Abstract
Meiosis is unique to germ cells and occurs in a sex-specific manner. The genes regulating meiotic initiation in either sex are yet to be fully elucidated. Recent studies have revealed the importance of retinoic acid and one of its target genes, Stra8, in meiotic initiation in both sexes. Microarray analysis of whole murine embryonic ovary and postnatal testis time course data revealed a single peak of Stra8 expression in each organ at the onset of meiosis; at Embryonic Day 14.5 in the ovary and 10 days postpartum in the testis. In order to identify other genes involved in the initiation of meiosis in mammals, murine testis and ovary microarray data were examined more closely for transcripts with expression profiles similar to Stra8. Three such candidates include establishment of cohesion 1 homolog 2 (Esco2), encoding a protein essential for sister chromatid cohesion; SET domain, bifurcated 2 (Setdb2), the mouse ortholog of Eggless, which is essential for oogenesis in Drosophila; and ubiquitin-activating enzyme 6 (Uba6), a gene with fivefold higher expression in human and mouse testes than any other organ. In situ hybridization and immunohistochemistry or immunofluorescence were performed to localize Esco2, Setbd2, and Uba6 expression in the developing testis. The cellular expression pattern localized all three of these transcripts and their respective proteins to germ cells transitioning from mitosis to meiosis, hence supporting the hypothesis of their involvement in the initiation of meiosis. Future research will be directed at determining a specific role for these three proteins in germ cell differentiation.
Keywords: gamete biology, germ cells, meiosis, spermatogenesis, testis
Three genes, Esco2, Setdb2, and Uba6 show a Stra8-like microarray expression profile, and their proteins localize specifically to germ cells during the mitotic-to-meiotic transition.
INTRODUCTION
Germ cell development is an intricately regulated process of cellular differentiation transforming primordial germ cells to a sex-dependent cell type, oocytes in females and spermatozoa in males. Aberrant gene expression during male germ cell development can lead to abnormal sperm production and infertility. Meiosis is a cellular process unique to the differentiation of germ cells and occurs in a sex-specific manner. In the murine model, oogonia enter meiosis from Embryonic Day (E) 13.5 in the ovary and arrest in the dictyate stage of meiosis I just prior to birth [1]. In the male embryonic gonad, the gonocytes refrain from entering meiosis and instead arrest in mitosis from E14.5 until after birth [2, 3]. The progression of meiosis in both sexes then differs dramatically. Meiosis in the female mouse can be drawn out over an extended period of time, with the oocyte arresting at metaphase of meiosis II at ovulation and not completing its meiotic division until after fertilization [1]. In the male mouse, however, gonocytes resume proliferation just after birth, differentiate into proliferating spermatogonia, and then initiate meiosis at approximately 8 days postpartum (dpp) [4]. Meiosis is then completed quickly (approximately 10 days in the mouse), resulting in the production of four haploid spermatids, each of which undergoes the drastic cellular and morphological changes collectively known as spermiogenesis before being released as immature spermatozoa.
There is strong and extensive evidence to suggest that retinoic acid (RA), an active metabolite of vitamin A, is a key regulator of germ cell differentiation and meiosis in both the male and the female mammalian gonad. In the female embryo, RA, produced by the mesonephros, induces oogonia to enter meiosis, whereas the expression of Cyp26b1, a cytochrome p450 family member, triggers RA degradation in the embryonic testis, and the gonocytes remain in the mitotic cell cycle [3, 5, 6]. In vitro culture experiments have also demonstrated that meiosis can be initiated in E12.5 mouse testes cultured with RA [5, 6]. In neonatal male germ cell cultures, RA can drive the transition from undifferentiated to differentiated spermatogonia [7]. In the adult animal, vitamin A-deficient (VAD) male mice are infertile, with the seminiferous epithelium of these animals containing only type A spermatogonia and Sertoli cells [8]. Additionally, the injection of VAD mice with either retinol or RA triggers germ cell differentiation to begin in synchrony throughout the testis [8].
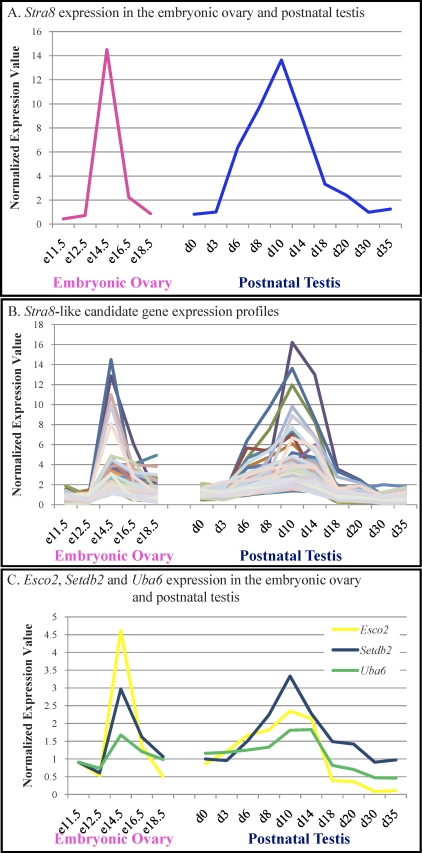
RA is thought to induce the onset of meiosis in both sexes through its target gene, Stra8. The localization and expression pattern of Stra8 supports this. Microarray analysis of embryonic ovary and postnatal testis developmental time courses [9, 10] identifies two ages at which Stra8 is highly expressed, E14.5 in the ovary and 10 dpp in the testis (Fig. 1A), aligning with the onset of meiosis in both sexes. In addition, Stra8 is expressed by A and B spermatogonia and preleptotene and leptotene spermatocytes with the highest level of expression detected in the adult mouse testis seminiferous epithelium at stages VII–VIII [11, 12]. Hence, Stra8 is present in premeiotic germs cells and is highly expressed when meiosis is first initiated and also at the stages of the seminiferous epithelium in the adult testis when germ cells are transitioning from mitosis to meiosis.
FIG. 1.
Identification of candidate regulators of the mitotic-to-meiotic transition. A) The Stra8 expression pattern throughout embryonic ovary and postnatal testis development. Pink, embryonic ovary; blue, postnatal testis. B) Microarray expression profiles for all 142 Stra8-like candidate transcripts. C) The embryonic ovary and postnatal testis expression profiles for Esco2 (yellow), Setdb2 (blue), and Uba6 (green). For A–C, raw or normalized expression values recorded from the Affymetrix chip are given on the Y axis, with the X axis depicting sample sex and/or age. e, embryonic day; d, postnatal day.
Further evidence for a STRA8 function in meiotic initiation has been derived from analysis of the Stra8 null mouse. Germ cells do not complete meiosis in either the male or the female Stra8-null mouse. Anderson et al. [13] reported that early mitotic divisions of spermatogonia appeared to occur normally; however, the formation of cells in meiotic prophase was blocked, and no markers of meiotic chromosome cohesion, synapsis, and recombination were detected [13]. Analysis of a Stra8-null male mouse line generated by Mark et al. [14] concluded that their mutant spermatocytes did undergo the initial stages of meiotic prophase (e.g., DNA replication and initiation of recombination); however, 24 hours following meiotic S phase, the spermatocyte chromosomes prematurely condensed, blocking meiotic progression [14]. Additionally, treating vitamin A-sufficient male mice with RA induced STRA8 expression and appeared to promote more synchronized premeiotic DNA replication [11]. These in vivo studies highlight the importance of STRA8 to germ cell differentiation; however, it is clear that Stra8 is not the only gene required for meiotic initiation.
The study presented here utilized our extensive microarray database detailing both testis- and ovary-expressed genes and our current understanding of STRA8 biology to identify candidate genes involved in the process of meiotic initiation. We focused on three different genes and their products—Esco2 (establishment of cohesion 1 homolog 2), Setdb2 (SET domain, bifurcated 2), and Uba6 (ubiquitin-like modifier activating enzyme 6)—and showed that their pattern of mRNA expression and protein localization support the hypothesis that they function in the transition from mitosis to meiosis.
MATERIALS AND METHODS
Animals and Tissues
All animal experiments were approved by Washington State University Animal Care and Use Committees and were conducted in accordance with the guiding principles for the care and use of research animals of the National Institutes of Health. BL/6–129 and CD1 mouse colonies were maintained in a temperature- and humidity-controlled environment with food and water provided ad libitum. BL/6–129 mice ranging from birth to adulthood (35–90 dpp) and CD1 time-mated pregnant female mice use in these studies were collected from these colonies. The animals were killed by decapitation (fetuses and 0–10 dpp) or asphyxiation followed by cervical dissociation (10 dpp, adult) and their testes or ovaries dissected. Fetal gonad tissues were collected from CD1 mice embryos staged by forelimb and hind limb morphology [15]. Tissue samples for RNA preparation and protein isolation were snap frozen immediately after collection and stored at −80°C until use. Tissues for in situ hybridization and immunohistochemistry or immunofluorescence were placed in Bouin fixative for 5 h (SETDB2) or 4% paraformaldehyde overnight (ESCO2 and UBA6) immediately after collection, then dehydrated through a graded ethanol series and embedded in paraffin. Sections of 3–5 μm were placed on Superfrost Plus slides (Menzel-Glaser).
Data Analysis
Array output was normalized via the robust multiarray method, and data analysis was conducted using GeneSpring version 7.3.1 (Agilent Technologies). Genes were considered Stra8-like if 1) the raw expression value was greater than 50 in both embryonic ovary E14.5 and postnatal testis 10-dpp samples, 2) the normalized value in E14.5 ovary was twofold greater than E12.5 ovary, and 3) the standard correlation value with Stra8 was greater than 0.9 in the embryonic ovary and the postnatal testis. A comparison of normalized expression values within the postnatal testis samples was not included in this analysis, as adding this comparison significantly reduced the number of genes on the list and removed some genes with known functions in meiosis. Genes were determined to be RA responsive by comparing the Stra8-like gene list to genes determined previously to be induced by RA in VAD animals (Snyder and Griswold, unpublished data). Gonad specific genes were determined by database and literature searches. Functional annotation cluster analysis was performed by using the tools of DAVID Bioinformatics Resources (http://david.abcc.ncifcrf.gov) [16]. Affymetrix Gene Chip Probe IDs identified in the microarray analysis as having a Stra8-like profile were uploaded to DAVID Bioinformatics Resources, and functional annotation clustering was used to examine biological process gene ontology (GO) terms defined as significantly overrepresented. Enrichment scores for each cluster are reported, as is the most biological relevant GO term in the cluster and the number of transcripts represented.
Northern Blotting
Northern blots were prepared on Hybond XL membrane (Amersham Biosciences) containing 10 μg of 10- and 90-dpp mouse total testis RNA according to the manufacturer's instructions. The PCR-derived cDNAs used to generate the DIG-cRNAs for Esco2, Setdb2, and Uba6 were [α_32P]dCTP-labeled using the Megaprime DNA labeling system (Amersham) as per the manufacturer's instructions and hybridized to the membranes at 42°C overnight. The membranes were washed to a stringency of 0.1× SSC and 0.1% SDS up to 50°C and exposed to x-ray film (Hyperfilm; Amersham) overnight at −80°C in Hyperscreen Intensifying Screen cassettes (Amersham).
In Situ Hybridization
In situ hybridization was used to localize candidate meiotic gene mRNAs on mouse testis sections as previously described [17]. PCR products were derived from adult mouse testis cDNA using the following primer sets: Esco2 (forward: 5′TTCTAGAGCTTGGCGGTGTT3′, reverse: 5′TGTTGGGTCAGAAAATGCAA3′), Setdb2 (forward: 5′AACAAATCAAGTGCGGTTCC3′, reverse: 5′TTCAAGGACAGTGGGGTTTC3′), Uba6 (forward: 5′GCCTGAAGTGAATGCTGACA3′, reverse: 5′ACTCTTCATGCCCAGGATTG3′). The PCR products were ligated into pGEMTEasy (Promega) and transformed into DH10β host cells. Positive subclones were identified via PCR, inserts verified by sequencing, and the plasmids used as PCR templates to derive products using M13 forward and reverse primers (Sigma-Aldrich) with the Esco2, Setdb2, and Uba6 probe region flanked by the T7 and SP6 promoter sequences. These PCR products were used as template for PCR-based production of DIG-cRNAs from the T7 and Sp6 promoters. Hybridization and washing were performed at 55°C. Both antisense and sense (negative control) cRNAs were used on each sample, in every experiment, for each set of conditions tested.
Western Blotting
Protein samples from mouse tissues were prepared by homogenization on ice in RIPA buffer (1% Nonidet P-40, 0.5% sodium deoxycholate, 0.1% SDS in PBS) in the presence of protease inhibitors (Protease Inhibitor Cocktail Set; Roche), as described previously [18]. Protein concentration was determined using the Hartree-Lowry protein assay [19], and 20 μg of total testis protein per lane for UBA6 and 60 μg per lane for ESCO2 and SETDB2 were loaded onto 12% SDS-PAGE gels with protein size standards (Page Ruler Prestained Protein Ladder; Fermentas). Following electrophoresis, the proteins were transferred to Hybond XL nitrocellulose membrane (Amersham), and all subsequent incubations were performed at room temperature unless otherwise stated. The membranes were blocked by incubation in 5% skim milk/TBS for 1 h. Primary antibodies were diluted in 5% skim milk/0.1% Tween-20/TBS, and incubations were performed overnight at 4°C (rabbit anti-UBA6 [IMGENEX; IMG-6168A], rabbit anti-SETDB2 [Abcam; ab5517], and rabbit anti-ESCO2 [Bethyl Laboratories; A301-689A]; all used at 1:3000) followed by incubation with HRP-coupled secondary antibodies (1:5000, diluted in 5% skim milk/0.1% Tween-20/0.01% SDS/TBS; Zymed) for 60 min. Antibody binding was detected by incubating membranes in Western lighting chemiluminesence reagent (Perkin Elmer) and exposure to x-ray film (Amersham). Each antibody was tested on at least two distinct protein homogenates for each sample age, with consistent results. For the juvenile ages, lysates were prepared from testes collected from multiple mice and then pooled. Each adult mouse testis protein lysate was prepared from one testis from different mice.
Immunohistochemistry and Immunofluorescence
Immunohistochemistry or immunofluorescence with anti-ESCO2, SETDB2, and UBA6 antibodies, also used in the Western blotting experiments, was performed essentially as previously described [20]. Antigen retrieval was performed in 0.01 M citrate (pH 6; ≥90°C maintained for 5 min), and primary antibodies were applied at 0.5–1.0 μg/ml for overnight incubation at room temperature in 0.1% bovine serum albumin/PBS. Control sections were incubated without primary antibody. Subsequent steps were performed at room temperature, with PBS washes between incubations. Primary antibody binding was detected using the AlexaFluor 488 goat anti-rabbit secondary antibody (Invitrogen; A-11008) for ESCO2 and SETDB2 and the biotinylated goat anti-rabbit antibody (Zymed) for UBA6. All secondary antibodies were applied to the sections at 1:500 dilution in 0.1% bovine serum albumin/PBS for 1 h. The staining pattern for ESCO2 and SETDB2 was then visualized and photographed, following mounting under coverslips using Vectashield with DAPI (Vector Laboratories), using a Leica confocal microscope at the Washington State University Imaging Facility. To visualize UBA6, slides were incubated with Vectastain Elite ABC kit (Vector Laboratories) according to the manufacturer's instructions. Antibody binding was detected as a brown precipitate following development with 3,3′-diaminobenzidine tetrahydrochloride (Sigma-Aldrich). The sections were mounted under glass coverslips in Permount (Fischer). Germ and somatic cell types were identified on the basis of their nuclear morphology and position within the developing gonad. In the postnatal testis, sections from at least 3 BL/6–129 animals were analyzed for protein localization, and each immunohistochemistry or immunofluorescence experiment was repeated with consistent results obtained.
RESULTS
Identification of Candidate Genes Involved in the Mitotic-to-Meiotic Transition
In order to identify potential regulators of the mitotic-to-meiotic transition in germ cells, we utilized our previously published embryonic ovary and postnatal testis age series microarray analyses [9, 10], publicly available through GEO Datasets on the NCBI Web site (http://www.ncbi.nlm.nih.gov), showing that Stra8 expression is highest at E14.5 in the embryonic ovary and 10 dpp in the postnatal testis (Fig. 1A). From our array analysis of time points between E11.5 and E18 in the embryonic ovary and 0 and 35 dpp in the postnatal testis, we generated a list of 162 probe sets representing 142 unique transcripts that had a raw microarray expression value of greater than 50 and a minimum correlation of 0.85 when their expression profiles were compared to that of Stra8 (Fig. 1B). The analysis tool DAVID (http://david.abcc.ncifcrf.gov) [16] was used to determine which ontology clusters were enriched in this list. Of the 162 probe sets, 98 were associated with significantly overrepresented GO terms in a biological process. Functional annotation clustering revealed clusters associated with DNA metabolic process (15 transcripts, enrichment score 4.26), sexual reproduction (14 transcripts, enrichment score 3.73), chromosome organization (14 transcripts, enrichment score 3.21), and cell cycle (16 transcripts, enrichment score 2.7).
In order to decide which genes or expressed sequence tags (ESTs) on this list were good candidates for further analyses, extensive database searches were performed for each transcript to determine whether they had a known function, whether they were specifically expressed in the gonad, and whether their expression is induced in response to RA. Functions and gonad expression specificities were assigned, where possible, based on literature searches and predicted protein domains. The Stra8-like target list was compared to a microarray analysis detailing genes induced in the testis in response to RA-treated adult VAD male mice (Snyder and Griswold, unpublished data) to determine whether any of the Stra8-like targets were RA responsive. Of the 142 genes, 26 were gonad specific, and 8 were induced in RA-treated VAD testes after a 24-h treatment.
The three genes chosen for the analysis detailed in this study were establishment of cohesion 1 homolog 2 (Esco2), SET domain bifurcated 2 (Setdb2), and ubiquitin-like modifier activating enzyme 6 (Uba6). The expression profile of each of these transcripts peaks at E14.5 in the embryonic ovary and at 10 dpp in the postnatal testis (Fig. 1C). The microarray profiles for Esco2, Setdb2, and Uba6 in these data sets displayed a high standard correlation with that of Stra8, being 0.933, 0.928, and 0.9, respectively.
Of these three selected candidates, only Esco2 was found to be RA responsive (Snyder and Griswold, unpublished data). To determine any retinoic acid response elements (RAREs) were present upstream of the transcriptional start sites of any of these genes, the 2-kb region before the transcription start site for each gene was analyzed for a consensus RARE (AGGTCAXXXXXAGGTCA). There were no RAREs present in the putative promoter region of any of the three selected genes.
ESCO2, an acetyltransferase, is essential for the proper cohesion of sister chromatids [21]. Natural human mutations in this gene lead to growth and mental retardation and cardiac and renal abnormalities, collectively known as Roberts syndrome [22, 23]; however, there are no published reports outlining the fertility of men with this syndrome. SETDB2, a histone lysine methyltransferase, is important for chromosome condensation and transcriptional repression [24] and is the mouse ortholog of Eggless, a gene essential to oogenesis in Drosophila [25]. UBA6, one of only two ubiquitin-activating E1 enzymes, has fivefold higher expression in the testis than any other mouse organ tested [26], and there are other enzymes involved in the ubiquitination pathway that are known to be testis specific [27–29]. Of these three genes, only Uba6 was toward the top of the list when it was ordered according to those transcripts with the greatest induction on meiotic initiation in both sexes. For each of these three genes, nothing is known about their cellular expression or localization patterns in the testis, let alone whether they contribute to the mitotic-to-meiotic transition in germ cells. To determine whether the products of these genes were present in the testis and in what cell types, Northern and Western blotting, in situ hybridization, and immunohistochemistry or immunofluorescecne were performed.
Esco2, Setdb2, and Uba6 mRNA and Protein Are Present in the Juvenile and Adult Testis
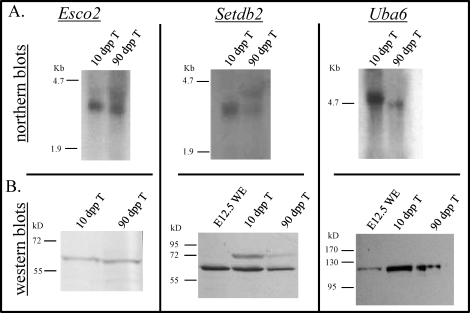
Northern blotting was performed to determine whether the in situ hybridization probes used for the detection of Esco2, Setdb2, and Uba6 mRNAs in this study were specific and also to validate that these transcripts were expressed in the testis. To examine each of these genes, total testis RNA samples were prepared from 10- and 90-dpp male mice. These ages were selected to ensure that genes involved in meiosis were being transcribed. Probing these two samples for Esco2 mRNA resulted in a single band at 3 kb (Fig. 2), which corresponds to its reported size. For Setdb2, a single band of 3 kb was present in the 10-dpp sample, with a band of the same size present in the 90-dpp sample as well as a second band at 3.6 kb (Fig. 2). The two Setdb2 bands seen in the 90-dpp testis sample are most likely splice variants. This suggests that a second Setdb2 transcript is expressed in the adult that is not present in the juvenile testis. A single band of 5 kb was also detected in both samples for Uba6, correlating to its reported size.
FIG. 2.
Meiotic initiation candidate expression in the postnatal testis. A) Northern blots illustrating the expression of Esco2, Setdb2, and Uba6 in the postnatal testis. Total mRNA from 10-day-postpartum (dpp) and adult mouse testes was hybridized with Esco2, Setdb2, and Uba6cDNA/cRNA probes. B) Western blots illustrating the expression of ESCO2, SETDB2, and UBA6 in the postnatal testis. Total protein lystates from Embryonic Day (E) 12.5 male and female whole mouse embryos and 10- and 90-dpp mouse testes were incubated with antibodies that recognize ESCO2, SETDB2, and UBA6 proteins. T, testis; WE, whole embryo.
Using commercially available antibodies, Western blotting was performed both to detect ESCO2, SETDB2, and UBA6 in total testis protein extracts and to verify antibody specificity. A band of 65 kD was present in each testis sample for ESCO2, similar to its theoretical molecular weight of 67 kD. The complete SETDB2 amino acid sequence was predicted to be 80 kD; however, a band of 72 kD was detected for this protein in each testis protein extract tested (Fig. 2B) as well as a second band in both postnatal testis samples at 65 kD. A single band of 120 kD was present in all total testis protein samples analyzed using an antibody raised against UBA6, correlating to its previously reported size [26] (Fig. 2B).
Esco2, Setdb2, and Uba6 mRNAs Are Specifically Expressed by Germ Cells in the Embryonic Gonad
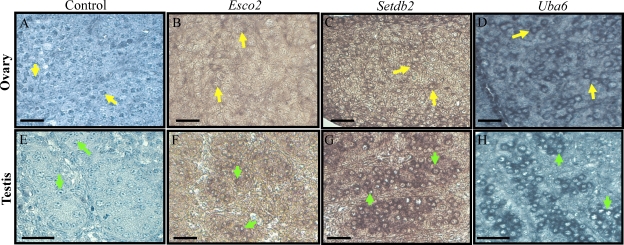
Given that all three candidates display a peak in expression at E14.5 in the embryonic ovary, in situ hybridization was performed to analyze the mRNA expression patterns of the three candidate genes in the murine embryonic ovary and testis. Sections of the embryonic testis were also included to determine whether the expression of these genes was specific to meiotic germ cells. Figure 3 displays cross sections of E14.5 ovaries and testes, hybridized with RNA probes specific for Esco2, Setdb2, or Uba6. All three mRNAs were detected specifically in oogonia in the ovary and surprisingly, also present in gonocytes in the testis (Fig. 3). There was no staining detected in any somatic cell type in either sex.
FIG. 3.
Esco2, Setdb2, and Uba6 expression in the Embryonic Day (E) 14.5 fetal gonad. In situ hybridization of the E14.5 mouse ovary (B–D) and testis (F–H) with Esco2, Setdb2, and Uba6 antisense cRNAs. Cells with positive signal are colored mauve. No counterstain was applied in B, C, F, and G so that the positive signal in these tissues could more easily be viewed. Sense controls for both testis and ovary are shown in A and E, respectively. Bars = 20 μm. The arrows in each panel point to positively stained germ cells: yellow arrows, oogonia; green arrows, gonocytes.
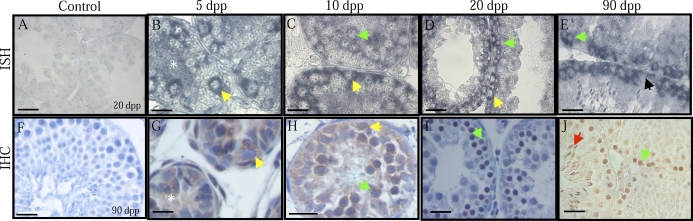
Esco2 Is Expressed by Multiple Testicular Cell Types but Localizes Specifically to the Nucleus of Spermatocytes
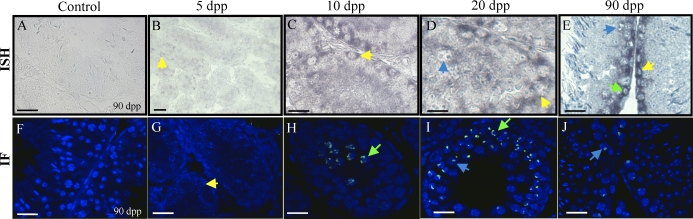
ESCO2 is an essential component of the sister chromatid cohesion complex. The function of this complex has been well studied in the mitotic cell cycle, and human Esco2 mutations result in Robert's syndrome [22]. It is currently unknown whether ESCO2 functions in meiosis or whether the natural human mutations found in this gene lead to human male infertility. In situ hybridization was performed to determine the cellular expression pattern for Esco2 transcript in the postnatal testis (Fig. 4). At each age tested, Esco2 transcript was present in spermatogonia, with mRNA also detected in spermatocytes at 10, 20, and 90 dpp (Fig. 4, C–E). At 5 dpp, Esco2 transcript was present in Sertoli cells (Fig. 4B), but no transcript could be detected in Sertoli cells from 10 dpp on. No stage-specific expression of Esco2 was apparent in the 90-dpp testis cross sections.
FIG. 4.
Esco2 expression and localization in the developing murine testis. A–E) In situ hybridization of 5-, 10-, 20-, and 90-day-postpartum (dpp) mouse testes with Esco2 antisense cRNAs. Sense cRNA control shown in A. F–J) Immunofluorescence detecting ESCO2 in 5-, 10-, 20-, and 90-dpp mouse testes. No secondary antibody control shown in F. Bars = 20 μm; yellow arrow, spermatogonia; green arrow, preleptotene spermatocytes; light blue arrow, pachytene spermatocytes.
Immunofluorescence on an age series of mouse testis cross sections with an antibody raised against ESCO2 demonstrated that this protein localized specifically to spermatocytes (Fig. 4). At 5 dpp, no ESCO2 was detected throughout the testis (Fig. 4G); however, at 10 dpp, ESCO2 was present diffusely throughout the nuclei of preleptotene and leptotene spermatocytes located in the center of the seminiferous epithelium and not located at the basement membrane (Fig. 4H). From 15 dpp on, ESCO2 was present in discreet domains within the nuclei of pachytene spermatocytes (Fig. 4, I and J; 15 dpp not shown). At no age was ESCO2 detected either in the Sertoli cells or in the interstitial space between tubules, and no staining was present on the negative control slides for both the in situ hybridization (Fig. 4A) and immunofluorescence experiments (Fig. 4F).
SETDB2 Is Detected Only in Germ Cells, but Its Transcript Is Also Present in Somatic Cells
Setdb2, a histone lysine methyltransferase involved in transcriptional repression, shows high sequence similarity to two transcripts critical to meiosis. It is the mammalian ortholog of Eggless, which is known to be essential for oogenesis and necessary for the correct formation of the egg chamber [25]. SETDB2 shares 20% homology with SUV39H1, also a histone lysine methyltransferase that is known to be essential to the progression of mammalian meiosis [30]. At 5 and 10 dpp, Setdb2 mRNA was present in spermatogonia and Sertoli cells (Fig. 5, B and C) and also faintly detected in spermatocytes at 20 dpp (Fig. 5D). This localization of Setdb2 to the mitotic and meiotic cell population was also conserved in the 90-dpp testis (Fig. 5E), with no transcript detected in either the haploid germ cells or the interstitial cells. As was the case for Esco2, there was no stage specificity apparent in the Setdb2 mRNA expression pattern in the 90-dpp mouse testis.
FIG. 5.
Setdb2 expression and localization in the developing murine testis. A–E) In situ hybridization of 5-, 10-, 20-, and 90-day-postpartum (dpp) mouse testes with Setdb2 antisense and sense (A) cRNAs. F–J) Immunofluorescence detecting SETDB2 in 5-, 10-, 20-, and 90-dpp mouse testes. No secondary antibody control shown in F. Bars = 20 μm; yellow arrow, spermatogonia; green arrow, preleptotene spermatocytes; light blue arrow, pachytene spermatocytes; red arrow, sperm tails; white asterisk, Sertoli cell cytoplasm.
SETDB2 and ESCO2 were present in the same cell types in the mouse testis, yet they localized to different regions within the cell. No SETDB2 was present at 5 dpp (Fig. 5G); however, at 10 and 20 dpp, staining was observed throughout the nuclei of the preleptotene spermatocytes, which had moved away from the basement membrane (Fig. 5H). At 20 dpp, SETDB2 was present in the cytoplasm of pachytene spermatocytes (Fig. 5I). In the adult mouse testis, SETDB2 was undetectable in the nuclei of spermatocytes but localized to the cytoplasm of the pachytene spermatocytes in a granular fashion (Fig. 5J). SETDB2 staining was never detected in any testicular somatic cell at any age tested or in the negative control in situ hybridization and immunofluorescent slides (Fig. 5, A and F).
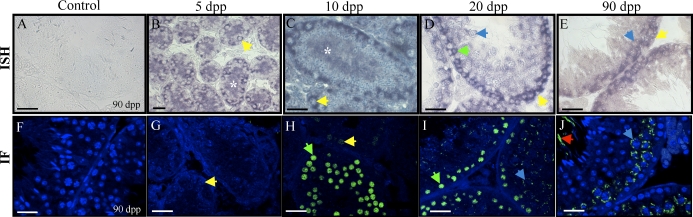
The mRNA Expression Pattern of Uba6 Corresponds to Its Protein Localization Pattern
Uba6 is one of two activators of the ubiquitination pathway. Until recently it was thought that there was only one E1 enzyme, UBA1, but the discovery that UBA6 can also activate ubiquitin for attachment to proteins for degradation has indicated that there may be a specificity associated with the activation of the ubiquitination pathway [31]. Even though the testis has been identified as having the highest levels of Uba6 transcript in both humans and mouse tissues [26], there are no reports outlining the cellular expression pattern of Uba6 within the testis. Uba6 was initially present in the somatic and germ cell populations in the neonatal testis but then became restricted to the germ cells as the animal aged (Fig. 6). At 5 dpp, Uba6 was present in both spermatogonia and Sertoli cells (Fig. 6B); however, at 10, 20, and 90 dpp, transcript was detected only in spermatogonia and preleptotene, leptotene, and zygotene spermatocytes (Fig. 6, C–E). As was observed with Setdb2, no transcripts were detected in either the haploid germ cells or the interstitial cells.
FIG. 6.
Uba6 expression and localization in the developing murine testis. A–E) In situ hybridization of 5-, 10-, 20-, and 90-day-postpartum (dpp) mouse testes with Uba6 antisense and sense (A) cRNAs. F–J) Immunohistochemistry detecting UBA6 in 5-, 10-, 20-, and 90-dpp mouse testes. No secondary antibody control shown in F. Bars = 20 μm; yellow arrow, spermatogonia; green arrow, preleptotene spermatocytes; red arrow, spermatozoa; black arrow, Sertoli cell; white asterisk, Sertoli cell cytoplasm.
The cellular localization of UBA6 protein matches its in situ hybridization expression pattern. UBA6 protein localized specifically to the cytoplasm of spermatogonia and Sertoli cells at 5 dpp (Fig. 6G). At 10 dpp, UBA6 was detectable in the cytoplasm of spermatogonia and preleptotene spermatocytes (Fig. 6H); however, at 20 and 90 dpp, UBA6 was present only in the nuclei of preleptotene, leptotene, and zygotene spermatocytes (Fig. 6, I and J). Protein was also detected in the spermatozoa at 90 dpp (Fig. 6J). No positive signal was detected in the negative controls for either the in situ hybridization or the immunohistochemistry experiments (Fig. 6, A and F).
DISCUSSION
This study utilized our microarray data set profiling the transcriptome of the embryonic ovary and postnatal testis and our current understanding of how RA induces meiotic initiation in both sexes to generate a list of candidate meiosis initiator genes. By identifying transcripts with a similar embryonic ovary and postnatal testis expression profile to Stra8, a marker of meiotic initiation induced by RA, we generated a short list of potential regulators of the mitotic-to-meiotic transition. The list consisted of genes that control a variety of different cell functions and included ESTs and hypothetical proteins with no known function.
It was encouraging to see transcripts in the list, such as Slc25a31 (known previously as Ant4), Tex15, and Rad51, as the products of these genes are known to function in regulating meiosis [32–34]. This supported our array analysis approach and hypothesis that genes exhibiting a Stra8-like expression profile may play a role in meiosis. Additionally, the DAVID analysis identified DNA metabolic process, sexual reproduction, chromosome organization, and cell cycle as being the most well-represented ontologies in our list of genes. However, commonly used markers of meiosis, such as Scyp3 and Rec8, were not present in our 142 gene candidates. The most plausible explanation is that Stra8 expression displays a distinct peak in both sexes, whereas genes such as Sycp3 are expressed when meiosis begins and their expression remains stable, so no specific peak in expression is present for these types of transcripts. This would result in a profile that is not similar to that of Stra8 and would not pass the constraints set in our selection criteria.
Of the 142 gene candidates, we chose three for further investigation. Esco2, Setdb2, and Uba6 were selected, as they had yet to be identified as regulators of meiosis; each either had—or were predicted to have—very distinct functions and were highly expressed in the postnatal testis, the main model of this study's focus. There were other genes on the list that displayed a higher induction of expression at meiotic initiation (e.g., Dazl), but the majority of these transcripts had known roles in meiosis. We chose to target proteins that had yet to be linked to meiosis for the extend analysis. In actuality, there are at least 20 other candidates in this list that warrant additional analysis and will be the focus of future research in our laboratory.
The observation that the mRNAs of all three of our selected candidate genes are present in gonocytes in the embryonic testis indicates that these proteins do not only play a role in meiotic initiation in the embryonic gonad. It is clear that all three of these proteins perform a function that could be utilized in many different cell types; however, our postnatal testis immunohistochemistry data imply that these proteins are specific to male germ cells after birth. Further investigation of their function in both embryonic and postnatal germ cells and whether they are also involved in postnatal ovary development will be the focus of future research.
This study represents the first investigation of a function for ESCO2 in germ cell development. A role for both ESCO1 and ESCO2 in the establishment of sister chromatid cohesion has been well established. A loss of either ESCO1 or ESCO2 in HEK293 cells leads to a block in cell division [35], and human mutations in Esco2 cause Robert's syndrome. Robert's syndrome leads to many different developmental and physical abnormalities [23], and most patients do not live to reach puberty. Normal life spans can be reached in patients with milder forms of the syndrome. Women of reproductive age with Robert's syndrome can achieve pregnancy; however, there appears to be an increased risk of spontaneous pregnancy loss associated with this disorder [23]. There are no published studies that investigate the fertility of men with Robert's syndrome. The localization data presented here indicate that ESCO2 is present only in the nucleus of the meiotic germ cells in the postnatal testis and localizes to a very specific domain of the pachytene spermatocyte nuclei. Future experiments will resolve which subnuclear domain contains ESCO2 in pachytene spermatocytes, and loss-of-function studies will determine whether ESCO2 is essential for germ cell development.
Of the three genes selected for further analysis, only Esco2 has been found to be responsive to RA treatment. No sequences resembling the RARE were present in the 2-kb sequence upstream of the transcriptional start sites of any of these three genes; however, the Esco2 promoter region does contain the necessary site for transcriptional activation through SP1 [36]. SP1 can activate transcription through forming a complex with RA receptor alpha [37], and SP1 sites have been shown to be essential for RA-induced transcription of Creb1 and Reln transcription [38, 39]. Hence the possibility that RA can induce Esco2 transcription through the SP1 sequence located in the Esco2 promoter needs to be investigated.
SETDB2 is an intriguing candidate regulator of murine meiosis and a member of the SET family of proteins. This family regulates gene expression through the methylation of histone lysines [24, 40] and are known to play important roles in the embryonic development of many different species [41]. The Drosophila ortholog of Setdb2, Eggless, is essential for female fly fertility [25]. As the name suggests, female flies homozygous for the null Eggless produce no oocytes, as oogenesis arrests in its early stages and reduced proliferation of the somatic cells required for egg chamber formation occurs. Our data indicate that Setdb2 is expressed in the mitotic and meiotic germ cell populations of the testis, intimating that it may be playing a role in remodeling chromatin in the transition of a germ cell from mitosis to meiosis. We also observed a shift in the intracellular localization of SETDB2 from nuclear in preleptotene spermatocytes at 10 and 20 dpp to cytoplasmic in pachytene spermatocytes from 15 dpp on. Further experiments will be required to determine whether this shift is indicative of a different role for SETDB2 during the first wave of meiotic initiation in the juvenile testis versus the adult testis and whether SETDB2 is localized to a specific organelle in the cytoplasm of the pachytene spermatocytes. Interestingly Suv39h2, another member of the SET domain-containing protein family and known to be expressed in spermatocytes [42], was also one of our 142 gene candidates. The inactivation of Suv39h2 in fetal male germ cells has recently been linked to the dynamic reorganization of heterochromatin as epigenetic reprogramming takes place in these cells [43], and we also saw expression of Setdb2 in fetal gonocytes (Fig. 3). Collectively, these data and observations suggest that there is much to learn from further investigation of SET protein functions in germ cell development.
Protein ubiquitination is a critical regulatory mechanism in biology, controlling fundamental processes such as cell cycle progression and DNA repair. Ubiquitin and ubiquitin-like modifiers are attached to target proteins through a well-characterized three-step enzymatic pathway of activation, conjugation, and ligation. Until recently, it was thought that only one E1 activation enzyme was expressed in mammalian cells. The identification of UBA6 as the second indicates that there may be cell- and tissue-specific pathways of ubiquitination [31]. Our expression data support the hypothesis that UBA6 plays a role in meiotic initiation but also implies that it may be functioning to regulate the mitotic cell cycle. Uba6 was expressed in oogonia at E14.5 and in spermatogonia and preleptotene, leptotene, and zygotene spermatocytes at 10 dpp, patterns that support its role in meiotic initiation. An intracellular localization shift similar to that observed for SETDB2 was also observed for UBA6 between 10 and 20 dpp. It is possible that this cytoplasmic-to-nuclear transition relates to a change of function as the germ cells differentiate, or it could be due to a lack of the required nuclear transport proteins in the spermatogonia and preleptotene spermatocytes, which are made in the more differentiated germ cells so that UBA6 can be moved into the nucleus. Deletion of Uba6 in mice results in embryonic lethality [44]; hence, our future analysis of UBA6 function in spermatogenesis will include using conditional knockout mice to assess its role in different testicular cells types.
The data presented here represent the first study to utilize the expression pattern of a meiotic initiation marker gene (Stra8) to generate a list of candidate genes that may also be involved in meiosis. Known regulators of meiosis were among the candidates; however, the list also contained a large number of genes that had yet to be investigated in the context of germ cell development. This method of analysis proved successful, as the expression patterns for Esco2, Setdb2, and Uba6 all suggest roles for these genes in the mitotic-to-meiotic transition.
Acknowledgments
We would like to acknowledge the assistance of Derek Pouchnik and Lizhong Yang for their assistance with processing microarray data.
Footnotes
This research was supported by a Contraceptive Center Grant U54 42454 and by HD 10808 from NIH.
REFERENCES
- Byskov AG. Differentiation of mammalian embryonic gonad. Physiol Rev 1986; 66: 71 117 [DOI] [PubMed] [Google Scholar]
- McCarrey JR. Development of the germ cell. Despardins C, Ewing L. (Eds.), Cell and Molecular Biology of the Testis. New York: Oxford University Press; 1993: 58 89 [Google Scholar]
- Western PS, Miles DC, van den Bergen JA, Burton M, Sinclair AH. Dynamic regulation of mitotic arrest in fetal male germ cells. Stem Cells 2008; 26: 339 347 [DOI] [PubMed] [Google Scholar]
- Kerr JB, Loveland KL, O'Bryan MK, De Kretser DM. (Eds.), Cytology of the Testis and Intrinsic Control Mechanisms. New York: Elsevier; 2006. [Google Scholar]
- Bowles J, Knight D, Smith C, Wilhelm D, Richman J, Mamiya S, Yashiro K, Chawengsaksophak K, Wilson MJ, Rossant J, Hamada H, Koopman P. Retinoid signaling determines germ cell fate in mice. Science 2006; 312: 596 600 [DOI] [PubMed] [Google Scholar]
- Koubova J, Menke DB, Zhou Q, Capel B, Griswold MD, Page DC. Retinoic acid regulates sex-specific timing of meiotic initiation in mice. Proc Natl Acad Sci U S A 2006; 103: 2474 2479 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Q, Li Y, Nie R, Friel P, Mitchell D, Evanoff RM, Pouchnik D, Banasik B, McCarrey JR, Small C, Griswold MD. Expression of stimulated by retinoic acid gene 8 (Stra8) and maturation of murine gonocytes and spermatogonia induced by retinoic acid in vitro. Biol Reprod 2008; 78: 537 545 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Pelt AM, de Rooij DG. Synchronization of the seminiferous epithelium after vitamin A replacement in vitamin A-deficient mice. Biol Reprod 1990; 43: 363 367 [DOI] [PubMed] [Google Scholar]
- Small CL, Shima JE, Uzumcu M, Skinner MK, Griswold MD. Profiling gene expression during the differentiation and development of the murine embryonic gonad. Biol Reprod 2005; 72: 492 501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shima JE, McLean DJ, McCarrey JR, Griswold MD. The murine testicular transcriptome: characterizing gene expression in the testis during the progression of spermatogenesis. Biol Reprod 2004; 71: 319 330 [DOI] [PubMed] [Google Scholar]
- Zhou Q, Nie R, Li Y, Friel P, Mitchell D, Hess RA, Small C, Griswold MD. Expression of stimulated by retinoic acid gene 8 (Stra8) in spermatogenic cells induced by retinoic acid: an in vivo study in vitamin A-sufficient postnatal murine testes. Biol Reprod 2008; 79: 35 42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oulad-Abdelghani M, Bouillet P, Decimo D, Gansmuller A, Heyberger S, Dolle P, Bronner S, Lutz Y, Chambon P. Characterization of a premeiotic germ cell-specific cytoplasmic protein encoded by Stra8, a novel retinoic acid-responsive gene. J Cell Biol 1996; 135: 469 477 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson EL, Baltus AE, Roepers-Gajadien HL, Hassold TJ, de Rooij DG, van Pelt AM, Page DC. Stra8 and its inducer, retinoic acid, regulate meiotic initiation in both spermatogenesis and oogenesis in mice. Proc Natl Acad Sci U S A 2008; 105: 14976 14980 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mark M, Jacobs H, Oulad-Abdelghani M, Dennefeld C, Feret B, Vernet N, Codreanu CA, Chambon P, Ghyselinck NB. STRA8-deficient spermatocytes initiate, but fail to complete, meiosis and undergo premature chromosome condensation. J Cell Sci 2008; 121: 3233 3242 [DOI] [PubMed] [Google Scholar]
- Kaufman MH. The Atlas of Mouse Development. San Diego: Academic Press; 1992. [Google Scholar]
- Dennis G, Jr, Sherman BT, Hosack DA, Yang J, Gao W, Lane HC, Lempicki RA. DAVID: Database for Annotation, Visualization, and Integrated Discovery. Genome Biol 2003; 4: P3 [PubMed] [Google Scholar]
- Hogarth CA, Calanni S, Jans DA, Loveland KL. Importin alpha mRNAs have distinct expression profiles during spermatogenesis. Dev Dyn 2006; 235: 253 262 [DOI] [PubMed] [Google Scholar]
- Hogarth CA, Jans DA, Loveland KL. Subcellular distribution of importins correlates with germ cell maturation. Dev Dyn 2007; 236: 2311 2320 [DOI] [PubMed] [Google Scholar]
- Hartree EF. Determination of protein: a modification of the Lowry method that gives a linear photometric response. Anal Biochem 1972; 48: 422 427 [DOI] [PubMed] [Google Scholar]
- Loveland KL, Hogarth C, Szczepny A, Prabhu SM, Jans DA. Expression of nuclear transport importins beta 1 and beta 3 is regulated during rodent spermatogenesis. Biol Reprod 2006; 74: 67 74 [DOI] [PubMed] [Google Scholar]
- Dorsett D. Roles of the sister chromatid cohesion apparatus in gene expression, development, and human syndromes. Chromosoma 2007; 116: 1 13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vega H, Waisfisz Q, Gordillo M, Sakai N, Yanagihara I, Yamada M, van Gosliga D, Kayserili H, Xu C, Ozono K, Jabs EW, Inui K, et al. Roberts syndrome is caused by mutations in ESCO2, a human homolog of yeast ECO1 that is essential for the establishment of sister chromatid cohesion. Nat Genet 2005; 37: 468 470 [DOI] [PubMed] [Google Scholar]
- Goh ES, Li C, Horsburgh S, Kasai Y, Kolomietz E, Morel CF. The Roberts syndrome/SC phocomelia spectrum—a case report of an adult with review of the literature. Am J Med Genet A 2010; 152A: 472 478 [DOI] [PubMed] [Google Scholar]
- Falandry C, Fourel G, Galy V, Ristriani T, Horard B, Bensimon E, Salles G, Gilson E, Magdinier F. CLLD8/KMT1F is a lysine methyltransferase that is important for chromosome segregation. J Biol Chem 2010; 285: 20234 20241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clough E, Moon W, Wang S, Smith K, Hazelrigg T. Histone methylation is required for oogenesis in Drosophila. Development 2007; 134: 157 165 [DOI] [PubMed] [Google Scholar]
- Pelzer C, Kassner I, Matentzoglu K, Singh RK, Wollscheid HP, Scheffner M, Schmidtke G, Groettrup M. UBE1L2, a novel E1 enzyme specific for ubiquitin. J Biol Chem 2007; 282: 23010 23014 [DOI] [PubMed] [Google Scholar]
- Bedard N, Hingamp P, Pang Z, Karaplis A, Morales C, Trasler J, Cyr D, Gagnon C, Wing SS. Mice lacking the UBC4-testis gene have a delay in postnatal testis development but normal spermatogenesis and fertility. Mol Cell Biol 2005; 25: 6346 6354 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Z, Oughtred R, Wing SS. Characterization of E3Histone, a novel testis ubiquitin protein ligase which ubiquitinates histones. Mol Cell Biol 2005; 25: 2819 2831 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin H, Keriel A, Morales CR, Bedard N, Zhao Q, Hingamp P, Lefrancois S, Combaret L, Wing SS. Divergent N-terminal sequences target an inducible testis deubiquitinating enzyme to distinct subcellular structures. Mol Cell Biol 2000; 20: 6568 6578 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters AH, O'Carroll D, Scherthan H, Mechtler K, Sauer S, Schofer C, Weipoltshammer K, Pagani M, Lachner M, Kohlmaier A, Opravil S, Doyle M, et al. Loss of the Suv39h histone methyltransferases impairs mammalian heterochromatin and genome stability. Cell 2001; 107: 323 337 [DOI] [PubMed] [Google Scholar]
- Groettrup M, Pelzer C, Schmidtke G, Hofmann K. Activating the ubiquitin family: UBA6 challenges the field. Trends Biochem Sci 2008; 33: 230 237 [DOI] [PubMed] [Google Scholar]
- Brower JV, Rodic N, Seki T, Jorgensen M, Fliess N, Yachnis AT, McCarrey JR, Oh SP, Terada N. Evolutionarily conserved mammalian adenine nucleotide translocase 4 is essential for spermatogenesis. J Biol Chem 2007; 282: 29658 29666 [DOI] [PubMed] [Google Scholar]
- Yang F, Eckardt S, Leu NA, McLaughlin KJ, Wang PJ. Mouse TEX15 is essential for DNA double-strand break repair and chromosomal synapsis during male meiosis. J Cell Biol 2008; 180: 673 679 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staeva-Vieira E, Yoo S, Lehmann R. An essential role of DmRad51/SpnA in DNA repair and meiotic checkpoint control. EMBO J 2003; 22: 5863 5874 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hou F, Zou H. Two human orthologues of Eco1/Ctf7 acetyltransferases are both required for proper sister-chromatid cohesion. Mol Biol Cell 2005; 16: 3908 3918 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishihara M, Yamada M, Nozaki M, Nakahira K, Yanagihara I. Transcriptional regulation of the human establishment of cohesion 1 homolog 2 gene. Biochem Biophys Res Commun 2010; 393: 111 117 [DOI] [PubMed] [Google Scholar]
- Cheng YH, Yin P, Xue Q, Yilmaz B, Dawson MI, Bulun SE. Retinoic acid (RA) regulates 17beta-hydroxysteroid dehydrogenase type 2 expression in endometrium: interaction of RA receptors with specificity protein (SP) 1/SP3 for estradiol metabolism. J Clin Endocrinol Metab 2008; 93: 1915 1923 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong JS, Kim SW, Koo JS. Sp1 up-regulates cAMP-response-element-binding protein expression during retinoic acid-induced mucous differentiation of normal human bronchial epithelial cells. Biochem J 2008; 410: 49 61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y, Kundakovic M, Agis-Balboa RC, Pinna G, Grayson DR. Induction of the reelin promoter by retinoic acid is mediated by Sp1. J Neurochem 2007; 103: 650 665 [DOI] [PubMed] [Google Scholar]
- Dambacher S, Hahn M, Schotta G. Epigenetic regulation of development by histone lysine methylation. Heredity 2010; 105: 24 37 [DOI] [PubMed] [Google Scholar]
- Margueron R, Trojer P, Reinberg D. The key to development: interpreting the histone code? Curr Opin Genet Dev 2005; 15: 163 176 [DOI] [PubMed] [Google Scholar]
- O'Carroll D, Scherthan H, Peters AH, Opravil S, Haynes AR, Laible G, Rea S, Schmid M, Lebersorger A, Jerratsch M, Sattler L, Mattei MG, et al. Isolation and characterization of Suv39h2, a second histone H3 methyltransferase gene that displays testis-specific expression. Mol Cell Biol 2000; 20: 9423 9433 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshioka H, McCarrey JR, Yamazaki Y. Dynamic nuclear organization of constitutive heterochromatin during fetal male germ cell development in mice. Biol Reprod 2009; 80: 804 812 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiu YH, Sun Q, Chen ZJ. E1-L2 activates both ubiquitin and FAT10. Mol Cell 2007; 27: 1014 1023 [DOI] [PubMed] [Google Scholar]