Abstract
Border cell migration is a stereotyped migration occurring during the development of the Drosophila egg chamber. During this process, a cluster composed of six to eight follicle cells migrates between nurse cells toward the oocyte. Receptor tyrosine kinases (RTKs) are enriched at the leading edge of the follicle cells and establish the directionality of their migration. Endocytosis has been shown to play a role in the maintenance of this polarization; however, the mechanisms involved are largely unknown. In this study, we show that border cell migration requires the function of the small GTPases Rab5 and Rab11 that regulate trafficking through the early and the recycling endosome, respectively. Expression of a dominant negative form of rab11 induces a loss of the polarization of RTK activity, which correlates with a severe migration phenotype. In addition, we demonstrate that the exocyst component Sec15 is distributed in structures that are polarized during the migration process in a Rab11-dependent manner and that the down-regulation of different subunits of the exocyst also affects migration. Together, our data demonstrate a fundamental role for a plasma membrane–endosome trafficking cycle in the maintenance of active RTK at the leading edge of border cells during their migration.
Keywords: vesicular trafficking
During the development of multicellular organisms, complex cell migration events take place to organize the general body shape, tissues, and organs. Migration can be of different types and natures: Cells can migrate alone or in groups, and these groups can adopt different shapes and organizations (1). Border cell migration in the Drosophila melanogaster egg chamber is a potent model system to study the directed migration of a cluster of cells through a complex tissue (1, 2).
Directed migration implies that cells are able to integrate an extracellular signal that forms a gradient along the length of the cell. Migrating cells need to convert a subtle gradient into a robust intracellular polarization, which might be achieved through feedback loops (2). During border cell migration, the extracellular signals are ligands of receptor tyrosine kinases (RTK). The receptors on which they act are the epidermal growth factor receptor (EGFR) and PVR, which is the sole Drosophila homolog of the platelet-derived growth factor receptor and vascular endothelial growth factor receptor (3).
Endocytosis is the general name given to the process by which cells uptake extracellular material and plasma membrane proteins and lipids inside the cell (4). The endocytic pathway is composed of the different compartments reached successively by endocytosed material packed into vesicles at the plasma membrane. The first compartment reached by these vesicles is called the early or sorting endosome. From that compartment, proteins are sorted to different routes. Proteins can be sent to the degradative pathway composed of late endosomes and lysosomes. They can also be targeted back to the plasma membrane, either directly or via the slow recycling route through the recycling endosome compartment (5, 6). Different lines of evidence point to a fundamental role for endocytosis during cell migration (7). Indeed, key proteins involved in migration are regulated by the endocytic pathway, including cell attachment proteins, such as cadherins (8) and integrins (9), as well as guidance receptors during directed migration (10).
Trafficking between the different stations of the endocytic pathway is controlled by specific small GTPases of the Rab family (11). Rab5 regulates transport through the early endosome, Rab4 through the rapid recycling pathway, Rab11 through the recycling endosome, and Rab7 to late endosomes. Furthermore, another endosomal Rab protein has been recently characterized in Drosophila and was named RabX4 (12). Small GTPases can be locked into an active form or an inactive form by a single-amino-acid change. In the latter case, the Rab mutants have a dominant negative effect as they trap guanine nucleotide exchange factors (GEF) and thereby maintain their wild-type counterpart in an inactive form. Thus, expressing a dominant negative Rab causes a loss of function of this particular Rab protein and eventually blocks the transport step regulated by this Rab.
By using such dominant negative Rab proteins, we identified Rab5 and Rab11 as necessary for border cell migration. We focused our analysis on the role of Rab11 and of the recycling endosome in this process and found that this transport step is key for the maintenance of RTK activity at the leading edge of migrating cells. The exocyst is a complex of eight proteins involved in the delivery of material to the plasma membrane (13, 14). We found that the Sec15 subunit is polarized during cell migration. Because Sec15 is a Rab11 binding protein (15, 16), we tested further the relationship between these two proteins. We found that Sec15 polarity is controlled by rab11 activity and that sec15 and other subunits of the exocyst are necessary for the spatial restriction of RTK activity and for border cell migration.
Altogether, we show the requirement of a trafficking loop between the plasma membrane and the recycling endosome for collective cell migration in vivo. This loop regulates the spatial restriction of active RTK at the leading edge of the cell cluster, transforming the extracellular gradient into a robust intracellular polarity.
Results
Trafficking Through the Early Endosome and the Recycling Endosome Is Necessary for Border Cell Migration.
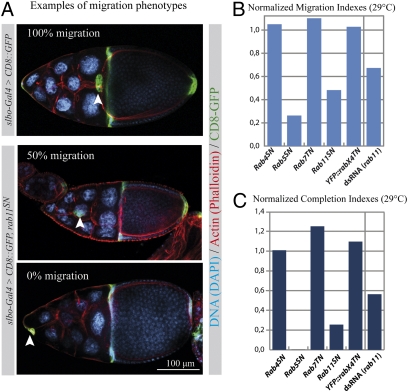
To determine the involvement of different endocytic compartments in border cell migration, we measured the distance traveled in the egg chamber by border cell clusters expressing dominant negative forms of Rab proteins (Fig. 1A and Fig. S1A). First, five endosomal Rab proteins (YFP::rab4SN, YFP::rab5SN, YFP::rab7TN, YFP::rab11SN, and YFP::rabX4TN) from the Hugo Bellen collection (12) were expressed in the border cell cluster. YFP::rab5SN was used as a positive control, because the rab5 regulator sprint was previously shown to be involved in border cell migration (10). Similar results were obtained using untagged versions of the dominant negative Rab proteins (Fig. S1A).
Fig. 1.
Expression of dominant negative forms of Rab5 and Rab11 blocks border cell migration. (A) Representative examples of border cell migration in a control stage 10 egg chamber (Top) and when border cells express rab11SN (Middle and Bottom). Border cells are additionally expressing CD8::GFP (green) and are stained with phalloidin (red) and DAPI (blue). The border cell clusters are indicated by arrowheads. Anterior is left and posterior is right. The percentage of migration compared with the total migration distance is indicated. (B and C) Normalized migration and completion indexes after expression of different dominant negative Rab proteins in border cells or after down-regulation of rab11 by dsRNA (26 < n < 105). Indexes were normalized to c306-Gal4 [dsRNA(rab11)]; slbo-Gal4 (YFP::rabX4TN); or slbo-Gal4, UAS CD8::GFP.
To confirm the involvement of rab11 during border cell migration, we down-regulated rab11 in the border cell clusters by double-stranded (ds)RNA expression. As for the dominant negative construct, a strong impairment of migration was observed (Fig. S1A). Trafficking through the Rab11-positive recycling endosome is thus necessary for proper border cell migration.
Comparison between different migration phenotypes is not easy because the process can be affected in many different ways. We introduce here indexes to quantify the strength of the phenotypes. First, the “migration index” (M.I.) is calculated by giving a proportional weight to the distance reached by the cluster in different egg chambers (SI Materials and Methods). This M.I. approximates the mean distance reached by border cell clusters for a specific genotype. Furthermore, this value has the advantage that it can be normalized (nM.I.) to a proper control, for example slbo-Gal4 (Fig. S1B and Fig. 1B). In addition, we measured the percentage of clusters having completed migration and named that value “completion index” (C.I.). This value can also be normalized (nC.I.) to the appropriate control (Fig. S1C and Fig. 1C). Both the nM.I. and the nC.I. confirmed that trafficking through the Rab5/early endosome and the Rab11/recycling endosome is critical for proper border cell migration.
Jak/STAT signaling is necessary for the specification and migration of the border cells (17). We verified that the migration phenotype resulting from impaired trafficking in the recycling endosome is not due to abnormal Jak/STAT signaling. For this, we determined if STAT is properly localized in the nucleus of border cells and furthermore whether its direct target, Slbo, is normally expressed. In both cases, we observed no differences between control border cells and when migration is impaired due to rab11SN expression (Fig. S1D). Thus, we conclude that the expression of dominant negative rab11 affects border cell migration independently of Jak/STAT signaling.
Trafficking Through the Recycling Endosome Regulates the Polarization of Active Receptor Tyrosine Kinases.
Previous work by the group of Pernille Rørth showed that sprint, a regulator of Rab5, plays a role in maintaining active RTKs at the leading edge of border cell clusters (10). Among the possible roles of sprint and rab5, a polarized recycling of receptors, or of a factor necessary to maintain the receptors at the leading edge, was proposed. Trafficking through the recycling endosome could thus be a station of such a transport cycle. Furthermore, other proteins involved in cell migration, in particular the adhesion proteins E-Cadherin (8) and Integrins (9), are known to be recycled. We tested the localization of these proteins in control and rab11SN-expressing border cells at the onset of migration (Fig. 2A). The distribution of E-Cadherin was similar to what was previously described (18): We saw an accumulation between polar cells, which was stronger at the leading edge than at the trailing edge. No difference between control and rab11SN-expressing border cells could be observed.
Fig. 2.
Rab11 regulates the spatial distribution of active RTKs and genetically interacts with their downstream target Rac1. (A) Representative images showing the distribution of β-Integrin, E-Cadherin, and pTyr (red) at the onset of migration in stage 9 control egg chambers and when rab11SN is expressed in border cells. Border cells are additionally expressing CD8::GFP (green) and are stained with DAPI (blue). A grayscale image of the red channel is shown for every image. A dashed line delimits the border cell cluster in the grayscale image. Anterior is left and posterior is right. (B) Quantification of the ratio of the mean fluorescent signal of CD8::GFP, E-Cadherin, β-Integrin, and pTyr determined in an area of the leading edge [F(P)] divided by the signal at the trailing edge [F(A), SI Materials and Methods] in control clusters (blue bars) and when rab11SN is expressed (red bars). Error bars are SEM (13 < n < 50; ***P < 0.005, Student's t test). (C) Normalized migration indexes for the indicated conditions. Hatched bars represent the difference between the expected indexes if the phenotypes were only additive and the experimental indexes. Indexes were normalized to slbo-gal4. Error bars are SEM (three individual experiments, total number of egg chambers: 92 < n < 247).
Although Integrins are dispensable for border cell migration (19), they are involved in many types of migration and are regulated by endocytosis and recycling (9). β-Integrin is the only Integrin that is expressed in border cells during migration (20). We observed a weak heterogeneous signal for β-Integrin. Again no difference between control and rab11SN-expressing border cells was observed (Fig. 2A).
Then, we assessed the distribution of phosphorylated Tyrosine (pTyr) that was previously used as a marker of RTK activity in border cells (10). As previously published, we saw a pronounced polarization of the signal with more pTyr at the leading edge compared with the trailing edge (Fig. 2A). The pattern of pTyr is similar to that of E-Cadherin, but is not restricted to the polar cells. However, in contrast to E-Cadherin, pTyr localization was changed after expression of rab11SN: Its polarization was completely abolished, suggesting that rab11 is controlling the spatial distribution of active RTK.
To confirm our observation, we quantified fluorescence intensity in an area of the plasma membrane at the leading edge and at the trailing edge of the border cell cluster (see SI Materials and Methods for details). We calculated the ratio of the leading edge signal [F(P)] divided by the trailing edge signal [F(A)] for the different proteins of interest (Fig. 2B, blue bars). We expected the CD8::GFP signal to be equally distributed at the plasma membrane of the border cell cluster. Indeed, for CD8::GFP, we obtained values close to 1 [F(P)/F(A) = 1.03 ± 0.04 SEM], as expected for a nonpolarized distribution. At the beginning of cell migration in control border cells, E-Cadherin was polarized at the leading edge, and the fluorescence ratio was ∼1.7 ± 0.17, confirming that the signal is enriched at the leading edge. In contrast, the β-Integrin signal was slightly enriched at the trailing edge and the ratio we obtained was 0.78 ± 0.04. Confirming our initial observation and published data, we found that pTyr staining is enriched at the leading edge [F(P)/F(A) = 1.6 ± 0.13]. Because the measured areas were determined in a nonbiased way, polar cells were present in some measurements. Polar cells do not participate in the migration process per se (21) but do contain high amounts of pTyr staining at the posterior end of their cortex, which could interfere with our measurements. Thus, we repeated the measurements while excluding the polar cells. The value obtained [F(P)/F(A) = 1.49 ± 0.11] confirmed that pTyr is enriched at the leading edge of the cells actively involved in the migration process.
We performed the same quantification in border cells expressing rab11SN (Fig. 2B, red bars). As expected, GFP was not significantly modified and still displayed equal anterior and posterior fluorescence intensities [ratio F(P)/F(A) = 1.1 ± 0.05]. Both β-Integrin posterior enrichment [ratio F(P)/F(A) = 0.80 ± 0.06] and E-Cadherin anterior enrichment [F(P)/F(A) = 1.57 ± 0.16] were not affected by rab11SN expression. In contrast, the polarity of the pTyr signal was completely abolished by the expression of rab11SN [F(P)/F(A) = 0.91 ± 0.11, or 0.93 ± 0.13 when the polar cells are excluded].
These data show that trafficking through the recycling endosome plays a critical role in the spatial restriction of RTK activity. This phenotype is not due to a simple loss of cell polarity because the polarity of E-Cadherin is maintained, but is specific to the polarized localization of RTK activity.
rab11 Genetically Interacts with the RTK's Downstream Target rac1.
To further explore the relationship between rab11 and RTK signaling, we manipulated simultaneously rab11 function and RTK activity. We found that perturbing rab11 activity by either removing one copy of rab11 (rab11ex1/+) or expressing a low amount of a dominant negative form has synergetic effects with some mutants of the RTK pathway. However, the redundancy of the ligands and receptors involved complicated our analysis. Hence, we decided to search for a genetic interaction between Rab11 and the common downstream target of the RTK pathways. Rac1 was recently shown to be that common target and to be necessary for border cell migration (22, 23). Indeed, we observed a strong synergetic effect between rab11 and two different mutant forms of rac1 (24): racL89 andracN17 (Fig. 2C).
Together with the previous data showing a loss of RTK activity after rab11SN expression, these results support that trafficking through the recycling endosome is necessary for the proper regulation of RTK activity during border cell migration, possibly through the regulaton of Rac1.
Rab11 Regulates the Polarization of the Exocyst Component Sec15 in Border Cells.
We next determined if Rab11-positive endosomes are polarized. To our surprise, we found using GFP and/or YFP fusions (12, 25, 26) that neither Rab11 nor Rab5 are polarized, whereas Rab4 and Rab7 are partially polarized (Fig. S2). We concluded that there is no correlation between endosomal compartments distribution and function during migration and that a polarized transport from the recycling endosome might be involved in the polarization of RTK activity.
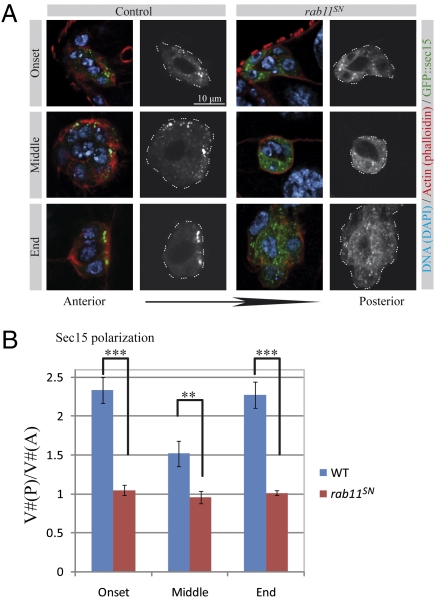
Polarized redelivery of endocytosed material is frequently mediated by the exocyst complex. The exocyst is composed of eight proteins and was initially identified in yeast where it fixes the position of exocytosis during bud formation (13). It has been implicated in exocytosis in many different polarized processes (14, 27, 28), including polarization of epithelial cells and asymmetric cell division in Drosophila (29–32) and cell migration in mammals (33). Sec15, a subunit of the exocyst, has been shown to directly interact with Rab11 (15, 16). To determine if the exocyst is involved in border cell migration, the distribution of a GFP::Sec15 fusion (31) was determined at different points of the migration process. Sec15 is strongly polarized at the onset and at the end of migration (Fig. 3A). Sec15 also displays a partial polarization in some egg chambers during the process of migration itself. We quantified this polarity by counting the number of vesicles localizing to the posterior half of the cluster [V#(P)] and to the anterior half of the cluster [V#(A)]. On the basis of the ratio of V#(P)/V#(A), a very strong polarization was observed at the onset and at the end of migration (Fig. 3B), whereas the polarization observed during the process of migration was weaker.
Fig. 3.
The exocyst component Sec15 is polarized in a Rab11-dependent manner during border cell migration. (A) Representative images showing the distribution of GFP::Sec15 at the onset, in the middle, and at the end of the migration process in control egg chambers and when rab11SN is expressed. Egg chambers were additionally stained with phalloidin (red) and DAPI (blue). A grayscale image of the green channel is shown for every image. A dashed line delimits the border cell cluster in the grayscale image. (B) Quantification of the ratio of Sec15 punctae that are distributed in the posterior half of the cluster [V#(P)] to the anterior half [V#(A)] at the onset, in the middle, and at the end of the migration process in control egg chambers (blue bars) and when rab11SN is expressed (red bars). (6 < n < 12, error bars are SEM; ***P < 0.005, **P < 0.01, Student's t test).
We hypothesized that this polarization of Sec15 might be controlled by rab11. To test this, we determined the distribution of GFP::Sec15 in border cells expressing rab11SN. At 25 °C, although rab11SN does not completely block migration, we observed a dramatic loss of Sec15 polarity (Fig. 3A), which was even more pronounced at 29 °C (Fig. 3B). These data show that Sec15 is polarized in a rab11-dependent manner during border cell migration.
The exocyst is known to be loaded on vesicles that are delivered to the plasma membrane and to define the position at the plasma membrane where these vesicles are delivered. To determine if the polarized distribution of Sec15 is vesicular, we performed time-lapse microscopy. We observed that during very active moments of migration, the polarity of Sec15 was lost and that this polarity was reestablished when the cells pause. Furthermore, time-lapse imaging revealed that the polarized Sec15 structures were static and not motile, as we would expect for transport vesicles (Movies S1 and S2 and Fig. S3). Some of these structures were observed for >90 min. These observations suggest that the polarized Sec15 structures are not transport vesicles, but that Sec15 may localize to a stable compartment or to a specialized domain of the plasma membrane.
The rab11-dependent polarization of stable Sec15 structures is consistent with the exocyst playing an important role in defining the recycling site of endocytosed material.
Exocyst Component sec15 Is Necessary for Border Cell Migration.
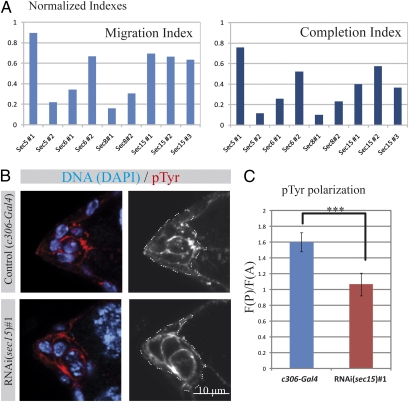
To further test if sec15 is necessary for border cell migration, its expression was down-regulated by dsRNA. We observed that the knock-down of sec15 by three different dsRNA trangenes (expressing two different constructs) against sec15 causes a strong migration phenotype, comparable to that obtained with rab11 RNAi (Fig. 4A). Therefore, we conclude that sec15 is directly involved in border cell migration. Next we determined if other subunits of the exocyst are also necessary for border cell migration. To this end, we performed RNAi against different subunits (sec5, sec6, and sec8). All of the dsRNA tested, except one (possibly due to inefficient knock-down of the target), induced migration phenotypes similar to or stronger than sec15 RNAi (Fig. 4A). To ascertain that the migration phenotype is due to a perturbation of the polarity of RTK signaling, we stained border cell clusters for pTyr and we measured the polarization of the signal as previously (Fig. 4 B and C). We observed that knock-down of sec15 induces a dramatic loss of pTyr polarization, similarly to the expression of rab11SN. Thus sec15 is necessary for proper cell migration and for regulating the spatial restriction of RTK activity. Altogether these data show that a complete trafficking cycle between the plasma membrane and the recycling endosome is necessary for border cell migration and for the spatial regulation of RTK activity. This cycle requires the functions of early endocytic steps (ref. 10 and this study), rab11 and sec15.
Fig. 4.
The exocyst component Sec15 is necessary for border cell migration. (A) Normalized migration and completion indexes (normalized to c306-Gal4) for clusters expressing dsRNA against the indicated gene (36 < n < 146). (B) Representative images showing the distribution of pTyr (red) at the onset of the migration process in stage 9 control egg chambers and when sec15 expression is down-regulated in border cells by dsRNA. Nuclei are stained with DAPI (blue). A grayscale image of the red channel is shown for every image. A dashed line delimits the border cell cluster in the grayscale image. Anterior is left and posterior is right. (C) Quantification of pTyr fluorescence ratio (Fig. 3) in control (blue bar) and when sec15 is down-regulated in border cells (red bar). (16 < n < 18, error bars are SEM; ***P < 0.005, Student's t test).
Discussion
Endocytosis Is a Key Regulator of Cell Migration.
During migration, the cell needs to rearrange its cytoskeleton, its plasma membrane content, and its interaction with other cells. Many of these features can be controlled by vesicular trafficking. For example, Integrins, Cadherins, and other cell–cell or cell–matrix attachment proteins are transmembrane proteins tightly regulated by trafficking (7). Furthermore, the distribution of proteins and lipids at the plasma membrane is directly controlled by vesicular trafficking, as well as the localization of some actin remodeling proteins (34–36). During the process of border cell migration, endocytosis was recently shown to regulate the polarity of RTK activity (10). In this paper we show that the endocytic process plays a role in regulating the spatial localization of RTK activity by trafficking through the recycling endosome and by the polarized redelivery of endocytosed material to the plasma membrane.
“Plasma Membrane–Endosomes–Plasma Membrane” Cycle Spatially Restricts RTK Activity at the Leading Edge.
The key endocytic proteins previously involved in border cell migration—Sprint, Cbl, and Shibire—were shown to regulate the polarization of RTK activity during border cell migration (10). Different possible mechanisms were proposed to explain their action and were not addressed in this landmark article. Recently, it was shown that both the degradative pathway and the recycling pathway might be involved in this process (37). Thus, at least two models, which are not mutually exclusive, could explain the role of endocytosis in establishing this polarity. First, active RTKs could be endocytosed and degraded when diffusing away from the leading edge. Second, polarized recycling of endocytosed active RTKs could concentrate these active receptors at the leading edge. From our experiments we can conclude that the recycling of active RTKs or of a cofactor at the plasma membrane is necessary for border cell migration. Furthermore, we demonstrate that the slow recycling route, through the recycling endosome, is used and that polarized redelivery at the plasma membrane is mediated by the exocyst subunit sec15.
Are RTKs Recycled Themselves?
It seems logical to think that RTKs, or active RTKs, are the cargo transported through this endocytic cycle. However, the identity of the protein being recycled remains to be determined. Indeed, we have no indication that RTKs are recycled. We performed immunofluorescence staining of the EGFR. The signal obtained was diffuse and inconclusive in both control and rab11SN-expressing border cells. In addition, if active RTKs were trafficking through the recycling endosome, we would expect to see the pTyr in endocytic vesicles marked by Rab11. However, we have never observed such a colocalization, and neither have others (37). These data do not rule out a potential recycling of RTKs, because they could be present in the recycling endosome in quantities below detection levels or in an inactive form. However, our data suggest that the main cargo of this trafficking cycle is of another nature. This cargo could be a plasma membrane diffusion barrier, because polarized cells, such as epithelial cells and neurons, maintain different membrane domains, which rely on such barriers: the tight junctions (38) and the axon hillock, respectively (39). Moreover, diffusion barriers have been proposed to define plasma membrane domains in migrating cells (40, 41). These diffusion barriers appear to be linked to the actin cytoskeleton, but their exact nature is unknown. Because E-cadherin is involved in cell migration (18), it would have been an ideal candidate to play such a role, but it is unaffected by rab11SN expression.
Another possibility is that endocytosis acts indirectly. For example, it might regulate key components of the plasma membrane or of the cytoskeleton. Recent evidence has shown that endocytosis and recycling can play a critical role in creating a positive feedback loop during polarity establishment in the budding yeast (35, 36). In this particular case, endocytosis is critical for the localization of regulators of small GTPases of the Rho family. Furthermore, mammalian Tiam1, a GDP-to-GTP exchange factor (GEF) for Rac, has recently been shown to localize to endosomes, leading to the loading of active Rac at the plasma membrane through an endocytic-recycling cycle (34). Interestingly, we found a robust genetic interaction between Rab11 and Rac1, which directs border cell migration (22). Until now, two Rac-GEFs, myoblast city and elmo, have been involved in border cell migration, but not the Drosophila Tiam1 homolog still life (42). Further studies will be necessary to determine if Rac1 is the main cargo of the endocytic-recycling cycle that regulates border cell migration.
Recycling as a Universal Polarization Mechanism.
In the past few years, trafficking via the recycling endosome has been involved in the establishment or rearrangement of cell polarity in various events. In particular, a role for the recycling endosome has been observed when a rapid and dramatic rearrangement of the cell organization is required (28), including cellularization, cell–cell boundary rearrangement, asymmetric cell division, and cell migration. Trafficking through the recycling endosome is an ideal mechanism to polarize a cell rapidly, because it hijacks material already available in the cell at a new location. Furthermore, it is a very efficient mechanism to reinforce polarity by feedback loops. Similarly the exocyst plays a key role in the majority of these cell polarizations. In the case of cell migration, the recycling endosome may transform the diffuse extracellular gradient of RTK ligand into a robust intracellular polarization of RTK activity that is crucial for directed migration.
Cell Migration and Endocytic Recycling in Mammals.
There is much evidence that the function of the recycling endosome in the regulation of directed migration is conserved in mammals. The mammalian homologs of Drosophila rab11 are Rab11A and -B and Rab25. They have been directly implicated in the migration of cancerous cells and in the formation of metastasis, a cell migration event resembling border cell migration (43–45). Rab11 effectors are also involved in mammalian cell migration (46–48). More specifically, PDGF receptor-dependent cell migration has been shown to be regulated by endocytosis in a mammalian cell culture assay (49) and the recycling endosome has been indirectly implicated in the regulation of migration guided by the EGFR (47). Given the involvement of the recycling endosome in so many processes, targeting its function to reduce metastasis is unlikely to be efficient. However, identifying the main cargo of this recycling cycle could help identify more specific targets for drugs blocking the formation of metastasis.
Materials and Methods
Fly Genetics.
The generation of dominant negative rab4, rab7, and rab11 is described in SI Materials and Methods. YFP fusion to dominant negative Rab proteins, YFP and GFP fusions to wild-type Rab proteins, and dominant negative Rab protein (12, 25, 26) constructs were expressed in the border cell cluster using one copy of slbo-Gal4 (50) or slbo-Gal4, UAS-CD8::GFP. dsRNA constructs from the Vienna Drosophila RNAi Center (51) were expressed in flies using c306-Gal4 (52). Other stocks used were grk3/CyO (53), EgfrE1/Cyo (54), rab11ex1/TM3 (55), UAS-racL89.6 (24), and UAS-GFP::sec15 (31). For the experiments to determine genetic interactions, flies were maintained at 25 °C and at 21 °C for RacN17, because at 25 °C it completely blocks migration (23). Flies were maintained at 29 °C for the expression of dominant negative Rab proteins in other experiments. For RNAi experiments, flies were maintained at 18 °C until adulthood to avoid lethality and then maintained at 29 °C for at least 24 h before dissection.
Tissue Staining and Antibodies.
Image Acquisition.
Images from fixed tissues were acquired using a LSM 510 META inverted confocal microscope (Zeiss), using either a 20× objective or a 63× objective, and by standard methods (see SI Materials and Methods for details and for the procedure used for image quantification).
Migration and Completion Indexes.
Live Imaging.
Living egg chambers were prepared for real-time imaging of border cell migration as described (56). Image acquisition is described in SI Materials and Methods.
Supplementary Material
Acknowledgments
We thank Marc Therrien, Hugo Bellen, Denise Montell, Pernille Rørth, Vic Small, Juergen Knoblich, the Developmental Studies Hybridoma Bank, the Bloomington Stock Collection, and the Vienna Drosophila RNAi Center for fly stocks and antibodies. We are grateful to Marc Therrien, Sébastien Carréno, Vincent Archambault, and David Hipfner for critical reading of the manuscript. We also thank Christelle Ogoudikpe for technical assistance. This work was supported by grants from the Canadian Institutes for Health Research to G.E. (MOP-84515). G.E. holds a Canada Research Chair (Tier II) in Vesicular Trafficking and Cell Signaling.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1010795108/-/DCSupplemental.
References
- 1.Montell DJ. Morphogenetic cell movements: Diversity from modular mechanical properties. Science. 2008;322:1502–1505. doi: 10.1126/science.1164073. [DOI] [PubMed] [Google Scholar]
- 2.Rørth P. Collective guidance of collective cell migration. Trends Cell Biol. 2007;17:575–579. doi: 10.1016/j.tcb.2007.09.007. [DOI] [PubMed] [Google Scholar]
- 3.Rørth P. Initiating and guiding migration: Lessons from border cells. Trends Cell Biol. 2002;12:325–331. doi: 10.1016/s0962-8924(02)02311-5. [DOI] [PubMed] [Google Scholar]
- 4.Doherty GJ, McMahon HT. Mechanisms of endocytosis. Annu Rev Biochem. 2009;78:857–902. doi: 10.1146/annurev.biochem.78.081307.110540. [DOI] [PubMed] [Google Scholar]
- 5.Grant BD, Donaldson JG. Pathways and mechanisms of endocytic recycling. Nat Rev Mol Cell Biol. 2009;10:597–608. doi: 10.1038/nrm2755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gruenberg J, Stenmark H. The biogenesis of multivesicular endosomes. Nat Rev Mol Cell Biol. 2004;5:317–323. doi: 10.1038/nrm1360. [DOI] [PubMed] [Google Scholar]
- 7.Ulrich F, Heisenberg CP. Trafficking and cell migration. Traffic. 2009;10:811–818. doi: 10.1111/j.1600-0854.2009.00929.x. [DOI] [PubMed] [Google Scholar]
- 8.Delva E, Kowalczyk AP. Regulation of cadherin trafficking. Traffic. 2009;10:259–267. doi: 10.1111/j.1600-0854.2008.00862.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ramsay AG, Marshall JF, Hart IR. Integrin trafficking and its role in cancer metastasis. Cancer Metastasis Rev. 2007;26:567–578. doi: 10.1007/s10555-007-9078-7. [DOI] [PubMed] [Google Scholar]
- 10.Jékely G, Sung HH, Luque CM, Rørth P. Regulators of endocytosis maintain localized receptor tyrosine kinase signaling in guided migration. Dev Cell. 2005;9:197–207. doi: 10.1016/j.devcel.2005.06.004. [DOI] [PubMed] [Google Scholar]
- 11.Zerial M, McBride H. Rab proteins as membrane organizers. Nat Rev Mol Cell Biol. 2001;2:107–117. doi: 10.1038/35052055. [DOI] [PubMed] [Google Scholar]
- 12.Zhang J, et al. Thirty-one flavors of Drosophila rab proteins. Genetics. 2007;176:1307–1322. doi: 10.1534/genetics.106.066761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.TerBush DR, Maurice T, Roth D, Novick P. The Exocyst is a multiprotein complex required for exocytosis in Saccharomyces cerevisiae. EMBO J. 1996;15:6483–6494. [PMC free article] [PubMed] [Google Scholar]
- 14.Wu H, Rossi G, Brennwald P. The ghost in the machine: Small GTPases as spatial regulators of exocytosis. Trends Cell Biol. 2008;18:397–404. doi: 10.1016/j.tcb.2008.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wu S, Mehta SQ, Pichaud F, Bellen HJ, Quiocho FA. Sec15 interacts with Rab11 via a novel domain and affects Rab11 localization in vivo. Nat Struct Mol Biol. 2005;12:879–885. doi: 10.1038/nsmb987. [DOI] [PubMed] [Google Scholar]
- 16.Zhang XM, Ellis S, Sriratana A, Mitchell CA, Rowe T. Sec15 is an effector for the Rab11 GTPase in mammalian cells. J Biol Chem. 2004;279:43027–43034. doi: 10.1074/jbc.M402264200. [DOI] [PubMed] [Google Scholar]
- 17.Silver DL, Geisbrecht ER, Montell DJ. Requirement for JAK/STAT signaling throughout border cell migration in Drosophila. Development. 2005;132:3483–3492. doi: 10.1242/dev.01910. [DOI] [PubMed] [Google Scholar]
- 18.Niewiadomska P, Godt D, Tepass U. DE-Cadherin is required for intercellular motility during Drosophila oogenesis. J Cell Biol. 1999;144:533–547. doi: 10.1083/jcb.144.3.533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Devenport D, Brown NH. Morphogenesis in the absence of integrins: Mutation of both Drosophila beta subunits prevents midgut migration. Development. 2004;131:5405–5415. doi: 10.1242/dev.01427. [DOI] [PubMed] [Google Scholar]
- 20.Dinkins MB, Fratto VM, Lemosy EK. Integrin alpha chains exhibit distinct temporal and spatial localization patterns in epithelial cells of the Drosophila ovary. Dev Dyn. 2008;237:3927–3939. doi: 10.1002/dvdy.21802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Han DD, Stein D, Stevens LM. Investigating the function of follicular subpopulations during Drosophila oogenesis through hormone-dependent enhancer-targeted cell ablation. Development. 2000;127:573–583. doi: 10.1242/dev.127.3.573. [DOI] [PubMed] [Google Scholar]
- 22.Wang X, He L, Wu YI, Hahn KM, Montell DJ. Light-mediated activation reveals a key role for Rac in collective guidance of cell movement in vivo. Nat Cell Biol. 2010;12:591–597. doi: 10.1038/ncb2061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Geisbrecht ER, Montell DJ. A role for Drosophila IAP1-mediated caspase inhibition in Rac-dependent cell migration. Cell. 2004;118:111–125. doi: 10.1016/j.cell.2004.06.020. [DOI] [PubMed] [Google Scholar]
- 24.Luo L, Liao YJ, Jan LY, Jan YN. Distinct morphogenetic functions of similar small GTPases: Drosophila Drac1 is involved in axonal outgrowth and myoblast fusion. Genes Dev. 1994;8:1787–1802. doi: 10.1101/gad.8.15.1787. [DOI] [PubMed] [Google Scholar]
- 25.Wucherpfennig T, Wilsch-Bräuninger M, González-Gaitán M. Role of Drosophila Rab5 during endosomal trafficking at the synapse and evoked neurotransmitter release. J Cell Biol. 2003;161:609–624. doi: 10.1083/jcb.200211087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Emery G, et al. Asymmetric Rab 11 endosomes regulate delta recycling and specify cell fate in the Drosophila nervous system. Cell. 2005;122:763–773. doi: 10.1016/j.cell.2005.08.017. [DOI] [PubMed] [Google Scholar]
- 27.Hsu SC, TerBush D, Abraham M, Guo W. The exocyst complex in polarized exocytosis. Int Rev Cytol. 2004;233:243–265. doi: 10.1016/S0074-7696(04)33006-8. [DOI] [PubMed] [Google Scholar]
- 28.Emery G, Knoblich JA. Endosome dynamics during development. Curr Opin Cell Biol. 2006;18:407–415. doi: 10.1016/j.ceb.2006.06.009. [DOI] [PubMed] [Google Scholar]
- 29.Beronja S, et al. Essential function of Drosophila Sec6 in apical exocytosis of epithelial photoreceptor cells. J Cell Biol. 2005;169:635–646. doi: 10.1083/jcb.200410081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Classen AK, Anderson KI, Marois E, Eaton S. Hexagonal packing of Drosophila wing epithelial cells by the planar cell polarity pathway. Dev Cell. 2005;9:805–817. doi: 10.1016/j.devcel.2005.10.016. [DOI] [PubMed] [Google Scholar]
- 31.Jafar-Nejad H, et al. Sec15, a component of the exocyst, promotes notch signaling during the asymmetric division of Drosophila sensory organ precursors. Dev Cell. 2005;9:351–363. doi: 10.1016/j.devcel.2005.06.010. [DOI] [PubMed] [Google Scholar]
- 32.Langevin J, et al. Drosophila exocyst components Sec5, Sec6, and Sec15 regulate DE-Cadherin trafficking from recycling endosomes to the plasma membrane. Dev Cell. 2005;9:365–376. doi: 10.1016/j.devcel.2005.07.013. [DOI] [PubMed] [Google Scholar]
- 33.Spiczka KS, Yeaman C. Ral-regulated interaction between Sec5 and paxillin targets Exocyst to focal complexes during cell migration. J Cell Sci. 2008;121:2880–2891. doi: 10.1242/jcs.031641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Palamidessi A, et al. Endocytic trafficking of Rac is required for the spatial restriction of signaling in cell migration. Cell. 2008;134:135–147. doi: 10.1016/j.cell.2008.05.034. [DOI] [PubMed] [Google Scholar]
- 35.Howell AS, et al. Singularity in polarization: Rewiring yeast cells to make two buds. Cell. 2009;139:731–743. doi: 10.1016/j.cell.2009.10.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Marco E, Wedlich-Soldner R, Li R, Altschuler SJ, Wu LF. Endocytosis optimizes the dynamic localization of membrane proteins that regulate cortical polarity. Cell. 2007;129:411–422. doi: 10.1016/j.cell.2007.02.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Janssens K, Sung HH. Rorth P. Direct detection of guidance receptor activity during border cell migration. Proc Natl Acad Sci USA. 2010;107:7323–7328. doi: 10.1073/pnas.0915075107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Shin K, Fogg VC, Margolis B. Tight junctions and cell polarity. Annu Rev Cell Dev Biol. 2006;22:207–235. doi: 10.1146/annurev.cellbio.22.010305.104219. [DOI] [PubMed] [Google Scholar]
- 39.Winckler B, Forscher P, Mellman I. A diffusion barrier maintains distribution of membrane proteins in polarized neurons. Nature. 1999;397:698–701. doi: 10.1038/17806. [DOI] [PubMed] [Google Scholar]
- 40.Vasanji A, Ghosh PK, Graham LM, Eppell SJ, Fox PL. Polarization of plasma membrane microviscosity during endothelial cell migration. Dev Cell. 2004;6:29–41. doi: 10.1016/s1534-5807(03)00397-6. [DOI] [PubMed] [Google Scholar]
- 41.Weisswange I, Bretschneider T, Anderson KI. The leading edge is a lipid diffusion barrier. J Cell Sci. 2005;118:4375–4380. doi: 10.1242/jcs.02551. [DOI] [PubMed] [Google Scholar]
- 42.Bianco A, et al. Two distinct modes of guidance signalling during collective migration of border cells. Nature. 2007;448:362–365. doi: 10.1038/nature05965. [DOI] [PubMed] [Google Scholar]
- 43.Yoon SO, Shin S, Mercurio AM. Hypoxia stimulates carcinoma invasion by stabilizing microtubules and promoting the Rab11 trafficking of the alpha6beta4 integrin. Cancer Res. 2005;65:2761–2769. doi: 10.1158/0008-5472.CAN-04-4122. [DOI] [PubMed] [Google Scholar]
- 44.Cheng KW, et al. The RAB25 small GTPase determines aggressiveness of ovarian and breast cancers. Nat Med. 2004;10:1251–1256. doi: 10.1038/nm1125. [DOI] [PubMed] [Google Scholar]
- 45.Palmieri D, Bouadis A, Ronchetti R, Merino MJ, Steeg PS. Rab11a differentially modulates epidermal growth factor-induced proliferation and motility in immortal breast cells. Breast Cancer Res Treat. 2006;100:127–137. doi: 10.1007/s10549-006-9244-6. [DOI] [PubMed] [Google Scholar]
- 46.Jing J, Tarbutton E, Wilson G, Prekeris R. Rab11-FIP3 is a Rab11-binding protein that regulates breast cancer cell motility by modulating the actin cytoskeleton. Eur J Cell Biol. 2009;88:325–341. doi: 10.1016/j.ejcb.2009.02.186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Caswell PT, et al. Rab-coupling protein coordinates recycling of alpha5beta1 integrin and EGFR1 to promote cell migration in 3D microenvironments. J Cell Biol. 2008;183:143–155. doi: 10.1083/jcb.200804140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zhang J, et al. RCP is a human breast cancer-promoting gene with Ras-activating function. J Clin Invest. 2009;119:2171–2183. doi: 10.1172/JCI37622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kawada K, et al. Cell migration is regulated by platelet-derived growth factor receptor endocytosis. Mol Cell Biol. 2009;29:4508–4518. doi: 10.1128/MCB.00015-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Rørth P, et al. Systematic gain-of-function genetics in Drosophila. Development. 1998;125:1049–1057. doi: 10.1242/dev.125.6.1049. [DOI] [PubMed] [Google Scholar]
- 51.Dietzl G, et al. A genome-wide transgenic RNAi library for conditional gene inactivation in Drosophila. Nature. 2007;448:151–156. doi: 10.1038/nature05954. [DOI] [PubMed] [Google Scholar]
- 52.Brand AH, Perrimon N. Targeted gene expression as a means of altering cell fates and generating dominant phenotypes. Development. 1993;118:401–415. doi: 10.1242/dev.118.2.401. [DOI] [PubMed] [Google Scholar]
- 53.Schüpbach T. Germ line and soma cooperate during oogenesis to establish the dorsoventral pattern of egg shell and embryo in Drosophila melanogaster. Cell. 1987;49:699–707. doi: 10.1016/0092-8674(87)90546-0. [DOI] [PubMed] [Google Scholar]
- 54.Baker NE, Rubin GM. Effect on eye development of dominant mutations in Drosophila homologue of the EGF receptor. Nature. 1989;340:150–153. doi: 10.1038/340150a0. [DOI] [PubMed] [Google Scholar]
- 55.Dollar G, Struckhoff E, Michaud J, Cohen RS. Rab11 polarization of the Drosophila oocyte: A novel link between membrane trafficking, microtubule organization, and oskar mRNA localization and translation. Development. 2002;129:517–526. doi: 10.1242/dev.129.2.517. [DOI] [PubMed] [Google Scholar]
- 56.Prasad M, Jang AC, Starz-Gaiano M, Melani M, Montell DJ. A protocol for culturing Drosophila melanogaster stage 9 egg chambers for live imaging. Nat Protoc. 2007;2:2467–2473. doi: 10.1038/nprot.2007.363. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.