Abstract
The proton-coupled folate transporter (PCFT; SLC46A1) mediates folate transport into enterocytes in the proximal small intestine; pcft loss-of-function mutations are the basis for hereditary folate malabsorption. The current study explored the roles of Asp residues in PCFT function. A novel, homozygous, loss-of-function mutation, D156Y, was identified in a child of Pakistani origin with hereditary folate malabsorption. Of the 6 other conserved Asp residues, only one, D109, is shown to be required for function. D156Y, along with a variety of other substitutions at this site (Trp, Phe, Val, Asn, or Lys), lacked function due to instability of the PCFT protein. Substantial function was preserved with Glu, Gly, and, to a lesser extent, with Ser, Thr, and Ala substitutions. This correlated with PCFT bio-tinylated at the cell surface. In contrast, all D109 mutants, including D109E, lacked function irrespective of pH (4.5, 5.5, and 7.4) or substrate concentration (0.5-100μM), despite surface expression comparable to wild-type PCFT. Hence, D156 plays a critical role in PCFT protein stability, and D109, located in the first intracellular loop between the second and third transmembrane domains, is absolutely required for PCFT function.
Introduction
The folate B9 vitamins are essential cofactors for one-carbon metabolic reactions required for de novo synthesis of nucleotides, methionine, and for methylation reactions.1 These hydrophilic molecules require specific membrane-transport processes to reach the metabolic machinery within cells. The major transporter that delivers folates to systemic tissues at their ambient neutral pH is the reduced folate carrier (RFC).1,2 High-affinity binding proteins mediate the transport of folates into cells by an endocytic process.3,4 Folate absorption at the acid microenvironment of the proximal small intestinal brush-border membrane was recently shown to be mediated by a proton-coupled folate transporter (PCFT; SLC46A1; NP_54200).5 PCFT's critical role in this process, along with transport across the blood:choroid plexus:cerebrospinal fluid (CSF) barrier, was established by the demonstration of loss-of-function mutations in the pcft gene in subjects with the autosomal recessive disorder, hereditary folate malabsorption (HFM; Online Mendelian Inheritance in Man [OMIM] No. 229050).5,6 Since then, additional subjects with hereditary folate malabsorption (HFM) and loss-of-function PCFT mutations have been reported by this and other groups.7–12 Mechanisms of folate transport and homeostasis were recently reviewed.1
PCFT uses a proton gradient to drive the uphill transport of folates into cells; transport is optimal at low pH, is electrogenic, and is accompanied by cellular acidification. As the pH is increased, there is a progressive decrease in the influx maximum velocity (Vmax) and increase in the influx Michaelis-Menten constant (Km). However, transport persists even at neural pH in the absence of a pH gradient driven, at least in part, by the voltage gradient across the cell membrane.5,13–16
Information on specific residues that play an important role in function is emerging. H281 in the seventh transmembrane domain is a determinant of folate binding through its impact on proton binding. H247, in the third large intracellular loop, appears to form a hydrogen bond with S172 in the second intracellular loop, thereby regulating access to the translocation pathway.17 E185, in fifth transmembrane domain (TMD), was found to be essential for proton coupling.16 Most recently, the 12-transmembrane-domain PCFT secondary structure was confirmed by the substituted cysteine-accessibility method, and a sulfhydryl bond between cysteines in 2 extracellular loops was defined.18 Studies on PCFT mutations associated with HFM also provide insights into residues critical for carrier function. The R113 residue was shown to be mutated in 2 subjects with HFM, and its absence results in a profound loss of function irrespective of the substituted residue.6,8,19
The current study encompasses an evaluation of the role of Asp residues in PCFT function. Only 2 of the 7 conserved Asp residues were found to be important. D156, mutated in a patient with HFM, was shown to play a critical role in protein stability; D109 is absolutely required for function.
Methods
Patient
The female patient was born in Pakistan to consanguineous parents. Her mother declined taking supplementary folic acid during her pregnancy and, at the time of delivery, had a hemoglobin level of 9.8 g/dL. At 2.5 months of age, the infant developed megaloblastic anemia and was initially treated with blood transfusions, oral folic acid, and injections of vitamins. Anemia persisted, and at the age of 8 months, after she had moved to Canada, the patient developed diarrhea and lethargy. Her hemoglobin level was 6.6 g/dL, mean corpuscular volume 83 fL, and serum folate 7.2 nmol/L (normal, 11.8-59.4 nmol/L); RFC folate was low, < 117 nmol/L (normal, 582-2701 nmol/L). Her vitamin B12 level was normal. Bone marrow biopsy was hypercellular, with both megaloblastic and dysplastic changes. Oral folic acid was continued, but she had frequent episodes of vomiting and/or diarrhea. At 11 months of age, the patient developed seizures. A head computed tomography demonstrated hyperdense cortical/subcortical areas in both the posterior parietal and occipital lobes. By 12 months of age, her weight and length had reached a plateau, dropping from the 25th and 50th percentiles, respectively, to the third percentile. Head circumference was at the third percentile. At 13 months of age, serum and red blood cell folate levels were normal on continued oral folic acid supplementation, but a cerebrospinal fluid (CSF) folate level obtained for the first time was < 5 nmol/L (normal, 2-3× the normal serum folate concentration). Serum immunoglobulins were normal. The seizures resolved with anticonvulsant therapy and supplementation with 1.6 mg/kg/d of 5-formyl-THF (tetrahydrofolate) orally. Two months later, the CSF folate level increased to 21 nmol/L, but the CSF:serum folate ratio remained < 0.5. A magnetic resonance image of the head at 15 months of age showed normal gray-white matter differentiation with no signal abnormality on the standard sequences. At the age of 18 months, the patient was above the 95th percentile for weight, had normal developmental milestones, and a normal neurologic assessment, except for some speech delay. At present, the patient's folate supplementation is 5 mg/d of parenteral 5-formyl-THF.
Identification of a PCFT mutation in a patient with HFM
This study was approved by the Albert Einstein College of Medicine's Clinical Committee of Investigation in accordance with the Declaration of Helsinki. After written informed consent was obtained, peripheral blood was collected from the patient and family members. Genomic DNA was isolated at the Albert Einstein College of Medicine DNA Isolation and Cell Expansion Core. Each of the 5 PCFT exons, with their splice acceptors and donor sites, were amplified by polymerase chain reaction (PCR) using primers reported previously.5 PCR products were analyzed on an agarose gel, purified by a gel purification kit (GE Healthcare) and sequenced at the Albert Einstein College of Medicine Cancer Center Genomics Shared Resource.
Chemicals
Methotrexate (MTX-disodium salt, (3′, 5′, 7-[3H](N)) was obtained from Moravek Biochemicals Inc. and purified by liquid chromatography before use as previously described.20 Unlabeled MTX was purchased from Sigma-Aldrich; EZ-Link Sulfo-NHS-LC-Biotin (sulfosuccin-imidyl-6-[biotinamido] hexanoatep) was from Pierce Biotechnology; streptavidin-agarose beads was from Fischer Scientific; and protease-inhibitor cocktail was from Roche Applied Science. MG132 (N-CBZ-Leu-Leu-Leu-AL) was purchased from Sigma-Aldrich, and bafilomycin A1 was from LC Laboratories.
Construction of mutant plasmids by site-directed mutagenesis
Mutations were introduced into PCFT cDNA with the Quick change II XL site-directed mutagenesis kit (Stratagene). Primers for each mutation are listed in Table 1. Mutations in the plasmid constructs were verified by DNA-sequencing analysis. A PCFT pcDNA3.1(+) expression vector, which encodes hemagglutinin (HA)–tagged human PCFT at the C-terminus, was used as the template for all site-directed mutants.21
Table 1.
Forward primers used for site-directed mutagenesis of Asp mutants
| D156E | GCCCTCCTCGGCGAGTTCGGTGGCCTTC |
| D156N | GCCCTCCTCGGCAACTTCGGTGGCCTTC |
| D156K | GCCCTCCTCGGCAAATTCGGTGGCCTTC |
| D156A | GCCCTCCTCGGCGCCTTCGGTGGCCTTC |
| D156Y | GCCCTCCTCGGCTATTTCGGTGGCCTTC |
| D156S | GCCCTCCTCGGCTCCTTCGGTGGCCTTC |
| D156W | GCCCTCCTCGGCTGGTTCGGTGGCCTTC |
| D156F | GCCCTCCTCGGCTTCTTCGGTGGCCTTC |
| D156C | GCCCTCCTCGGCTGCTTCGGTGGCCTTC |
| D156T | GCCCTCCTCGGCACTTTCGGTGGCCTTC |
| D156V | GCCCTCCTCGGCGTTTTCGGTGGCCTTC |
| D156G | GCCCTCCTCGGCGGTTTCGGTGGCCTTC |
| D109E | GGAGCTTGGAGCGAAAGTGTGGGCCGC |
| D109N | GGAGCTTGGAGCAACAGTGTGGGCCGC |
| D109K | GGAGCTTGGAGCAAAAGTGTGGGCCGC |
| D109A | GGAGCTTGGAGCGCCAGTGTGGGCCGC |
| D109G | GGAGCTTGGAGCGGTAGTGTGGGCCGC |
| D109S | GGAGCTTGGAGCTCCAGTGTGGGCCGC |
| D54K | CGCTTCAGCGCCAAACTCGGCTACAATG |
| D72K | AACCGCAGCGCGAAACCCACCATGCAGGAA |
| D170K | GCGTCCGTGGCAAAAGTCAGCTCCAGTCGC |
| D286K | TTTGGGGCCCAGAAAATCTTAACCCTT |
| D331E | TACTGCCTGGCCGAAGCCTGGGTAGCT |
| D331N | TACTGCCTGGCCAACGCCTGGGTAGCT |
| D331K | TACTGCCTGGCCAAAGCCTGGGTAGCT |
| D331A | TACTGCCTGGCCGCCGCCTGGGTAGCT |
Cell lines, cell-culture conditions, and transient transfection
HeLa-R1-11 cells are a more stable subclone of the HeLa R1 cell line that lacks both RFC and PCFT expression, the former due to a genetic deletion,22 the latter due to methylation of the promoter and loss of gene copies.23 R1-11 cells have been maintained in RPMI 1640 medium supplemented with 5% fetal bovine serum, 100 U/mL penicillin, and 100 μg/mL streptomycin at 37°C in a humidified atmosphere of 5% CO2. For transient transfection, R1-11 cells (3.5 × 105/vial or 6.0 × 105/well) were seeded into 17-mm liquid scintillation vials or 6-well plate and grown for 48 hours. The mutant cDNA constructs (0.8 μg or 2 μg of each plasmid) were transiently transfected into the cells with Lipofectamine 2000 (Invitrogen). The cells were processed for transport or Western blot analysis 2 days later.
Membrane-transport analyses (functional characterization)
A technique for rapid measurement of [3H]-MTX influx was employed.24 R1-11 transfectants were washed twice with 2 mL of HEPES (N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid)–buffered saline (HBS) (20mM HEPES, 140mM NaCl, 5mM KCl, 2mM MgCl2, and 5mM dextrose at pH 7.4) and incubated in the same buffer in a water bath (37°C) for 20 minutes. The buffer was then aspirated after which transport buffer, 500 μL of HBS or MES (2-[morpholino]ethanesulfonic acid)–buffered saline (MBS; 20mM MES, 140mM NaCl, 5mM KCl, 2mM MgCl2, and 5mM dextrose) containing [3H]-MTX was added. [3H]-MTX uptake was halted after 1 minute by the addition of 10 volumes of ice-cold HBS buffer at pH 7.4, an interval over which uptake was unidirectional. Cells were then washed 3 times with ice-cold HBS buffer, after which 500 μL of 0.2M NaOH was added, and cells were digested by incubation for 30-45 minutes at 65°C. A portion of the hydrolysate was then transferred to a vial, fluor was added, and intracellular radioactivity was assessed on a liquid scintillation spectrometer. The protein content of another portion of hydrolysate was determined using the Pierce kit. Influx was expressed as pmoles of [3H]-MTX per milligram of protein per minute, and data were reported as mean ± SEM from at least 3 independent experiments performed on different days.
Cell surface biotinylation assay and Western blot analysis
These assays have been described previously.16 Briefly, 2 days after transfection, cells were washed twice with PBS (2 mL) at pH 8.0 and treated with 1 mg/mL EZ-Link Sulfo-NHS-LC-Biotin in PBS (pH 8.0) at room temperature for 30 minutes. Cells were washed twice and treated with hypotonic buffer (0.5mM Na2HPO4 and 0.1mM EDTA [ethylenediaminetetraacetic acid] at pH 7.0), containing protease inhibitors, and kept on ice for 30 minutes. Cells were then scraped from the plates and centrifuged at 14 000 rpm for 15 minutes at 4°C. The membrane fraction was pelleted and resuspended in 400 μL of lysis buffer (0.1% sodium dodecyl sulfate [SDS], 1% Triton X-100, 1mM EDTA, 150mM NaCl, 20mM Tris; pH 7.4), containing protease inhibitors, for 30-120 minutes at 4°C (rotating in a cold room). After centrifugation at 14 000 rpm at 4°C for 15 minutes, 25 μL of supernatant was taken from each sample for Western blot analysis (designated as the crude membrane-protein fraction). The remaining supernatant was mixed with streptavidin-agarose beads (50 μL, that had been prewashed 3 times with lysis buffer) overnight at 4°C. The beads were washed 4 times with the lysis buffer (500 μL) containing protease inhibitors. After the final wash, beads-bound protein was stripped by heating for 5 minutes at 95° in 2× SDS-polyacrylamide gel electrophoresis (PAGE) loading buffer, containing dithiothreitol (DTT), and loaded directly on polyacrylamide gels. The crude membrane fraction was mixed with 2× SDS-PAGE loading buffer (1:1), containing DTT, before Western blot analysis. For the crude membrane samples, blots were first probed with a rabbit β-actin antibody (#4967L; Cell Signaling Technology), then stripped with stripping buffer (100mM 2-β-mercaptoethanol, 2% SDS, 62.5mM Tris-HCl; pH 6.7) and reprobed with anti-HA antibody (H6908; Sigma-Aldrich). For biotinylated samples, blots were directly probed with anti-HA antibody.
Results
Identification of a D156Y mutation in a subject with HFM
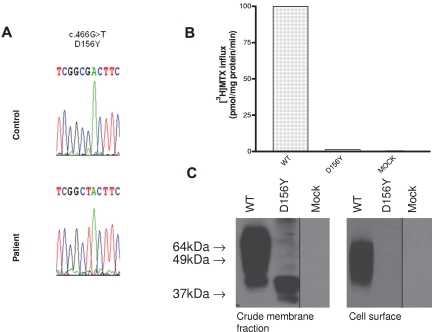
A novel pcft mutation was identified in the patient described above (c466 G>T) located in exon 2 at position “562” of the cDNA sequence (NM_080669), as indicated in Figure 1A. Both parents were heterozygous for the same mutation. This resulted in a homozygous Asp to Tyr substitution at the D156 residue (NP 54 200.2). As shown in Figure 1B, no transport function could be detected in HeLa R1-11 cells transfected with the D156Y mutant. On Western blot of the mutated PCFT, HA-tagged at the C-terminus, a protein with a lower molecular weight (MW) than wild-type PCFT was identified in the crude cell-membrane fraction; however, no protein was detected at the cell surface (Figure 1C).
Figure 1.
Identification of a novel D156Y PCFT mutation in a subject with HFM and its expression as well as transport function. (A) A chromatogram showing a homozygous mutation in the PCFT gene in a subject with HFM. (B) [3H]-MTX (0.5μM) influx over 1 minute at pH 5.5 and 37°C in Hela-R1-11 cells transiently transfected with wild-type PCFT, D156Y-PCFT, or the vector (mock). (C) Western blot analysis of cells transiently transfected with wild-type PCFT, the D156Y mutant, or vector: crude cell membrane fraction (left); biotinylated protein at the cell surface (right). Vertical lines have been inserted to indicate repositioned gel lanes.
Initial screening of PCFT Asp mutants
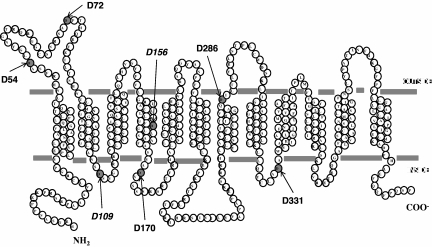
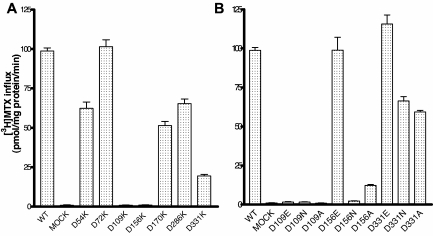
PCFT contains 7 aspartic acid residues. Six are fully conserved (D72, D109, D156, D170, D286, and D331) among species (to xenopus and zebrafish); one is semiconserved (D54). The location of these residues is illustrated in Figure 2, based upon the current understanding of the PCFT secondary structure.18 D156 is located in the fourth TMD. To develop a broader understanding of the role of Asp residues in PCFT function, PCFT mutants with a drastic change to the opposite-charged Lys were evaluated by the assessment of [3H]-MTX influx at pH 5.5 in cells transiently transfected with these constructs. As indicated in Figure 3A, the only mutants that were not functional were D109K, and, as expected, the residue mutated in the subject with HFM (D156K). Hence, D54, D72, D170, and D286 are not required for function, since a marked change at these positions preserved more than 50% of activity. Approximately 25% of activity was observed for the D331K mutant.
Figure 2.
A topologic model for human PCFT.18 All 7 Asp residues are indicated. D54 is semiconserved; D72, D109, D156, D170, D286, and D331 are fully conserved among species (monkey, horse, rat, mouse, dog, cow, opossum, xenopus, and zebra fish). D156 is mutated in the subject with HFM.
Figure 3.
Functional assessment of PCFT aspartate mutants. [3H]-MTX influx was assessed at pH 5.5 and 37°C over 1 minute at a concentration of 0.5μM in Hela-R1-11 transfectants. (A) Initial screening of Asp mutants. (B) Studies focused on D109, D156, and D331 residues replaced with Glu, Asp, or Ala. Data are the mean ± SEM from 3 independent experiments.
To further explore the role and structural requirements of the D109, D156, and D331 residues, each was replaced with Glu, Asn, or Ala. As indicated in Figure 3B, an important role for D331 was excluded by the retention of function, despite replacement with a neutral (Ala) or polar (Asn) residue. Full function was retained with the like-charged D331E substitution. No function was retained with any of the substitutions at the D109 residue, including replacement with the like-charged Glu. On the other hand, replacement of the D156 residue with Glu fully preserved function, replacement with Ala preserved approximately 15% of function, and substitution with Asn resulted in a complete loss of activity. Therefore, only D109 and D156 appeared to be critical for PCFT function; further studies focused on these 2 residues.
Expression and function of D156 PCFT mutants
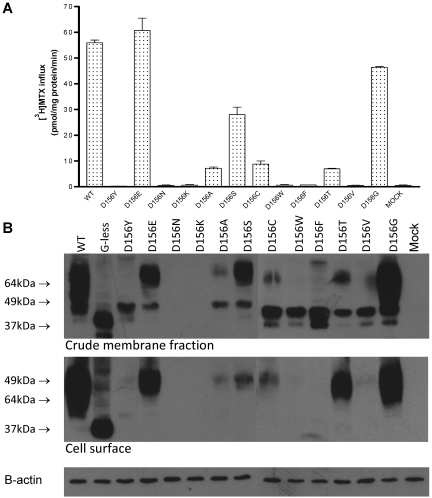
In addition to D156Y, -E, -N, -K, and -A, 7 more mutants were generated at this position. As indicated in Figure 4A, substantial function was retained with replacement by Gly (80%) and, to an appreciable—though lesser—extent, with serine (50%). A low level (∼ 15%) of activity was observed with Ala, Cys, and Thr. Substitution with Tyr, Phe, and Val resulted in a complete loss of activity. Hence, while a negative charge is preferred at this site, nearly full function can be achieved with neutral and polar replacements.
Figure 4.
Expression and function of D156 PCFT mutants. (A) [3H]-MTX influx was performed at 0.5μM and pH 5.5 over 1 minute. Data are the mean ± SEM from 3 independent experiments performed on different days. (B) Western blot assay of the crude cell-membrane fraction (top panel) and biotinylated protein at the cell surface (middle panel). β-actin was the loading control (bottom panel). These blots are representative of 3 experiments and were performed on 2 gels at the same time.
PCFT expression in both the crude cell-membrane fraction and at the cell surface was assessed for all 12 D156 mutants. It can be seen that little or no protein could be detected at the MW of wild-type PCFT in the crude membrane fractions, or at the cell surface after biotinylation, for the mutants that lack function (Figure 4B). On the other hand, when some function was preserved, protein was detected both in the crude membrane fraction and at the cell membrane. The mutants with the greatest function tended to have the highest levels of biotinylated protein (D156E, -S, and -G). For most of the mutants, except D156N and D156K, a protein of lower MW than wild-type PCFT was detected. This band approximated the MW of deglycosylated PCFT (G-less) in the crude membrane fraction, but not at the plasma membrane, indicating that this protein failed to traffic to the surface of the cell.
Further studies were designed to address whether these bands represent deglycosylated forms of PCFT or degraded products of the protein. D156Y was selected as a representative mutant. Instead of the HA tag at the C-terminus, this mutant was HA-tagged at the N-terminus. As previously documented, wild-type and deglycosylated-PCFT activities remain the same, regardless of the position of HA tag.21 No mutant protein could be detected on Western blot of the crude membrane fraction (data not shown). Hence, the low MW forms detected with this mutant and, presumably, with the other mutants are not deglycosylated PCFT; rather, these represent the degradation products of the mutant proteins.
Possible roles for ubiquitination and proteasomal degradation in the instability of the mutated D156Y PCFT protein were assessed with MG132 (proteasome inhibitor) and bafilomycin A1 (ubiquitin inhibitor). To optimize concentrations of these inhibitors, growth inhibition was assessed 24 hours after the treatment of R1-11 cells at various inhibitor concentrations; 0.5μM MG132 and 10nM bafilomycin A1 resulted in approximately 20% inhibition of cell growth and were used at these concentrations. In 3 separate experiments, treatment of cells transfected with the D156Y PCFT, wild-type PCFT, or the vector with these reagents did not result in any change in [3H]-MTX influx (data not shown). Further studies, described below, were then undertaken to determine whether the decrease in function of D156G and D156S mutants could be attributed entirely to decreased PCFT expression at the plasma membrane or whether there was, in addition, impaired intrinsic function/properties of the mutated carrier.
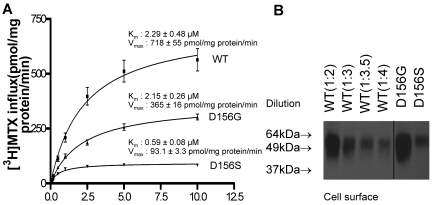
Influx kinetics mediated by D156 mutant PCFTs
Figure 5 illustrates [3H]-MTX influx kinetics mediated by D156G and D156S PCFT mutants. The influx Km for D156G was unchanged, but was decreased by a factor of approximately 4 for D156S, compared with wild-type PCFT, consistent with a substantial increase in the affinity of the latter mutant for its substrate. The Vmax was decreased for both mutants, but to a greater extent for the Ser than Gly mutant, in comparison to wild-type PCFT, generally consistent with the levels of expression at the cell surface. To more quantitatively compare the levels of protein, biotinylated protein was diluted with loading buffer before electrophoresis. A 1:2 dilution (1 part lysate + 1 part buffer) of the wild-type PCFT produced a band similar to that of D156G; a 1:3.5 dilution of wild-type PCFT produced a band similar to that of D156S, further supporting the likelihood that Vmax differences were largely due to differences in the levels of protein at the cell surface. Hence, amino acid substitutions at this site do not appear to impair folate substrate binding nor carrier function.
Figure 5.
MTX influx kinetics mediated by D156 PCFT mutants. (A) [3H]-MTX influx kinetics mediated by D156G and D156S PCFT, compared with wild-type PCFT. Influx was assessed at pH 5.5 at 37°C over 1 minute with [3H]-MTX concentrations of 0.1, 0.2, 0.5, 1, 2.5, 5, and 10μM. Data are the mean ± SEM from 3 independent experiments. (B) Western blot of biotinylated protein obtained from cells transfected with wild-type PCFT and the D156G and D156S mutants. Wild-type PCFT was diluted to more accurately quantify, by comparison, levels of mutant PCFTs. Ten μL of the biotinylated wild-type PCFT protein fraction was added to 10, 20, 25, and 30 μL of 2× SDS-loading buffer, described as 1:2, 1:3, 1:3.5, and 1:4 dilutions, respectively. The data are representative of 2 independent experiments. A vertical line has been inserted to indicate a repositioned gel lane.
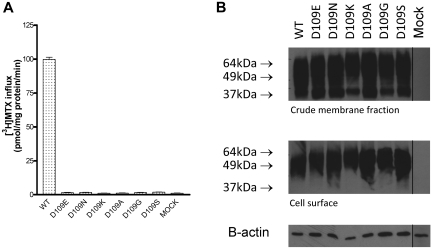
Expression and function of D109 PCFT mutants
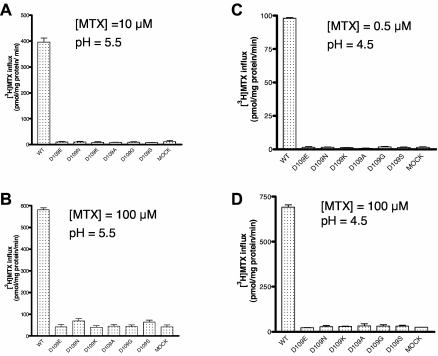
Six PCFT mutants were studied at the D109 position; the results were entirely different from what was observed for the D156 mutants. As indicated in Figure 6A, substitutions of D109 with -E, -N, -K, -A, -G, and -S residues resulted in a complete loss of function at pH 5.5 when the [3H]-MTX concentration was 0.5μM; this was the case even with the highly conservative -E mutant. Further, no transport activity was detected for these mutants, in comparison to the mock-transfected cells, at MTX concentrations of 10μM MTX at pH 5.5 (Figure 7A) or at 100μM MTX (Figure 7B) at pH 5.5, excluding the possibility that the loss of function is due to a decrease (of at least 200-fold) in PCFT affinity for MTX. To determine whether the loss of activity might be associated with a decrease in proton binding to the carrier, as observed for other PCFT mutants,17 the pH of transport buffer was decreased to 4.5; still, no activity could be detected for any of the mutants at a MTX level of 0.5μM (Figure 7C) or 100μM (Figure 7D). The trivial increase in uptake for the -N and -S mutants at 100μM MTX at pH 5.5 did not reach statistical significance and was not observed at all at pH 4.5. Nor was MTX influx preserved at pH 7.4, excluding defective proton coupling as a basis for the loss of activity of the D109 mutants (data not shown). Finally, in contrast to the D156 mutants, there was no decrease in protein expression in the crude membrane fraction nor in the level of surface biotinylation for any of the D109 mutants, thereby excluding alterations in protein stability or trafficking as a basis for the profound loss of function (Figure 6B).
Figure 6.
Functional properties of D109 PCFT mutants. (A) [3H]-MTX influx (0.5μM) over 1 minute at pH 5.5. Data are the mean ± SEM from 3 independent experiments. (B) Western blot assay of the crude cell-membrane fraction (top panel) and biotinylated protein (middle panel) of D109 PCFT mutants. β-actin was the loading control (bottom panel). The graph shown is a representative of 2 independent experiments. The vertical line between the D109S and Mock lanes indicates repositioned gel lanes.
Figure 7.
Impact of MTX concentration and pH on the activity of PCFT D109 mutants. (A) [3H]-MTX influx (10μM) over 1 minute at pH 5.5. (B) [3H]-MTX influx (100μM) at pH 5.5. (C) [3H]-MTX influx (0.5μM) at pH 4.5. (D) [3H]-MTX influx (100μM) at pH 4.5.
Discussion
This laboratory recently cloned PCFT and established the physiologic role this transporter plays in intestinal folate absorption and transport of folates across the blood:choroid plexus:CSF barrier by the defects in these processes in subjects with HFM.5–7 In the current study, the role of Asp residues was examined, one of which (D156Y) was shown to be mutated in a subject with HFM. Despite the fact that 5 Asp residues are fully conserved beyond D156, only one other residue, D109, was found to be important for function. However, the role of these 2 residues, and the impact of mutations at these sites, was very different.
The D156 residue, located in the mid-portion of the fourth TMD, is critical for protein stability. Loss of stability was observed with a variety of polar, neutral, or positively charged mutants. However, stability and trafficking to the cell membrane was substantially preserved with the Gly (relatively polar) or, to a lesser extent, with the Ser (polar) substitutions. It would appear that small size and polarity were associated with retention of stability. Low MW bands were observed with D156 mutants; this was also observed with deglycosylated forms of PCFT that are fully functional.21 However, these bands were shown to be degradation products, based upon the observation that low MW forms were detected only when PCFT mutants were HA-tagged at the C-terminus. The data suggest that loss of transport function can be attributed largely, if not entirely, to the extent of protein degradation. The influx Km for the Gly mutant was unchanged, whereas the decrease in influx Km for Ser mutant was consistent with an increased, rather than decreased, affinity for MTX. Differences in maximum velocity observed with these substitutions were due predominantly to differences in the level of PCFT protein at the cell surface. Hence, mutations at this site do not appear to alter the rate of conformational change during carrier cycling.
It is of interest that Asp residues in the Leishmania FT1 folate transporter, D514 and D529 (located in the eleventh TMD and sixth extracellular loop, respectively), are both required for protein stability.25 Substitution with Val resulted in a complete loss of protein, but stability was fully retained with a Glu substitution, similar to what was observed with D156Y. Extensive site-directed mutagenesis of the D156 PCFT suggests that polar substitutions and compact size favor stability. Hence, Gly (D156G) preserved the majority of transport function and polar residues, save for the larger Asn, sustained some function. Thr and Cys residues tend to form hydrogen bonds with carbonyl oxygen atoms in the preceding turn of helices, of particular importance for intrinsic membrane-transport proteins.26 The preservation of activity with the Gly and polar substitutions exclude participation of the D156 residue in a salt bridge.
The properties of the D109 residue were quite different. No substitution, irrespective of charge or polarity, preserved function. Indeed, even the most conservative substitution to Glu lacked function. In contrast to the D156 mutants, each D109 mutant protein was expressed and detected at the cell surface at levels comparable to that of wild-type PCFT. Activity could not be detected even at high MTX concentrations, excluding the possibility that loss of function was due to an increase in influx Km to at least 2 orders of magnitude above the wild-type PCFT influx Km. Likewise, a decrease in the affinity of a proton-binding site, that allosterically alters the conformation of the folate-binding pocket, as a factor was excluded by the lack of function even when the proton concentration was increased by an order of magnitude by decreasing the pH to 4.5. This is in contrast to what was observed for the H281A mutant, in which there was marked restoration of function with a similar increase in proton con-centration.17 Further, the lack of preservation of activity at pH 7.4 excludes a role for this residue in proton coupling. Based upon these findings, the D109 residue is essentially irreplaceable.
D109 is located in the first intracellular loop between the second and third TMDs. Mutations in this loop at R113 (R113S, R113C) were detected in 2 unrelated families with HFM,6,8 and, when this residue was mutated to a variety of other amino acids, there was a marked decrease in function, although some activity was preserved with like-charged substitutions.19 Hence, it would appear that the first intracellular loop plays an important role in PCFT function. This loop encompasses a D109XXGRR113 sequence required for PCFT trafficking to the apical membrane of polarized Caco2 and MDCK cells.27 Mutation of multiple residues to Ala (DSVAAA, AAAAAA) resulted in a trafficking defect in these cell lines. However, the current study demonstrates that in nonpolarized HeLa cells, mutation of the D109 residue does not alter protein expression, stability, or trafficking to the plasma membrane. It is not clear as to whether a single mutation at this or other sites in this loop would alter trafficking to the apical membrane of polarized cells.
The basis for the critical role the D109 residue plays in PCFT function is unknown. Multiple alignments of 81 nonhomologous protein families indicate that the Asp residue is more highly conserved than any other amino acid due to its short side chain, high charge density, strong polar interactions, and rigidity.28 Even Gly, which is small, highly compact and with polar properties, did not substitute for Asp, consistent with extreme spatial requirements at this site. Hence, in the absence of the Asp residue, substrate either does not bind to the carrier and/or oscillation of the carrier between its inward and outward facing conformations, intrinsic to the alternative-access mechanism, is impaired.29,30
HFM manifests within the first few months of life and, untreated, may lead to death in early infancy.31 However, it is not unusual for subjects to live for a year or more before appropriate therapy is instituted, although the chances of neurologic involvement and other complications increase as the interval between birth and treatment is increased. There are multisystem clinical manifestations of HFM. Patients always present with anemia, usually megaloblastic, and sometimes accompanied by neutropenia and thrombocytopenia. In some patients, the clinical presentation is dominated by an immune deficiency syndrome associated with hypogammaglobulinemia and infections, such as Pneumocystis jiroveci.6,9 Neurologic disorders characterized by developmental delays are common; seizures are less frequent, but can represent a major clinical challenge, particularly when treatment has been delayed. This patient, for whom adequate treatment was substantially delayed, developed seizures at 11 months of age. The clinical picture of HFM has been the subject of recent reviews.31,32
The neurologic consequences of the loss of PCFT function is associated with a failure of folate transport across the choroid plexus, where this carrier is highly expressed at the basolateral membrane.33 This results in very low levels of folate within the CSF. Even when the blood folate level is normalized, CSF folate levels remain low, as was the case in this patient. Indeed, the end point for treatment of HFM should be a CSF folate level that is comparable to the normal level for a child the age of the patient, which requires achieving supranormal blood levels. For instance, the normal CSF level for children age 0-1 year is ∼ 115nM, decreasing to ∼ 85nM by age 5 and ∼ 6nM by age 19.34 Beyond PCFT, 2 other folate transporters are expressed in the choroid plexus: (1) RFC at the apical membrane in apposition to the CSF35 and (2) folate receptor-α (FR-α), mainly expressed at the apical membrane, but present elsewhere as well in choroid plexus ependymal cells.1 While the role of RFC is not clear, it is clear that FR-α is required for folate transport across this epithelium, but the impact of loss of this protein differs temporally from what is observed when PCFT function is lost in HFM. Three families have now been reported with loss-of-function FR-α mutations.36,37 These subjects, with normal folate blood levels, develop neurologic signs of folate deficiency beyond the second year of life associated with very low CSF folate levels. The basis for this delay is not clear. The normal folate blood levels may have a protective effect, delaying neurologic damage; alternatively, other routes of transport across the choroid plexus may compensate for the absence of FR-α during the early years of life.
Acknowledgments
This work was supported by a grant from National Institutes of Health (CA-82621).
Footnotes
An Inside Blood analysis of this article appears at the front of this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Authorship
Contribution: D.S.S. performed the majority of laboratory experiments and wrote the manuscript; S.H.M. performed initial sequencing of the patient's mutation and functional analysis; L.R. provided the patient's history and blood specimen; R.Z. and I.D.G. equally contributed to guiding the design of the studies and editing of the manuscript; and A.F. provided bioinformatics support for this study.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: I. David Goldman, Departments of Medicine and Molecular Pharmacology, Albert Einstein College of Medicine, Chanin Two, 1300 Morris Park Ave, Bronx, NY 10461; e-mail: i.david.goldman@einstein.yu.edu.
References
- 1.Zhao R, Matherly LH, Goldman ID. Membrane transporters and folate homeostasis: intestinal absorption and transport into systemic com-partments and tissues. Expert Rev Mol Med. 2009;11:e4. doi: 10.1017/S1462399409000969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Matherly LH, Hou Z. Structure and function of the reduced folate carrier: a paradigm of a major facilitator superfamily mammalian nutrient transporter. Vitam Horm. 2008;79C:145–184. doi: 10.1016/S0083-6729(08)00405-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Salazar MD, Ratnam M. The folate receptor: what does it promise in tissue-targeted therapeutics? Cancer Metast Rev. 2007;26(1):141–152. doi: 10.1007/s10555-007-9048-0. [DOI] [PubMed] [Google Scholar]
- 4.Kamen BA, Smith AK. A review of folate receptor alpha cycling and 5-methyltetrahydrofolate accumulation with an emphasis on cell models in vitro. Adv Drug Deliv Rev. 2004;56(8):1085–1097. doi: 10.1016/j.addr.2004.01.002. [DOI] [PubMed] [Google Scholar]
- 5.Qiu A, Jansen M, Sakaris A, et al. Identification of an intestinal folate transporter and the molecular basis for hereditary folate malabsorption. Cell. 2006;127(5):917–928. doi: 10.1016/j.cell.2006.09.041. [DOI] [PubMed] [Google Scholar]
- 6.Zhao R, Min SH, Qiu A, et al. The spectrum of mutations in the PCFT gene, coding for an intestinal folate transporter, that are the basis for hereditary folate malabsorption. Blood. 2007;110(4):1147–1152. doi: 10.1182/blood-2007-02-077099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Min SH, OH SY, Karp GI, Poncz M, Zhao R, Goldman ID. The clinical course and genetic defect in the PCFT in a 27-year-old woman with hereditary folate malabsorption. J Pediatr. 2008;153(3):435–437. doi: 10.1016/j.jpeds.2008.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lasry I, Berman B, Straussberg R, et al. A novel loss of function mutation in the proton-coupled folate transporter from a patient with hereditary folate malabsorption reveals that Arg 113 is crucial for function. Blood. 2008;112(5):2055–2061. doi: 10.1182/blood-2008-04-150276. [DOI] [PubMed] [Google Scholar]
- 9.Borzutzky A, Crompton B, Bergmann AK, et al. Reversible severe combined immunodeficiency phenotype secondary to a mutation of the proton-coupled folate transporter. Clin Immunol. 2009;133(3):287–294. doi: 10.1016/j.clim.2009.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Atabay B, Turker M, Ozer EA, Mahadeo K, Diop-Bove NK, Goldman ID. Mutation of the proton-coupled folate transporter gene (PCFT-SLC46A1) in Turkish siblings with hereditary folate malabsorption. Pediatr Hematol Oncol. 2010;27(8):614–619. doi: 10.3109/08880018.2010.481705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Meyer E, Kurian MA, Pasha S, Trembath RC, Cole T, Maher ER. A novel PCFT gene mutation (p.Cys66LeufsX99) causing hereditary folate malabsorption. Mol Genet Metab. 2010;99(3):325–328. doi: 10.1016/j.ymgme.2009.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mahadeo K, Diop-Bove N, Shin D, et al. Properties of the Arg376 residue of the proton-coupled folate transporter (PCFT-SLC46A1) and a glutamine mutant causing hereditary folate malabsorption. Am J Physiol Cell Physiol. 2010;299(5):C1153–C1161. doi: 10.1152/ajpcell.00113.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Umapathy NS, Gnana-Prakasam JP, Martin PM, et al. Cloning and functional characterization of the proton-coupled electrogenic folate transporter and analysis of its expression in retinal cell types. Invest Ophthalmol Vis Sci. 2007;48(11):5299–5305. doi: 10.1167/iovs.07-0288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhao R, Qiu A, Tsai E, Jansen M, Akabas MH, Goldman ID. The proton-coupled folate transporter (PCFT): impact on pemetrexed transport and on antifolate activities as compared to the reduced folate carrier. Mol Pharmacol. 2008;74(3):854–862. doi: 10.1124/mol.108.045443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Qiu A, Min SH, Jansen M, et al. Rodent intestinal folate transporters (SLC46A1): secondary structure, functional properties, and response to dietary folate restriction. Am J Physiol Cell Physiol. 2007;293(5):C1669–C1678. doi: 10.1152/ajpcell.00202.2007. [DOI] [PubMed] [Google Scholar]
- 16.Unal ES, Zhao R, Goldman ID. Role of the glutamate 185 residue in proton translocation mediated by the proton-coupled folate transporter SLC46A1. Am J Physiol Cell Physiol. 2009;297(1):C66–C74. doi: 10.1152/ajpcell.00096.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Unal ES, Zhao R, Chang MH, Fiser A, Romero MF, Goldman ID. The functional roles of the His247 and His281 residues in folate and proton translocation mediated by the human proton-coupled folate transporter, SLC46A1. J Biol Chem. 2009;284(26):17846–17857. doi: 10.1074/jbc.M109.008060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhao R, Unal ES, Shin DS, Goldman ID. Membrane topologicical analysis of the proton-coupled folate transporter (PCFT-SLC46A1) by the substituted cysteine accessibility method. Biochemistry. 2010;49(13):2925–2931. doi: 10.1021/bi9021439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lasry I, Berman B, Glaser F, Jansen G, Assaraf YG. Hereditary folate malabsorption: a positively charged amino acid at position 113 of the proton-coupled folate transporter (PCFT/SLC46A1) is required for folic acid binding. Biochem Biophys Res Commun. 2009;386(3):426–431. doi: 10.1016/j.bbrc.2009.06.007. [DOI] [PubMed] [Google Scholar]
- 20.Zhao R, Babani S, Gao F, Liu L, Goldman ID. The mechanism of transport of the multitargeted antifolate, MTA-LY231514, and its cross-resistance pattern in cell with impaired transport of methotrexate. Clin Cancer Res. 2000;6(9):3687–3695. [PubMed] [Google Scholar]
- 21.Unal ES, Zhao R, Qiu A, Goldman ID. N-linked glycosylation and its impact on the electrophoretic mobility and function of the human proton-coupled folate transporter (HsPCFT). Biochim Biophys Acta. 2008;1178(6):1407–1414. doi: 10.1016/j.bbamem.2008.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhao R, Gao F, Hanscom M, Goldman ID. A prominent low-pH methotrexate transport activity in human solid tumor cells: contribution to the preservation of methotrexate pharmacological activity in HeLa cells lacking the reduced folate carrier. Clin Cancer Res. 2004;10(2):718–727. doi: 10.1158/1078-0432.ccr-1066-03. [DOI] [PubMed] [Google Scholar]
- 23.Diop-Bove NK, Wu J, Zhao R, Locker J, Goldman ID. Hypermethylation of the human proton-coupled folate transporter (SLC46A1) minimal transcriptional regulatory region in an antifolate-resistant HeLa cell line. Mol Cancer Ther. 2009;8(8):2424–2431. doi: 10.1158/1535-7163.MCT-08-0938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sharif KA, Goldman ID. Rapid determination of membrane transport parameters in adherent cells. BioTechniques. 2000;28(5):926–928. 930, 932. doi: 10.2144/00285st06. [DOI] [PubMed] [Google Scholar]
- 25.Dridi L, Haimeur A, Ouellette M. Structure-function analysis of the highly conserved charged residues of the membrane protein FT1, the main folic acid transporter of the protozoan parasite, Leishmania. Biochem Pharmacol. 2010;79(1):30–38. doi: 10.1016/j.bcp.2009.07.019. [DOI] [PubMed] [Google Scholar]
- 26.Gray TM, Matthews BW. Intrahelical hydrogen bonding of serine, threonine, and cysteine residues within alpha-helices and its relevance to membrane-bound proteins. J Mol Biol. 1984;175(1):75–81. doi: 10.1016/0022-2836(84)90446-7. [DOI] [PubMed] [Google Scholar]
- 27.Subramanian VS, Marchant JS, Said HM. Apical membrane targeting and trafficking of the human proton-coupled transporter in polarized epithelia. Am J Physiol Cell Physiol. 2008;294(1):C233–C240. doi: 10.1152/ajpcell.00468.2007. [DOI] [PubMed] [Google Scholar]
- 28.Fiser A, Simon I, Barton GJ. Conservation of amino acids in multiple alignments: aspartic acid has unexpected conservation. FEBS Lett. 1996;397(2-3):225–229. doi: 10.1016/s0014-5793(96)01181-7. [DOI] [PubMed] [Google Scholar]
- 29.Guan L, Kaback HR. Lessons from lactose permease. Annu Rev Biophys Biomol Struct. 2006;35:67–91. doi: 10.1146/annurev.biophys.35.040405.102005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nie Y, Sabetfard FE, Kaback HR. The Cys154–>Gly mutation in LacY causes constitutive opening of the hydrophilic periplasmic pathway. J Mol Biol. 2008;379(4):695–703. doi: 10.1016/j.jmb.2008.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Geller J, Kronn D, Jayabose S, Sandoval C. Hereditary folate malabsorption: family report and review of the literature. Medicine (Baltimore) 2002;81(1):51–68. doi: 10.1097/00005792-200201000-00004. [DOI] [PubMed] [Google Scholar]
- 32.Mahadeo K, Min SH, Diop-Bove N, Kronn D, Goldman ID. GeneReviews [Internet]. In: Pagon RA, Bird TC, Dolan CR, Stephens K, editors. Seattle (WA): University of Washington, Seattle; 1993. [Accessed May 6, 2010]. http://www.ncbi.nlm.nih.gov/bookshelf/br.fcgi?book=gene&part=folate-mal. [Google Scholar]
- 33.Zhao R, Min SH, Wang Y, Campanella E, Low PS, Goldman ID. A role for the proton-coupled folate transporter (PCFT; SLC46A1) in folate receptor-mediated endocytosis. J Biol Chem. 2009;284(7):4267–4274. doi: 10.1074/jbc.M807665200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Verbeek MM, Blom AM, Wevers RA, Lagerwerf AJ, van de Geer J, Willemsen MA. Technical and biochemical factors affecting cerebrospinal fluid 5-MTHF, biopterin and neopterin concentrations. Mol Genet Metab. 2008;95(3):127–132. doi: 10.1016/j.ymgme.2008.07.004. [DOI] [PubMed] [Google Scholar]
- 35.Wang Y, Zhao R, Russell RG, Goldman ID. Localization of the murine reduced folate carrier as assessed by immunohistochemical analysis. Biochim Biophys Acta. 2001;1513(1):49–54. doi: 10.1016/s0005-2736(01)00340-6. [DOI] [PubMed] [Google Scholar]
- 36.Steinfeld R, Grapp M, Kraetzner R, et al. Folate receptor alpha defect causes cerebral folate transport deficiency: a treatable neurodegenerative disorder associated with disturbed myelin metabolism. Am J Hum Genet. 2009;85(3):354–363. doi: 10.1016/j.ajhg.2009.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cario H, Bode H, Debatin KM, Opladen T, Schwarz K. Congenital null mutations of the FOLR1 gene: a progressive neurologic disease and its treatment. Neurology. 2009;73(24):2127–2129. doi: 10.1212/WNL.0b013e3181c679df. [DOI] [PubMed] [Google Scholar]