Abstract
Regulation of growth of ovarian theca-interstitial tissues is essential for normal ovarian development and function. Reactive oxygen species are involved in modulation of signal transduction pathways, including regulation of tissue growth and apoptosis. Previously, we have demonstrated that antioxidants inhibit proliferation of theca-interstitial cells. This report evaluates the effects of antioxidants on apoptosis of rat theca-interstitial cells. The cells were cultured in chemically defined media without or with vitamin E succinate and ebselen. Apoptosis was evaluated by cytochemical assessment of nuclear morphology, activity of executioner caspases 3 and 7, and determination of staining with annexin V in combination with propidium iodide. Both tested antioxidants induced significant morphological changes consistent with apoptosis, including chromatin condensation, nuclear shrinkage, and pyknosis. Antioxidants also induced other hallmarks of apoptosis including increased activity of caspases 3/7 as well as increased staining with annexin V. The present findings demonstrate that antioxidants with distinctly different mechanisms of action induce a series of events consistent with the process of apoptosis in ovarian mesenchyme. These observations may be of translational-clinical relevance, providing mechanistic support for the use of antioxidants in the treatment of PCOS, a condition associated with excessive growth and activity of theca-interstitial cells.
Keywords: antioxidants, apoptosis, ovary, theca-interstitial cells
The antioxidants vitamin E succinate and ebselen induce apoptosis of ovarian theca-interstitial cells in a concentration-dependent fashion.
INTRODUCTION
Regulation of growth of ovarian mesenchymal tissues is essential for normal ovarian development and function. Under pathological conditions, such as polycystic ovary syndrome (PCOS), ovarian mesenchyma (thecal and interstitial tissues) is hyperplastic [1]. One possible explanation of this observation, or at least a significant contributor, may be related to the presence of increased oxidative stress demonstrated in several studies on women with PCOS [2–4]. Excessive growth of tissues may be due to increased cellular proliferation and/or reduced apoptosis. Our previous studies have demonstrated that moderate oxidative stress results in stimulation of theca-interstitial proliferation, while antioxidants, including vitamin E succinate and ebselen, reduce proliferation [5]. These observations are consistent with the concept that reactive oxygen species (ROS) at moderate levels may function as second messengers, stimulating signal transduction pathways and inducing increased cell growth and reduction of apoptosis [6, 7].
Effects of moderate levels of ROS often closely resemble actions of insulin, an important modulator of growth and apoptosis in many cell types, including theca-interstitial cells [8, 9]. Indeed, we have shown that in theca-interstitial cells, moderate oxidative stress as well as insulin activate MAPK3/1 and RPS6KB1 [10]. Antiapoptotic cell survival promoting signaling of insulin includes activation of RPS6KB1 [11]. Collectively, in view of the previously mentioned considerations, we hypothesized that antioxidants may reduce growth of the theca-interstitial compartment by a mechanism involving induction of apoptosis.
This study was designed to evaluate effects of antioxidants on several hallmarks of apoptosis: abnormal nuclear morphology, activity of executioner caspases 3/7, and staining with annexin V. In this report we present novel evidence demonstrating proapoptotic effects of vitamin E succinate and ebselen on ovarian mesenchymal tissues.
MATERIALS AND METHODS
Animals and Tissues
Ovarian theca-interstitial cells were obtained from Sprague-Dawley rats. The animals were obtained at the age of 25 days (Taconic Farms, Germantown, NY) and housed in an air-conditioned environment with a 12L:12D photoperiod. Commencing on Day 28 of age, the animals received daily injections of 17β-estradiol (1 mg/0.3 ml sesame oil s.c.; for 3 days) in order to stimulate ovarian growth and development of antral follicles [12, 13]. At the age of 31 days (24 h after the last injection of estradiol), the animals were anesthetized using ketamine (i.p.) and xylazine (i.p.) and killed by intracardiac perfusion with 0.9% saline. Following saline perfusion the ovaries were collected. All treatments and procedures were in accordance with accepted standards of human animal care as outlined in the NIH Guide for the Care and Use of Laboratory Animals and a protocol approved by the Yale University Animal Care Committee and Animal Care and Use Committee at the University of California, Davis.
Theca-interstitial cells were dissected and purified as described previously [8, 14]. Briefly, enzymatically digested ovarian tissues were filtered, and the cells were isolated using a discontinuous Percoll gradient. The purity of the theca-interstitial cell preparation has been previously evaluated immunohistochemically [8]. Viability of the cells was routinely in the 85%–95% range.
Cell Culture and Reagents
All incubations were carried out for up to 24 h at 37°C in an atmosphere of 5% CO2 in humidified air, in serum-free McCoys 5a medium (with antibiotics, 0.1% BSA and 2 mmol/L l-glutamine). The cells were incubated in absence or in the presence of vitamin E succinate (VES) or ebselen. Chemicals were obtained from Sigma Chemical Co. (St. Louis, MO); cell culture reagents were purchased from Grand Island Biological Co. (Grand Island, NY).
Determinations of Apoptosis
Apoptosis was evaluated by three methods: cytochemical assessment of nuclear morphology, activity of executioner caspases 3 and 7, and determination of staining with annexin V.
Cytochemical assessment of apoptotic death was carried out on cells fixed in 2% neutral-buffered paraformaldehyde (30 min at 4°C) and stained with DNA-binding fluorescent dye (4,6-diamidino-2-phenylindole [DAPI]) at a final concentration of 20 μg/ml for 15 min at room temperature in the dark. The cells were evaluated using fluorescence microscopy with an ultraviolet light filter. An observer was blinded to treatments. Morphological criteria of apoptosis described previously were applied [15, 16]. Findings of nuclear condensation (brightly fluorescent pyknotic nuclei), and multiple, densely stained chromatin fragments of nearly spherical shape (apoptotic bodies) were used to identify apoptotic cells.
The activity of caspases 3 and 7 was determined using fluorescent kit (Apo-ONE Homogenous Caspase 3/7; Promega, Madison, WI). Simultaneously, the number of living cells was determined in the same wells using fluorescent method (Cell Titer Blue kit; Promega). Determinations of caspases 3/7 activities and the number of viable cells were performed using fluorescence plate reader Wallac Counter Victor 2 (Perkin Elmer, Waltham, MA).
Determination of Annexin V was performed using a Vybrant Apoptosis Assay kit (Promega) detecting phosphatidylserine of Annexin V as a marker of apoptosis and using propidium iodide as a marker for dead cells. Each determination was performed using at least 150 000 cells; 50 μl of binding buffer were added to each sample followed by Alexa Fluor-Annexin V reagent. After a short incubation, propidium iodide was added followed by a binding buffer; the samples were then run on a FACScan flow cytometer. At least 10 000 events were evaluated per sample. Positive and negative controls were run before experimental samples. Results were analyzed using WinMidi v.2.8 freeware (http://facs.scripps.edu).
Statistical Analysis
Values represent means ± SEM. Statistical analysis was performed using analysis of variance followed by pairwise comparisons using the Bonferroni correction.
RESULTS
Cytochemical Determination of Apoptosis
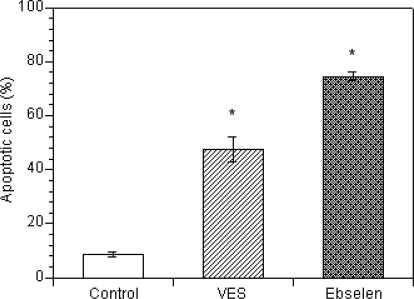
Figure 1 summarizes the effects of vitamin E succinate and ebselen on nuclear morphology of ovarian theca-interstitial cells, as evaluated by DAPI staining. In the absence of antioxidants, 8.5 ± 1% of cells showed chromatin condensation and nuclear shrinkage consistent with apoptosis. In the presence of vitamin E succinate, the rate of these morphological features of apoptosis increased 5.6-fold, while in the presence of ebselen, it increased 8.8-fold.
FIG. 1.
Effects of vitamin E succinate (VES; 30 μM) and ebselen (30 μM) on apoptosis detected by DAPI staining following a 24-h incubation in culture slides for in situ analysis. Cultures were carried out in chemically defined media. Each bar represents the mean (± SEM); * denotes means significantly different from control (P < 0.05).
Activity of Caspases 3/7
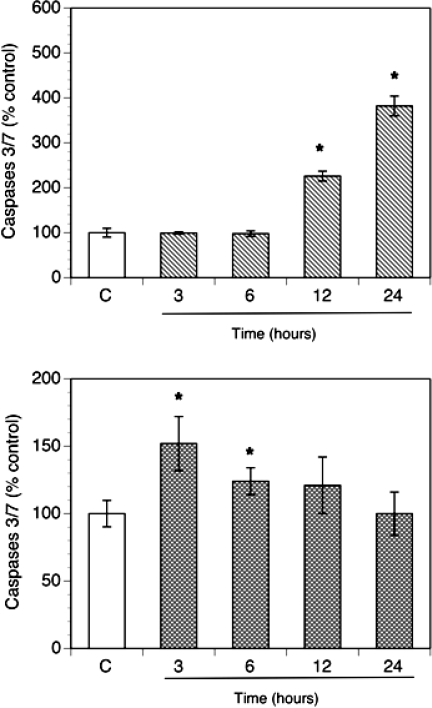
To further study effects of antioxidants on apoptosis, activity of effector-executioner caspases 3/7 was evaluated. In order to characterize a time course of the effects, the cells were incubated for up to 24 h without (control) or with antioxidants. Apoptosis was quantified by detection of the activity of caspases 3/7 expressed per number of viable cells (Fig. 2). Vitamin E succinate had no significant effect at 3 and 6 h but significantly stimulated apoptosis at 12 and 24 h with the greatest amount of apoptosis observed at 24 h (382 ± 22% of control). In contrast, ebselen induced an early wave of apoptosis with the most profound effect at 3 h (152 ± 10% of control).
FIG. 2.
Time course of effects of vitamin E succinate (100 μM; upper panel) and ebselen (30 μM; lower panel) on caspases 3/7 in culture. The cells were plated in 96-well plates at a density of 25 000 cells per well. Cultures were carried out in chemically defined media for up to 24 h. Activity of caspases 3/7 was calculated per number of living cells and expressed as percentage of control (means ± SEM); * denotes means significantly different from control (P < 0.05).
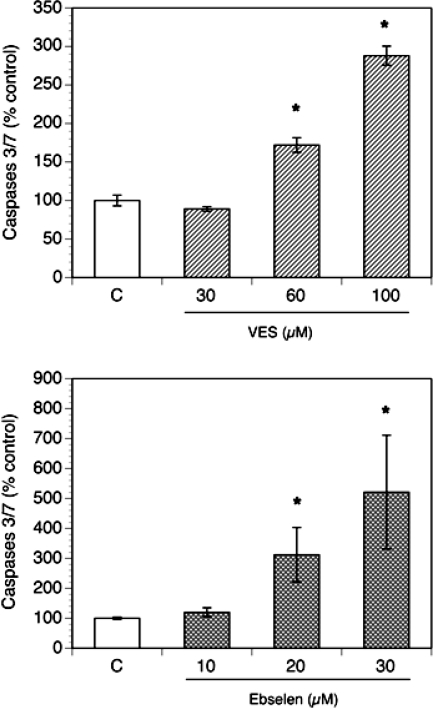
Based on these observations, in the subsequent experiments, vitamin E succinate-induced apoptosis was tested after 24 h, while that induced by ebselen was tested after 3 h. As presented in Figure 3, both vitamin E succinate and ebselen induced a concentration-dependent increase of caspase 3/7 activity.
FIG. 3.
Concentration-dependent effects of vitamin E succinate (VES; 30–100 μM, 24-h incubations) and ebselen (10–30 μM; 3-h incubations) on activity of caspases 3/7 in culture. The cells were plated in 96-well plates at a density of 25 000 cells per well. Cultures were carried out in chemically defined media. Activity of caspases 3/7 was calculated per number of living cells and expressed as percentage of control (means ± SEM); * denotes means significantly different from control (P < 0.05).
Annexin V
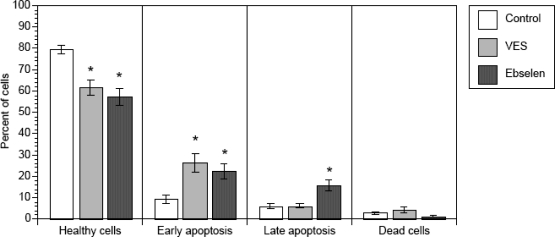
Another marker of apoptosis involves staining using annexin V, a calcium-dependent phospholipid binding protein with high affinity for phosphatidylserine, a plasma membrane phospholipid. During the process of apoptosis, phosphatidylserine is translocated from the inner to the outer leaflet of the plasma membrane. Early and late apoptosis was detected by simultaneous flow cytometry assessment of staining with annexin V and propidium iodide (Fig. 4). It is apparent that the proportion of healthy cells declined in the presence of vitamin E succinate (by 23% vs. control) and in the presence of ebselen (by 28% vs. control). In parallel, exposure to vitamin E succinate and ebselen led to an increase in the proportion of early apoptotic cells by 2.8-fold and 2.4-fold, respectively. Ebselen also increased the proportion of late apoptotic cells by 2.6-fold. Vitamin E succinate had no significant effect on late apoptosis.
FIG. 4.
Effects of vitamin E succinate (VES; 100 μM) and ebselen (30 μM) on apoptosis detected by flow cytometry by identification of staining with annexin V and propidium iodide (PI). Theca interstitial cells were either cultured in the absence of additives or exposed for the last 24 h to VES or exposed for the last 3 h to ebselen. Cells were considered healthy in the absence of staining for annexin V or PI; early apoptotic in the presence of staining for annexin V and absence of staining for PI. Late apoptosis was identified in the presence of staining for both annexin V and PI. Dead cells stained only for PI. The results are presented as percentage of cells (± SEM); * denotes means significantly different from control (P < 0.05).
DISCUSSION
To our knowledge, this is the first report describing induction of apoptosis by antioxidants in ovarian mesenchyme. Apoptosis, often referred to as programmed cell death, is an important and tightly regulated mechanism of cell deletion, playing a major role in the physiologic control of cell population [17]. This process is distinctly different from necrosis, typically a premature cell death induced by nonphysiologic disturbances, such as thermal insults or metabolic poisons.
Both antioxidants tested in the present study induced a series of effects consistent with apoptotic death of theca-interstitial cells, including alteration in the appearance of the nucleus, activation of executioner caspases, and changes in the morphology of the plasma membrane. DAPI staining of the nucleus identifies events occurring in the latter stages of apoptosis and detects chromatin concentration in association with nuclear shrinkage and ultimately pyknosis-fragmentation of the nucleus [18]. Quantification of activation of executioner caspases 3 and 7 identifies early stages of apoptosis, while detection of staining for annexin V in combination with staining for propidium iodide identifies both early and late apoptosis [19, 20].
It is apparent that both ebselen and vitamin E succinate induced theca-interstitial apoptosis; however, the effects of ebselen were observed earlier than vitamin E succinate. These differences may be related to specifics of the mode of action of each of these compounds. Vitamin E scavenges free radicals, while ebselen is a seleno-organic compound with little free radical scavenging capability but possessing glutathione peroxidase-like activity [21]. Furthermore, ebselen has been shown to inhibit the activity of the main cellular source of ROS outside the mitochondria: NADPH oxidase [22].
Review of the literature reveals that effects of antioxidants on apoptosis depend on the specifics of experimental conditions. Antioxidants have been shown to induce apoptosis in several biological systems under basal conditions but typically protect from apoptosis induced by significant oxidative stress or chemotherapeutic agents, such as doxorubicin [23–26]. Reactive oxidants, such as peroxynitrite, have been shown to mediate both apoptosis and necrosis, while ebselen interacts with peroxynitrite and provides efficient protection against these effects [27, 28]. Such divergent proapoptotic and antiapoptotic actions of antioxidants are still not well understood. One possible explanation reconciling these actions of antioxidants may be that the cells attain the lowest level of apoptosis in the presence of a certain optimal level of ROS and that apoptosis increases when the cells are exposed to either too high or too low levels of ROS.
The present observations indicate that theca-interstitial cells are sensitive to the level of ROS and that reduction of oxidants triggers apoptosis. These findings may be of translational-clinical relevance to PCOS, a condition associated with excessive growth and activity of theca-interstitial cells. It is tempting to speculate that antioxidants may be of potential therapeutic value and that one of the possible mechanisms of their action may be related to reduction of oxidative stress and apoptosis. This concept is supported by clinical studies demonstrating beneficial effects of one of the antioxidants, N-acetylcysteine, on various aspects of PCOS, including restoration of gonadal function [29–31]. However, administration of N-acetylcysteine has been studied on relatively few patients, and while no significant side effects were reported in the previously mentioned studies on women with PCO, other reports have indicated that N-actylcysteine use may be associated with a broad range of adverse reactions, including nausea, vomiting, anaphylactoid reactions, and, rarely, ECG abnormalities or status epilepticus [32].
In another study, treatment of PCOS using rosiglitazone resulted in reduction of oxidative stress as well as improvement of other endocrine and metabolic parameters of this condition [33]. Metformin, another commonly used treatment of PCOS, has been shown to reduce oxidative stress, possibly in part by reduction of NADPH oxidase activity [34, 35]. In at least two studies on women with PCOS, including a randomized placebo-controlled trial, metformin also reduced ovarian volume and/or stromal to total ovarian area [36, 37]. One of the possible mechanisms of these effects of rosiglitazone and metformin may be related to reduction of oxidative stress and consequent antioxidant-like action on ovarian theca-interstitial cells.
It is essential to acknowledge, however, that actions of reactive oxygen species and antioxidants on the ovary are not limited to theca-interstitial cells but include also an intricate pattern of effects on a broad range of ovarian tissues and functions, including granulosa cells, oocytes, steroidogenesis, follicular growth, and apoptosis [38, 39]. In particular, inhibition of oxidative stress suppressed apoptosis of granulosa cells in cultured follicles [38], indicating that effects of antioxidants on apoptosis are cell-type dependent.
In conclusion, the results of this study provide evidence that in theca-interstitial cells, two antioxidants with distinctly different mechanisms of action induce a series of events consistent with the process of apoptosis. These findings may explain, at least in part, previous observations that antioxidants inhibit proliferation of theca-interstitial cells [5]. Antioxidants may be of therapeutic value in treatment of conditions associated with excessive growth of ovarian mesenchyma such as PCOS. The role of oxidative stress and antioxidants on possible modulation of steroidogenesis, the key function of ovarian theca-interstitial cells, should be addressed in future studies.
Footnotes
Supported by grant R01-HD050656 from the Eunice Shriver National Institute of Child Health and Human Development (to A.J.D.).
REFERENCES
- Hughesdon PE. Morphology and morphogenesis of the Stein-Leventhal ovary and of so called “hyperthecosis.” Obstet Gynecol Surv 1982; 37: 59 77 [DOI] [PubMed] [Google Scholar]
- Sabuncu T, Vural H, Harma M. Oxidative stress in polycystic ovary syndrome and its contribution to the risk of cardiovascular disease. Clin Biochem 2001; 34: 407 413 [DOI] [PubMed] [Google Scholar]
- Fenkci V, Fenkci S, Yilmazer M, Serteser M. Decreased total antioxidant status and increased oxidative stress in women with polycystic ovary syndrome may contribute to the risk of cardiovascular disease. Fertil Steril 2003; 80: 123 127 [DOI] [PubMed] [Google Scholar]
- Kuscu NK, Var A. Oxidative stress but not endothelial dysfunction exists in non-obese, young group of patients with polycystic ovary syndrome. Acta Obstet Gynecol Scand 2009; 88: 612 617 [DOI] [PubMed] [Google Scholar]
- Duleba AJ, Foyouzi N, Karaca M, Pehlivan T, Kwintkiewicz J, Behrman HR. Proliferation of ovarian theca-interstitial cells is modulated by antioxidants and oxidative stress. Hum Reprod 2004; 19: 1519 1524 [DOI] [PubMed] [Google Scholar]
- Clement MV, Pervaiz S. Reactive oxygen intermediates regulate cellular response to apoptotic stimuli: an hypothesis. Free Radical Res 1999; 30: 247 252 [DOI] [PubMed] [Google Scholar]
- del Bello B, Paolicchi A, Comporti M, Pompella A, Maellaro E. Hydrogen peroxide produced during gamma-glutamyl transpeptidase activity is involved in prevention of apoptosis and maintainance of proliferation in U937 cells. FASEB J 1999; 13: 69 79 [DOI] [PubMed] [Google Scholar]
- Duleba AJ, Spaczynski RZ, Olive DL, Behrman HR. Effects of insulin and insulin-like growth factors on proliferation of rat ovarian theca-interstitial cells. Biol Reprod 1997; 56: 891 897 [DOI] [PubMed] [Google Scholar]
- Spaczynski RZ, Tilly JL, Mansour A, Duleba AJ. Insulin and insulin-like growth factors inhibit and luteinizing hormone augments ovarian theca-interstitial cell apoptosis. Mol Hum Reprod 2005; 11: 319 324 [DOI] [PubMed] [Google Scholar]
- Kwintkiewicz J, Spaczynski RZ, Foyouzi N, Pehlivan T, Duleba AJ. Insulin and oxidative stress modulate proliferation of rat ovarian theca-interstitial cells through diverse signal transduction pathways. Biol Reprod 2006; 74: 1034 1040 [DOI] [PubMed] [Google Scholar]
- Jonassen AK, Mjos OD, Sack MN. p70s6 kinase is a functional target of insulin activated Akt cell-survival signaling. Biochem Biophys Res Commun 2004; 315: 160 165 [DOI] [PubMed] [Google Scholar]
- Richards JS, Ireland JJ, Rao MC, Bernath GA, Midgley AR, Jr, Reichert LE., Jr Ovarian follicular development in the rat: hormone receptor regulation by estradiol, follicle stimulating hormone and luteinizing hormone. Endocrinology 1976; 99: 1562 1570 [DOI] [PubMed] [Google Scholar]
- Rao MC, Midgley AR, Jr, Richards JS. Hormonal regulation of ovarian cellular proliferation. Cell 1978; 14: 71 78 [DOI] [PubMed] [Google Scholar]
- Magoffin DA, Erickson GF. An improved method for primary culture of ovarian androgen-producing cells in serum-free medium: effect of lipoproteins, insulin, and insulin-like growth factor-I. In Vitro Cell Dev Biol 1988; 24: 862 870 [DOI] [PubMed] [Google Scholar]
- Huppertz B, Frank HG, Kaufmann P. The apoptosis cascade—morphological and immunohistochemical methods for its visualization. Anat Embryol (Berl) 1999; 200: 1 18 [DOI] [PubMed] [Google Scholar]
- Stadelmann C, Lassmann H. Detection of apoptosis in tissue sections. Cell Tissue Res 2000; 301: 19 31 [DOI] [PubMed] [Google Scholar]
- Kerr JF, Wyllie AH, Wyllie AF, Currie AR, Currie AR. Apoptosis: a basic biological phenomenon with wide-ranging implications in tissue kinetics. Br J Cancer 1972; 26: 239 257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doonan F, Cotter TG. Morphological assessment of apoptosis. Methods 2008; 44: 200 204 [DOI] [PubMed] [Google Scholar]
- Ballou LR, Laulederkind SJ, Rosloniec EF, Raghow R. Ceramide signalling and the immune response. Biochim Biophys Acta 1996; 1301: 273 287 [DOI] [PubMed] [Google Scholar]
- van Engeland M, Nieland LJ, Ramaekers FC, Schutte B, Reutelingsperger CP. Annexin V-affinity assay: a review on an apoptosis detection system based on phosphatidylserine exposure. Cytometry 1998; 31: 1 9 [DOI] [PubMed] [Google Scholar]
- Maiorino M, Roveri A, Ursini F. Antioxidant effect of Ebselen (PZ 51): peroxidase mimetic activity on phospholipid and cholesterol hydroperoxides vs free radical scavenger activity. Arch Biochem Biophys 1992; 295: 404 409 [DOI] [PubMed] [Google Scholar]
- Wakamura K, Ohtsuka T, Okamura N, Ishibashi S, Masayasu H. Mechanism for the inhibitory effect of a seleno-organic compound, Ebselen, and its analogues on superoxide anion production in guinea pig polymorphonuclear leukocytes. J Pharmacobiodyn 1990; 13: 421 425 [DOI] [PubMed] [Google Scholar]
- Neuzil J, Weber T, Terman A, Weber C, Brunk UT. Vitamin E analogues as inducers of apoptosis: implications for their potential antineoplastic role. Redox Rep 2001; 6: 143 151 [DOI] [PubMed] [Google Scholar]
- Yang CF, Shen HM, Ong CN. Ebselen induces apoptosis in HepG(2) cells through rapid depletion of intracellular thiols. Arch Biochem Biophys 2000; 374: 142 152 [DOI] [PubMed] [Google Scholar]
- Kotamraju S, Konorev EA, Joseph J, Kalyanaraman B. Doxorubicin-induced apoptosis in endothelial cells and cardiomyocytes is ameliorated by nitrone spin traps and ebselen: role of reactive oxygen and nitrogen species. J Biol Chem 2000; 275: 33585 33592 [DOI] [PubMed] [Google Scholar]
- Yoshizumi M, Kogame T, Suzaki Y, Fujita Y, Kyaw M, Kirima K, Ishizawa K, Tsuchiya K, Kagami S, Tamaki T. Ebselen attenuates oxidative stress-induced apoptosis via the inhibition of the c-Jun N-terminal kinase and activator protein-1 signalling pathway in PC12 cells. Br J Pharmacol 2002; 136: 1023 1032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonfoco E, Krainc D, Ankarcrona M, Nicotera P, Lipton SA. Apoptosis and necrosis: two distinct events induced, respectively, by mild and intense insults with N-methyl-D-aspartate or nitric oxide/superoxide in cortical cell cultures. Proc Natl Acad Sci U S A 1995; 92: 7162 7166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sies H, Arteel GE. Interaction of peroxynitrite with selenoproteins and glutathione peroxidase mimics. Free Radic Biol Med 2000; 28: 1451 1455 [DOI] [PubMed] [Google Scholar]
- Fulghesu AM, Ciampelli M, Muzj G, Belosi C, Selvaggi L, Ayala GF, Lanzone A. N-acetyl-cysteine treatment improves insulin sensitivity in women with polycystic ovary syndrome. Fertil Steril 2002; 77: 1128 1135 [DOI] [PubMed] [Google Scholar]
- Rizk AY, Bedaiwy MA, Al-Inany HG. N-acetyl-cysteine is a novel adjuvant to clomiphene citrate in clomiphene citrate-resistant patients with polycystic ovary syndrome. Fertil Steril 2005; 83: 367 370 [DOI] [PubMed] [Google Scholar]
- Masha A, Manieri C, Dinatale S, Bruno GA, Ghigo E, Martina V. Prolonged treatment with N-acetylcysteine and L-arginine restores gonadal function in patients with polycystic ovary syndrome. J Endocrinol Invest 2009; 32: 870 872 [DOI] [PubMed] [Google Scholar]
- Sandilands EA, Bateman DN. Adverse reactions associated with acetylcysteine. Clin Toxicol (Phila) 2009; 47: 81 88 [DOI] [PubMed] [Google Scholar]
- Yilmaz M, Bukan N, Ayvaz G, Karakoc A, Toruner F, Cakir N, Arslan M. The effects of rosiglitazone and metformin on oxidative stress and homocysteine levels in lean patients with polycystic ovary syndrome. Hum Reprod 2005; 20: 3333 3340 [DOI] [PubMed] [Google Scholar]
- Hou X, Song J, Li XN, Zhang L, Wang X, Chen L, Shen YH. Metformin reduces intracellular reactive oxygen species levels by upregulating expression of the antioxidant thioredoxin via the AMPK-FOXO3 pathway. Biochem Biophys Res Commun 2010; 396: 199 205 [DOI] [PubMed] [Google Scholar]
- Piwkowska A, Rogacka D, Jankowski M, Dominiczak MH, Stepinski JK, Angielski S. Metformin induces suppression of NAD(P)H oxidase activity in podocytes. Biochem Biophys Res Commun 2010; 393: 268 273 [DOI] [PubMed] [Google Scholar]
- Genazzani AD, Battaglia C, Malavasi B, Strucchi C, Tortolani F, Gamba O. Metformin administration modulates and restores luteinizing hormone spontaneous episodic secretion and ovarian function in nonobese patients with polycystic ovary syndrome. Fertil Steril 2004; 81: 114 119 [DOI] [PubMed] [Google Scholar]
- Romualdi D, Giuliani M, Cristello F, Fulghesu AM, Selvaggi L, Lanzone A, Guido M. Metformin effects on ovarian ultrasound appearance and steroidogenic function in normal-weight normoinsulinemic women with polycystic ovary syndrome: a randomized double-blind placebo-controlled clinical trial. Fertil Steril 2010; 93: 2303 2310 [DOI] [PubMed] [Google Scholar]
- Tilly JL, Tilly KI. Inhibitors of oxidative stress mimic the ability of follicle-stimulating hormone to suppress apoptosis in cultured rat ovarian follicles. Endocrinology 1995; 136: 242 252 [DOI] [PubMed] [Google Scholar]
- Yacobi K, Tsafriri A, Gross A. Luteinizing hormone-induced caspase activation in rat preovulatory follicles is coupled to mitochondrial steroidogenesis. Endocrinology 2007; 148: 1717 1726 [DOI] [PubMed] [Google Scholar]