Biomineralized materials are highly organized composites with hierarchical structures, in which the basic building blocks (apatite crystals) are generally in the nanometer size range to ensure optimum physical and biological functions (1). The biological mechanisms of tissue development have attracted a great deal of recent attention in fields ranging from biology and chemistry to materials science and bioengineering (2, 3). It is well known that the organic component of bone acts as an important regulator of lattice orientation, particle size, and size distribution in biomineralization processes, but the molecular recognition details at inorganic crystal interfaces are poorly understood (4). Recently, most investigations have been focused on the effects of macromolecules such as carboxylate-rich proteins on hydroxyapatite nucleation and growth because complex interactions and changes of conformation are involved (5, 6). The role of small organic molecules such as citrate was deemphasized and was assumed to be simply that of adsorption on the crystal growth steps, resulting in inhibition of crystal growth (7, 8). However, there is still much to be learned about the mechanisms of crystal size control in biological systems. New insight about the role of citrate is emerging, as illustrated by a study in PNAS (9), in which strongly bound citrate molecules were identified as playing critical roles in interfering with crystal thickening and stabilizing apatite nanocrystal sizes in bone.
The report, by Schmidt-Rohr and colleagues (9), focuses on a quantitative analysis of citrate adsorbed on bone surfaces by the use of advanced multi-NMR spectroscopy and distance measurements. It demonstrates that the citrate, strongly immobilized on the surface of bone, accounts for 5.5 wt% of the organic matter in bone. The density of the citrate coverage is approximately one molecule per (2 nm)2, which implies that approximately one sixth of the available apatite surface area in bone is covered by citrate molecules. The long axes of the citrate molecules are nearly parallel to the apatite surface, with their three carboxylate groups at distances of 0.3–0.45 nm from the apatite surface. The suggested reasons for the ability of the citrate molecules to control the thickness of apatite nanocrystals are the relatively large amounts of citrate in the organic component of bone and the matching of spacings between carboxylate groups in the citrate molecule with those of calcium ions along the apatite c-axis. The impressive new picture proposed for the interaction of citrate molecules with apatite surfaces adds to our understanding of a number of basic problems involved in the complex bone biomineralization process.
An understanding of hierarchical biomineral structure construction, size control, and order of self-assembly must involve the roles of organic components of bone such as collagen, carboxylate-rich protein, and citrate molecules in calcium phosphate nucleation and crystal growth. Previously, it was known only that most carboxylate-rich proteins and collagen could promote nucleation by decreasing the induction time (10); citrate molecules inhibit nucleation by interacting with calcium ions (7, 8). However, they can all inhibit crystal growth by binding on the crystal surfaces. Other details, such as the mechanism of size control in vivo, have not been satisfactorily elucidated. Furthermore, there seems to be a predetermined size of the inorganic nanocrystal corresponding to the optimal mechanical property for the biomineral nanocomposite material. Size control mechanisms in biological systems, for example relating to growth initiation and the mechanisms of growth inhibition, are still not clear. On the basis of this unique model of citrate binding to apatite, we can propose mechanisms for crystal size control in bone as follows.
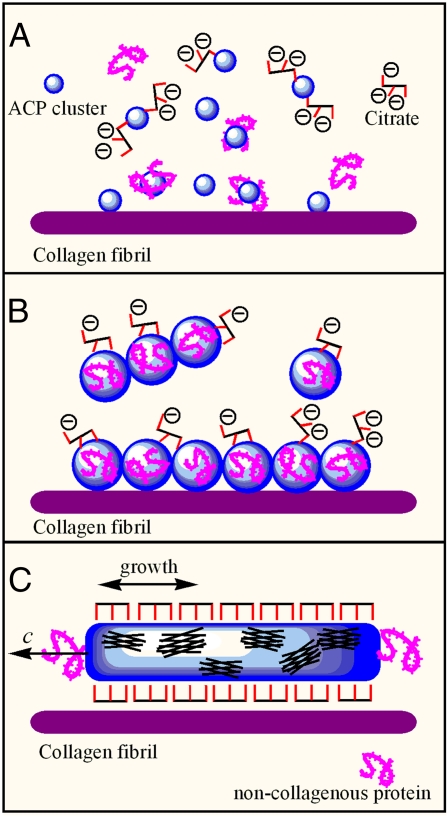
Recent investigations of the first-formed mineral phase of bone show that amorphous calcium phosphate (ACP) is a major component, and the question arises as to how citrate influences the early ACP phase formation (11). Generally, in supersaturated calcium phosphate solutions, the relatively stable ACP clusters are formed first (12, 13). At the early stage of nucleation in the presence of citrate, for the small size and amorphous structure of the early clusters, only some citrate molecules can partly bind with the cluster surface, inhibit further aggregation, and increase the induction time, as shown in Fig. 1A. In the later nucleation stage, the ACP clusters become larger because of aggregation, which can be promoted by the presence of noncollagenous protein. At the same time, mineralized collagen fibrils promote the self-assembly of oriented small amorphous clusters on the collagen surfaces (14, 15). At this stage, some unstable tiny prenuclei form within the large amorphous clusters, and the surface area occupied by bound citrate molecules increases slightly, as shown in Fig. 1B. Although some citrate molecules can interact with the amorphous clusters, the binding area density is low, owing to the continuing mismatch between the spacing of the terminal carboxylate groups in citrate and the structural parameter of the amorphous clusters. Binding of citrate molecules on the ACP surfaces can slow down but not stop the nucleation process. After nucleus formation, the area density of citrate binding on the crystal surfaces increases because the spacing of carboxylate groups in citrate matches that of calcium ions along the c-axis in apatite (10). Thus, crystal growth in the thickness direction is inhibited but continues in the longitudinal direction. The initial nucleus formation can be induced by noncollagenous proteins, which means that the c-axis of the nucleus follows the macromolecular chain direction. In this way, the nanocrystal thickness control and oriented crystal growth are achieved with the cooperation of citrate and proteins, as shown in Fig. 1C.
Fig. 1.
Schematics of nanocrystal size control involving citrate. (A) In the early nucleation stage, citrate can partly bind with ACP clusters and inhibit their further aggregation. (B) In the later nucleation stage, the ACP clusters become larger because of aggregation, which can be promoted by the presence of noncollagenous protein. At this time, some unstable tiny prenuclei form within the larger amorphous clusters, and the surface area density of citrate binding undergoes a small increase. (C) After apatite nanocrystal formation, the (10Ī0) surface is fully covered by citrate due to space matching. The crystal growth in the [10Ī0] direction is inhibited. At the same time, the surface binding with citrate raises the interfacial compatibility between apatite crystals and the collagen layer.
This citrate binding model can help us to understand the mechanism of apatite morphology control in vivo; for example, why the crystal morphology of apatite in bone is plate-like, whereas it is rod-like in human tooth enamel. The amount of citrate in both biological environments is quite different. In bone, it is relatively large at 5 wt% of the organic components. After apatite nanocrystal formation, the (10Ī0) surface is fully covered by citrate because of space matching. The crystal growth in the [10Ī0] direction is inhibited, but the citrate effect on other crystal surfaces is very small owing to poor space matching. Thus, after crystal growth, the (10Ī0) crystal face becomes predominant, resulting in plate-like morphology. However, the amount of citrate in saliva is
Approximately one sixth of the available apatite surface area in bone is covered by citrate molecules.
much lower than in bone, but the concentration of fluoride ion is relatively high, and this ion readily exchanges with the hydroxyl ion of apatite. As a result, the substitution of fluoride ion for hydroxyl ions brings about a reduction in the volume of the unit cell, and the lattice becomes more dense (16), which results in the spacing mismatch between citrate and fluorapatite in teeth. Thus, the final crystal displays hexagonal rod-like morphology in tooth enamel, the hardest tissue in vivo.
The discovery of strongly bound citrate on the surfaces of apatite nanocrystals may help us to understand why bone nanocomposites have good mechanical properties and interfacial compatibility. In citrate, both terminal carboxylate groups can interact with calcium ions at a distance of 0.3 nm, and all citrate carbons are found at approximately 0.4 nm from the top phosphate layer; the long axes of the citrate molecules are nearly parallel to the apatite surface, and the citrate methylene groups face outward. This unique high binding structure reduces the hydrophilic character of the surface, making it more compatible with the nonpolar proline and alanine residues of the collagen matrix. In this way, it decreases the interfacial energy between the thickness-confined nanocrystal and collagen layer and provides good interfacial compatibility. It also can help direct apatite nanocrystal growth, with the c-axis oriented along the long axis of a collagen fibril (11, 17, 18). This may be a reason for the formation of layer-by-layer composite structures frequently found in biological systems such as bones and teeth.
The implications of the current studies will be helpful for our understanding of complex biomineralization processes. The interesting role of citrate binding on apatite nanocrystal size control is a biologically inspired lesson from nature, which can be developed into an advanced strategy to control material fabrication. To achieve this, further research will be needed on the complex kinetic processes of citrate combination with collagenous and noncollagenous proteins in the regulation of nucleation and crystal growth.
Acknowledgments
Our laboratory work is supported by National Institutes of Health Research Grant DE003223 (to G.H.N.).
Footnotes
The authors declare no conflict of interest.
See companion article on page 22425.
References
- 1.Weiner S, Wagner HD. The material bone: Structure-mechanical function relations. Annu Rev Mater Sci. 1998;28:271–298. [Google Scholar]
- 2.Stupp SI, Braun PV. Molecular manipulation of microstructures: Biomaterials, ceramics, and semiconductors. Science. 1997;277:1242–1248. doi: 10.1126/science.277.5330.1242. [DOI] [PubMed] [Google Scholar]
- 3.Long JR, et al. A peptide that inhibits hydroxyapatite growth is in an extended conformation on the crystal surface. Proc Natl Acad Sci USA. 1998;95:12083–12087. doi: 10.1073/pnas.95.21.12083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tang R, Wang L, Nancollas GH. Size-effects in the dissolution of hydroxyapatite: an understanding of biological demineralization. J Mater Chem. 2004;14:2341–2346. [Google Scholar]
- 5.Deshpande AS, Fang PA, Simmer JP, Margolis HC, Beniash E. Amelogenin-collagen interactions regulate calcium phosphate mineralization in vitro. J Biol Chem. 2010;285:19277–19287. doi: 10.1074/jbc.M109.079939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yang Y, Cui Q, Sahai N. How does bone sialoprotein promote the nucleation of hydroxyapatite? A molecular dynamics study using model peptides of different conformations. Langmuir. 2010;26:9848–9859. doi: 10.1021/la100192z. [DOI] [PubMed] [Google Scholar]
- 7.Hartles RL. Citrate in mineralizaed tissues. Adv Oral Biol. 1964;1:225–253. doi: 10.1016/b978-1-4832-3117-4.50014-0. [DOI] [PubMed] [Google Scholar]
- 8.Johnsson M, Richardson CF, Sallis JD, Nancollas GH. Adsorption and mineralization effects of citrate and phosphocitrate on hydroxyapatite. Calcif Tissue Int. 1991;49:134–137. doi: 10.1007/BF02565136. [DOI] [PubMed] [Google Scholar]
- 9.Hu Y-Y, Rawal A, Schmidt-Rohr K. Strongly bound citrate stabilizes the apatite nanocrystals in bone. Proc Natl Acad Sci USA. 2010;107:22425–22429. doi: 10.1073/pnas.1009219107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wang L, Guan X, Du C, Moradian-Oldak J, Nancollas GH. Amelogenin promotes the formation of elongated apatite microstructures in a controlled crystallization system. J Phys Chem C Nanomater Interfaces. 2007;111:6398–6404. doi: 10.1021/jp0675429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mahamid J, Sharir A, Addadi L, Weiner S. Amorphous calcium phosphate is major component of the forming fin bones of zebrafish: Indications for an amorphous precursor phase. Proc Natl Acad Sci USA. 2008;105:12748–12753. doi: 10.1073/pnas.0803354105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Combes C, Rey C. Amorphous calcium phosphates: Synthesis, properties and uses in biomaterials. Acta Biomater. 2010;6:3362–3378. doi: 10.1016/j.actbio.2010.02.017. [DOI] [PubMed] [Google Scholar]
- 13.Wang L, Nancollas GH. Calcium orthophosphates: Crystallization and dissolution. Chem Rev. 2008;108:4628–4669. doi: 10.1021/cr0782574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bradt JH, Mertig M, Teresiak A, Pompe W. Biomimetic mineralization of collagen by combined fibril assembly and calcium phosphate formation. Chem Mater. 1999;11:2694–2701. [Google Scholar]
- 15.Olszta MJ, et al. Bone structure and formation: A new prespective. Mater Sci Eng Rep. 2007;58:77–116. [Google Scholar]
- 16.Aoba T. The effect of fluoride on apatite structure and growth. Crit Rev Oral Biol Med. 1997;8:136–153. doi: 10.1177/10454411970080020301. [DOI] [PubMed] [Google Scholar]
- 17.De Jong WF. The mineral substance in bone (Translated from French) Recl Trav Chim Pays Bas. 1926;45:445–448. [Google Scholar]
- 18.Jackson SF. The fine structure of developing bone in the embryonic fowl. Proc R Soc Lond B Biol Sci. 1956;146:270–280. doi: 10.1098/rspb.1957.0010. [DOI] [PubMed] [Google Scholar]