Abstract
Initial orthostatic hypotension is common in children. Isometric handgrip increases arterial pressure, central blood volume, cardiac output and total peripheral resistance. We show that in 14 subjects with initial orthostatic hypotension, isometric handgrip coupled with standing abolished symptoms of initial orthostatic hypotension and minimized decreases in BP and CO with standing.
Keywords: Orthostatic intolerance, Exercise pressor reflex, cardiac output, mean arterial pressure, heart rate
Active standing can induce large transient decreases in blood pressure (BP) and cardiac output (CO) within 15 seconds in healthy subjects, termed “Initial Orthostatic Hypotension”, due to rapid translocation of blood from the thorax to dependent body parts before normal neurovascular compensation occurs (2). Physical counter-maneuvers (leg crossing, gluteal clenching) can increase BP and arterial resistance after orthostatic intolerance has occurred (3). If performed too late, they may not prevent loss of postural tone and consciousness. Isometric handgrip evokes the exercise pressor reflex (EPR) that raises BP by increasing CO. We hypothesized that isometric handgrip preformed prior to and during standing, could increase CO; prevent hypotension and symptoms of OI with standing.
METHODS
We studied 14 subjects (7 males, 7 females; 15-22 yr (median=17), weight 65 ± 12 kg, height 175 ± 11 cm and BMI 21 ± 4 kg/m2) with initial orthostatic hypotension, dizziness upon standing, without systemic disease, vasovagal faint, or chronic orthostatic intolerance. Initial orthostatic hypotension was defined as a transient decrease in systolic BP exceeding 40 mmHg, or a decrease in diastolic BP exceeding 20 mmHg, that occurred within 15 seconds of standing, with cerebral hypoperfusion, dizziness, neurocognitive loss, pallor, headache and nausea. Informed consent was obtained.
BP, HR, and CO were continuously monitored with finger arterial plethysmography (Finometer, FMS, Amsterdam, Netherlands). Subjects remained supine for 15 min and then stood for 5 min while monitoring BP, HR, and CO. After 5 min, subjects returned to supine for 15 min recovery. Subjects then performed a maximum isometric contraction with their non-dominant arm for 1 min using a hand dynamometer (Lafayette Instruments, IN). After an additional 15 min, subjects performed maximum HG for 30 seconds, then stood for 5 min while isometric handgrip continued. Subjects returned to the supine position after 5 min of standing.
Mean arterial pressure (MAP) = (1/3 * systolic BP) + (2/3 * diastolic BP). Total peripheral resistance (TPR) = MAP/CO. Data were compared between supine, stand, and stand plus isometric handgrip. MAP, CO, TPR and HR were analyzed using ANOVA for repeated measures with Bonferroni post-test for multiple comparisons if findings were significant. All values are mean ± SE. Statistical significance was set at P<0.05.
RESULTS
All subjects completed the protocol and none fainted. Supine values were: MAP 82 ± 7 mmHg, CO 3.7 ± 1.2 L/min, HR 68 ± 14 beats/min and TPR 25 ± 11 Woods units (mmHg/L/min). All subjects reported OI symptoms during standing, including dizziness, lightheadedness, nausea, diminished postural tone, heat, and palpitations.
Figure 1 shows a representative response of standing; MAP decreased 70%, HR increased 64%, CO decreased by 14%, and TPR increased 88%. With isometric handgrip, MAP decreased 12 %, HR increased 68 %, CO increased 80%, and TPR decreased by 60% compared with baseline values.
Figure 1.
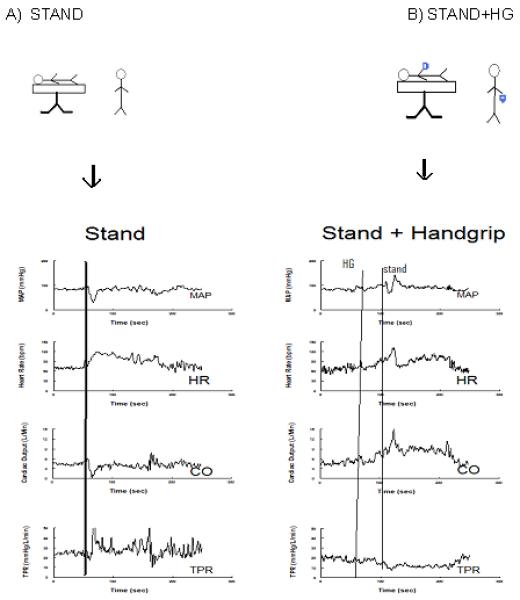
Effects of HG on standing in a representative subject. MAP, HR, CO, TPR from subject undergoing the orthostatic stress of standing (left). MAP, HR, CO, TPR from same subject undergoing orthostatic stress of standing after initiating the 80% maximum HG maneuver (right). MAP increases from baseline with handgrip and the decrease in MAP is somewhat reduced. Changes in CO, HR and TPR are blunted during stand + HG compared with stand alone.
For all subjects with stand alone, MAP decreased 42 ± 10 % (P<0.01), HR increased 62 ± 18 % (P<0.01), TPR was unchanged 17 ± 21 % (P =0.6 5) and CO decreased 33 ± 17 % (P <0.05). Standing with isometric handgrip (Figure 2) resulted in MAP decrease of 31 ± 9 % (P<0.01), HR increased 33 ± 17 % (P<0.01), TPR decreased 30 ± 15 % (P<0.01) and CO decreased 2 ± 14 % (P<0.05).
Figure 2.
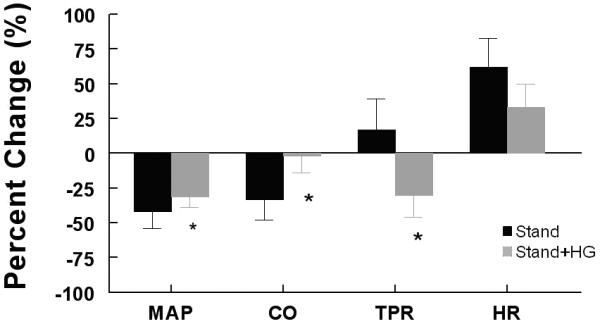
Percent change of MAP, HR, TRP and CO averaged over all subjects. Stand alone is in black, stand + HG is in grey. HG blunts the decrease in MAP, eliminates the decrease in CO and reverses the change in TPR such that TPR increases on average with stand alone, but decreases on average after HG.
This shows standing plus isometric handgrip significantly (P<0.05) attenuated the decline in MAP and CO and decreased TPR. HR was not different. In all subjects, symptoms with standing were absent when combined with isometric handgrip.
Figure 3 (available at www.jpeds.com) shows during stand alone, MAP decreased by 35 ± 3 mmHg, CO decreased 2.0 ± .5 L/min and TPR increased by 4 ± 7 Woods unit during standing. During stand HR increased 40 bpm. The decrease in CO was greatly reduced during stand plus isometric handgrip associated with a reduced fall in MAP. TPR decreased during stand plus isometric handgrip.
Figure 3.
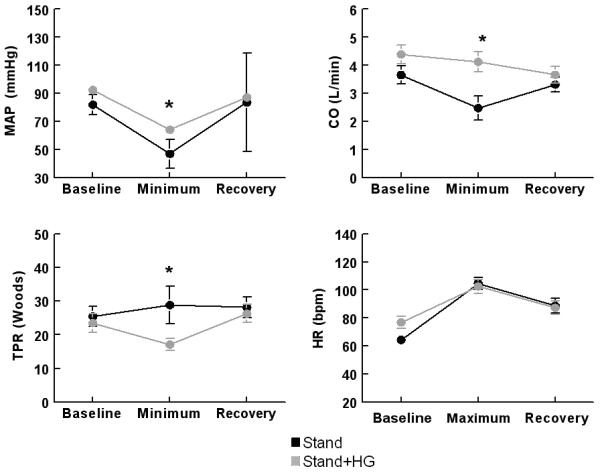
Absolute values of MAP, CO, (top) and TPR, HR (bottom) during stand (black) and during stand+HG (gray) averaged over all subjects. HG blunts the decrease in MAP, eliminates the decrease in CO and reverses the change in TPR such that TPR increases on average with stand alone, but decreases on average after HG. Note that baseline MAP, CO and HR are increased for stand + HG due to the effects of HG on supine hemodynamics.
DISCUSSION
Initial orthostatic hypotension is not classic orthostatic hypotension (OH). OH is persistent hypotension within 3 minutes of standing (7). Initial orthostatic hypotension occurs immediately, transiently and lasts <1 minute. Although OH occurs with autonomic failure, initial orthostatic hypotension is seen in healthy young subjects with autonomic competence (6). BP in OH decreases steadily but not abruptly and in initial orthostatic hypotension it decreases abruptly, reaching a nadir within 15 seconds as noted in all our subjects.
Subjects with initial orthostatic hypotension experience cerebral and retinal hypoperfusion (blackout), loss of postural tone and evanescent loss of consciousness. Unlike syncope, middle cerebral artery velocity is usually maintained (12). These presyncopal findings are short-lived and recovery occurs while subjects remain upright. In vasovagal syncope, fainting typically results from protracted standing associated with severe, persistent hypotension and often bradycardia. Dehydration, skeletal muscle pump dysfunction (4), decreased venous hydrostatic pressure (5), and drugs or conditions that inhibit sympathetic capabilities can exacerbate initial orthostatic hypotension.
Standing rapidly displaces ~700 ml of blood (8) from the chest to dependent venous vascular beds. Initial orthostatic hypotension is caused by a delay in the neurovascular response to this translocation (1, 5). In the young, this occurs within seconds, before effective neurovascular compensation occurs, resulting in pooling, decreased venous return, decreased CO, and hypotension.
Isometric handgrip evokes the EPR causing vagal withdrawal, sympathetic activation, and increased HR, BP and CO without changing TPR. The addition of EPR as isometric handgrip before standing prevents hypotension through cardiac and peripheral vascular sympathetic stimulation, thereby stabilizing CO and abolishing postural symptoms.
All subjects previously reported symptoms of initial orthostatic hypotension and experienced symptoms during standing with an average decrease of 35 mmHg MAP. Decreases in BP reflect systemic vasodilation caused by volume dependent activation of cardiopulmonary baroreflexes (10). However, we did not observe increased vasodilation previously (2) and present data showed a 17 % increase in TPR. In our subjects, standing caused venous return reductions as previously reported (7).
We showed that with standing plus isometric handgrip, the hypotension and low CO with active standing are attenuated. Others have shown that initiation of the EPR is dependent upon force of contraction (11), thus maximum HG was used to evoke the ERP. Although EPR causes sympathetic activation and parasympathetic withdrawal (9), BP seldom increases because baroreflexes prevent this. Standing plus isometric handgrip “pre-activates” the EPR; this with the baroreflex unloading of orthostasis potentiates EPR's effects resulting in more rapid neurovascular compensation.
There were some important limitations. Standing most accurately duplicates daily symptoms of orthostasis under experimental conditions, compared with the use of a tilt-table. Standing however, may affect data collected during the first few seconds due to positional changes, patient movement and muscle pump activation.
We studied young healthy subjects, thus our findings may not be applicable to all age groups and those with underlying diseases. We will continue to recruit subjects for this study as the number of study subjects in this work is 14 and increased enrollment should strengthen our findings.
Our data have immediate practical applications for children with initial orthostatic hypotension; fist clenching, before and during standing suppresses hemodynamic changes caused by standing and reduces symptoms associated with initial orthostatic hypotension in the young.
List of Abbreviations
- EPR
Exercise Pressor Reflex
- MAP
Mean Arterial Pressure
- CO
Cardiac Output
- TPR
Total Peripheral Resistance
- OI
Orthostatic intolerance
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The authors declare no conflicts of interest.
References
- 1.Wieling W, Krediet CTP, Van Dijk N, Linzer M, Tschakovsky ME. Initial orthostatic hypotension; review on a forgotten condition. Clin. Sci. 2007;112:157–165. doi: 10.1042/CS20060091. [DOI] [PubMed] [Google Scholar]
- 2.Stewart JM. Transient orthostatic hypotension is common in adolescents. J Pediatr. 2002;140:418–424. doi: 10.1067/mpd.2002.122643. [DOI] [PubMed] [Google Scholar]
- 3.Brignole M, Croci F, Menozzi C, Solano A, Donateo P, Oddone D, Puggioni E, Lolli G. Isometric arm counter-pressure maneuvers to abort impending vasovagal syncope. J Am Coll Cardiol. 2002;40:2053–2059. doi: 10.1016/s0735-1097(02)02683-9. [DOI] [PubMed] [Google Scholar]
- 4.Rowell LB. Human Cardiovascular Control. Oxford: 1993. [Google Scholar]
- 5.Sheriff DD, Rowell LB, Scher AM. Is rapid rise in vascular conductance at onset of dynamic exercise due to muscle pump? Am J Physiol. 1993;93:H1227–H1234. doi: 10.1152/ajpheart.1993.265.4.H1227. [DOI] [PubMed] [Google Scholar]
- 6.Sheriff DD, Nadland IH, Toska K. Hemodynamic consequences of rapid changes in posture in humans. J appl Physiol. 2007;103:452–458. doi: 10.1152/japplphysiol.01190.2006. [DOI] [PubMed] [Google Scholar]
- 7.Tanaka H, Yamaguchi H, Matushima R, Tamai H. Instantaneous orthostatic hypotension in children and adolescents: A new entity of orthostatic intolerance. Ped Research. 1999;46:691. doi: 10.1203/00006450-199912000-00022. [DOI] [PubMed] [Google Scholar]
- 8.Van Dijk N, De Bruin I, Gisolf J, De Bruin-Bon R, Linzer M, Van Lieshout JJ, Wieling W. Hemodynamic effects of leg crossing and skeletal muscle tensing during free standing in patients with vasovagal syncope. J Appl Physiol. 2005;98:584–590. doi: 10.1152/japplphysiol.00738.2004. [DOI] [PubMed] [Google Scholar]
- 9.Kaufman MP, Forster HV. Reflexes controlling circulatory, ventilatory and airway responses to exercise. Handbook of Physiol. 1996;10(sec12):381–447. [Google Scholar]
- 10.Tanaka H, Sjoberh BJ, Thulesius O. Cardiac output and blood pressure during active and passive standing. Clin Physiol. 1996;16:157–170. doi: 10.1111/j.1475-097x.1996.tb00565.x. [DOI] [PubMed] [Google Scholar]
- 11.McCloskey DI, Mitchell JH. Reflex cardiovascular and respiratory responses originating in exercising muscle. J of Physiol. 1972;224:173–186. doi: 10.1113/jphysiol.1972.sp009887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Thomas KN, Cotter JD, Galvin SD, Williams MJA, Willie CK, Ainslie PN. Initial Orthostatic Hypotension is unrelated to Orthostatic Tolerance in Healthy Young Subjects. Am J Physiol. 2009;107:506–17. doi: 10.1152/japplphysiol.91650.2008. [DOI] [PubMed] [Google Scholar]


