleavage sites, thus improving the accuracy of hairpin formation from the prenicked substrate.
Cleavage of a single substrate in the presence of Mn2+ was also tested. This condition allows nicking and hairpinning to occur without the formation of a paired complex. Here, the C-terminal regions of RAG1 and RAG2 did not inhibit hairpin formation on either intact 12RSS or 23RSS (not shown) or on a prenicked 12RSS substrate (Fig. 4C), indicating that the negative effect was specific to the more stringent conditions that require a paired complex.
Reversal of PHD-Dependent Inhibition by H3K4me3 Peptide.
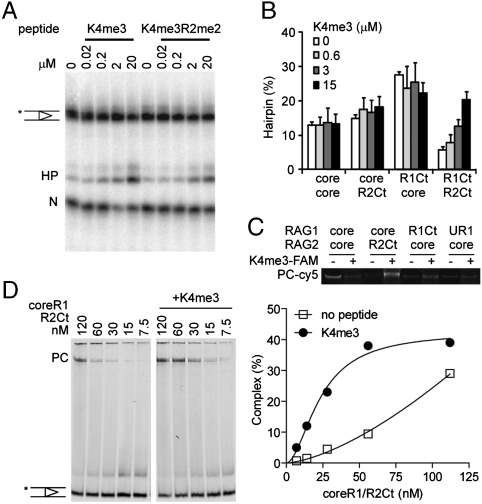
Shimazaki et al. have shown that DNA hairpin formation by the RAG1 and RAG2 core proteins was stimulated by a trimethylated Histone H3 peptide (H3K4me3) and unaffected by the nonmodified peptide (13). We asked whether this stimulation could overcome the R2Ct-dependent inhibition of hairpin formation and complex formation characterized in our experiments. Methylated peptides, either H3K4me3 or H3K4(me3)R2(symmetrical me2) (12), were able to stimulate hairpin formation by R1Ct/R2Ct in a dose-dependent manner to the level achieved with R1Ct/coreR2 (Fig. 5 A and B). Significant stimulation of hairpin formation was only seen in RAG2 C-terminus combinations where RAG1-Ct was also present (Fig. 5B).
Fig. 5.
Rescue of PHD-mediated inhibition of complex formation and hairpin formation with H3K4me3 peptide. (A) Addition of varying concentrations of H3K4me3 and H3K4(me3)R2(me2) peptide to the cleavage reactions (containing 10 nM RAG1Ct/RAG2Ct) rescued PHD inhibition of hairpin formation in a dose-dependent manner. (B) Summary of H3K4me3 titration on other RAG1/RAG2 combinations, average from 4 experiments (+SD error bars). (C) Qualitative assessment of peptide binding to paired complex. Fluorescent labeled peptide colocalized with the stable 12/23 complex formed with cy5 labeled 12RSS. (D) 2 μM H3K4me3 stabilized the precleavage complex formed with the RAG1/RAG2-PHD combinations (concentrations in nM). The amount of complex is expressed as a percentage of total substrate in each lane. Binding curve of the substrate binding +/- peptide is displayed.
To test whether the peptide was targeting the PHD-containing combinations specifically, we took advantage of a fluorescent label on the peptide in a binding reaction with cy5-labeled 12RSS and unlabeled 23RSS. The presence of the peptide was detected only in the DNA complexes formed with R2Ct, demonstrating the ability of the peptide to bind to the PHD domain in the precleavage complex (Fig. 5C).
The peptide also greatly enhanced the formation of the precleavage complex by coreR1 with R2Ct (Fig. 5D). An enhancement of complex formation was similarly detected with other PHD-containing combinations but not with coreR2 (not shown).
PHD-Dependent Inhibition of Strand-Transfer Activity Is Rescued by H3K4me3.
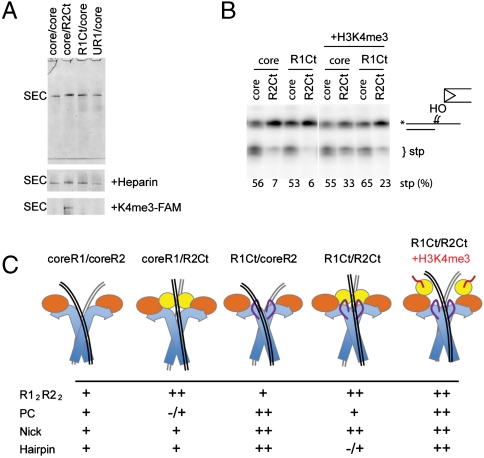
To study the effects of the PHD domain and other noncore regions of RAG1 on postcleavage complex formation, stability, and strand-transfer activity, the signal-end complexes (SEC) made with various RAG1/2 combinations were purified using an on-column reaction (18). This time HMGB1 was included to obtain enough complex to analyze (Fig. 6A). The postcleavage complex using core RAG1/2 is far more stable than the precleavage complex and remains intact even when 1 mg/mL heparin is added to the sample. Like the core constructs, all of the resulting SEC species with noncore domains added were stable when heparin was added (Fig. 6A). There were no significant differences in SEC stability between the constructs.
Fig. 6.
Properties of postcleavage complex with noncore domains. (A) Coomassie stained native gel of purified SEC produced by an on-column reaction, without and with heparin challenge (second panel). The third panel shows the fluorescent H3K4me3 peptide colocalized with SEC that contained PHD. (B) Effect of noncore domains on strand-transfer activity of postcleavage complexes. The target DNA is a 3′ overhang substrate that is cleaved when SEC transposes the signal end at the ds/ss junction. On addition of H3K4me3 the inhibition by RAG2Ct was alleviated. (C) Schematic model for inhibition mechanism supported by data: (i) The incomplete catalytic domain of core R1/coreR2 allows flexibility of substrates. (ii) The PHD domain of RAG2 alters coding flank contacts and restricts the position of the transesterification site. (iii) The R1Ct increases affinity with the signal sequence. (iv) Together the RAG1 and RAG2 C-terminal regions produce a tighter complex with 12/23RSS that restricts the distortions required for hairpinning. (v). When H3K4me3 is bound, the PHD is released from its inhibitory position allowing hairpinning to proceed. The arrangement of domains and DNAs was elucidated previously by electron microscopy (18).
Previous reports have described an inhibitory function of the C-terminus of RAG2 on RAG-mediated transposition (16, 19, 20). We assessed the contribution of this region and the other noncore domains on transposition activity. Using equivalent amounts of each SEC, strand transfer was detected by a previously characterized reaction using a radiolabeled 3′-overhang substrate (21). Here, strand transfer of the signal ends into the single strand—double strand junction results in the cleavage of the overhanging strand. We found that R2Ct inhibited the reaction by nearly 10-fold, whereas the other constructs had no effect (Fig. 6B, UR1/coreR2 SEC not shown). Unlike inhibition of hairpinning, inhibition of transposition mediated by the RAG2 C-terminus was independent of the presence of R1Ct in the complex.
Inhibition of transposition by the C-terminus of RAG2 has been ascribed to less efficient target capture (possibly indirectly by retaining the hairpins after cleavage) (16, 20). In our experiment the column purification separates the SEC from 12RSS hairpin DNA (18). Because H3K4me3 peptide binding to coreR1/R2Ct was capable of improving DNA-binding properties at the coding flank (Fig. 3D) it was plausible that peptide binding might also open up the target DNA-binding site and therefore stimulate strand-transfer activity. Indeed, the strand-transfer activity of coreR1/R2Ct was stimulated by the presence of peptide (Fig. 6B) whereas the peptide had no effect on other noncore domains. This specificity was demonstrated by the binding of the fluorescent labeled peptide to SECs containing PHD on a native gel (Fig. 6A). Remarkably, the potentially hazardous transposition activity of the postcleavage complex can also be stimulated by the methylated histone.
Discussion
In this study we investigated the effect of each region adjoining the catalytically essential cores of RAG1 and RAG2 on expression levels, protein–DNA complex formation and cleavage activity. We found that far from being superfluous to the function of the core enzyme, the noncore domains significantly affected the properties of the complex.
Studying the coexpression of various truncations, we identified an N-terminal region (1–238) of RAG1 that greatly reduced RAG1/2 transient expression in HEK293 cells. A graduated expression level between full length, 218–1040, 238–1040, and 265–1040 RAG1 suggests that multiple sites in the N-terminal domain of RAG1 regulate its expression. Among other considerations, ubiquitination may be significant. Simkus et al. identified poly ubiquitination at K233 and K257 that might account for some degradation of RAG1(22). Our data support posttranslational modification of K233 (mutation of this residue increases expression) but also imply that other sites within 1–218 influence RAG levels. The E3 ligase activity of the RING finger has little effect on RAG expression levels in the absence of the upstream region. Control of RAG2 levels in the cell cycle has been attributed to phosphorylation of the T490 residue, triggering ubiquitin-dependent degradation via the Skp2/UFC pathway (8). In this expression system we found evidence of degradation of full length RAG2; the removal of the final 7 residues was sufficient to stabilize expressed RAG2 protein level even when T490 remained, agreeing with a previous study that suggested the extreme C-terminus promotes instability (16).
The C-terminal region of RAG2 alters the characteristics of the RAG core complex in three ways. First, it promotes the formation of heterotetrameric RAG1/RAG2, probably through additional interactions beyond coreR1/coreR2 (Fig. 2). This increased interaction may be responsible for the second characteristic, weakened DNA binding (Fig. 3A). Because the binding of precleaved substrates was not affected, we presume the C-terminus is altering the binding site for coding DNA, possibly through steric hindrance (Fig. 3B). Third, despite the formation of a synaptic complex when the C-terminus of RAG1 is present, this complex is not fully active in hairpinning although it is capable of the nicking step. Nicking and hairpinning were normal in the less stringent single-RSS conditions that rely on Mn2+ (Fig. 4C).
The reliance of the PHD-dependent inhibition on the presence of the RAG1 C-terminus gives this 32 amino acid region an important function. It is plausible that the final 32 amino acids in murine RAG1 may complete the catalytic domain and indirectly structure the inhibitory interaction site. Addition of R1Ct results in improved DNA binding and may strengthen heptamer contacts because the precleaved substrates also bind with greater affinity (Fig. 4B). Increased DNA contacts are likely to be an indirect effect of the C-terminal residues as its negative charge is not directly conducive to DNA binding.
A model for the mechanism of PHD-dependent inhibition and H3K4me3 rescue is proposed in Fig. 6C, using the domain arrangement based on the EM structure (18). It is possible that the PHD domain restricts the access to the DNA-binding site and the conformation of bound DNA in the catalytic domain (particularly around the cleavage site and coding flank), and so limits DNA binding and prevents distortions of the DNA helix required for hairpinning. Evidence for the RAG2 C-terminal region reducing flexibility at the active site can be seen in cleavage assays (Fig. 4 B and C). Here, fewer aberrant products from alternative break sites were detected when prenicked substrates were used. Restriction of the active site is aided by presence of the final 32 residues of RAG1, explaining why the full inhibition is reliant on the C-termini of both RAGs. The binding of the modified histone tail might cause a shift in the position of the PHD domain, allowing the coding flank to bind and distort for hairpinning. This model provides a plausible mechanism for the observed increase in activity of the RAG core proteins on H3K4me3 peptide binding that was reported earlier (13). Alleviation of inhibition was also observed with the doubly modified H3 peptide R2(me2S)K4(me3), which bound slightly tighter to the isolated RAG2 PHD domain (12). However, no significant difference in stimulation was seen under these conditions.
It has previously been proposed that RAG2 PHD could have an inhibitory effect on RAG activity. Full length RAG2 with mutations in the PHD domain that eliminate histone binding had a greater defect in recombination frequency than coreR2, indicating that without the stimulation of histone binding, the RAG2 C-terminus is inhibitory (11, 12). The inhibitory effects of the RAG C-terminal regions may provide an autoregulatory mechanism for limiting the amount of cleavage in the absence of the correct histone modification. Without this self-regulation RAG1/2, once expressed, might be free to cleave without control and damage other accessible regions of the genome. This may be particularly relevant at cryptic RSS sites to which RAG1/2 is capable of binding.
Whereas studies with full length RAG2 had revealed a regulatory function of the C-terminal domain in transposition, this had not been shown for the cleavage reaction. Previous in vitro studies of the core versus full length proteins failed to demonstrate the PHD-dependent inhibition of hairpinning, possibly because of the presence of HMGB1. The inhibitory effect of the PHD domain is less apparent when the stimulatory factor HMGB1 is included in the in vitro reactions. Excess HMGB1 might interfere with the formation of the “inactive” conformation.
We also found that the inhibition of transposition by the PHD domain of RAG2 could also be partially alleviated by the peptide. Whereas this inhibition was independent of the RAG1 C-terminal region, the improvement in activity with the peptide may indicate a similar change at the active site. It may be important to restrict the transposition activity of the postcleavage complex to reduce chromosomal translocations and potential oncogenesis. It is possible that the active site still excludes genomic DNA and that we only see strand-transfer activity with small flexible artificial substrates. We cannot exclude that other inhibitory measures exist.
PHD modules are common to several nuclear proteins, particularly transcription factors and histone demethylases. The RAG1/2 complex provides an example of a PHD domain that can alter enzyme function in response to histone-tail occupancy. Rather than being an inert distal tethering module, the PHD domain directly interacts with the catalytic portion of the enzyme to regulate its activity.
Methods
Proteins and DNA.
PCR products encoding RAG1 full length (1–1040), N-terminal deletions of 218, 238, or 265aa; and RAG2 full length (1–527), RAG2 C-terminal 7aa truncation (1–520), were inserted downstream of an N-terminal 8xHis-MBP-PreScission cassette that was introduced into pLEXm as reported for the core constructs (18, 23). The RAG1 constructs with the Nt and Ct alone were produced by switching the AgeI-SphI fragments of the full length and core construct. RAG1 K233M, K234M, K233A-K234A-K236A, K233R-K234R-K236R, and RAG2 T490A mutations were produced by Quickchange site-directed mutagenesis (Stratagene). The expression vectors were transiently cotransfected into HEK293GNTI 100 or 250 mL suspension cultures in Freestyle media (+1% FBS) using Polyethylenimine (PEI) (25 kD linear, Polysciences) at a final concentration of 1 mg/L of each DNA and 4 mg/L PEI. Intracellular proteins were copurified in parallel using amylose in batch. HMGB1 was produced as in a previous report (18). H3 peptides were synthesized as described earlier (12).
12RSS and 23RSS substrates were made by annealing DAR39-DAR40 and SR11-SR11R as before. Prenicked 12RSS and 23RSS were also formed from oligos (DAR42-DG10-DAR40) and (DAR42-DG4-DAR61) as were precleaved 12RSS (DG10-DG9) and 23RSS (DG2-DG4) (2, 18). Biotinylated 12RSS was made for SEC purification.
Expression Levels.
0.5 × 106 HEK293E cells were seeded in 6-well plates. The next day, culture medium was exchanged for 2 mL Hybridoma-SFM containing 1% FBS. A transfection mix of 20 μg/mL PEI and 5 μg/mL of each RAG plasmid in unsupplemented Hybridoma-SFM media (Invitrogen) was incubated at room temperature for 10 min., then 0.5 mL was added to each well (final 4∶1∶1 μg/mL PEI∶RAG1∶RAG2). Cells were harvested at 48 h and washed with PBS. Cells were lysed on ice for 10 min. in 250 μL lysis buffer (20 mM Tris-HCl pH7.5, 500 mM NaCl, 5% (v/v) glycerol, 0.5% Triton X-100 (v/v) 1 mM DTT, 0.4 mM 4-(2-Aminoethyl) benzenesulfonyl fluoride, 10 μM leupeptin, 1 μg/mL pepstatin and 1.4 μM aprotinin). The soluble fraction was loaded onto SDS-PAGE (no differences in relative RAG levels were detected between soluble fraction and the total lysate). The total protein concentrations measured by Coomassie Plus reagent (Pierce) differed by < 10%. A western blot using anti-MBP-HRP showed comparative expression levels. Similar trends of expression were seen using HEK293GNT1 cells in suspension.
DNA Cleavage Assays.
Assays were carried out in 25 mM 3-(4-Morpholino)propane sulfonic acid-KOH pH7.0, 30 mM KCl, 1% glycerol, 0.1 mg/mL BSA, 4 mM CaCl2, 1 mM DTT, and either 5 mM MgCl2 or 1 mM MnCl2. 5 nM 12RSS, 5 nM 23RSS, and 20 nM RAG1/2 (as a heterotetramer) were added and incubated for 1 h at 37 °C. Other additives are specified in the figure legends. Reactions were stopped using 1 vol (90% formamide-TBE), heated for 5 min. 95 °C, separated on a 12.5% TBE-urea gel, visualized by a phosphorimager and products were quantified using ImageQuantNL (GE Healthcare).
DNA-Binding Assays.
Assays were the same as reported (24), but used 5 nM DNA and titrations of RAG1/2. Where fluorescent 12RSS-cy5 was used (5′ labeled DG9 or DAR40), reactions were scaled up to 10 nM of each DNA. Gels were scanned within the glass plates using a Typhoon Trio. The peptide could also be visualized using the Fluorescein label.
SEC Purification.
A slightly modified procedure to that reported was performed using the same buffers (18). 50 nM biotinylated 12RSS, 50 nM 23RSS, 200 nM HMGB1 and 100 nM RAG1/2 in 2.5 mL binding buffer were incubated for 30 min. at 37 °C. 100 μL streptavidin-agarose resin were added and incubated for 1 h at room temperature. The precleavage complex-resin mix was poured into a column and washed with 3 mL binding buffer. 0.25 mL binding buffer containing 5 mM MgCl2 was added to initiate the cleavage reaction, and incubated for a further hour at 37 °C. The elution and two 0.25 mL washes (containing additional 100 mM potassium glutamate) were pooled and concentrated to 100 μL by Microcon ultrafiltration (YM100, Millipore). SEC was quantified using A260 and A280 measurements and assessed on native PAGE (Tris-acetate in 0.5X Tris-glycine running buffer).
Strand-Transfer Activity.
3′ overhang substrate (21) was added to 30 nM normalized SEC (diluted by 1 volume H2O) to a final concentration of 20 nM and incubated for 1 h at 37 °C. Reaction products were analyzed on a 15% TBE-urea gel (Invitrogen).
Complex Analysis.
50 μL protein (A280 ∼ 0.2) was loaded onto Superose 6 at 50 μL/ min in running buffer comprising 20 mM Tris-HCl pH 7.5, 500 mM NaCl, 1% (v/v) glycerol 1 mM DTT. Chemical cross-linking with Bis(Sulfosuccinimidyl) suberate (BS3, Pierce) was performed at room temperature for 1 h. Cross-linking was stopped with 100 mM Tris-HCl and SDS loading buffer and separated immediately on Tris-Acetate gels (Invitrogen). Crosslinked species (initially identified from RAG1, RAG2 and RAG1/2 controls, not shown) were stained with Coomassie blue.
Acknowledgments.
This work was supported by the intramural research program of the National Institute of Diabetes and Digestive and Kidney Diseases, National Institutes of Health.
Footnotes
The authors declare no conflict of interest.
References
- 1.Gellert M. V(D)J recombination: RAG proteins, repair factors, and regulation. Annu Rev Biochem. 2002;71:101–132. doi: 10.1146/annurev.biochem.71.090501.150203. [DOI] [PubMed] [Google Scholar]
- 2.McBlane JF, et al. Cleavage at a V(D)J recombination signal requires only RAG1 and RAG2 proteins and occurs in two steps. Cell. 1995;83:387–395. doi: 10.1016/0092-8674(95)90116-7. [DOI] [PubMed] [Google Scholar]
- 3.Steen SB, Han JO, Mundy C, Oettinger MA, Roth DB. Roles of the “dispensable” portions of RAG-1 and RAG-2 in V(D)J recombination. Mol Cell Biol. 1999;19:3010–3017. doi: 10.1128/mcb.19.4.3010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Talukder SR, Dudley DD, Alt FW, Takahama Y, Akamatsu Y. Increased frequency of aberrant V(D)J recombination products in core RAG-expressing mice. Nucleic Acids Res. 2004;32:4539–4549. doi: 10.1093/nar/gkh778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Curry JD, Schlissel MS. RAG2’s non-core domain contributes to the ordered regulation of V(D)J recombination. Nucleic Acids Res. 2008;36:5750–5762. doi: 10.1093/nar/gkn553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Akamatsu Y, et al. Deletion of the RAG2 C terminus leads to impaired lymphoid development in mice. Proc Natl Acad Sci USA. 2003;100:1209–1214. doi: 10.1073/pnas.0237043100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kuo TC, Schlissel MS. Mechanisms controlling expression of the RAG locus during lymphocyte development. Curr Opin Immunol. 2009;21:173–178. doi: 10.1016/j.coi.2009.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jiang H, et al. Ubiquitylation of RAG-2 by Skp2-SCF links destruction of the V(D)J recombinase to the cell cycle. Mol Cell. 2005;18:699–709. doi: 10.1016/j.molcel.2005.05.011. [DOI] [PubMed] [Google Scholar]
- 9.West KL, et al. A direct interaction between the RAG2 C terminus and the core histones is required for efficient V(D)J recombination. Immunity. 2005;23:203–212. doi: 10.1016/j.immuni.2005.07.004. [DOI] [PubMed] [Google Scholar]
- 10.Matthews AG, et al. RAG2 PHD finger couples histone H3 lysine 4 trimethylation with V(D)J recombination. Nature. 2007;450:1106–1110. doi: 10.1038/nature06431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Liu Y, Subrahmanyam R, Chakraborty T, Sen R, Desiderio S. A plant homeodomain in RAG-2 that binds Hypermethylated lysine 4 of histone H3 is necessary for efficient antigen-receptor-gene rearrangement. Immunity. 2007;27:561–571. doi: 10.1016/j.immuni.2007.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ramon-Maiques S, et al. The plant homeodomain finger of RAG2 recognizes histone H3 methylated at both lysine-4 and arginine-2. Proc Natl Acad Sci USA. 2007;104:18993–18998. doi: 10.1073/pnas.0709170104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shimazaki N, Tsai AG, Lieber MR. H3K4me3 stimulates the V(D)J RAG complex for both nicking and hairpinning in trans in addition to tethering in cis: implications for translocations. Mol Cell. 2009;34:535–544. doi: 10.1016/j.molcel.2009.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ji Y, et al. The in vivo pattern of binding of RAG1 and RAG2 to antigen receptor loci. Cell. 2010;141:419–431. doi: 10.1016/j.cell.2010.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jones JM, Gellert M. Autoubiquitylation of the V(D)J recombinase protein RAG1. Proc Natl Acad Sci USA. 2003;100:15446–15451. doi: 10.1073/pnas.2637012100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Elkin SK, Matthews AG, Oettinger MA. The C-terminal portion of RAG2 protects against transposition in vitro. EMBO J. 2003;22:1931–1938. doi: 10.1093/emboj/cdg184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Swanson PC. The bounty of RAGs: Recombination signal complexes and reaction outcomes. Immunol Rev. 2004;200:90–114. doi: 10.1111/j.0105-2896.2004.00159.x. [DOI] [PubMed] [Google Scholar]
- 18.Grundy GJ, et al. Initial stages of V(D)J recombination: The organization of RAG1/2 and RSS DNA in the postcleavage complex. Mol Cell. 2009;35:217–227. doi: 10.1016/j.molcel.2009.06.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Swanson PC, Volkmer D, Wang L. Full-length RAG-2, and not full-length RAG-1, specifically suppresses RAG-mediated transposition but not hybrid joint formation or disintegration. J Biol Chem. 2004;279:4034–4044. doi: 10.1074/jbc.M311100200. [DOI] [PubMed] [Google Scholar]
- 20.Tsai CL, Schatz DG. Regulation of RAG1/RAG2-mediated transposition by GTP and the C-terminal region of RAG2. EMBO J. 2003;22:1922–1930. doi: 10.1093/emboj/cdg185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nishihara T, et al. In vitro processing of the 3′-overhanging DNA in the postcleavage complex involved in V(D)J joining. Mol Cell Biol. 2004;24:3692–3702. doi: 10.1128/MCB.24.9.3692-3702.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Simkus C, Bhattacharyya A, Zhou M, Veenstra TD, Jones JM. Correlation between recombinase activating gene 1 ubiquitin ligase activity and V(D)J recombination. Immunology. 2009;128:206–217. doi: 10.1111/j.1365-2567.2009.03101.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Aricescu AR, Lu W, Jones EY. A time- and cost-efficient system for high-level protein production in mammalian cells. Acta Crystallogr D. 2006;62:1243–1250. doi: 10.1107/S0907444906029799. [DOI] [PubMed] [Google Scholar]
- 24.Jones JM, Gellert M. Ordered assembly of the V(D)J synaptic complex ensures accurate recombination. EMBO J. 2002;21:4162–4171. doi: 10.1093/emboj/cdf394. [DOI] [PMC free article] [PubMed] [Google Scholar]