Abstract
AIM: To analyze the correlation between cytokine-induced killer (CIK) cells adoptive immunotherapy and cancer-related death in gastric cancer patients.
METHODS: One hundred and fifty-six gastric cancer patients after operation at the Third Affiliated Hospital of Soochow University were enrolled in this study. Their clinical data including demographic characteristics, operation time, tumor size, pathological type and staging, tumor metastasis, outcome of chemotherapy or CIK cells adoptive immunotherapy, survival time or time of death were collected with a standard structured questionnaire. Kaplan-Meier method was used to estimate the median survival time, and the 2- and 5- year survival rates. Hazard risk (HR) and 95% confidence interval (95% CI) of CIK cells adoptive immunotherapy for gastric cancer were calculated using the two-stage time-dependent covariates Cox model.
RESULTS: The survival time of gastric cancer patients was longer after CIK cells adoptive immunotherapy than after chemotherapy (χ2 = 10.907, P = 0.001). The median survival time of gastric cancer patients was also longer after CIK cells adoptive immunotherapy than after chemotherapy (49 mo vs 27 mo, P < 0.05). The 2- and 5-year survival rates of gastric cancer patients were significantly higher after CIK cells adoptive immunotherapy than after chemotherapy (73.5% vs 52.6%, 40.4% vs 23.9%, P < 0.05). A significant difference was observed in the survival curve for patients who received CIK cells adoptive immunotherapy (0, 1-10, 11-25, and over 25 frequencies) (χ2 = 14.534, P = 0.002). The frequencies of CIK cells adoptive immunotherapy were significantly related with the decreasing risk of death in gastric cancer patients after adjustment for sex and age of the patients, tumor stage and relapse (HR = 0.54, 95% CI: 0.36-0.80) when the first stage Cox model was used to define the subjects who remained alive beyond 36 mo as survivors. However, no correlation was observed between the frequencies of death in CIK cells adoptive immunotherapy and the risk of gastric cancer patients (HR = 1.09, 95% CI: 0.63-0.89) when the second stage Cox model was used to define the subjects who survived for more than 36 mo as survivors.
CONCLUSION: The survival time of the gastric cancer patients treated with chemotherapy combined with CIK cells adoptive immunotherapy is significantly longer than that of the patients treated with chemotherapy alone and increasing the frequency of CIK cells adoptive immunotherapy seems to benefit patients more.
Keywords: Immunotherapy, Cytokine-induced killer cells, Gastric cancer, Survival analysis, Probability
INTRODUCTION
Gastric cancer is one of the most common causes of cancer-related death in China[1]. Its incidence in Jiangsu Province of China is particularly high, and its mortality rate is much higher than the national average[2]. The early clinical detection rate of gastric cancer is less than 15%, and about 85% cases of gastric cancer are at the advanced stage when their gastric cancer is diagnosed[3]. Surgery is the standard treatment procedure for localized and resectable gastric cancer[4]. However, surgery alone does not improve the 5-year survival rate of local advanced gastric cancer patients[3]. Although standardized surgical resection and adjuvant therapeutic modalities are available for gastric cancer, the survival rate of advanced gastric cancer patients remains very low after operation[5]. About 60% of gastric cancer patients usually experience local recurrence and metastasis to other organs[6]. It has been demonstrated that local recurrence and distant metastasis constitute a major problem in the failure of cancer therapies[7]. Therefore, considerable efforts are needed to improve the current therapeutic modalities and to explore new therapies. In recent years, immune therapy has become the fourth important treatment modality for malignant tumors following surgery, radiotherapy and chemotherapy[8-10].
A number of adoptive immunotherapy with killer cells have been reported, including lymphokine-activated killer cells[11], tumor infiltrating lymphocytes[12], or anti-CD3 monoclonal antibody-induced killer cells[13]. However, their therapeutic efficacy is limited due to their low anti-tumor activities[14]. At present, cytokine-induced killer (CIK) cells are a new type of anti-tumor effector cells, which can proliferate rapidly in vitro, with a stronger anti-tumor activity and a broader target tumor spectrum than the reported anti-tumor effector cells[10,15]. Moreover, CIK cells can regulate and enhance immune function[16]. Studies have reported the level of tumor markers, change in cellular immune functions, exploration of molecule targets and a short-term efficacy in gastric cancer patients after chemotherapy plus CIK cells immunotherapy despite some side effects[17-19]. However, the relation between the frequencies of CIK cells immunotherapy and its clinical efficacy has not been examined. In the present study, data obtained from 156 gastric cancer patients in fitting multivariate Cox model showed that more frequencies of CIK cells immunotherapy could improve the survival rate of gastric cancer patients.
MATERIALS AND METHODS
Patients
One hundred and fifty-six primary gastric cancer patients after operation at the Third Affiliated Hospital of Soochow University (Jiangsu Province, China) were enrolled in this study. Those who did not meet the inclusion criteria, or had other tumors were excluded.
A standard questionnaire was designed to collect the data from the patients, including demographic characteristics, operation time, tumor size and location, pathological type and staging, tumor metastasis, outcome of chemotherapy or CIK cells immunotherapy. Meanwhile, time of relapse and death of the patients was recorded. Patients received 6 cycles of chemotherapy before CIK cells immunotherapy. Some patients underwent CIK cells immunotherapy due to cancer recurrence during chemotherapy. Recurrence of gastric cancer was defined when local, peritoneal or distant metastasis was detected at any site during chemotherapy[20]. The study was conducted according to the principles of the Declaration of Helsinki. All patients gave their informed consent prior to inclusion in the study. The study was approved by the Ethics Committee of the Third Affiliated Hospital of Soochow University.
Eighty-one patients (62 males, 19 females) at the age of 59.9 ± 10.5 years with a median age of 60.5 years who underwent chemotherapy alone served as chemotherapy group (group I), and those (60 males at the age of 62.4 ± 10.8 years with a median age 60.5 years, 15 females at the age of 51.0 ± 10.7 years with a median age of 50 years) who received chemotherapy plus CIK cells immunotherapy served as treatment group (group II) (Table 1).
Table 1.
Distribution of demographic and clinical characteristics in two groups
| Demographic and clinical features | n |
Groups, n (%) |
χ2 | P | |
| Group I | Group II | ||||
| Sex | |||||
| Men | 122 | 62 (76.5) | 60 (80) | 0.273 | 0.601 |
| Women | 34 | 19 (23.5) | 15 (20) | ||
| Age (yr) | |||||
| ≤ 45 | 14 | 7 (8.6) | 7 (9.3) | 0.047a | 0.977 |
| 45 < age ≤ 60 | 71 | 36 (44.5) | 35 (46.7) | ||
| > 60 | 71 | 38 (46.9) | 33 (44.0) | ||
| Tumor siteb | |||||
| Gastric cardia | |||||
| Yes | 58 | 30 (37.0) | 28 (37.3) | 0.002 | 0.970 |
| No | 98 | 51 (63.0) | 47 (62.7) | ||
| Gastric body | |||||
| Yes | 64 | 35 (43.2) | 29 (38.7) | 0.332 | 0.564 |
| No | 92 | 46 (56.8) | 46 (61.3) | ||
| Gastric antrum | |||||
| Yes | 36 | 17 (21.0) | 19 (25.3) | 0.414 | 0.520 |
| No | 120 | 64 (79.0) | 56 (74.7) | ||
| Tumor sizec (cm) | |||||
| < 5 | 76 | 43 (59.7) | 33 (55.0) | 0.299 | 0.585 |
| ≥ 5 | 56 | 29 (40.3) | 27 (45.0) | ||
| Histological typec | |||||
| Differentiated | 52 | 28 (35.4) | 24 (64.6) | 0.002 | 0.962 |
| Poorly-differentiated | 94 | 51 (38.5) | 43 (64.2) | ||
| Invasionc | |||||
| Yes | 112 | 55 (76.4) | 57 (89.1) | 3.745 | 0.053 |
| No | 24 | 17 (23.6) | 7 (10.9) | ||
| Lymph node metastasisc | |||||
| Yes | 100 | 55 (76.4) | 45 (70.3) | 0.643 | 0.423 |
| No | 36 | 17 (23.6) | 19 (29.7) | ||
| Relapse | |||||
| Yes | 98 | 58 (28.4) | 40 (46.7) | 5.566 | 0.018 |
| No | 58 | 23 (71.6) | 35 (53.3) | ||
| Pathological grade | |||||
| 1 | 2 | 1 (1.3) | 1 (1.3) | 2.976 | 0.395 |
| 2 | 27 | 18 (22.2) | 9 (12.0) | ||
| 3 | 92 | 44 (54.3) | 48 (64.0) | ||
| 4 | 35 | 18 (22.2) | 17 (22.7) | ||
| Tumor stage | |||||
| I | 14 | 9 (11.1) | 5 (6.7) | 2.129a | 0.546 |
| II | 22 | 12 (14.8) | 10 (13.3) | ||
| III | 102 | 49 (60.5) | 53 (70.7) | ||
| IV | 18 | 11 (13.6) | 7 (9.3) | ||
cmh2 χ2-test;
Two cases of gastric cancer involving gastric cardia and body;
Missed cases.
Preparation of CIK cells and treatment
Peripheral blood mononuclear cells (PBMC) were collected with a COBE spectra blood cell separator (Gambro BCT, Inc., Lakewood, USA). Viability of PBMC was assessed by trypan blue exclusion. PBMC (2.0 × 106 /mL) were plated onto 6-well dishes (Nunc, Denmark) and cultured with medium I containing RPMI 1640 in the presence of 1.0 × 106 U/L human interferon-γ (IFN-γ, Shanghai Fosun Pharma Co., China), 5.0 × 105 U/L recombinant human interleukin-2 (IL-2, Shangdong Quangang Pharmaceutical Co., China), 10% inactivated human serum, 25 mmol/L HEPES, 2 mmol/L L-glutamine, 100 U/mL penicillin and 100 μg/mL streptomycin. The cells were incubated in a humidified atmosphere containing 5% CO2 at 37°C. Monoclonal antibody (MAb) against CD3 (100 μg/L, Antibody Diagnostic Inc., USA) and IL-1α (1.0 × 105 U/L, Promega) were added after 24 h culture. Supernatant was aspirated and the cells were cultured in Medium II in the absence of INF-γ after another 48 h culture. The cells were transferred to Kolle flasks (Nunc, Denmark) and cultured in the same medium after 1 wk. The medium was changed every 3 d. The cytotoxic activity was assayed as previously described[17,21]. Patients in group I were treated with oxaliplatin (120 mg/m2 D1, 5-Fu 400 mg/m2 CIV 24 h D1-5, CF 200 mg/m2 D1-5) as previously described[22] with the doses adjusted according to the toxicity. Patients in group II received CIK cells immunotherapy as previously described[17] after 6 cycles of chemotherapy, with 1.0 × 109 CIK cells transfused into the patients within 1 h.
Statistical analysis
All data were loaded into the Epidata3.0 database with double-check, and analyzed with the SAS software package (version 9.13; SAS Institute, Cary, NC, USA). The data were expressed as mean ± SD. χ2-test or cmh2 χ2-test was used to compare the difference in balance between the concerned clinical indexes and to find the confounding factors between the two groups. Survival data were analyzed using Kaplan-Meier method and log-rank test to estimate the median survival time, 2- and 5-year survival rates, and to determine if the survival curves for the two groups were different.
When the frequency of CIK cells immunotherapy and the survival time were introduced into the Cox model, the interaction item was significantly associated with the death of gastric cancer patients (wald-χ2 = 4.946, P = 0.0261). A two-stage time-dependent Cox model was established to precisely estimate the hazard risk (HR) and 95% confidence interval (95% CI) of the association between the frequency of CIK cells immunotherapy and the death of gastric cancer patients. Because the median survival time of gastric cancer patients was about 36 mo, 36 mo was used as the optimum cutoff point.
The first stage Cox model involved 154 patients with a survival time of over 36 mo who were defined as survivors. Otherwise, the survival status was the same as the original definition.
The second stage Cox model only involved 56 patients with a survival time longer than 36 mo, and their survival status was defined as the original definition.
Pearson correlation test was performed between Schoenfeld residual of the frequencies of CIK cells immunotherapy and the survival time of gastric cancer patients to determine whether the frequency of CIK cells immunotherapy is a time-dependent variable in the two Cox models[23].
RESULTS
Distribution of demographic and clinical characteristics in two groups
No statistical difference was found in sex and age of the patients, tumor site, histological type, invasion depth, lymph node metastasis, pathological grade, tumor size and stage between the two groups. However, the number of patients was significantly greater in group II with recurrent disease than in group I (46.7% vs 28.4%, χ2 = 5.566, P = 0.018) (Table 1), suggesting that more patients with relapse should receive CIK cells immunotherapy.
Demographic and clinical characteristics of patients after CIK cells immunotherapy
No statistical difference was observed in sex and age of the patients, tumor site, histological type, invasion depth, lymph node metastasis, pathological grade or tumor size after CIK cells immunotherapy (0, 1-10, 11-25, and over 25 frequencies). However, a significant difference was found in cancer recurrence and stage after CIK cells immunotherapy (Table 2).
Table 2.
Distribution of demographic and clinical characteristics in group II
| Demographic and clinical features | n |
Frequency of CIK cells immunotherapy, n (%) |
χ2 | P | |||
| 0 | 1-10 | 11-25 | > 25 | ||||
| Sex | |||||||
| Men | 122 | 62 (76.5) | 39 (84.8) | 13 (86.7) | 8 (57.1) | 5.573 | 0.134 |
| Women | 34 | 19 (23.5) | 7 (15.2) | 2 (13.3) | 6 (42.9) | ||
| Age (yr) | |||||||
| ≤ 45 | 14 | 7 (8.6) | 5 (10.9) | 1 (6.6) | 1 (7.1) | 1.153 | 0.979 |
| 45 < age ≤ 60 | 71 | 36 (44.4) | 20 (43.5) | 7 (46.7) | 8 (57.1) | ||
| > 60 | 71 | 38 (47.0) | 21 (45.6) | 7 (46.7) | 5 (35.8) | ||
| Tumor sitea | |||||||
| Gastric cardia | |||||||
| Yes | 58 | 30 (37.0) | 17 (37.0) | 5 (33.3) | 6 (42.9) | 0.290 | 0.962 |
| No | 98 | 51 (63.0) | 29 (63.0) | 10 (66.7) | 8 (57.1) | ||
| Gastric body | |||||||
| Yes | 64 | 35 (43.2) | 19 (41.3) | 5 (33.3) | 5 (35.7) | 0.691 | 0.875 |
| No | 92 | 46 (56.8) | 27 (58.7) | 10 (66.7) | 9 (64.3) | ||
| Gastric antrum | |||||||
| Yes | 36 | 17 (21.0) | 10 (21.7) | 4 (26.7) | 5 (35.7) | 1.614 | 0.656 |
| No | 120 | 64 (79.0) | 36 (78.3) | 11 (73.3) | 9 (64.3) | ||
| Tumor sizec (cm) | |||||||
| < 5 | 76 | 43 (59.7) | 17 (45.9) | 7 (58.3) | 9 (81.8) | 4.834 | 0.184 |
| ≥ 5 | 56 | 29 (40.3) | 20 (54.1) | 5 (41.7) | 2 (18.2) | ||
| Histological typec | |||||||
| Differentiated | 52 | 28 (35.4) | 12 (30.0) | 6 (40.0) | 6 (50.0) | 1.760 | 0.624 |
| Poorly-differentiated | 94 | 51 (64.6) | 28 (70.0) | 9 (60.0) | 6 (50.0) | ||
| Invasionc | |||||||
| Yes | 112 | 55 (76.4) | 38 (90.5) | 10 (90.9) | 9 (81.8) | 4.226 | 0.238 |
| No | 24 | 17 (23.6) | 4 (9.5) | 1 (9.1) | 2 (18.2) | ||
| Lymph node metastasisc | |||||||
| Yes | 100 | 55 (76.4) | 31 (75.6) | 7 (58.3) | 7 (63.6) | 2.371 | 0.499 |
| No | 36 | 17 (23.6) | 10 (24.4) | 5 (41.7) | 4 (36.4) | ||
| Relapse | |||||||
| Yes | 98 | 58 (71.6) | 31 (67.4) | 8 (53.3) | 1 (7.1) | 15.633 | 0.0004 |
| No | 58 | 23 (28.4) | 15 (32.6) | 7 (46.7) | 13 (92.9) | ||
| Pathological grade | |||||||
| 1 | 2 | 1 (1.2) | 1 (2.2) | 0 (0.0) | 0 (0.0) | 2.976b | 0.3953 |
| 2 | 27 | 18 (22.2) | 8 (17.4) | 0 (0.0) | 1 (7.1) | ||
| 3 | 92 | 44 (54.3) | 25 (54.4) | 12 (80.0) | 11 (78.6) | ||
| 4 | 35 | 18 (22.2) | 12 (26.0) | 3 (20.0) | 2 (14.3) | ||
| Tumor stage | |||||||
| I | 14 | 9 (11.1) | 3 (6.5) | 0 (0.0) | 2 (14.2) | 13.66b | 0.0386 |
| II | 22 | 23 (28.4) | 3 (6.5) | 1 (6.7) | 6 (42.9) | ||
| III | 102 | 38 (46.9) | 34 (73.9) | 13 (86.7) | 6 (42.9) | ||
| IV | 18 | 11 (13.6) | 6 (13.1) | 1 (6.6) | 0 (0.0) | ||
Tumor sites in some cases were repeated;
Because the theoretical value is less than 1, χ2 was performed for patients who received cytokine-induced killer (CIK) cells immunotherapy at the frequencies of 11-25 and > 25, and for those who underwent CIK cells immunotherapy at the frequencies of 1-10, 11-25 and > 25;
Missed cases.
Survival time of patients in two groups
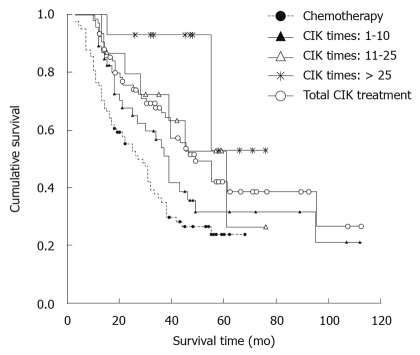
The survival time of gastric cancer patients in group II was much longer than that of those in group I (χ2 = 10.907, P = 0.001, Figure 1). The median survival time of patients in group II was also longer than that of those in group I (49 mo vs 27 mo).
Figure 1.
Survival rates of patients after cytokine-induced killer cells immunotherapy plus chemotherapy and chemotherapy alone. CIK: Cytokine-induced killer.
Two- and 5-year survival rates of patients in two groups
The 2- and 5-year survival rates of patients in group II were significantly higher than those of patients in group I (73.5% vs 52.6%, 40.4% vs 23.9%, P <0.05) (Table 3).
Table 3.
Two- and five-year survival rates of gastric cancer patients in two groups
| Groups | Survival rate (%) | 95% CI | u-value | P |
| 2-yr | ||||
| Chemotherapy | 52.6 | 41.7-63.6 | 2.721 | 0.007 |
| CIK treatment | 73.5 | 63.3-83.7 | ||
| 5-yr | ||||
| Chemotherapy | 23.9 | 13.5-34.3 | 1.913 | 0.0526 |
| CIK treatment | 40.4 | 27.1-53.7 |
CIK: Cytokine-induced killer; 95% CI: 95% confidence interval.
Survival time of patients after CIK cells immunotherapy
Because the CIK cells immunotherapy seemed effective against gastric cancer, whether the frequency of CIK cells immunotherapy affects its efficacy was determined. The survival curve was obviously higher for the patients after CIK cells immunotherapy plus chemotherapy than after chemotherapy alone. The survival time of gastric cancer patients was significantly longer after CIK cells immunotherapy than after chemotherapy (χ2 = 14.534, P = 0.002, Figure 1).
Time-dependent Cox model analysis of CIK cells immunotherapy and prognosis of patients
The frequency of CIK cells immunotherapy was a strong time-dependent variable. A significant difference was observed in the survival time and the frequency of CIK cells immunotherapy between the two models (χ2 = 27.990, P < 0.0001). However, it could not stratify the theoretical hypothesis of proportional hazard model. Hence, we tried to improve the analyzing results of the Cox model by dividing the patients into two stages with a relatively short survival time. Because of the relatively short interval time, the frequency of CIK cells immunotherapy may be a time-independent variable and can satisfy the assumption of proportional hazard model. The median survival time (36 mo) of the patients was used as the optimum cut-point.
In the first stage Cox model, the frequency of CIK cells immunotherapy was not a time-dependent variable, thus refusing the hypothesis of non proportional hazard model by Pearson correlation test between Schoenfeld residual of CIK cells immunotherapy frequency and survival time (r = 0.04, F = 0.10, P = 0.751). After the patients who remained alive beyond 36 mo after treatment were defined as survivors, the frequency of CIK cells immunotherapy was significantly associated with the decreasing risk of death in gastric cancer patients after adjustment for sex and age of the patients, tumor stage and relapse (HR = 0.54, 95% CI: 0.36-0.80) (Table 4).
Table 4.
First stage time-dependent multivariate Cox model analysis of cytokine-induced killer cells immunotherapy at different frequencies and prognosis of the patients1,2 (n = 1543)
| Variables4 | β | sβ | Wald-χ2 | P-value | HR | 95% CI |
| No. of CIK infusion | -0.620 | 0.200 | 9.592 | 0.002 | 0.54 | 0.36-0.80 |
| Sex | 0.294 | 0.298 | 0.969 | 0.325 | 1.34 | 0.75-2.41 |
| Age | 0.508 | 0.200 | 6.492 | 0.011 | 1.66 | 1.12-2.46 |
| Tumor stages | 0.739 | 0.202 | 13.377 | 0.0003 | 2.10 | 1.40-3.11 |
| Relapse (yes or no) | 3.363 | 0.719 | 21.848 | < 0.0001 | 28.87 | 7.05-118.25 |
>All patients with survival time of longer than 36 mo (median value for total patients) were defined as the survivors;
Pearson correlation test between Schoenfeld residual of cytokine-induced killer (CIK) cells immunotherapy frequency and survival time (r = 0.01, F = 0.01, P = 0.936);
Missed patients;
Variable value definition in the Cox model (frequency of CIK cells immunotherapy: 0 = 0 time, 1 = 1-10 times, 2 = 11-25 times, 3 = more than 25 times; Sex: 1 = man, 2 = woman), age (0 = ≤ 45 yr, 1 = about 60 yr, 2 = over 60 yr), tumor stage (0 = stage I, 1 = stage II, 2 = stage III, 3 = stage IV), relapse (1 = yes, 0 = no). HR: Hazard risk; 95% CI: 95% confidence interval.
In the second stage Cox model, the frequency of CIK cells immunotherapy was not a time-dependent variable (r = 0.307, F = 1.98, P = 0.176).When the second stage Cox model involving only 56 patients with a survival time of over 36 mo was fitted, no association was observed between the frequency of CIK cells immunotherapy and the survival time of gastric cancer patients (HR = 1.09, 95% CI: 0.63-0.89) (Table 5).
Table 5.
Second stage time-dependent multivariate Cox model analysis of cytokine-induced killer cells immunotherapy at different frequencies and prognosis of patients1,2 (n = 56)
| Variables3 | β | sβ | Wald-χ2 | P-value | HR | 95% CI |
| Number of CIK infusion | 0.089 | 0.280 | 0.102 | 0.750 | 1.09 | 0.63-1.89 |
| Sex | 0.676 | 0.619 | 1.191 | 0.275 | 1.97 | 0.58-6.62 |
| Age | -0.318 | 0.442 | 0.518 | 0.472 | 0.73 | 0.31-1.73 |
| Tumor stages | -0.471 | 0.341 | 1.909 | 0.167 | 0.62 | 0.32-1.22 |
| Relapse (yes or no) | 2.203 | 0.558 | 15.582 | < 0.0001 | 9.05 | 3.03-27.02 |
This model only involving the patients with a survival time longer than 36 mo. The survival rate of each patient was similar;
Pearson correlation test between Schoenfeld residual of cytokine-induced killer (CIK) cells immunotherapy frequency and survival time (r = 0.307, F = 1.98, P = 0.176);
Variable value definition in the Cox model (frequency of CIK cells immunotherapy: 0 = 0 time, 1 = 1-10 times, 2 = 11-25 times, 3 = more than 25 times; Sex: 1 = man, 2 = woman), age (0 = ≤ 45 yr, 1 = about 60 yr, 2 = over 60 yr), tumor stage (0 = stage I, 1 = stage II, 2 = stage III, 3 = stage IV), relapse (1 = yes, 0 = no). HR: Hazard risk; 95% CI: 95% confidence interval.
DISCUSSION
In China, gastric cancer patients are usually diagnosed at a relatively advanced stage with metastasis to other organs[24]. A number of treatment modalities are available for gastric cancer, such as surgical resection combined with chemotherapy[25,26], radiotherapy[27,28], thermotherapy[29,30] and/or traditional Chinese medicine[31,32]. However, the 5-year survival rate of advanced gastric cancer patients is still very poor[33]. It has been shown that cellular immunotherapy can promote host anti-cancer immunity, thus prolonging the survival time of gastric cancer patients[34]. Treatment of gastric cancer with autologous CIK cells is one of the promising cellular immunotherapies[35].
The results of the present study demonstrate that CIK cells immunotherapy plus chemotherapy for gastric cancer has more potential benefits than chemotherapy alone. Therefore, the effect of adjuvant cellular therapies for gastric cancer has drawn more attention of oncologists. It has been shown that adjuvant radiotherapy and chemotherapy for gastric cancer after curative resection can improve the disease-free and overall survival time of gastric cancer patients[25,36]. Residual tumor cells after chemotherapy can be removed by the host immune system[37]. However, several cycles of chemotherapy can decrease the immune functions of gastric cancer patients[38], and have been suspected to be one of the reasons for the high relapse rate of gastric cancer after postoperative systemic chemotherapy. Immunotherapy can directly kill cancer cells and boost the immune responses against the tumor[39]. Therefore, immunotherapy should be beneficial for gastric cancer patients, and not conducive to the growth of tumor cells.
In the present study, CIK cells were obtained upon culturing PBMC in the presence of IFN-γ, IL-2, anti-CD3MAb, and IL-1α[13]. This method allows us to generate a large number of CIK cells. In addition, the anti-tumor cell activity of CIK cells is stronger than that of anti-tumor effector cells[16]. The effector cells in our culture are believed to be CD3+CD56+. CIK cells have a higher survival rate, proliferation capacity, and killing activity than their target cells[40,41], and can secrete a variety of cytokines, which further enhance the cytotoxicity of immune effector cells[42] and change the tumor microenvironment to favor cancer eradication. In addition, CIK cells can kill both autologous and allogeneic tumor cells[43,44], as well as multi-drug resistance cells and FasL-positive cells[45,46]. Accordingly, CIK cells immunotherapy combined with chemotherapy may have a synergistic effect.
Several studies[17,47-49] showed that CIK cells immunotherapy can significantly improve the immune functions of cancer patients, such as an increase in CD3+CD56+ level. However, the clinical data are not enough to demonstrate the effectiveness of CIK cells immunotherapy. The results of this retrospective study, based on the follow-up of 156 gastric cancer patients, show that the frequency of CIK cells immunotherapy can significantly prolong the survival time of gastric cancer patients.
However, the frequency of CIK cells immunotherapy may significantly decrease the risk of cancer-related death in gastric cancer patients. The common Cox model was not preferred in our analysis because the balance test showed that tumor relapse and stage were different in patients of the two groups. There are two reasons that support our conclusion. First, except for tumor relapse and stage, the balance test showed the following factors, including age and sex of the patients, tumor size, site and invasiveness, lymph node metastasis and pathological grade did not affect us to assign the gastric cancer patients into groups I and II. Second, due to the fact that the frequency of CIK cells immunotherapy was a time-dependent variable in the Cox model, a two-stage time-dependent Cox model adjustment was made for some confounding factors (tumor relapse and stage), thus the false results were avoided when the common Cox model was used to make our analysis more reliable.
After the postoperative adjuvant chemotherapy, most residual tumor cells sensitive to chemotherapy were removed. Chemotherapy may suppress the immune function and therefore immunotherapy is necessary for boosting immunity. It was reported that the number of CD3+ cells and the CD4+/CD8+ ratio are significantly lower in most gastric cancer patients than in healthy controls after chemotherapy[17]. In the present study, the number of CD3+ and CD4+ cells was significantly increased, while the number of CD8+ cells was declined and the CD4+/CD8+ ratio was increased after CIK cells immunotherapy, suggesting that CIK cells also have an immune modulating function in addition to their anti-tumor function[50]. Single CIK cells immunotherapy has an in vivo effect against gastric cancer for about one month[51]. In contrast, the number of CD3+ cells and the CD4+/CD8+ ratio maintain at a high level after three cycles of CIK cells immunotherapy[17].
Our study has a few limitations. First, it was a retrospective cohort/observational study rather than a strictly-designed randomized trial. Since the patients were not assigned into CIK cells immunotherapy group and chemotherapy group, imbalance of certain clinical factors between the two groups could not be avoided. Second, there were some unknown potential reasons for choice of treatment regimens, which might affect our conclusion, despite the fact that the balance test was performed and some confounding factors were adjusted in the Cox model. Therefore, a randomized clinical trial is necessary to justify the benefit of CIK cells immunotherapy for gastric cancer. The benefit of radiochemotherapy and S1 chemotherapy for gastric cancers, established in a recent clinical trial[52], is important to determine whether CIK cells immunotherapy provides additional benefit when it is used in combination with radiotherapy and chemotherapy.
In conclusion, more frequencies of CIK cells are necessary for gastric cancer. The survival time of gastric cancer patients is significantly longer after chemotherapy plus CIK cells immunotherapy than after chemotherapy alone.
COMMENTS
Background
Gastric cancer is one of the most common causes of cancer-related death in China. Although standardized surgical resection and numerous adjuvant therapeutic modalities are available for gastric cancer, the postoperative survival rate of advanced stage cancer patients remains very low. In recent years, immune therapy for malignant tumors has become the fourth important tumor treatment modality following surgery, radiotherapy and chemotherapy. Cellular immunotherapy can promote host anti-cancer immunity, thus prolonging the survival time of gastric cancer patients. Treatment with autologous cytokine-induced killer (CIK) cells is one of the promising cellular immunotherapies.
Research frontiers
A number of adoptive cells immunotherapy have been reported, including using lymphokine activated killer cells, tumor infiltrating lymphocytes, and anti-CD3 monoclonal antibody-induced killer cells. However, their therapeutic efficacy is limited due to their low anti-tumor activities. CIK cells, a new type of anti-tumor effector cells, can proliferate rapidly in vitro, with a stronger anti-tumor activity and a broader spectrum of tumor targets than the reported anti-tumor effector cells. Moreover, CIK cells can regulate and enhance immune function.
Innovations and breakthroughs
CIK cells immunotherapy can decrease levels of tumor markers, change immune functions, and achieve a short-term efficacy against gastric cancer. However, the relation between the frequency of CIK cells immunotherapy and its clinical efficacy has not been examined. In the present study, data obtained from 156 gastric cancer patients were used in fitting multivariate Cox model, showing that more frequencies of CIK cells immunotherapy improve the survival rate of gastric cancer patients.
Applications
The survival time of gastric cancer patients was significantly longer after chemotherapy plus CIK cells immunotherapy than after chemotherapy alone, and more frequencies of CIK cells immunotherapy benefited gastric cancer patients more. This strategy can be applied in treatment of gastric cancer.
Terminology
CIK cells are cytokine-induced killer cells and a new type of anti-tumor effector cells, which can proliferate rapidly in vitro with a stronger anti-tumor activity and a broader spectrum of tumor targets than the reported anti-tumor effector cells. Moreover, CIK cells can regulate and enhance immune function.
Peer review
This study showed beneficial effect of CIK cells immunotherapy on gastric cancer, thus improving the 2- and 5-year survival rates of gastric cancer patients. The study is well designed and the data are believable.
Footnotes
Supported by The National Natural Science Foundation of China, No. 30872176, 30950022 and 30972703; grants of Jiangsu Province and Soochow University Medical Development Foundation, No. EE126765
Peer reviewer: Ming Li, Associate Professor, Tulane University Health Sciences Center, 1430 Tulane Ave Sl-83, New Orleans, LA 70112, United States
S- Editor Tian L L- Editor Wang XL E- Editor Lin YP
References
- 1.Shang J, Pena AS. Multidisciplinary approach to understand the pathogenesis of gastric cancer. World J Gastroenterol. 2005;11:4131–4139. doi: 10.3748/wjg.v11.i27.4131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Shen X, Zhang J, Yan Y, Yang Y, Fu G, Pu Y. Analysis and estimates of the attributable risk for environmental and genetic risk factors in gastric cancer in a Chinese population. J Toxicol Environ Health A. 2009;72:759–766. doi: 10.1080/15287390902841599. [DOI] [PubMed] [Google Scholar]
- 3.Varadhachary G, Ajani JA. Gastric cancer. Clin Adv Hematol Oncol. 2005;3:118–124. [PubMed] [Google Scholar]
- 4.Valentini V, Cellini F, Minsky BD, Mattiucci GC, Balducci M, D'Agostino G, D'Angelo E, Dinapoli N, Nicolotti N, Valentini C, et al. Survival after radiotherapy in gastric cancer: systematic review and meta-analysis. Radiother Oncol. 2009;92:176–183. doi: 10.1016/j.radonc.2009.06.014. [DOI] [PubMed] [Google Scholar]
- 5.Pisani P, Parkin DM, Bray F, Ferlay J. Estimates of the worldwide mortality from 25 cancers in 1990. Int J Cancer. 1999;83:18–29. doi: 10.1002/(sici)1097-0215(19990924)83:1<18::aid-ijc5>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- 6.Ajani JA. Evolving chemotherapy for advanced gastric cancer. Oncologist. 2005;10 Suppl 3:49–58. doi: 10.1634/theoncologist.10-90003-49. [DOI] [PubMed] [Google Scholar]
- 7.Catalano V, Labianca R, Beretta GD, Gatta G, de Braud F, Van Cutsem E. Gastric cancer. Crit Rev Oncol Hematol. 2005;54:209–241. doi: 10.1016/j.critrevonc.2005.01.002. [DOI] [PubMed] [Google Scholar]
- 8.Dougan M, Dranoff G. Immune therapy for cancer. Annu Rev Immunol. 2009;27:83–117. doi: 10.1146/annurev.immunol.021908.132544. [DOI] [PubMed] [Google Scholar]
- 9.Stroncek D, Berlyne D, Fox B, Gee A, Heimfeld S, Lindblad R, Loper K, McKenna D Jr, Rooney C, Sabatino M, et al. Developments in clinical cell therapy. Cytotherapy. 2010;12:425–428. doi: 10.3109/14653240903511952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hontscha C, Borck Y, Zhou H, Messmer D, Schmidt-Wolf IG. Clinical trials on CIK cells: first report of the international registry on CIK cells (IRCC) J Cancer Res Clin Oncol. 2010:Epub ahead of print. doi: 10.1007/s00432-010-0887-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rosenberg S. Lymphokine-activated killer cells: a new approach to immunotherapy of cancer. J Natl Cancer Inst. 1985;75:595–603. [PubMed] [Google Scholar]
- 12.Rosenberg SA, Spiess P, Lafreniere R. A new approach to the adoptive immunotherapy of cancer with tumor-infiltrating lymphocytes. Science. 1986;233:1318–1321. doi: 10.1126/science.3489291. [DOI] [PubMed] [Google Scholar]
- 13.Yun YS, Hargrove ME, Ting CC. In vivo antitumor activity of anti-CD3-induced activated killer cells. Cancer Res. 1989;49:4770–4774. [PubMed] [Google Scholar]
- 14.Takahashi H, Nakada T, Puisieux I. Inhibition of human colon cancer growth by antibody-directed human LAK cells in SCID mice. Science. 1993;259:1460–1463. doi: 10.1126/science.8451642. [DOI] [PubMed] [Google Scholar]
- 15.Schmidt-Wolf IG, Negrin RS, Kiem HP, Blume KG, Weissman IL. Use of a SCID mouse/human lymphoma model to evaluate cytokine-induced killer cells with potent antitumor cell activity. J Exp Med. 1991;174:139–149. doi: 10.1084/jem.174.1.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Schmidt-Wolf IG, Lefterova P, Mehta BA, Fernandez LP, Huhn D, Blume KG, Weissman IL, Negrin RS. Phenotypic characterization and identification of effector cells involved in tumor cell recognition of cytokine-induced killer cells. Exp Hematol. 1993;21:1673–1679. [PubMed] [Google Scholar]
- 17.Jiang J, Xu N, Wu C, Deng H, Lu M, Li M, Xu B, Wu J, Wang R, Xu J, et al. Treatment of advanced gastric cancer by chemotherapy combined with autologous cytokine-induced killer cells. Anticancer Res. 2006;26:2237–2242. [PubMed] [Google Scholar]
- 18.Jiang JT, Wu CP, Shi LR, Xu N, Deng HF, Lu MY, Ji M, Zhu YB, Zhang XG. Side effects during treatment of advanced gastric carcinoma by chemotherapy combined with CIK-cell transfusion in elderly people. Chin J Clin Oncol. 2008;5:79–82. [Google Scholar]
- 19.Jiang J, Zhu Y, Wu C, Shen Y, Wei W, Chen L, Zheng X, Sun J, Lu B, Zhang X. Tumor expression of B7-H4 predicts poor survival of patients suffering from gastric cancer. Cancer Immunol Immunother. 2010;59:1707–1714. doi: 10.1007/s00262-010-0900-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yoo CH, Noh SH, Shin DW, Choi SH, Min JS. Recurrence following curative resection for gastric carcinoma. Br J Surg. 2000;87:236–242. doi: 10.1046/j.1365-2168.2000.01360.x. [DOI] [PubMed] [Google Scholar]
- 21.Jiang JT, Wu CP, Deng HF, Lu MY, Li M, Wang H, Wu J. Compare cytotoxicity of cytokine-induced killer cells with tumor infiltrating lymphocytes in vitro. Jianyan Yixue. 2005;20:322–324. [Google Scholar]
- 22.De Vita F, Orditura M, Matano E, Bianco R, Carlomagno C, Infusino S, Damiano V, Simeone E, Diadema MR, Lieto E, et al. A phase II study of biweekly oxaliplatin plus infusional 5-fluorouracil and folinic acid (FOLFOX-4) as first-line treatment of advanced gastric cancer patients. Br J Cancer. 2005;92:1644–1649. doi: 10.1038/sj.bjc.6602573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Schoenfeld DA, Richter JR. Nomograms for calculating the number of patients needed for a clinical trial with survival as an endpoint. Biometrics. 1982;38:163–170. [PubMed] [Google Scholar]
- 24.Chen L, Tian H, Chen J, He ZG, Tao SF, Lokesh G, Peng SY. Surgical management of gastric stump cancer: a report of 37 cases. J Zhejiang Univ Sci B. 2005;6:38–42. doi: 10.1631/jzus.2005.B0038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kollmannsberger C, Budach W, Stahl M, Schleucher N, Hehr T, Wilke H, Schleicher J, Vanhoefer U, Jehle EC, Oechsle K, et al. Adjuvant chemoradiation using 5-fluorouracil/folinic acid/cisplatin with or without paclitaxel and radiation in patients with completely resected high-risk gastric cancer: two cooperative phase II studies of the AIO/ARO/ACO. Ann Oncol. 2005;16:1326–1333. doi: 10.1093/annonc/mdi252. [DOI] [PubMed] [Google Scholar]
- 26.Lin WL, Li DG, Chen Q, Lu HM. Clinical and experimental study of oxaliplatin in treating human gastric carcinoma. World J Gastroenterol. 2004;10:2911–2915. doi: 10.3748/wjg.v10.i19.2911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Abe M, Nishimura Y, Shibamoto Y. Intraoperative radiation therapy for gastric cancer. World J Surg. 1995;19:544–547. doi: 10.1007/BF00294720. [DOI] [PubMed] [Google Scholar]
- 28.Klautke G, Foitzik T, Ludwig K, Ketterer P, Klar E, Fietkau R. Neoadjuvant radiochemotherapy in locally advanced gastric carcinoma. Strahlenther Onkol. 2004;180:695–700. doi: 10.1007/s00066-004-9194-z. [DOI] [PubMed] [Google Scholar]
- 29.Zuo Y, Xu M, Shen D, Lu WD, Lu JF. [Postoperative intraperitioneal hyperthermic chemoperfusion combined with intravenous chemotherapy for 82 advanced gastric cancer patients] Zhonghua Zhongliu Zazhi. 2004;26:247–249. [PubMed] [Google Scholar]
- 30.Bozzetti F, Vaglini M, Deraco M. Intraperitoneal hyperthermic chemotherapy in gastric cancer: rationale for a new approach. Tumori. 1998;84:483–488. doi: 10.1177/030089169808400409. [DOI] [PubMed] [Google Scholar]
- 31.Yu QS, Zhang FZ, Tang XR. [Clinical study on early use of Chinese medicinal herbs and chemotherapy after operation of gastric cancer] Zhongguo Zhongxiyi Jiehe Zazhi. 1995;15:459–461. [PubMed] [Google Scholar]
- 32.Wang GT. [Treatment of operated late gastric carcinoma with prescription of strengthening the patient's resistance and dispelling the invading evil in combination with chemotherapy: follow-up study of 158 patients and experimental study in animals] Zhongxiyi Jiehe Zazhi. 1990;10:712–716, 707. [PubMed] [Google Scholar]
- 33.Yamaguchi K, Shimamura T, Hyodo I, Koizumi W, Doi T, Narahara H, Komatsu Y, Kato T, Saitoh S, Akiya T, et al. Phase I/II study of docetaxel and S-1 in patients with advanced gastric cancer. Br J Cancer. 2006;94:1803–1808. doi: 10.1038/sj.bjc.6603196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Herberman RB. Cancer therapy by biological response modifiers. Clin Physiol Biochem. 1987;5:238–248. [PubMed] [Google Scholar]
- 35.Margolin KA, Negrin RS, Wong KK, Chatterjee S, Wright C, Forman SJ. Cellular immunotherapy and autologous transplantation for hematologic malignancy. Immunol Rev. 1997;157:231–240. doi: 10.1111/j.1600-065x.1997.tb00986.x. [DOI] [PubMed] [Google Scholar]
- 36.Macdonald JS, Smalley SR, Benedetti J, Hundahl SA, Estes NC, Stemmermann GN, Haller DG, Ajani JA, Gunderson LL, Jessup JM, et al. Chemoradiotherapy after surgery compared with surgery alone for adenocarcinoma of the stomach or gastroesophageal junction. N Engl J Med. 2001;345:725–730. doi: 10.1056/NEJMoa010187. [DOI] [PubMed] [Google Scholar]
- 37.Haynes NM, van der Most RG, Lake RA, Smyth MJ. Immunogenic anti-cancer chemotherapy as an emerging concept. Curr Opin Immunol. 2008;20:545–557. doi: 10.1016/j.coi.2008.05.008. [DOI] [PubMed] [Google Scholar]
- 38.Wu K, Nie Y, Guo C, Chen Y, Ding J, Fan D. Molecular basis of therapeutic approaches to gastric cancer. J Gastroenterol Hepatol. 2009;24:37–41. doi: 10.1111/j.1440-1746.2008.05753.x. [DOI] [PubMed] [Google Scholar]
- 39.Larmonier N, Fraszczak J, Lakomy D, Bonnotte B, Katsanis E. Killer dendritic cells and their potential for cancer immunotherapy. Cancer Immunol Immunother. 2010;59:1–11. doi: 10.1007/s00262-009-0736-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hoffman DM, Gitlitz BJ, Belldegrun A, Figlin RA. Adoptive cellular therapy. Semin Oncol. 2000;27:221–233. [PubMed] [Google Scholar]
- 41.Lu PH, Negrin RS. A novel population of expanded human CD3+CD56+ cells derived from T cells with potent in vivo antitumor activity in mice with severe combined immunodeficiency. J Immunol. 1994;153:1687–1696. [PubMed] [Google Scholar]
- 42.Gritzapis AD, Dimitroulopoulos D, Paraskevas E, Baxevanis CN, Papamichail M. Large-scale expansion of CD3(+)CD56(+) lymphocytes capable of lysing autologous tumor cells with cytokine-rich supernatants. Cancer Immunol Immunother. 2002;51:440–448. doi: 10.1007/s00262-002-0298-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Johnson BE, Bridges JD, Sobczeck M, Gray J, Linnoila RI, Gazdar AF, Hankins L, Steinberg SM, Edison M, Frame JN, et al. Patients with limited-stage small-cell lung cancer treated with concurrent twice-daily chest radiotherapy and etoposide/cisplatin followed by cyclophosphamide, doxorubicin, and vincristine. J Clin Oncol. 1996;14:806–813. doi: 10.1200/JCO.1996.14.3.806. [DOI] [PubMed] [Google Scholar]
- 44.Scheffold C, Brandt K, Johnston V, Lefterova P, Degen B, Schöntube M, Huhn D, Neubauer A, Schmidt-Wolf IG. Potential of autologous immunologic effector cells for bone marrow purging in patients with chronic myeloid leukemia. Bone Marrow Transplant. 1995;15:33–39. [PubMed] [Google Scholar]
- 45.Schmidt-Wolf IG, Lefterova P, Johnston V, Scheffold C, Csipai M, Mehta BA, Tsuruo T, Huhn D, Negrin RS. Sensitivity of multidrug-resistant tumor cell lines to immunologic effector cells. Cell Immunol. 1996;169:85–90. doi: 10.1006/cimm.1996.0094. [DOI] [PubMed] [Google Scholar]
- 46.Verneris MR, Kornacker M, Mailänder V, Negrin RS. Resistance of ex vivo expanded CD3+CD56+ T cells to Fas-mediated apoptosis. Cancer Immunol Immunother. 2000;49:335–345. doi: 10.1007/s002620000111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Chen FX, Liu JQ, Zhang NZ, Gong XJ, Zhang GL, Xu YM, Zhou ZH, Wang T, Huang J. [Clinical observation on adoptive immunotherapy with autologous cytokine-induced killer cells for advanced malignant tumor] Ai Zheng. 2002;21:797–801. [PubMed] [Google Scholar]
- 48.Wu C, Jiang J, Shi L, Xu N. Prospective study of chemotherapy in combination with cytokine-induced killer cells in patients suffering from advanced non-small cell lung cancer. Anticancer Res. 2008;28:3997–4002. [PubMed] [Google Scholar]
- 49.Olioso P, Giancola R, Di Riti M, Contento A, Accorsi P, Iacone A. Immunotherapy with cytokine induced killer cells in solid and hematopoietic tumours: a pilot clinical trial. Hematol Oncol. 2009;27:130–139. doi: 10.1002/hon.886. [DOI] [PubMed] [Google Scholar]
- 50.Kim HM, Lim J, Yoon YD, Ahn JM, Kang JS, Lee K, Park SK, Jeong YJ, Kim JM, Han G, et al. Anti-tumor activity of ex vivo expanded cytokine-induced killer cells against human hepatocellular carcinoma. Int Immunopharmacol. 2007;7:1793–1801. doi: 10.1016/j.intimp.2007.08.007. [DOI] [PubMed] [Google Scholar]
- 51.Cho MY, Joh YG, Kim NR, Jung SI, Bae JW, Kim YC, Koo BH, Whang CW, Suh SO. T-lymphocyte subsets in patients with AJCC stage III gastric cancer during postoperative adjuvant chemotherapy. American Joint Committee on Cancer. Scand J Surg. 2002;91:172–177. doi: 10.1177/145749690209100207. [DOI] [PubMed] [Google Scholar]
- 52.Catalano V, Labianca R, Beretta GD, Gatta G, de Braud F, Van Cutsem E. Gastric cancer. Crit Rev Oncol Hematol. 2009;71:127–164. doi: 10.1016/j.critrevonc.2009.01.004. [DOI] [PubMed] [Google Scholar]