Abstract
Cross-sectional estimates of age-related changes in brain structure and function were compared with 6-y longitudinal estimates. The results indicated increased sensitivity of the longitudinal approach as well as qualitative differences. Critically, the cross-sectional analyses were suggestive of age-related frontal overrecruitment, whereas the longitudinal analyses revealed frontal underrecruitment with advancing age. The cross-sectional observation of overrecruitment reflected a select elderly sample. However, when followed over time, this sample showed reduced frontal recruitment. These findings dispute inferences of true age changes on the basis of age differences, hence challenging some contemporary models of neurocognitive aging, and demonstrate age-related decline in frontal brain volume as well as functional response.
Keywords: attrition, frontal lobe, multimodal, reorganization
Aging is associated with declining cognitive functioning (1–4) and changes in brain structure (5–8) and neurotransmission (9). In addition, functional neuroimaging studies have consistently revealed age-related alterations in task-induced brain activity (10, 11). Relative to younger adults, older adults tend to underrecruit occipital regions during cognitive task performance (12), and age-related differences are frequently observed in frontal cortex (12–14). In frontal cortex, the direction of differences has not only been age-related underrecruitment but overrecruitment (i.e., selective or stronger regional activity in older compared with younger adults).
Age-related overrecruitment has attracted much interest because it has been interpreted to reflect a potential for functional reorganization in the aging brain (15, 16), possibly in response to structural changes and accompanying cognitive decline (14). The fact that past demonstrations of frontal overrecruitment in aging were based on cross-sectional comparisons prevents strong conclusions, however. It is well documented that cross-sectional estimates of age-related cognitive decline can deviate from estimates based on longitudinal designs (17, 18). Similarly, comparison of cross-sectional and longitudinal analyses of structural brain integrity in aging revealed that longitudinal measures of shrinkage exceeded cross-sectional estimates (5).
Here, we report cross-sectional and longitudinal estimates of structural brain changes and age-related alterations in task-induced brain activity as measured by functional MRI (fMRI). Healthy adults ranging in age from 49–79 y performed a semantic categorization task that has been associated with frontal overrecruitment in aging in cross-sectional comparisons (19). Six years later, our participants underwent a second MRI session. Cross-sectional and longitudinal estimates were compared to assess whether they converged on similar patterns of age-related differences.
Results
Structural Imaging.
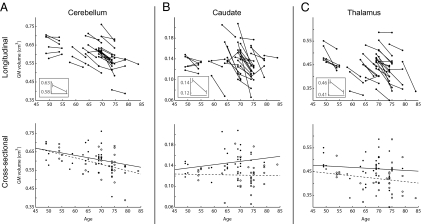
The longitudinal analysis of gray matter (GM) changes revealed marked volume reductions in several regions (Table S1), including the caudate and cerebellum. In addition, significant GM reduction was observed in the thalamus and in temporal and frontal cortices. The corresponding cross-sectional analyses did not show any significant age-related differences at the statistical level used in the longitudinal analysis. At a more liberal threshold, the results partially converged with the longitudinal findings by revealing age-related GM reduction in the frontotemporal cortex and cerebellum (Table S2), but there was no or weak support for reductions in the caudate and thalamus (Fig. 1).
Fig. 1.
Structural brain changes. (Upper Row) Longitudinal changes for (A) cerebellum, (B) caudate nucleus, and (C) thalamus. Each line represents gray matter volume for one participant at time points 1 and 2 for voxels that showed significant longitudinal change at the group level (see Table S1 for cluster size, MNI coordinates, and T values for each region); Insets show mean and SE. (Lower Row) Corresponding results from the cross-sectional analyses; solid circles and lines indicate time point 1, empty circles and dashed lines indicate time point 2. The cross-sectional results at time points 1 and 2 converged with the longitudinal findings in the cerebellum (r's = −0.32, −0.46) but not in the thalamus (r's = 0.21, −0.02) and the caudate (r's = −0.09, −0.17).
Longitudinal analysis of white matter (WM) changes revealed a distributed pattern of shrinkage. The strongest effects were seen in anterior, middle, and posterior parts of the corpus callosum, and additional effects were seen in the frontal, temporal, and parietal lobes (Table S3). The analyses of cross-sectional data revealed weaker and nonsignificant effects even at a more liberal uncorrected statistical threshold.
Functional Imaging.
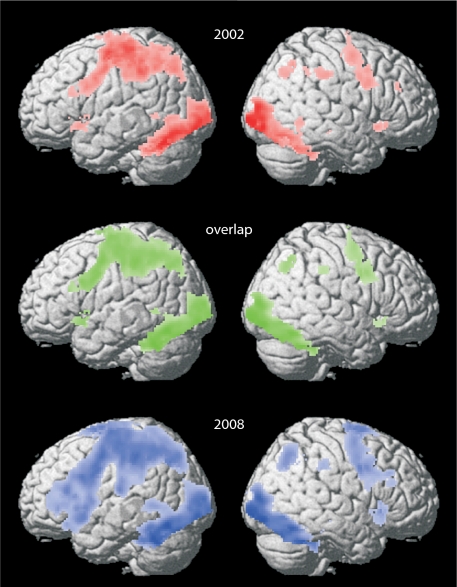
Analysis of brain regions associated with task performance showed highly overlapping results for both the 2002 and 2008 fMRI sessions (n = 38; Fig. 2). In keeping with previous findings (20), the categorization task was associated with increased blood oxygen level-dependent (BOLD) signal in a left hemisphere network that included the prefrontal cortex. In addition, several homologous sites in the right hemisphere were engaged, including right prefrontal regions (Fig. 2 and Table S4).
Fig. 2.
Brain activity for the categorization task during the first (Top, red) and second (Bottom, blue) time point. An extensive network including frontal regions were similarly activated during both time points (Middle, overlap in brain activity patterns across time points in green). Statistical threshold; P < 0.05 FWE corrected.
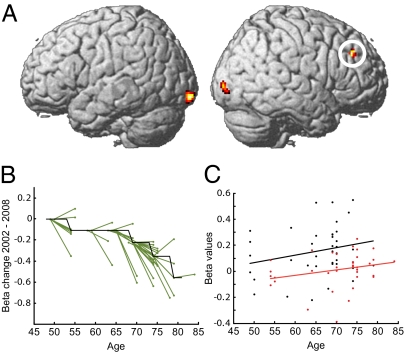
Longitudinal within-person comparisons of changes in BOLD signal revealed that a subset of the task-relevant regions displayed in Fig. 2 showed a time-related reduction (n = 38; Fig. 3A). This set of regions included areas in the occipital cortex and right prefrontal cortex. A plot of within-person changes in the right prefrontal cortex is presented in Fig. 3B. No longitudinal increases in BOLD signal were observed in the prefrontal cortex (time-related increases are reported in Table S5).
Fig. 3.
(A) Longitudinal changes in brain activity. The longitudinal analysis showed decreased activity in frontal (xyz = 26 38 30, t = 5.43) and occipital (xyz = −16 −100 −10, t = 5.09; xyz = 32 −90 −4, t = 5.07) cortex. (B) Differences in frontal cortex activity (beta values from the regression analysis) between time points plotted as a function of age (data from circled region in A). Black line, β values normalized to the median value for each preceding time bin, first time bin median set to 0; green lines, normalized β values for each individual at time points 1 and 2. The reduction of frontal activity between time points accelerated with increasing age. (C) Cross-sectional data for the same region as revealed by the longitudinal analysis showed the reversed pattern of increased brain activity as a function of age. Black dots and regression line from time point 1 (r = 0.24); red from time point 2 (r = 0.27).
A cross-sectional analysis of brain activity across all participants (n = 60) at the first test session revealed significant positive correlations with age in several brain regions, including the right frontal cortex (Fig. S1). Similarly, cross-sectional assessment of the age effect in the right frontal region in which longitudinal underrecruitment was found revealed trends toward overrecruitment at both the 2002 and 2008 sessions (n = 38; Fig. 3C). Thus, the cross-sectional estimates of age-related frontal changes deviated from the longitudinal estimate. Furthermore, at the 2002 session, increasing age was related to occipital underrecruitment (n = 38; Fig. S2).
Reconciling Cross-Sectional and Longitudinal Results.
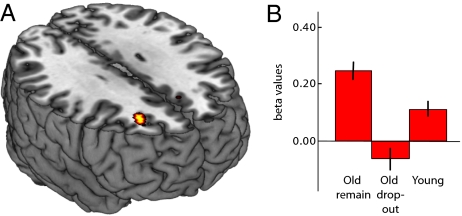
In view of the longitudinal finding of age-related frontal underrecruitment, a critical question is why overrecruitment was observed cross-sectionally. One possibility is that the elderly subjects who successfully completed two imaging sessions (and had been part of the longitudinal study for 20 y) constituted a high-performing subsample. Previous cross-sectional studies have shown frontal overrecruitment in high-performing but not low-performing elderly individuals (21). We tested this possibility by comparing older adults (>60 y at the first session; n = 28) who participated in both sessions with older adults who only took part in the first session (n = 16). Elderly adults who remained in the study had significantly higher episodic memory performance than those who dropped out (17, 22, 23) (Table S6) and also significantly higher right frontal activity at the first MRI session (Fig. 4A). In addition, the cross-sectional finding of age-related overrecruitment of the right frontal cortex (Fig. 3C and Fig. S1) was driven by the elderly subsample that took part in both fMRI sessions (Fig. 4B).
Fig. 4.
(A) Cross-sectional right frontal (xyz = 26 34 28) overrecruitment in high-functioning elderly. (B) Bar graph (mean and SE) of the right frontal response for subgroups of the sample. Elderly participants (>60 y) who stayed in the study across both sessions (Old remain) showed increased right-frontal activity at session 1 relative to age-matched participants who dropped out at session 2 (Old drop-out) and also relative to younger participants (Young, participants <60 y at session 1).
Longitudinal Structure–Function Fusion.
In a final set of analyses, functional and structural longitudinal data were integrated using the fusion independent component analysis (ICA) toolbox (24). Of chief interest was to examine whether the observation of diminished frontal cortex functioning could be accounted for by age-related local or distal changes in brain GM. Twenty components were estimated from the fused data; however, only one component turned out to be significantly different (P < 0.01) between the two time points and reflected time-related changes for both functional and structural data. Reduced functional activity was observed in several areas (Table S7), including the frontal and occipital regions that were identified in the univariate analyses. Structural GM change was observed in an extensive set of regions (Table S7). Critically, the regions showing atrophy included a right frontal region that overlapped with the area where significant functional decline was observed (Fig. S3).
Discussion
Taken together, the cross-sectional and longitudinal estimates of age-related changes in brain structure and functional activity converged in some ways, including age-related BOLD reductions in the occipital cortex (12) and GM reductions in the cerebellum and frontotemporal cortex (5, 25). Substantial differences were also revealed, notably in the direction of increased sensitivity of the longitudinal analytical approach. In addition, and most critically, the longitudinal analysis revealed an age-related activity reduction in the dorsal frontal cortex, whereas the cross-sectional analyses indicated overrecruitment in the same frontal region.
We obtained support that the inclusion of a relatively high-functioning elderly sample biased the cross-sectional results in the direction of frontal overrecruitment. However, when these older adults were followed longitudinally, a reduction in the frontal BOLD response was observed, suggesting that aging is associated with underrecruitment rather than overrecruitment of frontal cortex during cognitive tasks. Such a reduction could be related to basic working memory (26, 27) and attentional/executive processes, such as the ability to maintain the relevant task context (28), but more definite interpretation requires additional examination with a broader range of cognitive tasks.
The multivariate structure–function fusion analysis revealed functional and structural changes in several regions, including those observed in the univariate analyses, thus supporting the view of time-related activity reductions in the right frontal and occipital cortex. Importantly, in line with previous findings on age differences in attention (29), preliminary support was provided that age-related frontal atrophy accounted, at least in part, for the diminished frontal response, whereas the occipital functional change did not relate to any local structural changes in the presently examined age range.
Our findings add to previous demonstrations of discrepancies between results derived from longitudinal compared with cross-sectional designs. Discrepancies may be in the direction of either earlier or later onset of significant age-related decline. Here, as in previous imaging studies (5), the longitudinal approach was more sensitive in detecting brain changes, whereas past longitudinal analysis of cognitive changes indicated later onset of episodic memory decline (17). In the latter study, cohort differences in educational attainment accounted for the cross-sectional impression of early onset of cognitive decline. In addition to cohort differences, discrepancies between longitudinal and cross-sectional data may be driven by effects of attrition and practice on the longitudinal data. Given that the elderly adults who dropped out demonstrated underrecruited right frontal cortex already at baseline, it is unlikely that selective attrition accounted for the longitudinal observation of age-related frontal underrecruitment. Practice or, more generally, test–retest effects can have a substantial impact on cognition (17) and functional brain activity (20). Regional brain activity can be reduced by practice, which might translate into an apparent age effect in a longitudinal study. Here, however, we found no evidence that practice, holding age constant, influenced the level of right frontal activity (Fig. S4).
In conclusion, most if not all existing evidence that frontal cortex is overrecruited in advanced age is based on cross-sectional data, but the present set of results challenges inferences of true age changes based on analyses of age differences. Thus, our demonstration of age-related underrecruitment rather than overrecruitment of the frontal cortex has implications for contemporary theories of neurocognitive aging.
Methods
Participants.
All subjects were part of the BETULA Prospective Cohort Study on memory, health, and aging (2). BETULA is an ongoing longitudinal study containing cognitive and medical data for nearly 4,500 subjects. From the parent sample, 60 cognitively intact subjects (49–79 y of age, mean and SD: 66.0 ± 8.1 y, 37 women) were recruited for the baseline study in 2002 (30). Because the initial study concerned genetic variation in relation to brain function, as many as 50% of the participants were carriers of at least one ApoE ε4 allele. All subjects were right-handed and native Swedish speakers with no neurological or psychiatric problems. They were also nondemented, scored ≥24 on the Mini-Mental State Examination (31), and reported normal or corrected to normal vision. All participants were paid for their participation, and signed the informed consent form, which was in accordance with guidelines of the Swedish Council for Research in the Humanities and Social Sciences.
Of the 60 subjects who participated in the baseline study, 38 completed the 2008 follow-up study (55–84 y of age, mean and SD: 71.0 ± 8.1 y, 63% of the initial subject pool, 55% were carriers of at least one ApoE ε4 allele). The excluded participants ranged in age from 56 to 80 (73.1 ± 7.7) y. Reasons for exclusion included death (4 subjects, 18.2%); dementia (2 subjects, 9.1%); and serious illness (5 subjects, 22.7%), including stroke, blindness, pacemaker operation, and severe back pain. One additional subject was excluded from some follow-up analyses because of a contaminated structural image. The episodic memory performance (based on a cluster of tasks; cf. ref. 30) of the participants who completed both test sessions was compared with normative data (1), and all subjects except 1 performed within or above 1 SD of the mean of age-matched controls.
Analyses of behavioral data acquired outside the scanning room showed a weak trend toward a decline in episodic and semantic memory (Table S6). During scanning, on both test sessions, the participants performed the categorization task at a high level of accuracy and with little change in response times across the two assessments (Table S6).
A comparison (restricted to subjects older than 60 y at baseline) of those who remained in the study (mean age = 69.3 y, n = 29) and those who dropped out (mean age = 71.5 y, n = 16) revealed that those who dropped out performed worse on the episodic memory tasks [t(43) = 1.66, P = 0.05 (one-tailed)] and also on the postscan recognition memory test [t(41) = 2.79, P < 0.01 (one-tailed)] (Table S6), which is in line with previous demonstrations of attrition bias (17, 22, 23).
MRI Protocol.
All images at both baseline and follow-up were collected on the same 1.5-T Philips Intra scanner (Philips Medical System) with identical pulse sequences (regular scanner upgrades were done between the 2002 and 2008 scans). Functional T2*-weighted images (33 slices with a slice thickness of 3.9 mm) were acquired with a single-shot gradient echo planar imaging sequence [repetition time = 3,000 ms, echo time = 50 ms, field of view (FOV) = 22 × 22 cm2, flip angle (FA) = 90°, pixel size = 3.44] used for assessment of BOLD contrast. Five dummy scans were performed preceding image acquisition to avoid signal saturation effects. Structural high-resolution T1-weighted images (124 slices, 1.8-mm thickness, FOV = 18 × 18 cm2, FA = 35°) were collected following the functional images.
Stimuli and Behavioral Procedure.
Full details of the experimental design are given by Lind et al. (30). In short, a semantic categorization task was administrated and fMRI was conducted to measure brain responses. Subjects categorized each of 160 words in a word list as either abstract or concrete. One half of the words (80 words) were familiar to the subjects because they had made abstract/concrete decisions twice preceding functional scanning: the first time outside the scanner and the second time 15–20 min later with shifted word order inside the scanner while the structural data were collected. During fMRI scanning, a blocked-task paradigm that included the experimental (categorization) condition (30 s) and a baseline (fixation) condition (21 s) was used. During categorization, subjects pressed one of the two buttons to give responses using the right index and middle fingers, whereas they viewed a cross-hair constantly displayed on the center of the screen during fixation. Four runs, each starting and ending with a brief fixation block (12 s), were administered. Each run consisted of four categorization blocks (per run) containing 10 words each: either 10 familiar (presented twice before) or 10 previously unseen words. Finally, a postscan yes/no recognition test was conducted 15–20 min after the scanning sessions during which the participants indicated whether a presented word was previously unseen or previously studied. The experimental design remained intact at follow-up, except that the postscan recognition test was partly performed inside the scanner.
MRI Data Analysis.
During both sessions (2002 and 2008), functional images were analyzed using Statistical Parametric Mapping software (SPM8; Wellcome Department of Cognitive Neurology, University College London, London, United Kingdom). All images were first corrected for slice timing to correct for acquisition time differences between slices. The slice-timing corrected images were then rigidly aligned to the first image volume to correct for a subject's movement. Finally, a within-subject rigid registration was conducted to align functional and structural images (structural MRI 2002/2008 to fMRI 2002/2008) together. Hence, the following extracted flow fields obtained through diffeomorphic anatomical registration using exponentiated lie algebra (DARTEL; a full description of the DARTEL procedure is presented in SI Text), which are computed from each structural datum, can be applied to corresponding fMRI data in the next stage (i.e., stage iv). (To integrate functional and structural data using a multimodal image analysis technique, it is necessary to align fMRI maps accurately with T1-weighted images normalized using DARTEL). The realigned images were simultaneously warped, subject by subject, to the DARTEL template using subject-specific flow fields computed from corresponding structural data using DARTEL (SI Text), normalized into a common Montreal Neurological Institute (MNI) space as defined by SPM8 (using normalize to MNI function), and finally smoothed using an 8.0-mm full-width at half-maximum Gaussian filter. Voxel-wise general linear models were set up for each subject to generate contrast images. Each condition was modeled as a box-car that was convolved with the hemodynamic response function, whereas the baseline condition was implicitly modeled. Contrast images for each subject at each session were taken into a second level paired t test to delineate intersubject variability (e.g., longitudinal changes) across the two time points (2002 and 2008). Local maxima with P < 0.0001 (uncorrected), with an extent threshold of 20 contiguous voxels (k ≥ 20), were considered to be statistically significant. For the cross-sectional analyses, one-sample t tests were implemented with age as a covariate of interest using a more liberal threshold of P < 0.001 (uncorrected). For the main effect of task, which included only those participants who stayed in the study for both test sessions, a threshold of 0.05 family-wise error (FWE)-corrected (k ≥ 20) was used.
In the structural voxel-based morphometry analyses of GM and WM images, a supervised protocol was conducted to process T1-weighted MRI scans using SPM8. The supervised protocol proceeded in several stages (details provided in SI Text). In short, the T1-weighted images were segmented, and each GM/WM segment was nonlinearly aligned to subject/group-specific templates and normalized to MNI using DARTEL, followed by smoothing. For the longitudinal analysis, voxel-wise paired t tests were carried out to test for longitudinal GM/WM differences. Additionally, to adjust for intergender variation in brain volume, total intracranial volume for each subject was computed by summing the GM, WM, and cerebrospinal fluid segments of the modulated warped images and set as a covariate of no interest. To control for multiple comparisons, P values were thresholded at P < 0.005 (false discovery rate correction) at the voxel level and P < 0.05 (FWE) at the cluster level. For the cross-sectional analyses, one-sample t tests with age as a covariate of interest were implemented for each time point with a more liberal threshold of P < 0.001 (uncorrected).
Structure–Function Fusion.
Joint ICA, fusion ICA, was used to analyze the BOLD signal and GM volume jointly. A statistical parametric mapping contrast image for semantic encoding and a GM segmentation image for each time point served as input for fusion ICA (detailed description of the whole procedure conducted in fusion ICA is provided in SI Text). As output, maximally independent components, including both structural and functional features, were considered to be significant if and only if they showed differences between the two time points at P < 0.01. Regional effects (Table S7) contributing to each feature of the significant components were converted to Z values and considered reliable at |z| > 3.5.
Supplementary Material
Acknowledgments
We thank the staff of the BETULA Project, R. Adolfsson, and the staff at the Umeå Center for Functional Brain Imaging. This study was supported by the Göran Gustafsson Award in Medicine (to L.N.), a grant from the Swedish Science Council (to L.N.), and a Wallenberg Scholar Grant from the Knut and Alice Wallenberg Foundation (to L.N.). The BETULA Project is supported by a grant from the Swedish Science Council (to L.-G.N. and L.N.).
Footnotes
The authors declare no conflict of interest.
*This Direct Submission article had a prearranged editor.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1012651108/-/DCSupplemental.
References
- 1.Nilsson L-G, et al. Betula: A prospective cohort study on memory, health and aging. Aging, Neuropsychology, and Cognition. 2004;11:134–148. [Google Scholar]
- 2.Nilsson L, et al. The Betula Prospective Cohort Study: Memory, health, and aging. Aging, Neuropsychology, and Cognition. 1997;4:1–32. [Google Scholar]
- 3.Schaie KW. The course of adult intellectual development. Am Psychol. 1994;49:304–313. doi: 10.1037//0003-066x.49.4.304. [DOI] [PubMed] [Google Scholar]
- 4.Verhaeghen P, Salthouse TA. Meta-analyses of age-cognition relations in adulthood: Estimates of linear and nonlinear age effects and structural models. Psychol Bull. 1997;122:231–249. doi: 10.1037/0033-2909.122.3.231. [DOI] [PubMed] [Google Scholar]
- 5.Raz N, et al. Regional brain changes in aging healthy adults: General trends, individual differences and modifiers. Cereb Cortex. 2005;15:1676–1689. doi: 10.1093/cercor/bhi044. [DOI] [PubMed] [Google Scholar]
- 6.Head D, et al. Differential vulnerability of anterior white matter in nondemented aging with minimal acceleration in dementia of the Alzheimer type: Evidence from diffusion tensor imaging. Cereb Cortex. 2004;14:410–423. doi: 10.1093/cercor/bhh003. [DOI] [PubMed] [Google Scholar]
- 7.Good CD, et al. Cerebral asymmetry and the effects of sex and handedness on brain structure: A voxel-based morphometric analysis of 465 normal adult human brains. NeuroImage. 2001;14:685–700. doi: 10.1006/nimg.2001.0857. [DOI] [PubMed] [Google Scholar]
- 8.Sullivan EV, Pfefferbaum A. Diffusion tensor imaging and aging. Neurosci Biobehav Rev. 2006;30:749–761. doi: 10.1016/j.neubiorev.2006.06.002. [DOI] [PubMed] [Google Scholar]
- 9.Bäckman L, Nyberg L, Lindenberger U, Li SC, Farde L. The correlative triad among aging, dopamine, and cognition: Current status and future prospects. Neurosci Biobehav Rev. 2006;30:791–807. doi: 10.1016/j.neubiorev.2006.06.005. [DOI] [PubMed] [Google Scholar]
- 10.Spreng RN, Wojtowicz M, Grady CL. Reliable differences in brain activity between young and old adults: A quantitative meta-analysis across multiple cognitive domains. Neurosci Biobehav Rev. 2010;34:1178–1194. doi: 10.1016/j.neubiorev.2010.01.009. [DOI] [PubMed] [Google Scholar]
- 11.Persson J, Nyberg L. Altered brain activity in healthy seniors: What does it mean? Prog Brain Res. 2006;157:45–56. doi: 10.1016/s0079-6123(06)57004-9. [DOI] [PubMed] [Google Scholar]
- 12.Davis SW, Dennis NA, Daselaar SM, Fleck MS, Cabeza R. Que PASA? The posterior-anterior shift in aging. Cereb Cortex. 2008;18:1201–1209. doi: 10.1093/cercor/bhm155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rajah MN, D'Esposito M. Region-specific changes in prefrontal function with age: A review of PET and fMRI studies on working and episodic memory. Brain. 2005;128:1964–1983. doi: 10.1093/brain/awh608. [DOI] [PubMed] [Google Scholar]
- 14.Persson J, et al. Structure-function correlates of cognitive decline in aging. Cereb Cortex. 2006;16:907–915. doi: 10.1093/cercor/bhj036. [DOI] [PubMed] [Google Scholar]
- 15.Cabeza R. Hemispheric asymmetry reduction in older adults: The HAROLD model. Psychol Aging. 2002;17:85–100. doi: 10.1037//0882-7974.17.1.85. [DOI] [PubMed] [Google Scholar]
- 16.Park DC, Reuter-Lorenz P. The adaptive brain: Aging and neurocognitive scaffolding. Annu Rev Psychol. 2009;60:173–196. doi: 10.1146/annurev.psych.59.103006.093656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rönnlund M, Nyberg L, Bäckman L, Nilsson LG. Stability, growth, and decline in adult life span development of declarative memory: Cross-sectional and longitudinal data from a population-based study. Psychol Aging. 2005;20:3–18. doi: 10.1037/0882-7974.20.1.3. [DOI] [PubMed] [Google Scholar]
- 18.Schaie KW. Intellectual Development in Adulthood: The Seattle Longitudinal Study. New York: Cambridge Univ Press; 1996. [Google Scholar]
- 19.Logan JM, Sanders AL, Snyder AZ, Morris JC, Buckner RL. Under-recruitment and nonselective recruitment: Dissociable neural mechanisms associated with aging. Neuron. 2002;33:827–840. doi: 10.1016/s0896-6273(02)00612-8. [DOI] [PubMed] [Google Scholar]
- 20.Wagner AD, Koutstaal W, Maril A, Schacter DL, Buckner RL. Task-specific repetition priming in left inferior prefrontal cortex. Cereb Cortex. 2000;10:1176–1184. doi: 10.1093/cercor/10.12.1176. [DOI] [PubMed] [Google Scholar]
- 21.Cabeza R, Anderson ND, Locantore JK, McIntosh AR. Aging gracefully: Compensatory brain activity in high-performing older adults. NeuroImage. 2002;17:1394–1402. doi: 10.1006/nimg.2002.1280. [DOI] [PubMed] [Google Scholar]
- 22.Baltes PB, Staudinger UM, Lindenberger U. Lifespan psychology: Theory and application to intellectual functioning. Annu Rev Psychol. 1999;50:471–507. doi: 10.1146/annurev.psych.50.1.471. [DOI] [PubMed] [Google Scholar]
- 23.Van Beijsterveldt CE, et al. Predictors of attrition in a longitudinal cognitive aging study: The Maastricht Aging Study (MAAS) J Clin Epidemiol. 2002;55:216–223. doi: 10.1016/s0895-4356(01)00473-5. [DOI] [PubMed] [Google Scholar]
- 24.Calhoun VD, et al. Method for multimodal analysis of independent source differences in schizophrenia: Combining gray matter structural and auditory oddball functional data. Hum Brain Mapp. 2006;27:47–62. doi: 10.1002/hbm.20166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fjell AM, et al. One-year brain atrophy evident in healthy aging. J Neurosci. 2009;29:15223–15231. doi: 10.1523/JNEUROSCI.3252-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nagel IE, et al. Performance level modulates adult age differences in brain activation during spatial working memory. Proc Natl Acad Sci USA. 2009;106:22552–22557. doi: 10.1073/pnas.0908238106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rypma B, D'Esposito M. Isolating the neural mechanisms of age-related changes in human working memory. Nat Neurosci. 2000;3:509–515. doi: 10.1038/74889. [DOI] [PubMed] [Google Scholar]
- 28.Milham MP, et al. Attentional control in the aging brain: Insights from an fMRI study of the stroop task. Brain Cogn. 2002;49:277–296. doi: 10.1006/brcg.2001.1501. [DOI] [PubMed] [Google Scholar]
- 29.Thomsen T, et al. Brain localization of attentional control in different age groups by combining functional and structural MRI. NeuroImage. 2004;22:912–919. doi: 10.1016/j.neuroimage.2004.02.015. [DOI] [PubMed] [Google Scholar]
- 30.Lind J, et al. Reduced functional brain activity response in cognitively intact apolipoprotein E epsilon4 carriers. Brain. 2006;129:1240–1248. doi: 10.1093/brain/awl054. [DOI] [PubMed] [Google Scholar]
- 31.Folstein MF, Folstein SE, McHugh PR. “Mini-mental state.” A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.