Abstract
Atmospheric oxygen (O2) is estimated to have varied greatly throughout Earth’s history and has been capable of influencing wildfire activity wherever fuel and ignition sources were present. Fires consume huge quantities of biomass in all ecosystems and play an important role in biogeochemical cycles. This means that understanding the influence of O2 on past fire activity has far-reaching consequences for the evolution of life and Earth’s biodiversity over geological timescales. We have used a strong electrical ignition source to ignite smoldering fires, and we measured their self-sustaining propagation in atmospheres of different oxygen concentrations. These data have been used to build a model that we use to estimate the baseline intrinsic flammability of Earth’s ecosystems according to variations in O2 over the past 350 million years (Ma). Our aim is to highlight times in Earth’s history when fire has been capable of influencing the Earth system. We reveal that fire activity would be greatly suppressed below 18.5% O2, entirely switched off below 16% O2, and rapidly enhanced between 19–22% O2. We show that fire activity and, therefore, its influence on the Earth system would have been high during the Carboniferous (350–300 Ma) and Cretaceous (145–65 Ma) periods; intermediate in the Permian (299–251 Ma), Late Triassic (285–201 Ma), and Jurassic (201–145 Ma) periods; and surprisingly low to lacking in the Early–Middle Triassic period between 250–240 Ma. These baseline variations in Earth’s flammability must be factored into our understanding of past vegetation, biodiversity, evolution, and biogeochemical cycles.
Keywords: forest fire, paleofire, fire history, deep time
Fire-prone ecosystems cover 40% of the Earth’s present land surface (1). Several of the world’s major biomes are strongly influenced by fire (2) (grasslands, savannas, Mediterranean shrubland, and boreal forests) where fire is considered to halve the Earth’s potential modern forest cover by preventing the growth of climax vegetation (2). By influencing terrestrial vegetation, fires significantly alter the flux of key nutrients that drive primary productivity. The frequency of fire is fundamentally influenced by the concentration of oxygen in the atmosphere, where an increase in O2 above the present-day level of 20.9% would make our planet significantly more flammable (3, 4). If past O2 levels were higher than the present levels, then vegetation biomass ought to have been drastically reduced by increased fire frequency (3, 4). Conversely, periods of lower O2 should suppress fire frequency, allowing the biomass of terrestrial vegetation to increase. Variations in the biomass of terrestrial vegetation have significant implications for global biodiversity, ecology, and biogeochemical cycles. It is, therefore, important to understand how variations in past O2 have controlled fire activity throughout Earth’s history.
Several studies have estimated O2 concentrations during Earth’s past. These studies reveal periods of both super- and subambient O2 (5–7) and, in some cases, superlow O2 (< 15% O2) (8). Such estimates point to periods in Earth’s history when fire activity could have been significantly enhanced, suppressed, or even entirely switched off. The close-knit relationship between O2 concentration and fire means that it is essential to understand what minimum value of O2 limits combustion in order to estimate whether or not fire has ever been switched off during times of low O2 in Earth’s past. Yet, despite much research on both fire and O2 over the past 30 y, it has not been possible to make estimates of the probability of burning or the potential for fires to start and spread throughout Earth’s history. This is largely because (a) there is a lack of appropriate data relating oxygen concentration to ignition and spread of natural fires and (b) while many modern forest-fire models exist, all are dependent on parameters that cannot yet be measured for the past.
The effect of O2 concentration upon the flammability of materials has been a subject of study for many decades. Many materials have been tested for their flammability in different atmospheres, ranging from gases, liquids, and polymers through to fabrics and natural plant-based materials (9–14). Belcher and McElwain’s (14) experiments revealed that a minimum of 15% O2 was required to initiate short-lived combustion in natural plant-based materials. However, for fire to spread in the natural environment it requires not only successful ignition but also self-sustaining combustion (not measured in ref. 14). This is because lightning strikes (the most common ignition source of wildfires) last for only an instant, meaning that a fire has to be ignited and become self-sustaining rapidly to enable it to spread in the natural world. It is also important to gather data on smoldering, as opposed to flaming, fires (15) because these two types of fire have different behaviors (15, 16) and because lightning strikes typically ignite smoldering fires (that may later lead to flaming fires) (17, 18). Smoldering fire is a slow, low-temperature, flameless form of combustion, which is the most persistent type of combustion. Biomass capable of sustaining such fires are trunks, litter, duff, humus, peat, coal seams, and soils with a significant organic fraction. Once ignited, such fires are difficult to extinguish (despite extensive rains or weather changes), can persist for long periods of time (years), and can spread over extensive areas of forest subsurface (19). An important difference between smoldering and flaming fires in the context of this work is that smoldering fires can be initiated with much weaker ignition sources than flaming fires (15). This means that in order to understand the ignition and spread of natural fires throughout Earth’s history, the level of O2 required for self-sustaining smoldering fires must be assessed.
Models designed to assess modern forest-fire ignition and spread are difficult to apply when estimating how ancient variations in O2 have controlled fire activity. For example, cellular automata models used to predict forest-fire spread usually rely on knowledge of meteorological conditions as well as the specifics of the terrain; e.g., slope (20). Such models include the rate of spread, the shape of the forest-fire front, fuel type, humidity, wind speed and direction, topography, fuel continuity, and distribution of fire brands (20, 21). These parameters are not only hard to estimate in deep time but also vary spatially and temporally such that it is not possible to estimate all such parameters on a global scale. Moreover, modern forest-fire models do not consider the influence of variations in O2. Therefore, flammability estimates are needed that consider O2 as the most important control on fire once fuel and an ignition source are provided. Development of a global-perspective estimate of potential fire activity based on estimated past O2 is required to assess the baseline intrinsic flammability of Earth’s ecosystems throughout geological time.
We have used a strong electrical ignition source (similar to the conditions reached after a lightning strike) to start smoldering fires, and we measured their self-sustaining propagation in a large-scale, realistic, low-oxygen atmosphere. The spread rate of smoldering combustion was measured using thermocouples, which tracked the movements of the exothermic reaction. Pure sphagnum moss peat was used as the fuel because it is highly flammable, easily ignitable, and burns in modern natural fires (15, 16). We accept that peat does not represent the global range of fuel available for fires, which has changed throughout the evolutionary history of terrestrial ecosystems, but represents in dry conditions one of the most easily ignitable naturally occurring fuels on the planet. Moreover, early buildups of plant debris in the current form of coal reveal that peat has a long geological history back to ∼400 Ma (22). Peat fires take place across all modern climate zones such that climate effects imposed on plant ecosystems can be considered less important. We therefore believe that smoldering peat represents the best experimental fuel available toward creating a global estimate of Earth’s baseline intrinsic flammability over the past 350 Ma.
We have used our experimental fire propagation data to fit linear relationships between fire spread rate vs. O2 and burn duration vs. O2. These relationships are used to parameterize a simple fire “invasion” (spread) model for a given concentration of O2. The model outputs an estimate of the proportion of area burned for a given concentration of O2. This output was then used to assess potential variations in fire activity throughout Earth’s history according to published estimates of O2 for the past 350 Ma (5, 6). The aim being to highlight times in Earth’s history when fire has been able to contribute to disruption of ecosystems and influence biogeochemical cycles, and to answer the question: Has fire ever been switched off?
Results and Discussion
Our experimental burns provided three key pieces of information that we have used to inform our model: (a) the lower limit of atmospheric oxygen required to allow a self-sustaining smoldering fire to occur, (b) the spread rate of a shallow smoldering fire in different O2 concentrations, and (c) the length of time that such a fire takes to burn a superficial layer (burn duration).
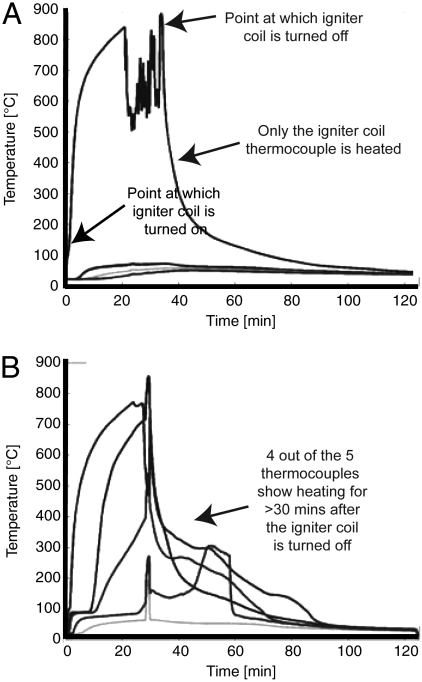
Fig. 1 shows the thermocouple traces for two experimental burns: one for 15% O2 (Fig. 1A) and the other for 16% O2 (Fig. 1B). The thermocouple positioned closest to the igniter (Fig. S1) measured the initial time when power was supplied to the coil, marked by a sharp rise in temperature, and the time when it was shut down (after 30 min), marked by a sudden fall in temperature. A failed ignition is characterized by a drop of all thermocouple readings as soon as the coil is turned off, with no thermocouple ever exceeding 200 °C. A successful ignition is characterized by a sustained smoldering front above 200 °C for a significant period after the igniter coil was shut down. These temperature criteria agree well with the detailed experimental measurements at ambient O2 in the same setup and with a similar peat in ref. 16. Temperature data from the thermocouples was used to quantify smoldering fire spread rates and burn durations under different O2 concentrations.
Fig. 1.
Thermocouple traces from the self-sustaining combustion experiments. (A) An example from 15% O2 and (B) an example from 16% O2. Each line on each graph represents the trace for a different thermocouple.
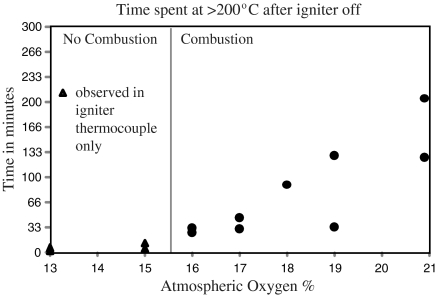
Fig. 2 shows the time the samples spent at > 200 °C after the igniter coil was shut down. At 13 and 15% O2, temperatures in excess of 200 °C were only observed for a very brief period in the thermocouple nearest the igniter. This represents heat retained by the igniter following shutdown. Above 15% O2, an increased duration of the time spent at 200 °C was observed in nonigniter thermocouples, revealing a self-sustaining smoldering front moving away from the igniter. This is highlighted in Fig. S1, which shows no self-sustaining front at 15% O2 but at 17% O2 shows high temperatures are sustained for up to 1 h after the igniter coil was shut down. These results reveal that self-sustaining smoldering combustion does not occur below 16% O2 and that we can assume, therefore, that if O2 has ever been lower than 16% in Earth’s history, then fire must have been switched off.
Fig. 2.
Time spent at > 200 °C after the igniter was turned off, showing self-sustained combustion occurring only at > 16% O2.
Temperatures in excess of 200 °C are maintained for at least 1 h after igniter coil shutdown at 17% O2, whereas temperatures fall rapidly following igniter coil shutdown at 15% O2 (Fig 2). A linear regression of the length of time (in minutes) that the samples burned for (burn duration, tburn) against oxygen concentration gives tburn = 26( ± 5)O2 - 380( ± 100) (Fig. S2) (where the numbers in brackets indicate the standard error).
The mass lost by the samples also reveals that self-sustaining combustion is only apparent from 16% O2 and above (Fig. S3A), although propagation appears to remain limited, and is only located around the igniter between 16–17% O2, with a sharp increase in mass loss being observed at 18% O2 and above. Peak and mean temperatures for the first 30 min after igniter coil shutdown reveal the same pattern, with high temperatures continuing in the peat above 16% O2 (Fig. S3 B and C). Moreover, peak and mean temperature of the peat appears to increase with increasing O2 (Fig. S3C). Spread rates were calculated using the time taken for the smolder front to propagate between thermocouples. This suggests that spread rate (SR, in units of mm/ min) increases with increasing O2 according to the equation SR = 0.11( ± 0.006)O2 - 0.15( ± 0.11) (Fig. S2B).
Estimation of the Prevalence of Fires over the Past 350 Ma.
Our model simulates a smoldering fire as ignited from a localized external source, which spreads through a finite amount of fuel (e.g., as in our experimental burns). We fit the model to our experimental data on spread rate and burn duration and use the model’s results for the proportion of area burned as a proxy of fire activity (Fig. 3). The two driving parameters in the model are the probability of local fire spread β and the probability of local fire extinction μ. Maximum and minimum values of β and μ are defined so that the range of model outputs across this parameter range easily includes the range of observed experimental results. We have randomly selected values of β and μ from within their range, run the model, and recorded the burn duration, spread rate, and proportion of available area that is burned from the simulation. This is repeated 106 times, to ensure that the whole (β, μ) parameter space is sampled. We then use the linear regression models (Fig. S2) to predict the burn duration and spread rate for an O2 concentration, and select all simulations whose results lay within 20% of these predictions (SI Methods). Finally, the distribution of fire activity/burn probability for a given O2 concentration is calculated from the selected model results.
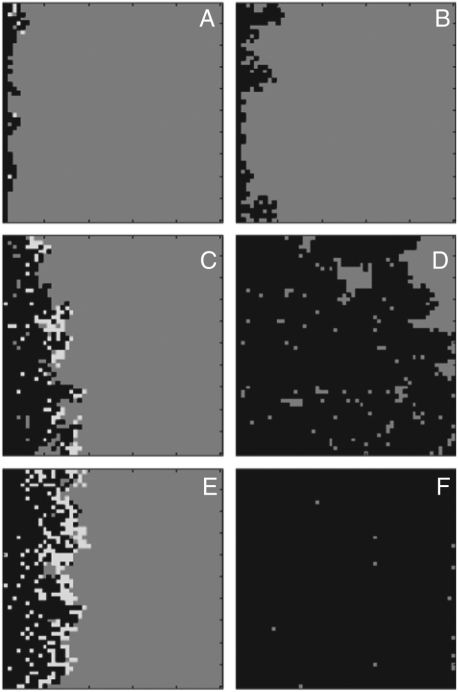
Fig. 3.
Examples of model output for three parameter sets. A and B roughly correspond to 18% O2 with β = 0.01 and μ = 0.04. C and D correspond to 21% O2 with β = 0.022 and μ = 0.04. E and F correspond to 24% O2 with β = 0.022 and μ = 0.02. A, C, and E show the model state after 150 time steps, and B, D, and F show the state at the end of the simulation. Gray squares are in the available-to-burn state, white squares are in the burning state, and black squares are in the burned-out state.
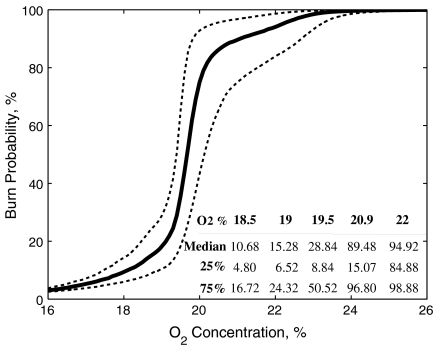
Fig. 4 shows the sigmoidal curve output from the model, which predicts the probability of an area being burned for different levels of O2. This reveals that the probability of an ignited fire spreading to neighboring cells (pixels in the model), and therefore the probability of a self-sustaining wildfire, is strongly dependent on the concentration of O2. From our experimental data we see that 16% O2 is the minimum that allows self-sustaining combustion; however, the model shows that such levels of atmospheric oxygen will allow < 10% of the area in the model to be ignited and burned. Between 18.5 and 19.5% O2, the probability of encountering burned cells begins to increase (see Fig. 3 and table inlay in Fig. 4). At 20.9% O2, there is ∼90% chance of encountering a burned area; in other words, 90% of the total area available has the potential to have been ignited and burned (see also Fig. 3). Above 20.9% O2, the increase slows and plateaus at 22% O2, where unburned regions become very rare (Figs. 3 and 4). This rapid transition is not an artifact of the model. It is supported not only by our own experimental data but is also consistent with findings of refs. 10 and 11, which showed a rapid rise in “ignition component” between 19–22% O2 (of paper at 10% moisture) and that this plateaued there after (Fig. S4A). We note that the linear increase in smoldering fire spread rates with O2, which we have been using in part to drive the model, are also consistent with refs. 10 and 12 (Fig. S4B) and the idea that a linear approximation can be drawn for fire frequency and its dependence on O2 (4). Moreover, if the model were more complex we would not expect different behavior because the model output has been seen to be relatively insensitive to changes to the spread rate and burn duration dependence in Fig S2. The sigmoidal behavior of the model has its roots in a phase transition that occurs at ∼20% O2. Below this threshold the fire rarely spreads across the entire arena, whereas above this threshold the fire spreads throughout the arena but leaves some regions of fuel unburned (resulting in the slower increase in burn probability as O2 increases). Such phase transitions are shared by related models (e.g., 21, 23, and 24). We therefore believe that the qualitative relationship between O2 and burn probability from the model is robust.
Fig. 4.
Model output of the probability of burning according to O2 concentration. Inlaid table shows the median probability of burning (%) and the 75 and 25% quartiles for O2 concentrations 18.5–22%.
Our model reveals that extensive wildfires may only be possible in Earth’s natural system above atmospheric oxygen levels of 19% O2. We highlight that this likely does not represent an overestimate because the data used to drive the model is based on (a) the most persistent type of fire (smoldering), and (b) a highly flammable fuel at very low moisture levels (∼15% dry weight).
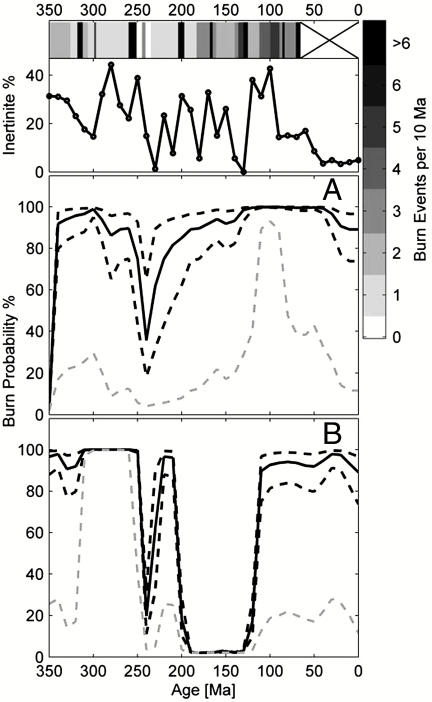
We have used the modeled relationship between burn probability and O2 to estimate fire activity over the past 350 Ma of Earth’s history. We have used two different published models of paleoatmospheric O2 (5, 6) as the record of O2 throughout this time. Fig. 5 shows the two output scenarios of fire activity over the past 350 Ma of Earth’s history. Output A uses O2 estimates from ref. 5, and output B uses ref. 6. We also include a qualitative record of fire activity using the palaeofire indicator data presented in refs. 14 and 25, and also an estimate of fire frequency from inertinite (charcoal) in coal/peats from ref. 7. Table 1 compares outputs A and B. Overall both outputs of fire activity appear to be broadly supported by the known record of fossil fires. The most striking feature of both outputs on Fig. 5 and in Table 1 is the very low fire activity between 250–240 Ma (median < 30%, lower 95% quantile < 5% estimated burn probability). This appears to be supported by the general lack of fossil fire evidence at this time and, in particular, two periods with no evidence of fossil fire. This is followed by a period of low yet rising fire activity (240–235 Ma) and is consistent with the relatively large amount of evidence of fossil fires. A major discrepancy occurs between the two output scenarios between 200–125 Ma, where output A estimates high levels of fire activity (> 80% burn probability) and output B estimates very low fire activity (0% burn probability). Output A appears best supported, whereas output B cannot be supported during this period based on the occurrences of fire in the fossil fire record. Both outputs also diverge at ∼350 Ma, where the evidence of fossil fires suggests that output B is better supported than output A. Both outputs estimate high fire activity in the Carboniferous. The Carboniferous period has abundant evidence of fossil fires from the tropical Euramerican mire systems (26). Moisture contents were likely to have been high in these tropical mires, and it is noted that fires are relatively rare in modern tropical forests (27). It is suggested that higher levels of O2 (5, 6) likely facilitated the spread and abundance of fires in these moist ecosystems (26). Both outputs appear to support the interpretation that paleoatmospheric O2 concentrations would have allowed for a high probability of large areas being burned. Both outputs estimate high fire activity during the Cretaceous. This time in Earth’s history also reveals the greatest number of literature reports of fossil fire evidence, supporting both output scenarios.
Fig. 5.
Two estimates of the probability of burning (%) throughout Earth’s history. Output A uses O2 estimates from ref. 5, and output B uses O2 estimates from ref. 6. Solid black line shows the median estimate, dark dashed line shows the interquartile range, and the light dashed line shows the lower 95% quartile. Grayscale band at the top of the plots is an estimate of fire activity expressed as number of burn events per 10 Ma, based on fossil fire indicators published in refs. 14 (250–65 Ma) and 25 (350–250 Ma). Top graph is the record of inertinite (fire) from ref. 7.
Table 1.
Comparison of the modeled outputs highlighting periods where the outputs agree/disagree and whether or not the fossil record of fires supports the outputs of the model
| Age, Ma |
Output A probability of burning |
Output B probability of burning |
Agreement between A and B? |
||
| Median, % | Lowest limit, 95% quartile | Median, % | Lowest limit, 95% quartile | ||
| 350–340 | 0* | 0* | 97‡ | 35† | No |
| 340–300 | > 95‡ | 20–30‡ | > 90‡ | 15–100‡ | Yes |
| 300–250 | > 90‡ | 15–30‡ | 100‡ | 100–40‡ | Yes |
| 250–240 | Falling ∼60–35‡ | < 10‡ | Falling 60–20‡ | 30–5‡ | Yes |
| 240–235 | Rising 30–50‡ | ∼5‡ | Rising 20–50‡ | ∼5‡ | Yes |
| 235–200 | Rising 50–85‡ | < 10* | 50–100 followed by sudden fall at 205 Ma to < 5* | 30– < 5* | First phase,Yes; second phase, No |
| 200–145 | Rising 85–95‡ | 10–25‡ | Prolonged < 5* | < 5* | No |
| 145–65 | 95–100‡ | > 95‡ | Rapid rise to 95‡ | 20–30* | Yes |
We accept that estimates of palaeofire indicators are not exact. This will be influenced by (a) collector effort, where certain geological periods will tend to have had increased focus on their study; (b) available terrestrial sediment outcrop; and (c) the prevalence of sediment types able to preserve fossil charcoals and fire indicators. We note, however, that it is well documented that fossil fire evidence is poor in the Early Triassic (250–240 Ma) (26). This includes the well-studied Molteno Formation, which yields an abundant fossil flora from numerous sedimentary environments but no charcoal (26, 28), and the petrified forest in Arizona, where charcoal is very rare (26). These highlight that this period, in particular, is well supported in literature as lacking in evidence of fire.
The Fire Window.
The term “fire window” (29) has been used to describe the limits of O2 necessary to support natural fires since the evolution of land plants. Our experimental data constrains the absolute lower limit of this window such that the minimum amount of O2 needed to ignite and maintain a self-sustaining wildfire is 16%. This is in contrast with previous estimates of 12% O2 (12). Our experimental data shows categorically that 16% O2 is required to allow ignition and self-sustaining combustion of dry natural fuel. Our model reveals that at O2 levels below 18.5%, fire activity will be very low (< 10% burn probability). Calculations of ignition in present-day levels of atmospheric oxygen suggest that fuel near the water saturation point (∼40% H2O) has a very low probability of ignition (10). Our model is based on data from fuels with ∼15% moisture (dry weight). We therefore strongly suggest that the lower limit of the fire window for the occurrence of moderately sized fires is more likely to be 18.5% O2. This implies that only vegetation in areas receiving very low rainfall and/or those that were seasonally very dry would have had a chance of ignition at O2 concentrations < 18% and that self-sustained combustion and burning could only occur across small areas.
Our data cannot indicate the upper limit of the fire window. At 22% O2, our model estimates that there is a very high probability of large areas being burned (> 90% burn probability). Therefore, at O2 concentrations > 22%, the amount of area burned would likely remain similar. The effects of high O2 (> 22%) most likely alter the importance of fire in wet ecosystems (e.g., the tropics, wetlands, and peatlands), as has been predicted for the Carboniferous (26). However, our model reveals that fossil charcoal abundance is likely to be a weak predictor of O2 concentrations above 22% O2 because of the strong sigmoidal relationship between O2 and fire (Fig. 4). Therefore, quantitative reconstructions of O2 from charcoal abundance (e.g., ref. 7) likely overestimate O2 concentrations. There is as yet no means to quantify fire return times in increased O2, although fire return times of every 1–5 y are estimated assuming 35% O2 (30). It is estimated that fires would reduce Earth’s current vegetation by ∼20% if O2 concentration rose to 35% (4). It has been suggested that periods of high O2 would only be possible if the Earth became much wetter (4). Otherwise, fires would pose a serious threat to the existence of any land vegetation because terrestrial ecosystems would be subject to continued conflagrations. This interpretation appears broadly consistent with the output from our model.
Fire Feedbacks to the Earth System.
The significance of fire is underrecognized within the Earth system despite the fact that fire is the most ubiquitous natural terrestrial disturbance. Fires influence ocean and land primary productivity by influencing the biogeochemical cycling of phosphorous (P). Enhanced soil erosion following loss of ground cover and erosion of P-rich ashes, produced from burning vegetation, unlock P from its terrestrial stores and release it into the ocean (31). Ocean primary productivity is limited primarily by nitrogen (N) and P (32). Organic material is transferred to the ocean floor on the death of marine algae. Not all the P and N in the organic material is released back to the ocean. Some material remains organic-bound and will eventually be buried and inaccessible in the ocean sediments. The ocean can be replenished with N from the atmosphere (32); however, P must be delivered from the land via riverine influx (33). This makes P the limiting nutrient of marine primary productivity. The role of fire in this land-ocean-atmosphere–driven system is to release terrestrially locked-up P into a bioavailable form.
There is a net loss of P from the land and a net gain of P in the oceans of the order of 560 Gg P y-1. Fires influence the supply of P via two routes: (a) aerosol inputs to the atmosphere via smoke and (b) increased weathering and influx of ash into rivers. Atmospheric deposition is the most important source of P for open ocean sites, whereas riverine flux/runoff dominates nearshore P fluxes. Wildfires are a major source of P to the atmosphere (0.25 Tg y-1) and can be shown to relate to fire season (34). Moreover, airborne ash particles have been shown to be important in increasing the nutrient content (including P) in surface waters (35). The whole Amazon Basin appears to be losing P to the atmosphere, and 23% of this flux can be attributed to fire (36). This P is being deposited in adjacent oceans and other regions downwind (36).
Our model (Fig. 4) reveals a sharp tipping point in the effect of O2 concentration on fire activity. The combination of our model with our experimental burns suggest that fire feedbacks to biogeochemical cycles would be greatly suppressed below 18.5% O2 and entirely switched off below 16% O2. Feedbacks would be rapidly enhanced between 19–22% O2 and would then depend on variations in fire return times thereafter. Fire-induced P fluxes would be high during the Carboniferous and Cretaceous periods; intermediate in the Permian, Late Triassic, and Jurassic; and low to lacking in the Early Middle Triassic. We note, however, that a negative feedback to fire-based P fluxes is expected at high levels of O2 (possible in parts of the Carboniferous and Cretaceous). Earth’s highest fire frequencies under current levels of O2 are mainly in equatorial dry areas and seasonally dry climates. If O2 significantly increased (> 25%), this would allow much larger areas of land to burn (even swamp and wet areas) so that fire frequencies would be increased in both tropical rainforest and arctic tundra. Such an increase in fire is expected to create large P-limited areas of land, which will decrease terrestrial net primary productivity (NPP) and ultimately decrease the flux of P into the ocean. The current human-induced increase in fire in the Amazon highlights this idea well, where in net terms 1.3 mg P m-2 y-1 is leaving the Amazon via the atmosphere because of fires. It is calculated that this is equivalent to the P required to sustain current levels of NPP for the Amazon for over 350 y (36). Human-induced fire activity mimics the effect of high O2 concentration and highlights the negative feedback that very high fire frequencies would have on terrestrial NPP.
Conclusions
Given a fuel and ignition source, O2 is the primary control on fire. Without O2, fires cannot exist. Our combustion experiments reveal that an absolute minimum of 16% O2 is required to allow self-sustaining, smoldering combustion in dry, highly flammable natural fuels. It is more likely that 18.5% O2 is required for the propagation of significant fires in a natural system. Using these data, we are able to produce estimates of fire activity throughout the past 350 Ma. Our model suggests that > 18.5% O2 is required to allow significant areas to be burned and that below 18.5% O2 less than 10% of a given area will likely be burned. Between 19 and 22% O2, the probability of burning rises rapidly to ∼90% and thereafter plateaus to 100%. It seems that there are tipping points on the oxygen scale that control the probability of large areas being burned. The rapidity of change between these two tipping points suggests that relatively small changes in O2 from the current ambient (20.9%) have the potential to bring about significant changes in burned areas. Using estimates of O2 for the past 350 Ma, we suggest that fires have had the potential to play a significant role in biogeochemical feedbacks throughout much of this time. Fire feedbacks may, however, have been greatly suppressed between 250 and 240 Ma, where all our output scenarios estimate a period of very low fire activity. It is important that fire be considered more fully as a driving force in studies of the Earth system. Moreover, the evolutionary and ecological significance of effectively switching off fire should be considered in future interpretations of the history of life on Earth.
Methods
Combustion Experiments.
All experiments were undertaken in the University College Dublin Péac facility within a controlled atmosphere walk-in chamber (Conviron BDW60) (see ref. 14). Pure sphagnum moss peat was chosen as a suitable fuel because its homogeneous thermal properties allowed moisture contents and packing ratios to be readily controlled between experiments. An experimental apparatus based on ref. 16 was used to perform the combustion experiments. Approximately 100 g of peat was placed in an insulated metal container 10 × 10 × 10 cm. The peat was approximately 5 cm in depth. The peat was not compressed, so as to maintain a low packing ratio and encourage the fires to spread. The peat was dried to ∼15% moisture (mean dry weight) to make it easily ignitable [natural peat is easily combustible at 115% moisture (dry weight) (16)]. Fuel moistures of 20% are common for fires in leaf litters (4). Therefore, we have tried to replicate the most flammable scenario with the easiest fire to start in the natural environment.
Natural fires are most commonly ignited by lightning strike; hence, the peat samples were ignited using a strong electrical ignition source. An electric coil of spiral diameter 10 mm and length 95 mm was placed at one end of the box at the base of the peat (Fig. S1). The coil was supplied with 100 W of electric power for a 30-min duration and then switched off. Smoldering combustion and propagation of the combustion front was measured using thermocouples. Five thermocouples were placed within the peat sample to a depth of 4 cm, with one placed directly next to the igniter coil itself (Fig. S1). The thermocouple next to the igniter measured the temperature of the peat surrounding the coil, and the other thermocouples measured the propagation of the self-sustained combustion front (via tracking changes in temperature). The thermocouple temperature data was recorded every 5 s by data loggers until the end of each experiment (2–5 h). Thermal-sequenced images and black-and-white videos were taken throughout the duration of the burns using an FLIR Systems S series ThermaCam and network web cameras. Combustion experiments were run in 13–20.9% O2 and in duplicate for each O2 level.
Fire Invasion Model.
We have used a cellular automata model for the invasion of a smoldering fire into a homogeneous arena of unburned fuel (mimicking the experimental conditions). This model is related to other discrete models of fire spread (20, 37) as well as to the lattice gas cellular automata of a susceptible-infected-recovered–type epidemic (38). The model considers a rectangular grid of 50 × 50 sites, which corresponds to an area 10 × 10 cm (replicating the area used in our experimental burns) and has a time step corresponding to 6 s. Each site can be in one of three states: (a) available to burn, (b) burning, and (c) burned out. The model has two parameters, β and μ. Fire spreads from a burning site to neighboring sites with probability β per time step (SI Methods). Each burning site has a probability μ per time step of becoming burned out, so the time that a site stays in the burning state follows a geometric distribution with mean 1/μ. Once a site is in the burned-out state, it never leaves this state.
We performed 106 simulations of this model with randomly selected values of β and μ from the ranges 0.005–0.05 and 0.01–0.2, respectively. This range gives model behavior that encompasses all the experimentally observed spread rates and burn durations. From each simulation we recorded the time taken for the fire to completely burn out (burn duration), the rate of spread of the fire across the area available, and the final proportion of sites burned. Using the experimentally derived relationships of O2 vs. spread rate and O2 vs. burn duration, we selected all simulations that lay within 20% of the predicted spread rate and burn duration for a given O2 concentration. The distribution of fire activity at this O2 concentration was then obtained from the proportions of sites burned for these selected simulations. Full details of the model can be found in SI Methods and Figs. S5–S8.
Supplementary Material
Acknowledgments.
We thank Matthew Haworth for his assistance while working in the Péac facility and two anonymous reviewers who materially improved the manuscript. C.M.B. and J.C.M. acknowledge funding through a European Union Marie Curie Excellence Grant (MEXT-CT-2006-042531). R.M.H. acknowledges funding through Engineering and Physical Sciences Research Council and International Fire Investigators and Consultants Ltd.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1011974107/-/DCSupplemental.
References
- 1.Chapin FS, Matson PA, Mooney HA. Principles of Terrestrial Ecosystem Ecology. New York: Springer; 2002. [Google Scholar]
- 2.Bond WJ, Wooward FI, Midgley GF. The global distribution of ecosystems in a world without fire. New Phytol. 2005;165:525–537. doi: 10.1111/j.1469-8137.2004.01252.x. [DOI] [PubMed] [Google Scholar]
- 3.Lovelock JE, Lodge JP. Oxygen in the contemporary atmosphere. Atmos Environ. 1972;6:575–578. [Google Scholar]
- 4.Lenton TM, Watson AJ. Redfield revisted 2. What regulates the oxygen content of the atmosphere? Global Biogeochem Cy. 2000;14:249–268. [Google Scholar]
- 5.Bergmann NM, Lenton TM, Watson AJ. COPSE: A new model of biogeochemical cycling over phanerozoic time. Am J Sci. 2004;304:397–437. [Google Scholar]
- 6.Berner RA. Phanerozoic atmospheric oxygen: New results using the GEOCARBSULF model. Am J Sci. 2009;309:603–606. [Google Scholar]
- 7.Glasspool IJ, Scott AC. Phanerozoic concentrations of atmospheric oxygen reconstructed from sedimentary charcoal. Nat Geosci. 2010;3:627–630. [Google Scholar]
- 8.Falkowski PG, et al. The rise of oxygen over the past 205 million years and evolution of large placental mammals. Science. 2005;309:2202–2204. doi: 10.1126/science.1116047. [DOI] [PubMed] [Google Scholar]
- 9.Lovelock JE, Margulis L. Atmospheric homeostasis by and for the biosphere: The Gaia hypothesis. Tellus. 1974;26:2–10. [Google Scholar]
- 10.Watson AJ. Reading, UK: University of Reading; 1978. Consequences for the biosphere of forest and grassland fires. PhD thesis. [Google Scholar]
- 11.Watson A, Lovelock JE, Margulis L. Methanogenesis, fires and the regulation of atmospheric oxygen. Biosystems. 1978;10:293–298. doi: 10.1016/0303-2647(78)90012-6. [DOI] [PubMed] [Google Scholar]
- 12.Wildman RA, et al. Burning of forest materials under late Paleozoic high atmospheric oxygen levels. Geology. 2004;32:457–460. [Google Scholar]
- 13.Xin Y, Khan MM. Flammability of combustible materials in reduced oxygen environment. Fire Safety J. 2007;42:536–547. [Google Scholar]
- 14.Belcher CM, McElwain JC. Limits for combustion in low O2 redefine paleoatmospheric predictions for the Mesozoic. Science. 2008;321:1197–1200. doi: 10.1126/science.1160978. [DOI] [PubMed] [Google Scholar]
- 15.Rein G. Smouldering combustion phenomena in science and technology. Int Rev Chem Eng. 2009;1:3–18. [Google Scholar]
- 16.Rein G, Cleaver N, Ashton C, Pironi P, Torero JL. The severity of smouldering peat fires and damage to the forest soil. Catena. 2008;74:304–309. [Google Scholar]
- 17.Drysdale D. An Introduction to Fire Dynamics. West Sussex, UK: Wiley–Blackwell; 1998. [Google Scholar]
- 18.Stott P. Combustion in tropical biomass fires: A critical review. Prog Phys Geog. 2000;24:355–377. [Google Scholar]
- 19.Mayer W. Geological clerics and Christian geologists. In: Kölbl-Ebert M, editor. Geology and Religion: A History of Harmony and Hostility. Vol. 310. London: Geol Soc London; 2009. pp. 197–209. [Google Scholar]
- 20.Alexandridis A, Vakalis D, Siettos CI, Bafas GV. A cellular automata model for forest fire spread prediction: The case of the wildfire that swept through Spetses Island in 1990. Appl Math Comput. 2008;204:191–201. [Google Scholar]
- 21.Encinas AH, Encinas LH, White SH, del Rey AM, Sanchez GR. Simulation of forest fire fronts using cellular automata. Adv Eng Softw. 2007;38:372–378. [Google Scholar]
- 22.Lapo AV, Druzdova IN. Phyterals of humic coals in the USSR. Int J Coal Geol. 1989;12:477–510. [Google Scholar]
- 23.Zekri N, Porterie B, Clerc J-P, Loraud J-C. Propagation in a two-dimensional weighted local small-world network. Phys Rev E. 2005;71:046121. doi: 10.1103/PhysRevE.71.046121. [DOI] [PubMed] [Google Scholar]
- 24.Grimmett G. Percolation. 2nd Ed. New York: Springer–Verlag; 1999. [Google Scholar]
- 25.Scott AC, Glasspool IJ. The diversification of paleozoic fire systems and fluctuations in atmospheric oxygen concentration. Proc Natl Acad Sci USA. 2006;103:10861–10865. doi: 10.1073/pnas.0604090103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Scott AC. The pre-Quaternary history of fire. Palaeogeogr Palaeocl. 2000;164:281–329. [Google Scholar]
- 27.Pyne SJ, Andrews PL, Laven RD. Introduction to Wildland Fire. New York: Wiley; 1996. [Google Scholar]
- 28.Anderson JM, Andreson HM, Cruickshank ARI. Late Triassic ecosystems of the Molteno/Lower Elliot biome of Southern Africa. Palaeontology. 1998;41:387–421. [Google Scholar]
- 29.Jones TP, Chaloner WG. Fossil charcoal, its recognition and palaeoatmospheric significance. Palaeogeogr Palaeocl. 1991;97:39–50. [Google Scholar]
- 30.Beerling DJ, Woodward FI, Lomas MR, Quack WP, Valdes PJ. The influence of Carboniferous palaeoatmospheres on plant function: An experimental and modelling assessment. Philos T R Soc B. 1998;353:131–139. [Google Scholar]
- 31.Kump LR. Terrestrial feedback in atmospheric oxygen regulation by fire and phosphorus. Nature. 1988;355:152–154. [Google Scholar]
- 32.Kump LR, McKenzie FT. Regulation of atmospheric O2 feedback in the microbial feedbag. Science. 1996;271:459–460. [Google Scholar]
- 33.Lenton TM. The role of land plants, phosphorus weathering and fire in the rise and regulation of atmospheric oxygen. Glob Change Biol. 2001;7:613–629. [Google Scholar]
- 34.Mahowald N, et al. Global distribution of atmospheric phosphorus sources, concentrations and deposition rates, and anthropogenic impacts. Glob Biogeochem Cy. 2008;22:1–19. [Google Scholar]
- 35.Spencer CN, Hauer FR. Phosphorus and nitrogen dynamics in streams during a wildfire. J N Am Benthol Soc. 1991;10:24–30. [Google Scholar]
- 36.Mahowald N, et al. Impact of biomass burning emissions and land use change on Amazonian atmospheric cycling and deposition of phosphorus. Glob Biogeochem Cy. 2005;19:1–15. [Google Scholar]
- 37.Karafyllidis I, Thanailakis A. A model for predicting forest fire spread using cellular automata. Ecol Model. 1997;99:87–97. [Google Scholar]
- 38.Fuks H, Lawniczak AT. Individual-based lattice model for the spatial spread of epidemics. Discrete Dyn Nat Soc. 2001;6:191–200. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.