Abstract
Apurinic/apyrimidinic (AP) sites are ubiquitous DNA lesions that are highly mutagenic and cytotoxic if not repaired. In addition, clusters of two or more abasic lesions within one to two turns of DNA, a hallmark of ionizing radiation, are repaired much less efficiently and thus present greater mutagenic potential. Abasic sites are chemically labile, but naked DNA containing them undergoes strand scission slowly with a half-life on the order of weeks. We find that independently generated AP sites within nucleosome core particles are highly destabilized, with strand scission occurring ∼60-fold more rapidly than in naked DNA. The majority of core particles containing single AP lesions accumulate DNA–protein cross-links, which persist following strand scission. The N-terminal region of histone protein H4 contributes significantly to DNA–protein cross-links and strand scission when AP sites are produced approximately 1.5 helical turns from the nucleosome dyad, which is a known hot spot for nucleosomal DNA damage. Reaction rates for AP sites at two positions within this region differ by ∼4-fold. However, the strand scission of the slowest reacting AP site is accelerated when it is part of a repair resistant bistranded lesion composed of two AP sites, resulting in rapid formation of double strand breaks in high yields. Multiple lysine residues within a single H4 protein catalyze double strand cleavage through a mechanism believed to involve a templating effect. These results show that AP sites within the nucleosome produce significant amounts of DNA–protein cross-links and generate double strand breaks, the most deleterious form of DNA damage.
Keywords: chromatin, oxidative damage, histone modification
Exogenously and endogenously produced agents continuously damage DNA. More than 70 types of damage have been identified, and significant effort is expended to determine which of these lesions are biologically important (1, 2). The apurinic/apyrimidinic (AP) site is a ubiquitous form of DNA damage that is produced in excess of 10,000 lesions per cell each day (3). This highly mutagenic lesion results from spontaneous hydrolysis of native and damaged nucleotides. AP sites are also formed as intermediates during base excision repair of alkylated and oxidized nucleotides and are themselves removed by multiple enzymatic pathways (4, 5). In mammalian cells incision of the AP’s 5′-phosphate by Ape1, followed by excision of the resulting 5′-terminal 2′-deoxyribose phosphate by DNA polymerase β is the major pathway for removing the lesion. Redundant repair pathways are reflective of the high mutagenic potential and large quantities of AP produced and are indicative of their physiological significance. Mammalian cells lacking Ape1, the major base excision repair protein responsible for incising AP sites, are embryonic lethal (6). Recent observations reinforce the perception that AP sites are cytotoxic. For instance, formation of bursts of AP sites by the natural product leinamycin is postulated to be the source of this antitumor agent’s cytotoxicity (7). In addition, increasing the lifetime of AP sites in cells via the expression of an inactive form of Ape1 enhances the cytotoxicity of DNA damaging drugs (8).
Prolonging the lifetime of DNA lesions increases the likelihood that they will be present during replication, resulting in mutations. Clustered lesions, consisting of two or more damage sites within ∼1.5 turns of the DNA duplex, are an important family of lesions produced in DNA exposed to γ-radiolysis. Bistranded clustered lesions are repaired much less efficiently than the respective isolated lesions by members of the base excision repair (BER) pathway. For example, a clustered lesion comprised of two AP sites on opposite strands that are displaced 5′ to one another significantly resists Ape1 incision in vitro (9). Moreover, AP sites that are part of clustered lesions persist in cells even after 1 d, whereas isolated lesions are repaired in 1 h (10, 11). The lifetimes of lesions in DNA are also affected by their rotational orientation within the nucleosome, which can limit their accessibility to repair proteins (12, 13). For electrophilic lesions such as AP, this enhances the possibility that chemical reactions will occur with neighboring nucleobases and/or protein residues to form DNA–DNA interstrand cross-links (14–16) and DNA–protein cross-links (DPCs), respectively (17, 18). DPCs require alternative repair pathways from BER (19–21). In addition, one DNA interstrand cross-link arising from an abasic lesion is misrepaired by nucleotide excision repair and transformed into an even more deleterious double strand break (22). Abasic sites are also prone to elimination, which results in strand scission and the release of an even more electrophilic α,β-unsaturated aldehyde (23, 24).
DNA of eukaryotic genomes is packaged into arrays of nucleosomes that comprise chromatin structure. The nucleosome core particle consists of 146–147 bp of DNA wrapped around a histone protein octamer containing two copies of four histone proteins (H2A, H2B, H3, and H4) (25). The lysine-rich N-terminal tails of the histones extend from the protein core, making various contacts with the DNA minor groove. The proximity of nucleosomal AP sites to these basic residues is expected to accelerate cleavage of the lesion relative to naked DNA (26–28). Previously, Povirk showed that the oxidized abasic lesion produced by bleomycin undergoes rapid strand scission when produced in chromatin (27). However, this reaction, and the behavior of nucleosomal abasic sites in general, are not well characterized. Therefore, we prepared nucleosome core particles containing precisely positioned AP sites using a stable photolabile precursor (4, Fig. 1C), which allowed for the complete generation of AP within 10 min. We find that isolated AP lesions in the nucleosome are at least 60 times more reactive than in naked DNA of the same sequence. This reaction involves formation of slowly reversible DNA–protein cross-links believed to involve Schiff base formation between AP sites and histone lysine residues. The enhanced lability of AP sites results in double strand breaks (DSBs) when they are part of a clustered lesion that resists BER. Importantly, the rate of double strand cleavage is faster than that expected based upon the reaction of two, isolated AP sites at the same positions. These data suggest Schiff base formation with one AP site accelerates reaction with a second lesion by acting as a template and increasing the effective molarity of a lysine side chain(s) in the vicinity of the second lesion. Moreover, these experiments reveal isolated AP lesions are efficient sources of DNA–protein cross-links, and that bistranded AP lesions, such as those produced by ionizing radiation, are substantial sources of double strand breaks in nucleosomal DNA.
Fig. 1.
Independent generation of AP sites within nucleosome core particles. (A) X-ray crystal structure of the α-satellite DNA nucleosome core particle (PDB ID code 1aoi) highlighting positions of AP incorporation. (B) A portion of the α-satellite DNA sequence showing where the AP sites are incorporated (see SI Text for complete DNA sequences). (C) Methods used for independently generating AP sites.
Results
Preparation of Nucleosome Core Particles Containing Site-Specific AP Sites.
The α-satellite palindrome sequence was chosen as a host for the AP lesion due to its well characterized structure (25, 29). In particular, the region near superhelical location (SHL) 1.5 (AP89, AP207) was chosen for AP incorporation because it is recognized as a hot spot for drug binding (Fig. 1A and B) (30, 31). This region is significantly bent and is in close proximity to the lysine-rich tails of histones H3 and H4. AP sites at positions 89 and 207 were generated via 10-min photolysis using long wavelength light (350 nm) of nucleosome core particle or naked DNA containing 4 (Fig. 1C). Short oligonucleotides containing 4 (Scheme S1) were prepared via solid phase chemical synthesis, and the 146-bp α-satellite DNA duplexes were constructed by ligating these with two longer biopolymers that were also chemically synthesized (Fig. S1). To ensure selective 5′-32P labeling of the strand containing 4, the DNA substrates were constructed such that the strand opposite the lesion was blocked synthetically at its 5′ end. The substrates containing the clustered AP lesion (3a–3d) were constructed such that either strand could be monitored independently using 32P. All 146-bp duplexes were purified by native PAGE, and nucleosome core particles were reconstituted via stepwise salt reduction using recombinant Xenopus laevis histone proteins expressed in Escherichia coli (32). Nucleosome reconstitutions containing less than 15% free DNA were used without further purification, and all values reported herein have been corrected to account for any remaining free DNA. The rotational orientation of each nucleosome core particle containing AP precursor 4 was determined through DNase I digestion (Fig. S2), which confirmed proper positioning of all substrates with respect to the X-ray structure (33, 34). Completeness of AP generation from 4 was confirmed by PAGE analysis, following alkaline treatment (Fig. S3). Photochemical generation of an AP site is advantageous over the commonly used hydrolysis of 2′-deoxyuridine (dU) by uracil DNA glycosylase (Fig. 1C) because the enzyme is inhibited by the presence of a nucleosome and its activity depends upon the rotational orientation of dU within the core particle (12, 13). Rapid generation of AP independent of its position within the core particle was particularly helpful when following the time course of reactions.
AP Lesions Display Accelerated Reactivity in Nucleosome Core Particles.
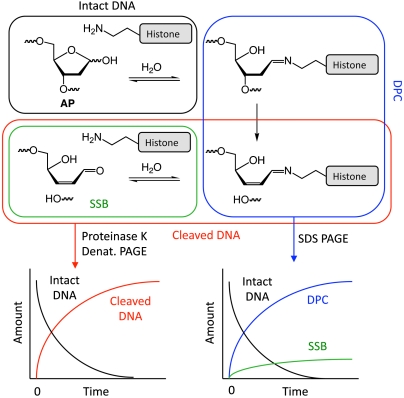
The reactivity of AP within nucleosome core particles was monitored via various forms of PAGE (Fig. 2). Analysis of samples by SDS PAGE, which dehybridizes noncovalently bound DNA–protein complexes, allowed for simultaneous quantification of intact DNA within core particles, DNA containing single strand breaks (SSBs) at the AP site, and DPCs. However, it does not distinguish DPCs containing a SSB from those in which the DNA is intact. To determine total strand scission regardless of the presence of DPCs, nucleosome core particles were digested with proteinase K and the DNA separated by denaturing PAGE. All samples were treated with the reducing agent NaBH4 prior to electrophoretic analysis, which minimized subsequent adventitious cleavage of AP. The reducing agent also trapped any DPCs resulting from Schiff base formation. In situ trapping of Schiff base intermediates was accomplished by incubating samples in the presence of NaBH3CN.
Fig. 2.
Strategy for using gel electrophoresis to analyze the reactivity of AP in nucleosome core particles. (Top) Expected cross-linking and cleavage reactions between an AP site and a lysine residue. (Bottom) Predicted detection of different products using two electrophoresis methods. Denaturing PAGE following histone digestion by proteinase K separates intact nucleosomal DNA (black) from cleaved DNA (red) whether or not the DNA was cross-linked to protein. SDS PAGE separates intact nucleosomal DNA (black), DNA–protein cross-links (DPC, blue), and cleaved DNA that is not cross-linked to protein (SSB, green). Please note that SDS PAGE does not distinguish between DPCs containing cleaved and intact DNA (blue).
Approximately 20% of intact, unmodified DNA remained in nucleosome core particle 1a after a 32-h incubation under physiological conditions (pH 7.5, 37 °C) (Fig. 3A). The majority of DNA in 1a (∼68%) was cross-linked to a histone protein(s). Denaturing PAGE analysis of proteinase K digested 1a revealed that > 80% of AP89 (Fig. 1B) had undergone elimination during the incubation (Fig. 3B), indicating that a significant amount of the DNA in the DPCs was cleaved. This was corroborated by subsequently analyzing the DNA from isolated DPCs by denaturing PAGE, which revealed ∼50% of the cross-linked DNA was cleaved within 1 h and that at least 90% was cleaved at any time after 17 h (Fig. S4). The product mixture was simplified by incubating core particle in the presence of NaBH3CN (10 mM). This resulted in a modest increase in overall DPC formation (∼85% after 32 h, Fig. S5) and a significant reduction of cleaved DNA in the DPCs (∼20%) (Fig. S4). These data strongly suggest that cleavage at AP89 is dependent on formation of a Schiff base and that hydrolysis of this intermediate following AP elimination is slow. Finally, despite rapid DNA–protein cross-link formation, no DNA interstrand cross-links were detected in any of these substrates (14).
Fig. 3.
Reaction of AP89 in a nucleosome core particle (1a) and naked DNA of the same sequence. (A) SDS PAGE analysis describing disappearance of intact DNA containing AP89 in nucleosome core particle 1a and growth of DPC and SSB as a function of time. (B) Comparison of the total amount of strand scission using denaturing PAGE analysis in naked, and nucleosomal DNA from 1a, following protease digestion.
The kinetics for disappearance of AP sites in nucleosome core particles 1a and 2a were approximated by ignoring the reversibility of initial Schiff base formation, and fitting the data to a first-order reaction (Table 1). The half-life of AP89 within the nucleosome was at least 60-fold shorter than in naked DNA (24). So little strand scission was observed in the naked DNA used to construct nucleosome core particle 1a under identical conditions (Fig. 3B) that the estimated rate constant (kDis ∼ 3.9 × 10-7 s-1, t1/2 ∼ 500 h) is likely a maximum, and the 60-fold acceleration of AP89 reactivity in the region of SHL 1.5 within the α-satellite nucleosome core particle is a minimum. AP was also destabilized in nucleosomes when introduced three nucleotides away on the opposite strand (AP207, 2a) (Table 1). The variation in reactivity between these two positions (∼4-fold) was small compared to the overall acceleration experienced in the nucleosome core particle.
Table 1.
Reactivity of AP sites in nucleosome core particles
| Substrate | Position | H4 tail | kDis, × 10-6 s-1 | t1/2 AP, h |
| 1a | AP89 | + | 22.7 ± 3.5 | 8.5 |
| 1b | AP89 | − | 7.5 ± 1.4 | 25.7 |
| 2a | AP207 | + | 6.1 ± 0.7 | 31.6 |
| 2b | AP207 | − | 3.7 ± 0.6 | 52.0 |
Structural data suggest the lysine-rich N-terminal tail of histone H4 is within proximity to the DNA at SHL 1.5 (25, 29). Therefore, core particles (1b, 2b) lacking 19 residues of the H4 tail (amino acids 1–19) were examined in order to determine what role (if any) the tail plays in the enhanced reactivity of AP at this location. In general, the reactivity of both AP sites at SHL 1.5 decreased in core particles lacking the H4 tail (Table 1). For instance, the rate constant for AP89 disappearance (kDis) decreased more than 3-fold when the reconstituted core particle contained a tailless H4 mutant (1b) (Fig. S5). The effect of removing the H4 tail on DPC formation correlated with the reduction noted in AP89 elimination (Fig. S5). The effect of removing the histone H4 tail was somewhat smaller on the reactivity of AP207. The rate constant describing AP207 disappearance decreased less than 2-fold when the H4 tail was removed (2b).
Clustered AP Lesions in the Nucleosome Accelerate Formation of DNA Double Stranded Breaks.
The reactivity of nucleosome core particles containing bistranded AP lesions displaced 5′ to one another by 3 nucleotides (3a, 3c) were examined (Fig. 4 and Fig. S6). The position of the AP sites in the nucleosomal DNA (89, 207) of these core particles are the same as in those containing isolated lesions (1, 2) and differ from one another only with respect to which strand of the DNA is radiolabeled (Fig. 1B). This spatial distribution of lesions was chosen because it significantly inhibits repair and can be expected to have a long lifetime in cellular DNA (9, 35). The rate constant for the disappearance of intact DNA in core particles 3a and 3c (also fit to first order) was essentially the same regardless of which strand was labeled (kDis ∼ 2.6 × 10-5 s-1) Furthermore, denaturing PAGE analysis of 3a and 3c following proteinase K digestion revealed that AP89 and AP207 underwent elimination at rates that were indistinguishable from one another when part of the bistranded lesion (Fig. S7), which is consistent with the lack of significant SSB accumulation in these substrates (Fig. 4). This suggests AP207 reacts ∼4-fold faster when it is part of a clustered lesion (3c) than when it is isolated in the core particle (2a). Importantly, naked DNA containing the bistranded lesion was not any more reactive than that containing a single AP site (Fig. S3). Although the reactivity of AP207 increased in the bistranded lesion, DNA–protein cross-link growth and stability were lower compared to that in nucleosome core particles containing an isolated AP site (Fig. 4A). Removal of the lysine-rich H4 tail also reduced the reactivity of AP89 and AP207 in the bistranded complexes (3b: kDis = 1.1 × 10-5 s-1; 3d: kDis = 8.5 × 10-6 s-1) (Fig. S6) to a similar extent (∼2.5-fold) as was observed in core particles containing isolated AP lesions (Table 1).
Fig. 4.
Reaction of a bistranded lesion (AP89, AP207) in a nucleosome core particle (3a). (A) Disappearance of intact core particle 3a and growth of DPC, SSB, and DSB as a function of time using SDS PAGE (without proteinase K treatment of aliquots). (B) Time dependence of the disappearance of intact nucleosome core particle 3a, and the growth of products, using SDS PAGE following proteinase K digestion of aliquots.
Enhanced AP site reactivity in the nucleosome core particle containing a clustered lesion gave rise to rapid double strand break formation (Fig. 4). Some double strand breaks are disguised by cross-linking to histone protein(s), but treatment of aliquots with proteinase K unmasked all strand scission (Fig. 4B). Double strand break formation proceeded rapidly after a short, barely discernible induction period between 0 and 6 h. More than 30% of the DNA underwent double strand cleavage in 6 h and double strand breaks reached more than 90% after incubating the nucleosome core particle (3a) for 32 h. Analysis of an aliquot removed after 32 h by native PAGE did not show any decomposition of the core particle (Fig. S6), indicating that double strand break formation did not result in nucleosome decomposition. Removing the lysine-rich tail of H4 reduced the efficiency of double strand break formation to a similar extent as it did the reaction of isolated AP lesions (Fig. S6).
Identification of Histone Proteins Involved in AP Cross-Linking.
Schiff base formation between AP sites and lysine residues were assumed to account for the accumulation of large amounts of histone–DNA cross-links during nucleosome core particle promoted DNA cleavage. In order to identify the protein(s) involved in cross-linking, we devised an assay that involved cross-link dependent transfer of the 32P-radiolabel from the DNA to the corresponding protein (Scheme 1) (Fig. S4). Core particles comprised of DNA containing 5′-32P-AP sites were incubated in the presence of NaBH3CN in order to trap DPCs involving a Schiff base at the site of the radiolabel. The DNA was subsequently digested with DNase I and nuclease P1, which leaves a short DNA fragment(s) containing the reduced, cross-linked 5′-32P-AP site bonded to the histone. The histone proteins were separated by SDS and Triton/Acid/Urea (TAU) PAGE and visualized with Coomassie blue staining, as well as by autoradiography (Fig. S4). Histone H4 was the major (> 95%) protein trapped upon incubation of nucleosome core particles containing AP89 whether (1a) or not (1b) the lysine-rich tail of H4 was present (Fig. 5A and Fig. S4). A relatively modest translocation of the AP site 3 nucleotides upstream on the opposite strand of the duplex (AP207, nucleosome core particle 2a) resulted in a slight decrease in cross-linking to H4 and a commensurate increase in H2A (+4%) and H3 (+2%) participation. However, removing the lysine-rich tail from H4 (2b) significantly reduced the amount of cross-linking between AP207 and this protein, which was compensated for by an increase in trapping by H3 but not H2A or H2B (Fig. 5B).
Scheme 1.
Method for determining protein(s) cross-linked with AP sites.
Fig. 5.
Identification of the histone proteins involved in DPC formation with AP sites in nucleosome core particles. (A) DPC formation with AP89 in the presence of wild-type (nucleosome core particle 1a) and tailless H4 protein (nucleosome core particle 1b). (B) DPC formation with AP207 in the presence of wild-type (nucleosome core particle 2a) and tailless H4 protein (nucleosome core particle 2b). See also Fig. S4.
The distribution of histone proteins involved in cross-linking of the bistranded lesion was very similar to those that react with isolated AP sites. Cross-linking to the individual AP sites was determined by generating the appropriate 5′-32P-AP in the top (3a, 3b, AP89) or bottom (3c, 3d, AP207) strand of the DNA within the nucleosome core particle (Fig. 1B). Overall, the majority of DPCs (> 85%) involved histone H4, regardless of the AP site being measured (Fig. 6A). Together, histones H4 and H3 account for more than 95% of the DPCs in wild-type core particles containing the bistranded lesion. The contribution of H3 to cross-linking AP89 in 3a was ∼11% compared to ∼3% in the substrate containing the isolated lesion (1a). Furthermore, the amount of cross-linking to H3 increased to more than 15% when the H4 tail was removed (3b). The effect of the H4 tail on cross-linking to AP207 was considerably larger in the bistranded lesion (3c, 3d). Cross-linking to H3 increased to more than 35% when the H4 tail was absent (3d), compared to ∼8% when the bistranded lesion was part of a nucleosome core particle composed of all wild-type proteins (3c).
Fig. 6.
Identification of the histone proteins involved in DPC formation with AP sites in the bistranded lesion. (A) DPC formation with AP89 (nucleosome core particle 3a, 3b) and AP207 (nucleosome core particle 3c, 3d) in the presence of wild-type (3a, 3c) and tailless H4 protein (3b, 3d). (B) Overall DNA–protein cross-link yield, and contribution by AP89 (3a) and AP207 (3c) to DPCs in the bistranded substrate when incubated in the presence of NaBH3CN. Individual contributions by AP89 and AP207 were determined by denaturing samples prior to gel electrophoresis.
Incubation of 3a (and 3c) was carried out in the presence of NaBH3CN in order to determine if simultaneous cross-linking of both AP sites to different residues of histone H4 was possible. The overall amount of DPCs in 3a (the strand containing AP89 is labeled) increased significantly in the presence of the reducing agent (∼93%, Fig. 6B). Importantly, the level of DPCs was unaffected when the DNA was denatured prior to analysis by SDS PAGE, indicating that every cross-linked duplex consisted of a covalent bond between AP89 and a histone. The level of DPCs to the strand containing AP207 (using nucleosome core particle 3c) was slightly lower under similar conditions. However, the summation of DPCs involving AP89 and AP207 under denaturing conditions was far greater than the overall amount of cross-linked duplex. Given that the majority of DPCs at AP89 and AP207 involve histone H4 (Fig. 6A), this indicates that both strands were simultaneously cross-linked to that protein in a significant portion of core particles.
Discussion
The mutagenicity of AP sites and the biological importance of their efficient repair are widely appreciated (4). More recently, AP lesions were shown to form interstrand cross-links within DNA, raising the possibility that potentially more deleterious lesions could be produced from them (14). In this work we report on the reactivity of individual AP lesions that were independently generated in a region of nucleosome core particles (SHL 1.5) that is a hot spot for DNA damage (30, 31). Although DNA–DNA cross-links were not detected when isolated AP sites were independently produced at defined sites in core particles, we observed an ∼60-fold increase in AP site reactivity within the nucleosome compared to free DNA, resulting in a reduction of the lesion’s half-life to hours (24). Isolated AP lesions rapidly led to DNA–protein cross-links and strand breaks. In addition, large amounts of DPCs persisted after AP elimination. In general, less is known about DPC repair than of other types of DNA lesions (21, 36). Even less is known about DPC repair in nucleosomes and how these lesions affect chromatin remodeling. The observations reported above suggest that site-specific incorporation of AP sites may be a good way of producing such systems for further study.
Kinetic and trapping experiments conducted on nucleosome core particles composed of wild-type or a mutant form of histone H4 lacking its lysine-rich tail (residues 1–19) show that this portion of the protein contributes to the accelerated cleavage of AP sites in the vicinity of SHL 1.5. Removal of the H4 tail results in only modest reduction in AP site cleavage compared to core particle comprised of wild-type histones. Furthermore, despite lacking 19 amino acid tail residues, the mutant H4 protein remains the major contributor to DPCs at AP89 and AP207. These data are consistent with significant involvement of histone H4 globular domain residues in the accelerated cleavage of AP sites. Examination of the nucleosome core particle crystal structure reveals that lysine residues 20 and 31 of histone H4 are in proximity to AP89 and AP207, respectively, making these residues attractive candidates for Schiff base formation. Other proteins may also participate in this reaction, which is evident by an increase in H3 cross-linking at AP207 upon removal of the H4 tail. AP207 is in closer proximity to the H3 tail than is AP89. Additional experiments involving site-specific mutagenesis of H4 and liquid chromatography/mass spectrometry of digested DPCs are required to determine exactly which lysine residues are responsible for these reactions.
Overall, the data are consistent with a mechanism (Scheme 2) in which strand scission within the core particles is accelerated via Schiff base formation. Cleavage via Schiff base formation is consistent with model studies on AP lesions and randomly generated oxidized abasic sites in chromatin, which suggested that the core histone proteins significantly destabilize such lesions (26–28, 37, 38). Presumably, Schiff base formation is reversible. However, the data for AP disappearance fitted well to a simple first-order process. This suggests that the rate of Schiff base hydrolysis, reverting to an AP site, is probably slow compared to its formation and subsequent reaction. The presence of large amounts of cleaved DNA in isolated DPCs formed throughout the reaction of 1a (AP89) indicated that strand scission was rapid upon Schiff base formation. Finally, the persistence of DPCs after DNA strand scission is also consistent with relatively slow Schiff base hydrolysis. Although a number of base excision repair proteins and DNA polymerases exhibit lyase activities toward AP sites (39–42), it is less common for proteins that are not enzymes to react with DNA. In a recent rare example of the latter, Ku excised AP sites from DNA via a Schiff base intermediate (43). Although AP cleavage in a nucleosome core particle also proceeds via Schiff base formation, we would be remiss not to acknowledge that minor contributions to strand scission by other mechanisms that do not involve direct attack by a lysine residue cannot be ruled out.
Scheme 2.
Reaction mechanism for the formation of a DPC and strand break from AP in a nucleosome core particle.
The lifetime of an isolated AP site in a nucleosome is vastly shortened compared to that in free DNA, but rates of DPC formation and strand scission are modest compared to anticipated repair rates in cells. Following oxidative treatment, AP site levels are restored to background levels (∼5 AP sites/106 nucleotides or ∼30,000 AP sites/cell) within 1 h in mammalian cells (11). We find that as many as 10% of the AP sites formed at SHL 1.5 resulted in DPCs with the nucleosome in the first hour. When one considers the above noted steady-state levels of AP sites per cell, it is likely that thousands of DNA-histone cross-links are formed per cell each day. Even greater production is expected in cells exposed to exogenous forms of oxidative stress. Moreover, clustered DNA lesions, which are a hallmark of ionizing radiation-induced damage, are repaired significantly more slowly than isolated lesions (9, 35, 44–46). Consequently, clustered lesions containing AP sites are longer lived and may have significant biological consequences including greater yields of highly deleterious double strand breaks in addition to the DPCs noted above (10, 47, 48).
The above data indicate that in addition to longer lifetimes, clustered lesions may be even more deleterious in cells due to increased reactivity. Although AP sites are destabilized in nucleosomal DNA, reaction at AP207 is accelerated ∼4-fold when it is part of a bistranded lesion compared to when it is the sole lesion in a core particle. The enhanced reactivity can be attributed to a templating effect between two or more lysine residues of histone H4, where formation of the first Schiff base increases the effective molarity of other lysine side chains with respect to the AP site on the opposing strand (Scheme 3). This proposal is supported by experiments in which we measured histone H4 cross-linking with AP89 (3a) and AP207 (3c) individually (when incubated in the presence of NaBH3CN). We determined that the sum total of the individual cross-links was greater than the total amount of cross-linked duplex (Fig. 6B). This indicates that both AP sites were cross-linked to the same protein molecule in a significant fraction of nucleosome core particles. The reduction in DNA–protein cross-linking and double strand break formation in the presence of tailless histone H4 illustrate the importance of the lysine-rich H4 tail on these processes.
Scheme 3.
Postulated templating effect for the reaction of a bistranded AP lesion with a single histone protein.
Accelerated double strand break formation from clustered abasic lesions in nucleosomes is biologically important in its own right, due to their highly deleterious nature. However, the lysines of the H4 tail that play such a large role in DNA cleavage at AP sites near SHL 1.5 are often modified in cells (49, 50). Lysine acetylation and permethylation will reduce the ability of these side chains to induce strand scission of DNA containing AP sites. The strong involvement of H4 tails may indicate a connection between double strand break formation and chromatin remodeling. It is well known that histone proteins undergo modification when DNA is damaged and this facilitates repair protein access to the damaged biopolymer (51, 52). Our observations raise the question whether it is possible that cells also modify H4 tails in response to DNA damage in order to protect against single and double strand breaks.
Materials and Methods
Nucleosome core particles were used directly in these experiments without adjustments in the concentration following reconstitution (10 mM Hepes, 60 mM NaCl, 1 mM EDTA, and 0.1 mM PMSF, pH 7.5). Nucleosome core particles containing 4 were photolyzed (350 nm) for 10 min at room temperature and immediately incubated at 37 °C for the duration of the time course experiment. For samples incubated in the presence of NaBH3CN (10 mM), the reducing reagent was added prior to photolysis. Aliquots were removed at appropriate times and divided into two portions. One portion was treated with proteinase K (2 μg/1 μL sample) for 5 min at room temperature and quenched by the addition of NaBH4 (0.1 M final). These samples were analyzed by 8% denaturing PAGE (acrylamide/bisacrylamide; 19∶1, 45% urea). The second portion was treated directly with NaBH4 (0.1 M final) and analyzed by 10% native SDS PAGE (acrylamide/bisacrylamide; 29∶1) as previously described. SDS PAGE analysis of aliquots from 3a–3d was carried out using a gel comprised of a 15% resolving layer (acrylamide/bisacrylamide; 29∶1) and a 3% stacking layer (acrylamide/bisacrylamide; 29∶1).
Supplementary Material
Acknowledgments.
We are grateful for support from the National Institute of General Medical Sciences (GM-063028 to M.M.G. and GM-084129 to G.D.B.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1012860108/-/DCSupplemental.
References
- 1.Cadet J, Douki T, Gasparutto D, Ravanat JL. Oxidative damage to DNA: Formation, measurement and biochemical features. Mutat Res. 2003;531:5–23. doi: 10.1016/j.mrfmmm.2003.09.001. [DOI] [PubMed] [Google Scholar]
- 2.Cooke MS, Evans MD, Dizdaroglu M, Lunec J. Oxidative DNA damage: Mechanisms, mutation, and disease. FASEB J. 2003;17:1195–1214. doi: 10.1096/fj.02-0752rev. [DOI] [PubMed] [Google Scholar]
- 3.Lindahl T. Instability and decay of the primary structure of DNA. Nature. 1993;362:709–715. doi: 10.1038/362709a0. [DOI] [PubMed] [Google Scholar]
- 4.Wilson DM, Bohr VA. The mechanics of base excision repair, and its relationship to aging and disease. DNA Repair. 2007;6:544–559. doi: 10.1016/j.dnarep.2006.10.017. [DOI] [PubMed] [Google Scholar]
- 5.Snowden A, Kow YW, Van Houten B. Damage repertoire of the Escherichia coli uvrabc nuclease complex includes abasic sites, base-damage analogues, and lesions containing adjacent 5′ or 3′ nicks. Biochemistry. 1990;29:7251–7259. doi: 10.1021/bi00483a013. [DOI] [PubMed] [Google Scholar]
- 6.Wilson DM, Thompson LH. Life without DNA repair. Proc Natl Acad Sci USA. 1997;94:12754–12757. doi: 10.1073/pnas.94.24.12754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Viswesh V, Gates K, Sun D. Characterization of DNA damage induced by a natural product antitumor antibiotic leinamycin in human cancer cells. Chem Res Toxicol. 2010;23:99–107. doi: 10.1021/tx900301r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.McNeill DR, Lam W, DeWeese TL, Cheng Y-C, Wilson DM. Impairment of ape1 function enhances cellular sensitivity to clinically relevant alkylators and antimetabolites. Mol Cancer Res. 2009;7:897–906. doi: 10.1158/1541-7786.MCR-08-0519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chaudhry MA, Weinfeld M. Reactivity of human apurinic/apyrimidinic endonuclease and Escherichia coli exonuclease III with bistranded abasic sites in DNA. J Biol Chem. 1997;272:15650–15655. doi: 10.1074/jbc.272.25.15650. [DOI] [PubMed] [Google Scholar]
- 10.Georgakilas AG, Bennett PV, Wilson DM, Sutherland BM. Processing of bistranded abasic DNA clusters in γ-irradiated human hematopoietic cells. Nucleic Acids Res. 2004;32:5609–5620. doi: 10.1093/nar/gkh871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Atamna H, Cheung I, Ames BN. A method for detecting abasic sites in living cells: Age-dependent changes in base excision repair. Proc Natl Acad Sci USA. 2000;97:686–691. doi: 10.1073/pnas.97.2.686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cole HA, Tabor-Godwin JM, Hayes JJ. Uracil DNA glycosylase activity on nucleosomal DNA depends on rotational orientation of targets. J Biol Chem. 2010;285:2876–2885. doi: 10.1074/jbc.M109.073544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hinz JM, Rodriguez Y, Smerdon MJ. Rotational dynamics of DNA on the nucleosome surface markedly impact accessibility to a DNA repair enzyme. Proc Natl Acad Sci USA. 2010;107:4646–4651. doi: 10.1073/pnas.0914443107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dutta S, Chowdhury G, Gates KS. Interstrand cross-links generated by abasic sites in duplex DNA. J Am Chem Soc. 2007;129:1852–1853. doi: 10.1021/ja067294u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Regulus P, et al. Oxidation of the sugar moiety of DNA by ionizing radiation or bleomycin could induce the formation of a cluster DNA lesion. Proc Natl Acad Sci USA. 2007;104:14032–14037. doi: 10.1073/pnas.0706044104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sczepanski JT, Jacobs AC, Greenberg MM. Self-promoted DNA interstrand cross-link formation by an abasic site. J Am Chem Soc. 2008;130:9646–9647. doi: 10.1021/ja8030642. [DOI] [PubMed] [Google Scholar]
- 17.Hashimoto M, Greenberg MM, Kow YW, Hwang J-T, Cunningham RP. The 2-deoxyribonolactone lesion produced in DNA by neocarzinostatin and other DNA damaging agents forms cross-links with the base-excision repair enzyme endonuclease III. J Am Chem Soc. 2001;123:3161–3162. doi: 10.1021/ja003354z. [DOI] [PubMed] [Google Scholar]
- 18.Nakano T, et al. DNA-protein cross-link formation mediated by oxanine. A novel genotoxic mechanism of nitric oxide-induced DNA damage. J Biol Chem. 2003;278:25264–25272. doi: 10.1074/jbc.M212847200. [DOI] [PubMed] [Google Scholar]
- 19.Minko IG, Zou Y, Lloyd RS. Incision of DNA–protein crosslinks by uvrabc nuclease suggests a potential repair pathway involving nucleotide excision repair. Proc Natl Acad Sci USA. 2002;99:1905–1909. doi: 10.1073/pnas.042700399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Reardon JT, Sancar A. Repair of DNA-polypeptide crosslinks by human excision nuclease. Proc Natl Acad Sci USA. 2006;103:4056–4061. doi: 10.1073/pnas.0600538103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Minko IG, et al. Initiation of repair of DNA-polypeptide cross-links by the UvrABC nuclease. Biochemistry. 2005;44:3000–3009. doi: 10.1021/bi0478805. [DOI] [PubMed] [Google Scholar]
- 22.Sczepanski JT, Jacobs AC, Van Houten B, Greenberg MM. Double strand break formation during nucleotide excision repair of a DNA interstrand cross-link. Biochemistry. 2009;48:7565–7567. doi: 10.1021/bi901006b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sugiyama H, et al. Chemistry of thermal degradation of abasic sites in DNA. Mechanistic investigation on thermal DNA strand cleavage of alkylated DNA. Chem Res Toxicol. 1994;7:673–683. doi: 10.1021/tx00041a013. [DOI] [PubMed] [Google Scholar]
- 24.Zheng Y, Sheppard TL. Half-life and DNA strand scission products of 2-deoxyribonolactone oxidative DNA damage lesions. Chem Res Toxicol. 2004;17:197–207. doi: 10.1021/tx034197v. [DOI] [PubMed] [Google Scholar]
- 25.Luger K, Mader AW, Richmond RK, Sargent DF, Richmond TJ. Crystal structure of the nucleosome core particle at 28 Å resolution. Nature. 1997;389:251–260. doi: 10.1038/38444. [DOI] [PubMed] [Google Scholar]
- 26.Bailly V, Verly WG. Possible roles of beta-elimination and delta-elimination reactions in the repair of DNA containing AP (apurinic/apyrimidinic) sites in mammalian cells. Biochem J. 1988;253:553–559. doi: 10.1042/bj2530553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bennett RAO, Swerdlow PS, Povirk LF. Spontaneous cleavage of bleomycin-induced abasic sites in chromatin and their mutagenicity in mammalian shuttle vectors. Biochemistry. 1993;32:3188–3195. doi: 10.1021/bi00063a034. [DOI] [PubMed] [Google Scholar]
- 28.Behmoaras T, Toulme J-J, Helene C. A tryptophan-containing peptide recognizes and cleaves DNA at apurinic sites. Nature. 1981;292:858–859. doi: 10.1038/292858a0. [DOI] [PubMed] [Google Scholar]
- 29.Davey CA, Sargent DF, Luger K, Maeder AW, Richmond TJ. Solvent mediated interactions in the solution of the nucleosome core particle at 1.9 Å resolution. J Mol Biol. 2002;319:1097–1113. doi: 10.1016/S0022-2836(02)00386-8. [DOI] [PubMed] [Google Scholar]
- 30.Kuduvalli PN, Townsend CA, Tullius TD. Cleavage by calicheamicin g1 of DNA in a nucleosome formed on the 5S RNA gene of Xenopus borealis. Biochemistry. 1995;34:3899–3906. doi: 10.1021/bi00012a005. [DOI] [PubMed] [Google Scholar]
- 31.Davey G, Wu B, Dong Y, Surana U, Davey CA. DNA stretching in the nucleosome facilitates alkylation by an intercalating antitumor agent. Nucleic Acids Res. 2010;38:2081–2088. doi: 10.1093/nar/gkp1174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Luger K, Rechsteiner TJ, Flaus AJ, Waye MMY, Richmond TJ. Characterization of nucleosome core particles containing histone proteins made in bacteria. J Mol Biol. 1997;272:301–311. doi: 10.1006/jmbi.1997.1235. [DOI] [PubMed] [Google Scholar]
- 33.Núnez ME, Noyes KT, Barton JK. Oxidative charge transport through DNA in nucleosome core particles. Chem Biol. 2002;9:403–415. doi: 10.1016/s1074-5521(02)00121-7. [DOI] [PubMed] [Google Scholar]
- 34.Gottesfeld JM, et al. Sequence-specific recognition of DNA in the nucleosome by pyrrole-imidazole polyamides. J Mol Biol. 2001;309:615–629. doi: 10.1006/jmbi.2001.4694. [DOI] [PubMed] [Google Scholar]
- 35.Paap B, Wilson DM, Sutherland BM. Human abasic endonuclease action on multilesion abasic clusters: Implications for radiation-induced biological damage. Nucleic Acids Res. 2008;36:2717–2727. doi: 10.1093/nar/gkn118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nakano T, et al. Nucleotide excision repair and homologous recombination systems commit differentially to the repair of DNA-protein crosslinks. Mol Cell. 2007;28:147–158. doi: 10.1016/j.molcel.2007.07.029. [DOI] [PubMed] [Google Scholar]
- 37.Mazumder A, et al. Stereochemical studies of the beta-elimination reactions at aldehydic abasic sites in DNA: Endonuclease III from Escherichia coli, sodium hydroxide, and Lys-Trp-Lys. Biochemistry. 1991;30:1119–1126. doi: 10.1021/bi00218a033. [DOI] [PubMed] [Google Scholar]
- 38.Mirzabekov AD, Shick VV, Belyavsky AV, Bavykin SG. Primary organization of nucleosome core particle of chromatin—sequence of histone arrangement along DNA. Proc Natl Acad Sci USA. 1978;75:4184–4188. doi: 10.1073/pnas.75.9.4184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Matsumoto Y, Kim K. Excision of deoxyribose phosphate residues by DNA polymerase beta during DNA repair. Science. 1995;269:699–702. doi: 10.1126/science.7624801. [DOI] [PubMed] [Google Scholar]
- 40.Garcia-Diaz M, Bebenek K, Kunkel TA, Blanco L. Identification of an intrinsic 5′-deoxyribose-5-phosphate lyase activity in human DNA polymerase λ. J Biol Chem. 2001;276:34659–34663. doi: 10.1074/jbc.M106336200. [DOI] [PubMed] [Google Scholar]
- 41.Prasad R, et al. Human DNA polymerase θ possesses 5′-DRP lyase activity and functions in single-nucleotide base excision repair in vitro. Nucleic Acids Res. 2009;37:1868–1877. doi: 10.1093/nar/gkp035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.David SS, Williams SD. Chemistry of glycosylases and endonucleases involved in base-excision repair. Chem Rev. 1998;98:1221–1261. doi: 10.1021/cr980321h. [DOI] [PubMed] [Google Scholar]
- 43.Roberts SA, et al. Ku is a 5′-dRP/AP lyase that excises nucleotide damage near broken ends. Nature. 2010;464:1214–1217. doi: 10.1038/nature08926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.O’Neill P, Wardman P. Radiation chemistry comes before radiation biology. Int J Radiat Biol. 2009;85:9–25. doi: 10.1080/09553000802640401. [DOI] [PubMed] [Google Scholar]
- 45.Mourgues S, Lomax ME, O’Neill P. Base excision repair processing of abasic site/single-strand break lesions within clustered damage sites associated with XRCC1 deficiency. Nucleic Acids Res. 2007;35:7676–7687. doi: 10.1093/nar/gkm947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sutherland BM, Bennett PV, Sidorkina O, Laval J. Clustered DNA damages induced in isolated DNA and in human cells by low doses of ionizing radiation. Proc Natl Acad Sci USA. 2000;97:103–108. doi: 10.1073/pnas.97.1.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Radford IR, Lobachevsky PN. Clustered DNA lesion sites as a source of mutations during human colorectal tumourigenesis. Mutat Res. 2008;646:60–68. doi: 10.1016/j.mrfmmm.2008.08.017. [DOI] [PubMed] [Google Scholar]
- 48.Blaisdell JO, Wallace SS. Abortive base-excision repair of radiation-induced clustered DNA lesions in Escherichia coli. Proc Natl Acad Sci USA. 2001;98:7426–7430. doi: 10.1073/pnas.131077798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Peleg S, et al. Altered histone acetylation is associated with age-dependent memory impairment in mice. Science. 2010;328:753–756. doi: 10.1126/science.1186088. [DOI] [PubMed] [Google Scholar]
- 50.Kouzarides T. Chromatin modifications and their function. Cell. 2007;128:693–705. doi: 10.1016/j.cell.2007.02.005. [DOI] [PubMed] [Google Scholar]
- 51.Fousteri M, van HA, Vargova H, Mullenders LHF. Repair of DNA lesions in chromosomal DNA. DNA Repair. 2005;4:919–925. doi: 10.1016/j.dnarep.2005.04.011. [DOI] [PubMed] [Google Scholar]
- 52.Vidanes GM, Bonilla CY, Toczyski DP. Complicated tails: Histone modifications and the DNA damage response. Cell. 2005;121:973–976. doi: 10.1016/j.cell.2005.06.013. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.