Abstract
Prions consist mainly of PrPSc, a pathogenic conformer of host-encoded PrPC. Prion populations with distinct phenotypes but associated with PrPSc, having the same amino acid sequence, constitute distinct strains. Strain identity is thought to be encoded by the conformation of PrPSc and to be maintained by seeded conversion. Prion strains can be distinguished by the cell panel assay, which measures their ability to infect distinct cell lines. Brain-derived 22L prions characteristically are able to infect R33 cells (i.e., are “R33 competent”), as well as PK1 cells in the presence of the inhibitor swainsonine (i.e. are “swa resistant”). Here we report that 22L prions retained their characteristic cell tropism and swa resistance when transferred from brain to R33 cells. However, when transferred from the R33 cells to PK1 cells, they gradually became R33 incompetent and swa sensitive, unless the transfer was in the presence of swa, in which case swa resistance and R33 competence were retained. PrPSc associated with swa-resistant/R33-competent and swa-sensitive/R33-incompetent prions had different conformational stabilities. When cloned R33-incompetent/swa-sensitive prions were again propagated in brain, their properties gradually reverted to those of the original brain-derived 22L prions. Our results support the view that 22L prion populations are heterogeneous and that distinct prion variants are selected in different cellular environments.
Keywords: evolution, scrapie cell assay, selection
We determine the susceptibility of a cell line to a prion strain by the standard scrapie cell assay (SSCA) (1, 2). In short, we expose cells to various dilutions of the prion sample, propagate them for three splits, and determine the proportion of PrPSc-containing cells by ELISA. We define as response index (RI) the reciprocal of the dilution that yields a designated proportion of infected cells under standard conditions (usually 300 PrPSc-positive cells/20,000 cells).
We discriminate murine prion strains by the cell panel assay (CPA) (3), which is based on the differential susceptibility of selected cell lines to individual prion strains. RML, 22L, ME7, and 301C prions can be distinguished by their relative RI values on a panel consisting of neuroblastoma-derived PK1 cells in the presence or absence of swainsonine (swa), neuroblastoma-derived R33 (or, recently, the more susceptible subclone R332H11) cells, fibroblastic LD9 and CNS-derived CAD cells. Swa, an inhibitor of complex glycosylation (4), suppresses infection of PK1 cells by RML but not by 22L prions (5).
As recently reported, when 22L prions were transferred from brain to cultured PK1 cells, the PK1 cell-adapted 22L variants gradually outgrew the original population, as documented by a profound change in their CPA characteristics: Although brain-derived 22L prions efficiently infected R33 cells (i.e., were R33 competent) and PK1 cells in the presence of swa (i.e., were swa resistant), the PK1 cell-adapted 22L prions were R33 incompetent (i.e., were 1.5–2 logs less infectious than brain-derived prions) and were swa sensitive. Importantly, propagation of 22L-infected PK1 cells in the presence of swa led to the selection of drug-resistant prion variants (5). Similarly, exposure of RML-infected mice or cell cultures to quinacrine resulted in the accumulation of quinacrine-resistant variants (6).
Here we show that, in contrast to the results obtained with PK1 cells, brain-derived 22L prions transferred to R33 cells remained R33 competent and swa resistant. When transferred from R33 cells to PK1 cells, the prion population became R33 incompetent and swa sensitive, but if transferred in the presence of swa it remained competent and resistant. When cloned R33-incompetent/swa-sensitive 22L prions were returned to brain, they eventually reacquired all characteristics of the original 22L prions. Importantly, the conformational stability of PrPSc associated with R33-competent/swa-resistant and R33-incompetent/swa-sensitive prions was shown to differ. Thus, distinct 22L variants, or substrains, are selected in several different environments and are associated with physicochemically diverse PrPSc.
Results
CPA Profiles of Brain-Adapted and R33 Cell-Adapted 22L Prions Are Similar.
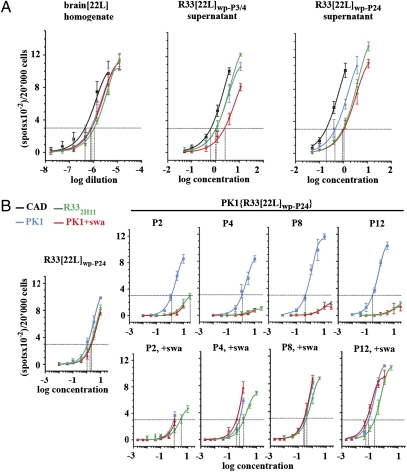
The nomenclature of prion-infected cells and tissues is explained in SI Text. We generated an uncloned population of 22L-infected R33 cells (R33[22L]wp) by exposing R33 cells to 22L-infected brain homogenate for 4 d and expanding the population for four splits; the populations after the third and fourth split were pooled to yield R33[22L]wp-P3/4. The CPA pattern of prions secreted by the R33[22L]wp-P3/4 population was similar to that of brain[22L] in that the prions were swa resistant and R33 competent and remained so even after 24 splits, about 100 doublings (R33[22L]wp-P24) (Fig. 1A). Thus, under the constraint of having to replicate in R33 cells, the prions retained R33 competence and remained swa resistant, perhaps because the traits are linked.
Fig. 1.
Transfer of prions from R33[22L]wp cells to PK1 cells entails a change in the CPA profile. (A) R33 cells were infected with a 10−3 dilution of 22L-infected brain homogenate for 4 d and propagated for three and four 1:20 splits to yield the uncloned populations R33[22L]wp-P3 and R33[22L]wp-P4, respectively. Conditioned media from the two cultures were combined. The R33[22L]wp population was propagated further for a total of twenty-four 1:20 splits to yield R33[22L]wp-P24. (B) PK1 cells were exposed to 20× concentrated conditioned medium from R33[22L]wp-P24 cells for 4 d and propagated in the presence or absence of 2 μg swa/mL for twelve 1:20 splits. In all cases conditioned media were concentrated 200-fold and assayed by the SSCA on PK1 cells with or without swa and on CAD and R332H11 cells. There was no change in properties of the prions secreted by the R33[22L]wp population even after 24 splits (A). After transfer to PK1 cells, the prions progressively lost R33 competence and swa resistance when cells were propagated in the absence of swa (B, Upper). However in the presence of swa (B, Lower), R33 competence and swa resistance were retained.
Transfer of Prions from a 22L-Infected R33 Population to PK1 Cells Results in Loss of R33 Competence and Swa Resistance.
When R33-competent, swa-resistant brain-derived 22L prions were propagated in PK1 cells, they gradually became R33 incompetent and swa sensitive (5). We therefore investigated whether the transfer of R33-competent, swa-resistant prions from R33[22L]wp-P24 cells to PK1 cells would result in a similar change. PK1 cells were exposed to R33[22L]wp-P24 prions for 4 d and split 1:20 after reaching confluence, about every 6 d. Fig. 1B Upper shows that in the course of about four 1:20 splits, the prions secreted by the PK1{R33[22L]wp-P24} cells indeed became swa sensitive and R33 incompetent. However, when the cells were propagated in the presence of swa, the secreted prions remained fully swa resistant and R33 competent, even after twelve 1:20 splits (Fig. 1B Lower). Thus, selection for swa resistance entailed R33 competence, again suggesting that the two properties are linked.
Prions from Distinct Cloned, 22L-Infected R33 Cell Lines May Differ in Their CPA Properties.
We asked whether the CPA characteristics of prions from 22L-infected R33 cell clones resembled those from 22L-infected R33 whole populations. Fig. 2 shows the CPA values from two independent assays of five 22L-infected R33 clones representative of 12 clones we had isolated and characterized. Although the CPA characteristics of clones R33[22L]AA8 and R33[22L]BB8 were similar to those of the uncloned 22L-infected R33 population (R33[22L]wp-P24) (Fig. 1A), namely R33 competence (RIR33/RIPK1 = 0.47) and swa resistance (RIPK1,swa/RIPK1 > 0.43), R33[22L]F3 was largely R33 incompetent (RIR33/RIPK1 ≤ 0.04) and swa sensitive (RIPK1,swa/RIPK1 <<0.04), whereas R33[22L]BA5 and R33[22L]E4 had intermediate properties. Prions secreted by the cloned, extensively passaged CAD[22L]A3 and PK1[22L]D3 cell lines failed to infect R33 cells even at the highest concentrations tested (RIR33/RIPK1 < 0.007) and were completely swa sensitive (Fig. S1).
Fig. 2.
CPA of 22L-infected R33 cell clones. Individual cell clones derived from populations of R33 cells infected with [22L] prions show diverse CPA patterns. (A) The CPAs of five representatives of the 12 R33[22L] clones range from complete swa resistance and R332H11 competence to almost complete swa sensitivity and R332H11 incompetence. (B) An independent repetition of the CPA using only PK1 cells, with or without swa, and R332H11 cells.
Thus, propagation of 22L prions in PK1 and CAD cells can lead to selection of swa-sensitive, R33-incompetent prion populations, whereas propagation in R33 cells results in populations that are forced to retain R33 competence unless, as shown below, the cells are variants permissive for R33-incompetent prions.
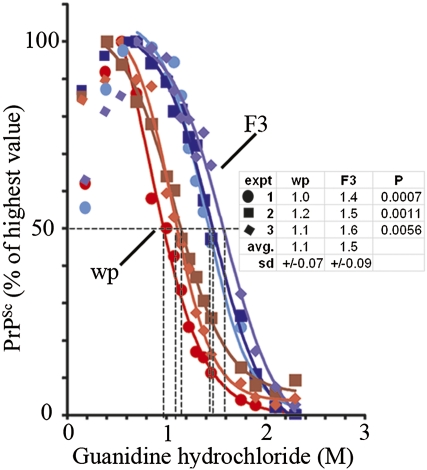
Conformational Stabilities of R33[22L]wp-P0 and R33[22L]F3 PrPSc Differ.
Distinct prion strains sometimes are associated with physicochemically distinct forms of PrPSc that can be characterized by the conformational stability assay (7); the molarity of guanidinium chloride at which 50% denaturation occurs is designated as the Gnd1/2 value. The 22L-infected R33 populations produced swa-resistant and R33-competent prions for up to 24 passages (Fig. 1A). PrPSc from a 22L-infected R33 cell population propagated for only three to four doublings (R33[22L]wp-P0) had a distinctly lower Gnd1/2 value (1.1 M) than PrPSc from R33[22L]F3 cells, which generated swa-sensitive and R33-incompetent prions (1.5 M) (Fig. 3).
Fig. 3.
Different conformational stabilities of PrPSc from R33[22L]wp-P0 and R33[22L]F3 cells. Lysate samples were exposed to the guanidinium chloride concentrations indicated for 15 min at 25 °C and, after guanidinium was adjusted to 0.2 M, were digested with proteinase K and analyzed by Western blotting. The signals were quantified as described in SI Methods. The curves were obtained from three independent experiments. In some cases, there was an increase in signal intensity after exposure to low guanidinium and proteinase K digestion, ascribed to increased exposure of epitope. The highest signal was set to 100%. The Gnd1/2 values averaged from three experiments were 1.1 M and 1.5 M for PrPSc from R33[22L]wp-P0 and R33[22L]F3 cells, respectively.
In some cases digestion of PrPSc from different strains with proteinase K (PK) yields protease-resistant moieties with different mobilities (8). Treatment of brain[22L] homogenates or R33[22L]F3 cell lysates with PK followed by deglycosylation with peptide N-glycosidase F did not yield fragments with different mobility (Fig. S2).
R33[22L]F3 Is an Unusual Clone.
The prions produced by R33[22L]F3 were swa sensitive and R33 incompetent (Fig. 2 A and B), raising the question as to how they could be propagated permanently in R33 cells. We suspected that the R33[22L]F3 cells might be exceptional and, unlike the parental R33 cells, susceptible to infection by the prions they were producing. We therefore cured R33[22L]F3 cells of infection by propagating them for 21 d (five 1:10 splits) in 5 μg pentosan polysulfate (PPS)/mL, a powerful inhibitor of prion replication (9), and then without PPS for 13 d (four splits); the cells were cured as assessed by PK-ELISA (<0.2% PrPSc-positive cells). Cured R33[22L]F3, but not R33 cells, were susceptible to infection by R33[22L]F3 and RML prions, albeit much less so than PK1 cells (Fig. 4). Thus, the R33[22L]F3 clone likely came about by infection of a variant R33 cell. Subclones of PK1 cells, which, like R33 cells are derived from N2a neuroblastoma cells, vary considerably in their susceptibility to RML and 22L (3). Apparently the R33[22L]F3 cells had assumed, to a moderate degree, PK1-like properties. It is likely that the 22L-infected R33 clones R33[22L]BA5 and R33[22L]E4 described above, which produced prion populations with intermediate swa resistance and R33 competence, varied to different degrees in their permissiveness for R33-incompetent prions.
Fig. 4.
“Cured” R33 cells recovered from the R33[22L]F3 clone have a modified CPA profile. R33[22L]F3 cells were cured of infection by propagation in the presence 5 μg PPS/mL for 13 d and assayed by the CPA. The cured cells were susceptible to infection by RML and R33[22L]F3 conditioned medium, albeit to a far lesser degree than PK1 cells, whereas R33 cells were fully resistant to both.
Although the R33[22L]F3 line was exceptional as regards the properties of the cells, the experiment showed that brain[22L]-derived prions, when propagated in a different environment, acquired a novel cell tropism and thus represented a 22L strain variant.
Prions Transferred from R33[22L]F3 Cells to Brain Reacquire the Original Brain[22L] Properties.
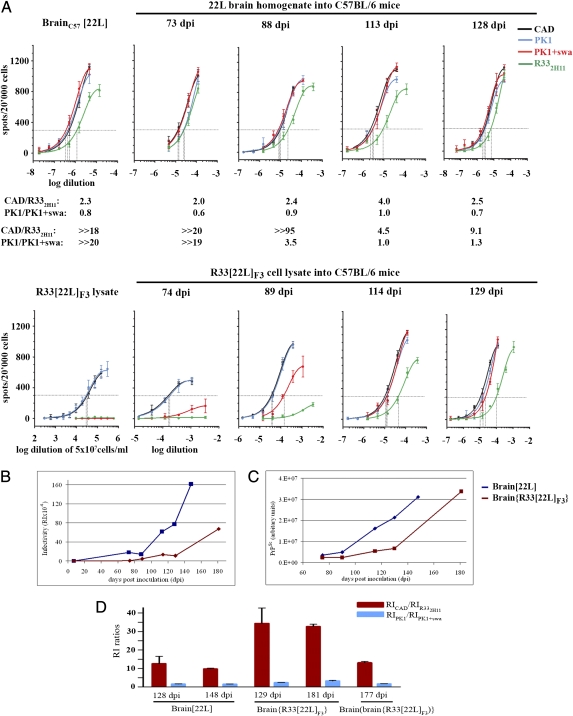
To determine whether the change in cell tropism exhibited by R33[22L]F3 prions was transient or permanent, we inoculated C57BL/6 mice intracerebrally (i.c.) with brain[22L] homogenate or R33[22L]F3 cell lysate containing similar amounts of PrPSc (Fig. S3) and harvested brains at different times after infection. The CPA profile of brain injected with brain[22L] homogenate remained unchanged over the entire observation period (Fig. 5A Upper). The response pattern of brain injected with R33[22L]F3 prions at 74 d after inoculation (dpi) still resembled that of the lysate, with no significant infection of R33 cells or of PK1 cells in the presence of swa (Fig. 4A Lower). Because infectivity caused by inoculum was undetectable in brain 1 d after i.c. injection and remained so for at least 2 wk (Fig. S4) (10), infectivity detected in brain inoculated with R33[22L]F3 at 74 dpi (RICAD cells = 6.0 × 103, equivalent to about 105.7 LD50 units/g brain) was newly synthesized and not caused by inoculum. At 89 dpi, partial resistance to swa on PK1 cells was evident (RIPK1/RIPK1,swa = 3.5), and at 114 dpi (RICAD =105, about 107.0 LD50 units/g brain) the sample was completely swa resistant (RIPK1/RIPK1/swa = 1) and R33 competent (RICAD/RIR33–2H1 = 4.5) (Fig. 5A Lower). Small differences in the ratios of RICAD/RIR33–2H11 (9.1 versus 2.5.) and RIPK1/RIPK1,swa (1.3 versus 0.7) between brains inoculated with R33[22L]F3 or brain[22L], harvested at days 129/128) (Fig. 5A), were reproduced in subsequent reanalyses (Fig. 5D and Fig. S5 B versus D, and G versus I).
Fig. 5.
Prions transferred from R33[22L]F3 cells to mouse brain gradually reacquire their original CPA profile. C57BL/6 mice were inoculated with amounts of 22L-infected brain homogenate (5.2 μg total protein) or R33[22L]F3 cell lysate (354 μg total protein, equivalent to 7.5 × 105 cells) that contained about the same amount of PrPSc (Fig. S3). At the time points indicated, four mice of each series were culled. Two brains were frozen for CPA analysis, and two were fixed for immunohistopathology. (A) (Upper) The CPAs of brains infected with brain[22L] were similar throughout the course of infection; the RIPK1/RIPK1,swa values fluctuated between 0.6 and 1.0. (Lower) Brains infected with R33[22L]F3 lysate showed little overall infectivity at 74 dpi; at 89 dpi there was partial swa resistance on PK1 cells and little response on R332H11 cells, but at 114 dpi there was complete swa resistance and R332H11 competence. (B) RIs measured on PK1 cells, as a function of time; data were taken from an independent set of SSCAs carried out on the same samples as in A but also including the terminal stage of disease. (C) PrPSc levels in brain homogenates in the same samples as above. Proteinase K-digested samples were subjected to Western blot analysis. Bands were recorded by CCD imaging and quantified. (D) The RICAD/RIR33,2H11 ratios are reproducibly higher for cell-derived 22L prions passaged once through brain (brain{R33[22L]F3}) than for “authentic” brain-derived 22L prions (brain[22L]) or for prions passaged twice through brain [brain(brain{R33[22L]F3})]. The data are from Fig. S5.
Thus, early after inoculation the prion population consisted mainly of cell-adapted prions, but as replication progressed, brain-adapted variants, either preexistent in the inoculum or arising during propagation, were amplified preferentially. Fig. 5 B and C depicts the time course of infectivity and PrPSc accumulation, respectively, in the brains of mice injected with brain[22L] homogenate or R33[22L]F3 lysate; strikingly, although about equivalent amounts of PrPSc had been injected (Fig S3), the accumulation of infectivity, as measured on PK1 cells, was much slower for R33[22L]F3 prions, reaching only about 15% of the brain[22L] value by 129 dpi. Moreover, mice injected with R33[22L]F3 lysate became terminally sick around 180 dpi, about 30 d later than the brain[22L] controls, when the infectivity level was about 40% of the controls. These data show that in mice R33[22L]F3 PrPSc has a lower specific infectivity than brain[22L] PrPSc and that, presumably, infectivity and pathogenicity increased significantly only as brain-adapted prions were selectively amplified.
Finally, C57BL/6 mice were inoculated with serial dilutions of (i) brain[22L] homogenate and (ii) brain{R33[22L]F3} homogenate (i.e., brain homogenate of terminally sick mice that had been inoculated with R33[22L]F3 lysates) to give brain(brain{R33[22L]F3}), that is, second passage of R33[22L]F3 prions in brain. Both groups became terminally sick in a dose-dependent fashion at about the same time, from about 146–149 d for the highest dose to 243–254 d for the lowest dose (Table 1). Fig. 5D and Fig. S5 show that the RICAD/RIR33 ratios, which reflect R33 competence, differed significantly for brain[22L] and brain{R33[22L]F3} homogenates, namely 9.8 ± 0.3 versus 32.7 ± 1.2 at terminal stage of disease. However, after the second mouse transfer the ratio was reduced to 13 ± 0.7, i.e., similar to that of brain[22L].
Table 1.
Incubation times of C57BL/6 mice inoculated with various dilutions of brain[22L] or brain{R33[22L]F3} homogenate
| Incubation time (dpi) |
||
| Inoculum dilution | Brain[22L] | Brain{R33[22L]F3} |
| 10−2 | 149 | 146 |
| 10−5 | 170 | 177 |
| 10−6 | 200 | 200 |
| 10−7 | 243 | 254 |
Four C57BL/6 mice each were inoculated with 30 μL of the brain homogenate dilutions indicated and were culled at the times indicated, when they exhibited clear symptoms of mouse scrapie.
The conformational stability assay indicated that the Gnd1/2 values for PrPSc from brain homogenate of the first and second mouse transmission were indistinguishable from “authentic” brain-derived 22L, about 1.3–1.4 M (Fig. S6), but were distinctly lower than 1.7 M for PrPSc from R33[22L]F3 lysate, which had been centrifuged and resuspended in PrP knockout brain homogenate to provide a similar milieu (the Gnd1/2 values determined in brain homogenate are slightly higher than those in cell lysate; Fig. 3). Thus, transmission of PrPSc from R33[22L]F3 cells through brain resulted in decreased conformational stability.
Immunohistopathology revealed that i.c. inoculation of mice with 1% homogenate of brain[22L] or of brain{R33[22L]F3} gave rise to indistinguishable PrPSc deposition patterns (Fig. S7 A and B). Both groups showed intense vacuolation of cerebellar cortex and loss of Purkinje cells, typical for 22L (Fig. S7C).
Thus, R33[22L]F3 prions propagated twice through mouse brain gradually reacquired the characteristics of the original brain[22L] prions by four independent criteria, namely cell tropism, incubation time, immunohistopathology, and conformational stability of the cognate PrPSc.
Discussion
The 22L prions transferred from brain to an R33 cell population retained R33 competence and swa resistance for at least 100 doublings; R33 competence was enforced by the host cells, and swa resistance apparently was linked to R33 competence. When prions were transferred from R33 to PK1 cells, the population gradually became R33 incompetent and swa sensitive, unless propagation was in the presence of swa, in which case swa resistance and R33 competence were retained. This result shows that, once the constraint imposed by R33 cells or by swa in PK1 cells is relaxed, the R33-incompetent variant outgrows its R33-competent and swa-resistant counterpart.
Surprisingly, the prions secreted by some 22L-infected R33 cell clones showed diverse CPA patterns, ranging from complete swa resistance and R33 competence in R33[22L]AA8 to complete swa sensitivity and R33 incompetence, as in R33[22L]F3. We discovered that the properties of R33 cells recovered by curing the R33[22L]F3 clone differed from those of the original R33 population, in that they had become more like those of PK1 cells, i.e., moderately permissive to RML and slightly permissive to R33[22L]F3-derived prions. We did not examine the other R33[22L] clones, but the diverse CPA patterns probably reflect different degrees of constraint imposed by variant R33 cells. As shown earlier, the susceptibility of PK1-derived subclones to prions can vary greatly (3).
It was of particular interest that when R33-incompetent, swa-sensitive R33[22L]F3-derived prions were returned to mouse brain, they gradually became R33 competent and swa resistant, suggesting that prions with a growth advantage in the host cell were being selected. Moreover, after the first passage through mice, the cell-derived 22L prion population had not yet fully acquired the original brain[22L] characteristics; this acquisition occurred only after the second mouse passage. It was shown earlier that various prion strains, after having been passaged in cell lines and then returned to brain, exhibited their original strain-specific incubation times and histopathological patterns (11–13). In all these cases, a change in the strain properties of the prions while propagating in cells could not be examined because appropriate tools, such as the CPA, were not available.
In summary, our data support the proposal that prion populations become heterogeneous because of mutation-like events and constitute “quasispecies” (14, 15), as documented for RNA phages (16) and animal RNA viruses and retroviruses (17), and that variants replicating most rapidly in a particular environment eventually become dominant (5, 18). The different conformational stability of PrPSc associated with swa-sensitive/R33-incompetent and swa-resistant/R33-competent prions suggests that variant-specific properties are enciphered by PrPSc (8, 19–21) and that prion “mutations” are the result of self-perpetuating conformational changes arising during propagation. Thus, prions, although devoid of a nucleic acid genome, are subject to mutation-like events and selective replication.
Methods
The 22L strain, cloned by two successive end-point dilutions, was from the TSE Resource Centre (5). The CPA is based on the SSCA performed as described in refs. 1–3, except that 5,000 cells were exposed to 1:3 serial dilutions of the prion preparation for 4 d. In addition, assays on PK1 cells were performed in the presence or absence of 2 μg swa/mL. During the course of this work, we isolated a subclone of R33, designated R332H11, which was about 10-fold more responsive to 22L than R33 and replaced R33 in the more recent CPAs.
Full methods and associated references are available in SI Methods.
Supplementary Material
Acknowledgments
We thank Y. Karapetyan for the immunohistopathology, C. A. Demczyk for assistance with cell culture, E. W. Smith for assistance with the SSCA, and A. Sherman for animal work. We thank C. Lasmezas for critical reading of the manuscript. The project was supported by National Institutes of Health Grant NSO59543 and by a grant from the Alafi Family Foundation (to C.W.).
Footnotes
The authors declare no conflict of interest.
*This Direct Submission article had a prearranged editor.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1013014108/-/DCSupplemental.
References
- 1.Klöhn PC, Stoltze L, Flechsig E, Enari M, Weissmann C. A quantitative, highly sensitive cell-based infectivity assay for mouse scrapie prions. Proc Natl Acad Sci USA. 2003;100:11666–11671. doi: 10.1073/pnas.1834432100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mahal SP, Demczyk CA, Smith EWJ, Klohn PC, Weissmann C. Assaying prions in cell culture: The standard scrapie cell assay (SSCA) and the scrapie cell assay in end point format (SCEPA) In: Hill AF, editor. Methods in Molecular Biology: Prions. Vol. 459. Totowa, NJ: Humana; 2008. [DOI] [PubMed] [Google Scholar]
- 3.Mahal SP, et al. Prion strain discrimination in cell culture: The cell panel assay. Proc Natl Acad Sci USA. 2007;104:20908–20913. doi: 10.1073/pnas.0710054104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Elbein AD, Solf R, Dorling PR, Vosbeck K. Swainsonine: An inhibitor of glycoprotein processing. Proc Natl Acad Sci USA. 1981;78:7393–7397. doi: 10.1073/pnas.78.12.7393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Li J, Browning S, Mahal SP, Oelschlegel AM, Weissmann C. Darwinian evolution of prions in cell culture. Science. 2010;327:869–872. doi: 10.1126/science.1183218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ghaemmaghami S, et al. Continuous quinacrine treatment results in the formation of drug-resistant prions. PLoS Pathog. 2009;5:e1000673. doi: 10.1371/journal.ppat.1000673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Peretz D, et al. A change in the conformation of prions accompanies the emergence of a new prion strain. Neuron. 2002;34:921–932. doi: 10.1016/s0896-6273(02)00726-2. [DOI] [PubMed] [Google Scholar]
- 8.Bessen RA, Marsh RF. Biochemical and physical properties of the prion protein from two strains of the transmissible mink encephalopathy agent. J Virol. 1992;66:2096–2101. doi: 10.1128/jvi.66.4.2096-2101.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Caughey B, Raymond GJ. Sulfated polyanion inhibition of scrapie-associated PrP accumulation in cultured cells. J Virol. 1993;67:643–650. doi: 10.1128/jvi.67.2.643-650.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Büeler H, et al. Mice devoid of PrP are resistant to scrapie. Cell. 1993;73:1339–1347. doi: 10.1016/0092-8674(93)90360-3. [DOI] [PubMed] [Google Scholar]
- 11.Birkett CR, et al. Scrapie strains maintain biological phenotypes on propagation in a cell line in culture. EMBO J. 2001;20:3351–3358. doi: 10.1093/emboj/20.13.3351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Arima K, et al. Biological and biochemical characteristics of prion strains conserved in persistently infected cell cultures. J Virol. 2005;79:7104–7112. doi: 10.1128/JVI.79.11.7104-7112.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Arjona A, Simarro L, Islinger F, Nishida N, Manuelidis L. Two Creutzfeldt-Jakob disease agents reproduce prion protein-independent identities in cell cultures. Proc Natl Acad Sci USA. 2004;101:8768–8773. doi: 10.1073/pnas.0400158101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Eigen M. On the nature of virus quasispecies. Trends Microbiol. 1996;4:216–218. doi: 10.1016/0966-842X(96)20011-3. [DOI] [PubMed] [Google Scholar]
- 15.Epstein IR, Eigen M. Selection and self-organization of self-reproducing macromolecules under the constraint of constant flux. Biophys Chem. 1979;10:153–160. doi: 10.1016/0301-4622(79)85035-8. [DOI] [PubMed] [Google Scholar]
- 16.Domingo E, Sabo D, Taniguchi T, Weissmann C. Nucleotide sequence heterogeneity of an RNA phage population. Cell. 1978;13:735–744. doi: 10.1016/0092-8674(78)90223-4. [DOI] [PubMed] [Google Scholar]
- 17.Domingo E, et al. Viruses as quasispecies: Biological implications. Curr Top Microbiol Immunol. 2006;299:51–82. doi: 10.1007/3-540-26397-7_3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Collinge J, Clarke AR. A general model of prion strains and their pathogenicity. Science. 2007;318:930–936. doi: 10.1126/science.1138718. [DOI] [PubMed] [Google Scholar]
- 19.Telling GC, et al. Evidence for the conformation of the pathologic isoform of the prion protein enciphering and propagating prion diversity. Science. 1996;274:2079–2082. doi: 10.1126/science.274.5295.2079. [DOI] [PubMed] [Google Scholar]
- 20.Legname G, et al. Continuum of prion protein structures enciphers a multitude of prion isolate-specified phenotypes. Proc Natl Acad Sci USA. 2006;103:19105–19110. doi: 10.1073/pnas.0608970103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Angers RC, et al. Prion strain mutation determined by prion protein conformational compatibility and primary structure. Science. 2010;328:1154–1158. doi: 10.1126/science.1187107. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.