Abstract
Ischemia/reperfusion (I/R) injury still represents an important cause of morbidity following hepatic surgery and limits the use of marginal livers in hepatic transplantation. Transient blood flow interruption followed by reperfusion protects tissues against damage induced by subsequent I/R. This process known as ischemic preconditioning (IP) depends upon intrinsic cytoprotective systems whose activation can inhibit the progression of irreversible tissue damage. Compared to other organs, liver IP has additional features as it reduces inflammation and promotes hepatic regeneration. Our present understanding of the molecular mechanisms involved in liver IP is still largely incomplete. Experimental studies have shown that the protective effects of liver IP are triggered by the release of adenosine and nitric oxide and the subsequent activation of signal networks involving protein kinases such as phosphatidylinositol 3-kinase, protein kinase C δ/ε and p38 MAP kinase, and transcription factors such as signal transducer and activator of transcription 3, nuclear factor-κB and hypoxia-inducible factor 1. This article offers an overview of the molecular events underlying the preconditioning effects in the liver and points to the possibility of developing pharmacological approaches aimed at activating the intrinsic protective systems in patients undergoing liver surgery.
Keywords: Apoptosis, Hepatocyte, Hypoxia, Ischemia/reperfusion, Liver surgery, Necrosis, Pharmacological preconditioning, Preconditioning, Survival pathways
INTRODUCTION
The understanding of the proteomic features associated with cell response to stresses is one of the present-day challenges in medical science. This knowledge is increasingly necessary to identify new molecular targets for therapeutic interventions. A turning-point on this matter has been the discovery that tissues already possess a number of inducible systems able to make them more resistant to a wide array of injuries. One of these adaptive responses is represented by the capacity of a non-lethal ischemia to modulate cell functions by increasing resistance to subsequent lethal ischemia/reperfusion[1,2]. Since its first description in the myocardium[1], this phenomenon, termed “ischemic preconditioning” (IP), has been the subject of rising interest in the scientific and medical communities. The effects of IP can be differentiated into early effects and late effects. The former, immediately follows the transient ischemia and involves the direct modulation of specific cell functions, while late effects are evident within 12-24 h from the transient ischemia and require the simultaneous activation of multiple stress-responsive genes associated with the synthesis of several proteins[2,3].
ISCHEMIA-REPERFUSION INJURY OF THE LIVER
Hepatic ischemia/reperfusion (I/R) injury occurs as a consequence of trauma and hemorrhagic shock as well as temporary clamping of the hepato-duodenal ligament during liver resection (Pringle manoeuvre).
I/R is the main factor responsible for primary graft non-function or malfunction following liver transplantation[3,4]. Even moderate reperfusion damage, which does not severely affect the graft, can impair long-term hepatic recovery and enhance patient susceptibility to infections and multiple organ failure[3,4]. The shortage of organs for liver transplantation, forces consideration of cadaveric and steatotic grafts (marginal grafts) which have a higher susceptibility to I/R injury[4]. Living donor liver transplantation (LDTL) is a promising alternative approach aimed at increasing the number of donor livers[5]. A major concern over the application of LDTL in adults is graft size disparity which is responsible for the appearance of the life threatening effects of the “small for size syndrome”[6]. “Small for size syndrome” can occur even when the critical mass for safe LDTL (40% of standard liver volume) is transplanted and this effect is related to the impaired regeneration of the reduced liver mass[7] induced by I/R[7,8].
LIVER ISCHEMIC PRECONDITIONING
Beside the heart, IP effects have been demonstrated in many other tissues[2,3]. Studies performed in rats and mice, showed that interruption of liver blood supply for 5-10 min followed by 10-15 min of reperfusion reduced hepatic injury during a subsequent extended period of ischemia followed by reperfusion[9-13]. These beneficial effects were particularly evident in fatty livers in which preconditioning almost halved transaminase release and histological evidence of necrosis[11]. The application of preconditioning protocols to rodent liver transplantations showed that IP applied before cold preservation, decreased transaminase release and sinusoidal endothelial cell killing in the graft, improving rat survival[12,13].
A further feature of hepatic IP was the capacity to promote hepatocyte regeneration. Hepatocyte proliferation in rats subjected to 70% hepatectomy is significantly reduced by 45 min of hepatic ischemia. Such an effect was entirely reverted by pre-exposure to IP[14]. Consistently, preconditioning procedures significantly enhanced liver regeneration in the experimental model of reduced-size rat liver transplantation[15,16].
MOLECULAR SIGNALS OF HEPATIC ISCHEMIC PRECONDITIONING
Despite a significant number of studies on liver preconditioning, knowledge on the mechanisms responsible for the induction of the “preconditioned” phenotype is still incomplete. Studies from our and other laboratories have indicated that the process of preconditioning implies the production of complex proteomic modifications within liver cells which are now beginning to be characterized.
Adenosine, adenosine triphosphate and nitric oxide as molecular inducers of hepatic preconditioning
“In vivo” and “in vitro” studies have clearly established that the onset of IP is triggered by the production of adenosine and by the subsequent stimulation of adenosine A2a receptor (A2aR)[9,17-21]. In particular, Peralta et al[9,17] showed that adenosine treatment reproduced the protective action of IP and that IP was reverted by adenosine deaminase and by the adenosine A2 receptor antagonist, 3,7-dimethyl-1-propargylxanthine. Pretreatment of rats with the adenosine A2 receptor agonist, CGS21680, but not with the adenosine A1 receptor agonist, N-phenyl-isopropyl adenosine, enhanced tolerance against IR damage[18]. By using primary rat hepatocytes preconditioned with 10 min of hypoxia plus 10 min of re-oxygenation, we confirmed that the extracellular release of adenosine induced hepatocyte protection by autocrine stimulation of the A2aR[20,21] (Figure 1). Indeed, studies in extra-hepatic and hepatic tissues have clearly shown that transient oxygen deprivation triggers the release of several metabolites including adenosine triphosphate (ATP)[2]. In the extracellular space, ATP is rapidly metabolized to adenosine via CD39 and CD73 ecto-nucleotidases[22,23] present on the extracellular portion of cell plasma membranes. In particular, ectoapyrase (CD39) converts ATP to adenosine monophosphate (AMP), while ecto-5′-nucleotidase (CD73) further degrades it to adenosine[24]. Thus, CD73 represents the major extracellular pathway for adenosine generation. Consistently targeted gene deletion or pharmacologic inhibition of CD73 was demonstrated to abolish hepatic protection by IP[19].
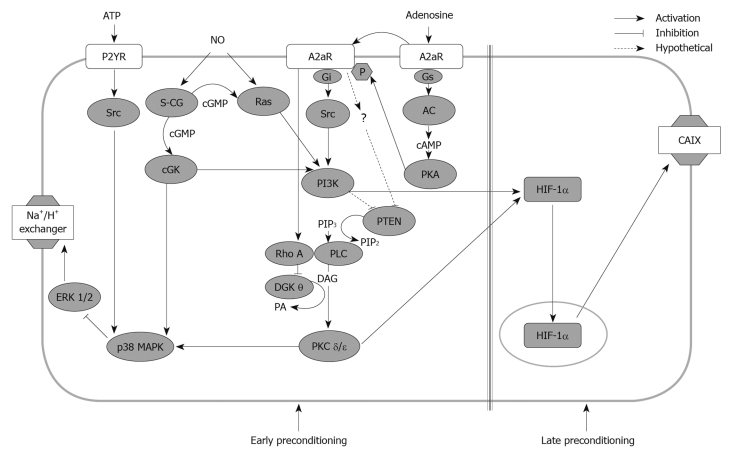
Figure 1.
Signalling pathways involved in the development of ischemic preconditioning in rat hepatocytes. Adenosine triphosphate (ATP), adenosine and nitric oxide (NO) act as inductors of hepatocyte preconditioning by modulating a network of constitutive and newly synthesized signal mediators. Some of these mediators play a common central role in hepatocyte cytoprotection. p38 MAP kinase (p38 MAPK) is a mediator of the cytoprotective effects of all three preconditioning stimuli. Phosphatidylinositol 3-kinase (PI3K) mediates both adenosine and NO early resistance to hypoxia. PI3K together with protein kinase C (PKC) δ and ε, also induces hepatocyte late resistance to hypoxia contributing to the normoxic activation of hypoxia-inducible factor 1 (HIF-1). Diacylglycerol kinase theta (DGKθ) and the phosphatase tensin-homologues-deleted from chromosome 10 (PTEN) which metabolize diacyglycerol and phosphatidylinositol, respectively, are inhibited during preconditioning to sustain activation of the diacylglycerol (DAG)-dependent PKC δ and ε and the PI3K-dependent signals. See text and Refs[21,25,27,28,32,35,37,43]. P2YR: Purinergic P2Y receptors; A2aR: Adenosine 2A receptors; S-CG: Soluble guanylate cyclase; cGMP: Cyclic guanosine monophosphate; cGK: cGMP-dependent kinase; AC: Adenylate cyclase; cAMP: Cyclic adenosine monophosphate; PIP3: Phosphatidylinositol-3-phosphate; PKA: Protein kinase A; CAIX: Carbonic anhydrase IX; PLC: Phospholipase C; PA: Phosphatidic acid.
Recent observations from our group also suggested that ATP itself could act as an additional trigger of liver preconditioning. We observed that the release of ATP from hepatocytes enhanced their tolerance to hypoxia independently from the generation of adenosine[24] (Figure 1). Such an effect was mimicked by treatment with the non-hydrolyzable ATP analogue adenosine-5’-O-(3-thiotriphosphate) (ATPγS) and involved the stimulation of the P2Y2 purinergic receptor[25].
Further evidence indicated that during IP, hepatic endothelial cells responded to adenosine stimulation by generating nitric oxide (NO) which contributed to the modulation of hepatocyte tolerance to I/R[3,17,26-28]. Indeed, the administration of NO donors promoted tolerance to I/R in the absence of adenosine, while NO synthase inhibitors reverted IP[17,26]. Similarly, the treatment of primary rat hepatocytes with the NO donor (Z)-1-{N-methyl-N-[6-(N-methyl-ammonio-hexyl) amino]} diazen-1-ium-1,2-diolate (NOC-9) reproduced hepatocyte resistance to hypoxic damage induced by IP, ATP or A2aR stimulation[27,28], suggesting that NO could act as an independent mediator of hepatic preconditioning[26].
Signalling pathways involved in adenosine and ATP- induced hepatoprotection
Using preconditioned rat hepatocytes, we observed that A2aR stimulation activated a cascade of intracellular signals involving Gi protein, phospholipase C (PLC), the novel isoforms of protein kinase C (PKC) δ and ε and p38 MAP kinase (p38 MAPK)[21] (Figure 1). The effective contribution of p38 MAPK in liver IP signalling was confirmed in vivo in mice where increased p38 MAPK phosphorylation was associated with tolerance against reperfusion injury[29]. Moreover, p38 MAPK inhibitors abolished resistance to I/R injury both “in vitro” and “in vivo”[21,30].
A2aRs are known to be typically coupled to Gs proteins that through adenylate-cyclase (A-C) stimulate protein kinase A (PKA)[31]. However, in an early study we excluded the involvement of PKA in mediating IP, as PKA pharmacological activation was devoid of protective action[21]. Subsequent research clarified this discrepancy, as we observed that A2aRs were actually coupled with Gs proteins and PKA[32]. PKA, however, by phosphorylating A2aR, shifted A2aR coupling from Gs proteins to Gi proteins and this led to the recruitment of the PLC-PKC pathway[32] (Figure 1). Interestingly, PKA phosphorylated A2aR only in the presence of its ligand (adenosine) and this explained why direct PKA activation in the absence of adenosine lacked protective activity[21,32] (Figure 1). The same research also highlighted the critical role of phosphatidylinositol-3-kinase (PI3K) in hepatic IP[32]. PI3Ks are a family of intracellular signal transducers that generate phosphatidylinositol (3,4,5)-triphosphate (PIP3), a second messenger that plays a central role in the regulation of cell proliferation, survival and metabolism[33]. In preconditioned hepatocytes, PI3K was activated upon A2aR engagement through Gi protein and Src kinase stimulation[32]. PI3K was shown to contribute to IP by promoting the activation of PLC and of PKC δ and ε (Figure 1)[32]. It is well known, however, that down-stream of PI3K, protein kinase B (PKB/AKT) is a key modulator of a variety of pro-survival and pro-regenerative signals[33]. Thus, the PI3K-PKB/AKT pathway likely represents an important pathway in the development of liver IP. Interestingly, PKB/AKT activation in connection with the development of tolerance to I/R was evident in rat hepatocytes and mouse livers[32,34] undergoing IP, as well as in preconditioned human liver grafts immediately after transplantation[35].
At present, the intracellular signals involved in ATP-dependent preconditioning are less well characterized. We reported that ATP-mediated activation of P2Y receptors was coupled with the phosphorylation of Src tyrosine kinase and of p38 MAP kinase that, in turn, inhibited the activation of ERK 1/2 consequent to hypoxic stress[25] (Figure 1).
Constitutive mediators of nitric oxide-induced cytoprotection
The signalling pathways responsible for the cytoprotective action of NO were investigated in rat hepatocytes treated with the NO donor, NOC-9, and then exposed to hypoxia. NOC-9-induced protection involved two parallel pathways. In one pathway, NO stimulated Ras GTPase, and in the other, NO directly activated the soluble guanylate cyclase (sGC) that by producing cyclic guanosine monophosphate (cGMP), stimulated the cGMP-dependent kinase (cGK) that also contributed to Ras GTPase activation[27,28]. Both the Ras and the cGK pathways then converged on the activation of PI3K, while only the sGC-cGK pathway was responsible for activating p38 MAPK (Figure 1)[27,28].
Negative regulators of liver preconditioning
It is increasingly clear that the development of hepatic IP requires the activation of a complex network of signals comprising cell-surface receptors, redox signals and a diverse array of protein kinases including PKCδ and PKCε. In preconditioned hepatocytes, the membrane recruitment and activation of PKCδ and PKCε was fully dependent on their direct interaction with diacylglycerol, generated by adenosine-induced activation of PLC-γ and diacylglycerol analogues which fully mimicked the activation of the signals that induce IP[3,21]. However, it is now clear that the accumulation of cellular diacylglycerol also depends on the rate of its metabolism to phosphatidic acid by diacylglycerol kinases (DGKs)[36]. In this regard, we recently observed that following IP or A2aR activation, the onset of hepatocyte tolerance to hypoxia was associated with a decrease in DGK activity[37]. Moreover, stimulation of A2aR specifically inhibited DGK isoform θ by activating RhoA-GTPase[37]. The pharmacological inhibition of DGKs has consistently led to a diacylglycerol-dependent activation of PKC δ/ε and of p38 MAPK. Moreover, both genetic and pharmacological inhibition of DGK θ induced cell tolerance to hypoxia[37]. Altogether these results unveiled a novel mechanism in the onset of hepatocyte preconditioning and demonstrated that the down-regulation of antagonist enzymes such as DGK was essential to obtain the diacylglycerol accumulation required to trigger PKC-mediated survival signals.
Similarly, preliminary data indicated that in parallel with the activation of PI3K, A2aR stimulation reduced the intracellular levels of the dual protein/lipid phosphatase tensin-homologues-deleted from chromosome 10 (PTEN) that inhibits PI3K-mediated signals by degrading phosphatidylinositol (3,4,5)-triphosphate[33]. We observed that PTEN inhibitors mimicked the induction of preconditioning (Cescon et al[35] unpublished results), while PKB/AKT activation and the clinical efficacy of IP in preconditioned human liver were fully explicated only in the presence of significant PTEN down-regulation.
Altogether these results demonstrated the importance of the down-modulation of key inhibitory enzymes for full activation of preconditioning responses. Moreover, these observations indicated the possible use of inhibitors of DGKs or PTEN as pharmacological inducers of hepatic preconditioning.
Nuclear transcription factors in liver preconditioning
As previously mentioned, the late effects of IP require the transcription of different stress-responsive genes and protein synthesis[2,3]. Growing evidence indicates that these responses are achieved by the coordinated activation of several transcription factors.
Nuclear factor-κB: Nuclear factor-κB (NF-κB) is typically devoted to the regulation of genes involved in inflammatory response and cell survival[38]. In experimental models of liver I/R, IP modifies NF-κB activity in different ways[29,39]. In one study, IP decreased NF-κB activity 1 h after reperfusion[39], and in another study, IP activated NF-κB during the ischemic period[29]. These contrasting results could be due to predominant NF-κB modulation in non-parenchymal vs parenchymal cells or to a differential regulation of NF-κB in the different phases of liver preconditioning. In addition, the NF-κB decrease during reperfusion was strictly related to a reduction in inflammatory cytokine expression[39], indicating a down-regulation of pro-inflammatory responses in Kupffer/sinusoidal endothelial cells. Conversely, NF-κB activation during ischemia was associated with the hepatoprotective action of IP[29], suggesting that, in hepatocytes, NF-κB-dependent genes contributed to survival responses.
Signal transducer and activator of transcription: The signal transducer and activator of transcription (STAT) transcription factors are a group of proteins implicated in the control of cell proliferation and survival processes[40]. IP induced the activation of the interleukin (IL)-6/STAT3 axis in liver and this pathway was involved in both cytoprotection and hepatic regeneration. On the one hand, as a result of hepatic preconditioning, NF-κB was shown to stimulate the expression of IL-6 and STAT3 that, in turn controlled cyclin beta1 synthesis and cell cycle progression[29]. On the other hand, studies with IL-6 null mice showed that the cytoprotective effects of IP against I/R injury depended on IL-6 signalling and were associated with hepatic STAT3 activation[41].
Hypoxia-inducible factor 1: Hypoxia-inducible factor 1 (HIF-1) is the main regulator of tissue adaptation to oxygen deprivation[42]. Active HIF-1 is a heterodimer consisting of an inducible HIF-1α subunit and a constitutively expressed HIF-1β subunit. HIF-1α is extremely labile in normoxia, as it is continuously degraded in proteasomes following hydroxylation, catalyzed by the oxygen-dependent HIF-prolyl-4-hydroxylase and arginyl-hydroxylase[42]. The lowering of intracellular oxygen prevents HIF-1α hydroxylation allowing its nuclear translocation and binding to hypoxic response elements of a number of genes regulating erythropoiesis, angiogenesis, glucose transport, glycolysis and cell survival[42]. Using preconditioned hepatocytes, we showed that HIF-1 activation was associated with the induction of a long lasting tolerance to hypoxic injury[43]. Furthermore, Amador and co-workers reported an increase in HIF-1α in concomitance with a lowering of hepatocyte apoptosis in human transplanted livers exposed to IP[44]. HIF-1 activation by IP was not due to the transient hypoxia occurring during the induction of preconditioning, but required A2aR activation[43]. This implicated an oxygen-independent mechanism in the regulation of HIF-1. Indeed, several reports demonstrated that a number of non-hypoxic stimuli (i.e. growth factors, cytokines, hormones and endotoxins) can activate HIF-1 in an oxygen-independent manner[45]. This process implies a PI3K- and PKC-dependent increase in the translation of HIF-1α mRNA, a process that shifts the synthesis/degradation balance towards HIF-1α accumulation[46]. We found that in hepatocytes, adenosine-dependent HIF-1 activation required the stimulation of both PI3K and PKC pathways[43]. This indicated that preconditioning stimuli, acting through the same survival pathways, could contextually lead to the early and late phase of response against cell injury.
Changes in the pattern of protein expression following liver preconditioning
Information concerning the genes modulated in response to liver IP is still limited. In accordance with the role of NO production as a trigger of IP, increased nitric oxide synthase expression was detected in preconditioned rat liver[47]. Microarray analysis of preconditioned human liver confirmed a significant increase in the amount of inducible nitric oxide synthase and also showed an increase in the anti-apoptotic protein, Bcl-2[48]. These analyses also showed that IL-1 receptor antagonist (IL-1Ra) was the most over-expressed gene in human preconditioned livers[48], in accordance with the anti-inflammatory effects of IP. Parallel studies investigating the gene expression pattern in preconditioned rat hepatocytes showed changes in 43 genes including those of the anti-inflammatory IL-10 and the antioxidant enzyme superoxide dismutase 2 (SOD2)[49]. In another study, a marked increase in SOD as well other endogenous antioxidants such as catalase (CAT) and glutathione peroxidase was also observed[50].
As previously mentioned, HIF-1 controls the expression of a variety of genes implicated in erythropoiesis, angiogenesis, glucose transport, glycolysis and cell survival[42]. In this context, we observed that the A2aR-dependent activation of HIF-1 in hepatocytes was associated with the expression of carbonic anhydrase IX (CAIX)[43], a transmembrane enzyme that by catalyzing bicarbonate production was implicated in preventing hepatocyte death (see later).
MOLECULAR MECHANISMS OF CELL RESISTANCE TO INJURY FOLLOWING HEPATIC PRECONDITIONING
The hepatoprotective effects of liver preconditioning impact on a number of different mechanisms. These include several processes acting against ischemia-induced damage as well as against reperfusion injury[2,3,50].
Protection against ischemic damage
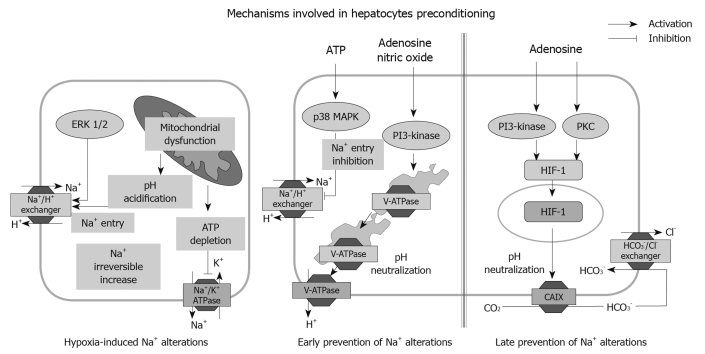
A decrease in hepatic energy state is the main cause of liver cell injury during ischemia. Oxygen deprivation causes loss of mitochondrial potential, ATP depletion and intracellular acidification which are turning points in the onset of irreversible liver cell injury[3,50,51]. In early research, we found that activation of the Na+/H+ exchanger in response to cellular acidosis combined with the inhibition of Na+ extrusion by the Na+/K+ ATPase, resulted in Na+ accumulation within hepatocytes[52] (Figure 2). Na+ overload was a critical step in hepatocyte damage during warm and cold hypoxia, as well as at the beginning of re-oxygenation, and its prevention markedly delayed the appearance of necrotic cell death[52-60]. Indeed, increased Na+ caused an irreversible influx of Ca2+ by activating the Na+/Ca2+ exchanger[56] and deranged cell volume regulatory mechanisms that ultimately led to osmotic hepatocyte lysis[53,58]. In rat hepatocytes, IP or treatment with A2aR agonists, ATP analogues or NO donors all protected against hypoxia-induced Na+ overload and such protection was causally associated with increased cell survival[20,21,25,27,28,43]. Interestingly, the maintenance of Na+ homeostasis was achieved both in the early phase of hepatocyte preconditioning[20,21,25,27,28], as well as in the late effects[43] (Figure 2). In the early phase of IP, inhibition of the Na+/H+ exchanger and activation of the vacuolar ATPase (V-ATPase) were mainly involved (Figure 2). Indeed, in hepatocytes treated with ATPγS, activation of the P2Y receptors/Src/p38MAPK axis inhibited ERK 1/2-mediated activation of the Na+/H+ exchanger responsible for Na+ influx during hypoxia[25] (Figure 2). Adenosine- and NO-dependent maintenance of Na+ homeostasis in preconditioned hepatocytes depended on p38 MAPK and PI3K signalling and involved the neutralization of intracellular pH achieved by the activation and translocation on plasma membrane of the V-ATPase (Figure 2). V-ATPase, acting as an alternative pH buffering system, extruded protons thus avoiding the activation of Na+-dependent transporters[20,27,28]. The mechanism of Na+ maintenance during the late phase of IP involved the HIF-1-mediated expression of CAIX in hepatocyte plasma membranes. The bicarbonate generated by CAIX was transferred to the cytosol through the Cl-/HCO3- exchanger and neutralized intracellular pH avoiding Na+ influx[42] (Figure 2). Beside these effects on Na+ homeostasis, during ischemia, IP also down-modulated hepatic energy metabolism by preserving the ATP and glycogen pools and limited lactate accumulation[61].
Figure 2.
Na+-dependent mechanisms involved in hepatocyte damage by hypoxia and their modulation by ischemic preconditioning. Hypoxia-induced adenosine triphosphate (ATP) depletion causes intracellular acidification, leading to inhibition of Na+/K+ ATPase and the activation of acid buffering systems (Na+/H+ exchanger). This leads to an increase in intracellular Na+ that precipitates irreversible hepatocyte damage. ATP-dependent signalling through purinergic P2Y receptors prevents Na+ accumulation by inhibiting the ERK 1/2-dependent activation of the Na+/H+ exchanger. Adenosine and nitric oxide (NO) activate the vacuolar proton ATPase that maintains intracellular pH avoiding activation of the Na+/H+ exchanger. Adenosine also induces the hypoxia-inducible factor 1 (HIF-1) target gene, carbonic anhydrase IX (CAIX), which converts CO2 into bicarbonate, that once transported into the hepatocytes through the Cl-/HCO3- exchanger, neutralizes the intracellular pH and prevents Na+ accumulation. See text and Refs[20,21,25,27,28,43,52,53,58]. P38 MAPK: p38 MAP kinase; PI3-kinase: Phosphatidylinositol 3-kinase; PKC: Protein kinase C.
Protection against reperfusion damage
Mitochondria are a major target of the damaging effects of reperfusion[51]. Oxygen re-admission promotes free radical formation by uncoupled mitochondria with consequent mitochondrial oxidative damage and swelling[62]. IP protected mitochondria from oxidative reperfusion damage[63] and preserved mitochondrial redox-state[64], thus attenuating the impairment of ATP synthesis occurring at reperfusion. IP also improved hepatic intracellular oxygenation[65], preserved sinusoidal wall integrity and avoided liver microcirculatory failure induced by I/R[64]. Together these actions preserved aerobic ATP synthesis maintaining the hepatic energy status during re-oxygenation.
During reperfusion, preconditioned livers also showed a significant reduction in oxidative damage[66,67]. This effect could be ascribed to the increased content of antioxidant enzymes such as SOD, CAT and GSPx[49,50], as well as the reduced generation of reactive oxygen species by mitochondria and inflammatory cells. In the latter context, several studies have outlined the capacity of liver preconditioning to reduce inflammatory responses associated with reperfusion. IP decreased leukocyte adhesion to sinusoidal endothelial cells, lowering post-ischemic neutrophil infiltration[68,69]. IP also attenuated the production of pro-inflammatory cytokines/chemokines during reperfusion[10,68,69]. Finally, pharmacological stimulation of A2aR inhibited the activation of hepatic natural killer T lymphocytes, a process that was causally associated with the protective action of IP against hepatic reperfusion damage[70].
An important consequence of IP was the prevention of hepatocyte and sinusoidal endothelial cell apoptosis[3]. Such an effect can be ascribed to the amelioration of oxidative damage, to the reduced production of pro-apoptotic cytokines as well as to a direct interference with apoptotic mechanisms. Indeed, the increase in PKB/Akt observed in preconditioned hepatocytes[32] represents an important anti-apoptotic signal, since PKB/Akt blocks apoptosis by interfering with Bad, caspase-9 and cFLIP functions[71]. NF-κB might also be implicated in the regulation of hepatocyte response to pro-apoptotic stimuli and the increase in NF-κB nuclear binding observed as early as 30 min after liver IP[29] should be considered in this context. It cannot be excluded that NO-mediated signals might also contribute to the anti-apoptotic action of preconditioning by preventing loss of mitochondrial potential, cytochrome c release and caspase activation[72].
In conclusion, the combined effects of liver preconditioning on energy status, ion homeostasis, oxidative stress, pro-apoptotic responses and inflammation could explain the reduction in hepatocyte and sinusoidal endothelial cell death observed in preconditioned livers exposed to I/R[3,51].
Induction of hepatic regeneration
One of the key issues in the possible exploitation of preconditioning on LDTL is related to its effects on hepatocyte proliferation. The mechanisms involved in the pro-regenerative effects of liver preconditioning are beginning to be elucidated. Hepatocyte growth factor (HGF) and transforming growth factor (TGF)-β are two cytokines that have opposite actions on liver regeneration, and promote and inhibit hepatocyte proliferation, respectively[73]. The capacity of IP to enhance liver regeneration after reduced-for-size transplantation was associated with increased HGF levels[15] and a lowering of TGF-β production[16]. These effects were causally related to a reduction in IL-1α and an increase in heat shock protein (HSP) 70 expression, respectively[15,16]. Furthermore, a recent study also associated the capacity of IP to attenuate injury in small-for-size liver grafts with the prevention of free radical production and mitochondrial dysfunction, through an increased expression of HSP90, a molecular chaperone that facilitates the mitochondrial import of Mn-SOD[74].
ISCHEMIC POST-CONDITIONING
The term ischemic post-conditioning refers to the capacity to prevent myocardial I/R injury by the application of brief cycles of ischemia during the reperfusion period after a sustained ischemic episode[75-77]. To date, the effects of post-conditioning in the liver have been reported in two studies. These studies showed that the application of brief ischemia in the early phase of reperfusion after rat liver transplantation, was associated with an amelioration of transaminase release and prevention of hepatocyte apoptosis[78-80]. These observations have new important clinical implications as these mechanisms may also act when hepatic damage has already started. In relation to the mechanisms involved in liver post-conditioning, preliminary results in our laboratory indicated that pharmacological post-conditioning with A2aR agonists induced PI3K activation and prevented post-ischemic damage in hepatocytes[81].
CLINICAL APPLICATIONS OF LIVER PRECONDITIONING
The clinical efficacy of hepatic preconditioning was clearly demonstrated in clinical trials performed in patients undergoing hemi-hepatectomy[4,82,83]. In these patients, IP obtained by 10 min of ischemia and 10 min of reperfusion before 30 min of inflow occlusion, significantly reduced transaminase release and ameliorated sinusoid endothelial cell apoptosis as compared to liver exposed to Pringle’s manoeuvre only[82,83]. These effects were particularly evident in patients with mild or moderate steatosis, but were not observed in subjects older that 60 years[82]. Considering the possible impact that preconditioning may have in attenuating the effects of long-term graft exposure to cold and warm ischemia during liver transplantation procedures[2,4], the therapeutic use of IP in this setting should have important outcomes. The application of IP in human liver transplantation from deceased donors has, however, demonstrated conflicting results[44,84-88]. Indeed, some studies have shown the efficacy of IP in ameliorating transaminase release and in reducing primary graft malfunctions, whereas others have not observed significant differences[44,84-87]. In an attempt to gain some insight into the possible reasons for the failure of IP to protect liver grafts against reperfusion injury, we investigated the intracellular signals activated by IP in transplanted livers from heart-beating deceased donors. The data obtained indicated that IP stimulated PI3K-mediated signals in only half of the grafts and such variability correlated with the clinical effectiveness of IP. Our data also suggested that it was the failure of PTEN down-modulation that likely contributed to the lack of PI3K response to IP[35]. These observations indicated the necessity to explore alternative procedures to surgical IP to overcome the variability of human grafts in activating preconditioning responses. In this regard, the pharmacological induction of liver preconditioning likely represents a more reliable technique for stimulating the intrinsic systems of cytoprotection in humans.
PHARMACOLOGICAL INDUCTION OF HUMAN LIVER PRECONDITIONING
The clinical potential of pharmacological liver preconditioning is clearly suggested by animal studies, however, only two trials have so far addressed this aspect. In one study, Lang and co-workers reported that patients receiving volatile NO during orthotopic liver transplantation displayed an accelerated restoration of liver function as compared to the control group[89]. In the other report, Beck-Schimmer and co-workers showed that preconditioning with the halogenated anaesthetic, sevoflurane, in 64 patients undergoing liver surgery significantly ameliorated transaminase release and the incidence of severe post-operative complications[90]. These observations are consistent with increasing data regarding the efficacy of sevoflurane preconditioning in preventing myocardial ischemia/reperfusion injury[90]. Nonetheless, the availability of several liver specific NO donors and of a variety of effective adenosine A2A receptor agonists[91-93] offers the possibility of extending the number of studies aimed at directly evaluating new approaches to pharmacological liver preconditioning in humans.
CONCLUSION
In spite of a large number of studies on liver preconditioning, general knowledge on this phenomenon is far from complete. The available data give some insight into the signalling pathways responsible for both the early and late responses of IP, as well as some of the cellular modifications involved in the hepatoprotective effects of preconditioning. Additional extracellular inductors and constitutive or newly synthesized mediators are, however, likely to be involved. Little is known about the proteomic changes associated with inhibition of the inflammatory responses and the promotion of hepatic regeneration. Further research is thus needed to clarify these aspects. In particular, preclinical studies are necessary to identify a panel of the most suitable targets of liver preconditioning whose modulation by means of pharmacological or genetic therapies will allow effective activation of endogenous hepatoprotective systems in patients.
Footnotes
Supported by The Regional Government of Piedmont, Italy (Carini, Fondi Ricerca Sanitaria Finalizzata, 2006, 2007; 2008, 2008 bis, 2009; Alchera, Fondi Ricerca Sanitaria Finalizzata, 2008 bis, 2009); and by the University “Amedeo Avogadro”
Peer reviewers: Ezio Laconi, MD, PhD, Professor of General Pathology, Department of Sciences and Biomedical Technologies, Unit of Experimental Pathology, University of Cagliari, Via Porcell, 4, IV Piano, 09125 Cagliari, Italy; Hussein M Atta, MD, PhD, Professor, Department of Surgery, Faculty of Medicine, Minia University, Misr-Aswan Road, El-Minia 61519, Egypt
S- Editor Sun H L- Editor Webster JR E- Editor Lin YP
References
- 1.Murry CE, Jennings RB, Reimer KA. Preconditioning with ischemia: a delay of lethal cell injury in ischemic myocardium. Circulation. 1986;74:1124–1136. doi: 10.1161/01.cir.74.5.1124. [DOI] [PubMed] [Google Scholar]
- 2.Hausenloy DJ, Yellon DM. Preconditioning and postconditioning: underlying mechanisms and clinical application. Atherosclerosis. 2009;204:334–341. doi: 10.1016/j.atherosclerosis.2008.10.029. [DOI] [PubMed] [Google Scholar]
- 3.Carini R, Albano E. Recent insights on the mechanisms of liver preconditioning. Gastroenterology. 2003;125:1480–1491. doi: 10.1016/j.gastro.2003.05.005. [DOI] [PubMed] [Google Scholar]
- 4.de Rougemont O, Lehmann K, Clavien PA. Preconditioning, organ preservation, and postconditioning to prevent ischemia-reperfusion injury to the liver. Liver Transpl. 2009;15:1172–1182. doi: 10.1002/lt.21876. [DOI] [PubMed] [Google Scholar]
- 5.Trotter JF, Wachs M, Everson GT, Kam I. Adult-to-adult transplantation of the right hepatic lobe from a living donor. N Engl J Med. 2002;346:1074–1082. doi: 10.1056/NEJMra011629. [DOI] [PubMed] [Google Scholar]
- 6.Dahm F, Georgiev P, Clavien PA. Small-for-size syndrome after partial liver transplantation: definition, mechanisms of disease and clinical implications. Am J Transplant. 2005;5:2605–2610. doi: 10.1111/j.1600-6143.2005.01081.x. [DOI] [PubMed] [Google Scholar]
- 7.Foschi D, Castoldi L, Lesma A, Musazzi M, Benevento A, Trabucchi E. Effects of ischaemia and reperfusion on liver regeneration in rats. Eur J Surg. 1993;159:393–398. [PubMed] [Google Scholar]
- 8.Selzner M, Camargo CA, Clavien PA. Ischemia impairs liver regeneration after major tissue loss in rodents: protective effects of interleukin-6. Hepatology. 1999;30:469–475. doi: 10.1002/hep.510300215. [DOI] [PubMed] [Google Scholar]
- 9.Peralta C, Hotter G, Closa D, Gelpí E, Bulbena O, Roselló-Catafau J. Protective effect of preconditioning on the injury associated to hepatic ischemia-reperfusion in the rat: role of nitric oxide and adenosine. Hepatology. 1997;25:934–937. doi: 10.1002/hep.510250424. [DOI] [PubMed] [Google Scholar]
- 10.Yoshizumi T, Yanaga K, Soejima Y, Maeda T, Uchiyama H, Sugimachi K. Amelioration of liver injury by ischaemic preconditioning. Br J Surg. 1998;85:1636–1640. doi: 10.1046/j.1365-2168.1998.00917.x. [DOI] [PubMed] [Google Scholar]
- 11.Serafín A, Roselló-Catafau J, Prats N, Xaus C, Gelpí E, Peralta C. Ischemic preconditioning increases the tolerance of Fatty liver to hepatic ischemia-reperfusion injury in the rat. Am J Pathol. 2002;161:587–601. doi: 10.1016/S0002-9440(10)64214-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Arai M, Thurman RG, Lemasters JJ. Contribution of adenosine A(2) receptors and cyclic adenosine monophosphate to protective ischemic preconditioning of sinusoidal endothelial cells against Storage/Reperfusion injury in rat livers. Hepatology. 2000;32:297–302. doi: 10.1053/jhep.2000.8896. [DOI] [PubMed] [Google Scholar]
- 13.Yin DP, Sankary HN, Chong AS, Ma LL, Shen J, Foster P, Williams JW. Protective effect of ischemic preconditioning on liver preservation-reperfusion injury in rats. Transplantation. 1998;66:152–157. doi: 10.1097/00007890-199807270-00002. [DOI] [PubMed] [Google Scholar]
- 14.Bedirli A, Kerem M, Pasaoglu H, Erdem O, Ofluoglu E, Sakrak O. Effects of ischemic preconditioning on regenerative capacity of hepatocyte in the ischemically damaged rat livers. J Surg Res. 2005;125:42–48. doi: 10.1016/j.jss.2004.11.028. [DOI] [PubMed] [Google Scholar]
- 15.Franco-Gou R, Peralta C, Massip-Salcedo M, Xaus C, Serafín A, Roselló-Catafau J. Protection of reduced-size liver for transplantation. Am J Transplant. 2004;4:1408–1420. doi: 10.1111/j.1600-6143.2004.00532.x. [DOI] [PubMed] [Google Scholar]
- 16.Franco-Gou R, Roselló-Catafau J, Casillas-Ramirez A, Massip-Salcedo M, Rimola A, Calvo N, Bartrons R, Peralta C. How ischaemic preconditioning protects small liver grafts. J Pathol. 2006;208:62–73. doi: 10.1002/path.1859. [DOI] [PubMed] [Google Scholar]
- 17.Peralta C, Hotter G, Closa D, Prats N, Xaus C, Gelpí E, Roselló-Catafau J. The protective role of adenosine in inducing nitric oxide synthesis in rat liver ischemia preconditioning is mediated by activation of adenosine A2 receptors. Hepatology. 1999;29:126–132. doi: 10.1002/hep.510290104. [DOI] [PubMed] [Google Scholar]
- 18.Nakayama H, Yamamoto Y, Kume M, Yamagami K, Yamamoto H, Kimoto S, Ishikawa Y, Ozaki N, Shimahara Y, Yamaoka Y. Pharmacologic stimulation of adenosine A2 receptor supplants ischemic preconditioning in providing ischemic tolerance in rat livers. Surgery. 1999;126:945–954. doi: 10.1016/s0039-6060(99)70037-1. [DOI] [PubMed] [Google Scholar]
- 19.Hart ML, Much C, Gorzolla IC, Schittenhelm J, Kloor D, Stahl GL, Eltzschig HK. Extracellular adenosine production by ecto-5'-nucleotidase protects during murine hepatic ischemic preconditioning. Gastroenterology. 2008;135:1739–1750.e3. doi: 10.1053/j.gastro.2008.07.064. [DOI] [PubMed] [Google Scholar]
- 20.Carini R, De Cesaris MG, Splendore R, Bagnati M, Albano E. Ischemic preconditioning reduces Na(+) accumulation and cell killing in isolated rat hepatocytes exposed to hypoxia. Hepatology. 2000;31:166–172. doi: 10.1002/hep.510310125. [DOI] [PubMed] [Google Scholar]
- 21.Carini R, De Cesaris MG, Splendore R, Vay D, Domenicotti C, Nitti MP, Paola D, Pronzato MA, Albano E. Signal pathway involved in the development of hypoxic preconditioning in rat hepatocytes. Hepatology. 2001;33:131–139. doi: 10.1053/jhep.2001.21050. [DOI] [PubMed] [Google Scholar]
- 22.Linden J. Molecular approach to adenosine receptors: receptor-mediated mechanisms of tissue protection. Annu Rev Pharmacol Toxicol. 2001;41:775–787. doi: 10.1146/annurev.pharmtox.41.1.775. [DOI] [PubMed] [Google Scholar]
- 23.Eltzschig HK, Eckle T, Mager A, Küper N, Karcher C, Weissmüller T, Boengler K, Schulz R, Robson SC, Colgan SP. ATP release from activated neutrophils occurs via connexin 43 and modulates adenosine-dependent endothelial cell function. Circ Res. 2006;99:1100–1108. doi: 10.1161/01.RES.0000250174.31269.70. [DOI] [PubMed] [Google Scholar]
- 24.Eltzschig HK, Weissmüller T, Mager A, Eckle T. Nucleotide metabolism and cell-cell interactions. Methods Mol Biol. 2006;341:73–87. doi: 10.1385/1-59745-113-4:73. [DOI] [PubMed] [Google Scholar]
- 25.Carini R, Alchera E, De Cesaris MG, Splendore R, Piranda D, Baldanzi G, Albano E. Purinergic P2Y2 receptors promote hepatocyte resistance to hypoxia. J Hepatol. 2006;45:236–245. doi: 10.1016/j.jhep.2006.02.017. [DOI] [PubMed] [Google Scholar]
- 26.Peralta C, Rull R, Rimola A, Deulofeu R, Roselló-Catafau J, Gelpí E, Rodés J. Endogenous nitric oxide and exogenous nitric oxide supplementation in hepatic ischemia-reperfusion injury in the rat. Transplantation. 2001;71:529–536. doi: 10.1097/00007890-200102270-00008. [DOI] [PubMed] [Google Scholar]
- 27.Carini R, Grazia De Cesaris M, Splendore R, Domenicotti C, Nitti MP, Pronzato MA, Albano E. Signal pathway responsible for hepatocyte preconditioning by nitric oxide. Free Radic Biol Med. 2003;34:1047–1055. doi: 10.1016/s0891-5849(03)00039-x. [DOI] [PubMed] [Google Scholar]
- 28.Carini R, Trincheri NF, Alchera E, De Cesaris MG, Castino R, Splendore R, Albano E, Isidoro C. PI3K-dependent lysosome exocytosis in nitric oxide-preconditioned hepatocytes. Free Radic Biol Med. 2006;40:1738–1748. doi: 10.1016/j.freeradbiomed.2006.01.013. [DOI] [PubMed] [Google Scholar]
- 29.Teoh N, Dela Pena A, Farrell G. Hepatic ischemic preconditioning in mice is associated with activation of NF-kappaB, p38 kinase, and cell cycle entry. Hepatology. 2002;36:94–102. doi: 10.1053/jhep.2002.33134. [DOI] [PubMed] [Google Scholar]
- 30.Amersi F, Shen XD, Anselmo D, Melinek J, Iyer S, Southard DJ, Katori M, Volk HD, Busuttil RW, Buelow R, et al. Ex vivo exposure to carbon monoxide prevents hepatic ischemia/reperfusion injury through p38 MAP kinase pathway. Hepatology. 2002;35:815–823. doi: 10.1053/jhep.2002.32467. [DOI] [PubMed] [Google Scholar]
- 31.Schulte G, Fredholm BB. Signalling from adenosine receptors to mitogen-activated protein kinases. Cell Signal. 2003;15:813–827. doi: 10.1016/s0898-6568(03)00058-5. [DOI] [PubMed] [Google Scholar]
- 32.Carini R, Grazia De Cesaris M, Splendore R, Baldanzi G, Nitti MP, Alchera E, Filigheddu N, Domenicotti C, Pronzato MA, Graziani A, et al. Role of phosphatidylinositol 3-kinase in the development of hepatocyte preconditioning. Gastroenterology. 2004;127:914–923. doi: 10.1053/j.gastro.2004.06.018. [DOI] [PubMed] [Google Scholar]
- 33.Cantley LC. The phosphoinositide 3-kinase pathway. Science. 2002;296:1655–1657. doi: 10.1126/science.296.5573.1655. [DOI] [PubMed] [Google Scholar]
- 34.Izuishi K, Tsung A, Hossain MA, Fujiwara M, Wakabayashi H, Masaki T, Billiar TR, Maeta H. Ischemic preconditioning of the murine liver protects through the Akt kinase pathway. Hepatology. 2006;44:573–580. doi: 10.1002/hep.21298. [DOI] [PubMed] [Google Scholar]
- 35.Cescon M, Carini R, Grazi G, Caraceni P, Alchera E, Gasloli G, Ravaioli M, Tuci F, Imarisio C, Dal Ponte C, et al. Variable activation of phosphoinositide 3-kinase influences the response of liver grafts to ischemic preconditioning. J Hepatol. 2009;50:937–947. doi: 10.1016/j.jhep.2008.11.016. [DOI] [PubMed] [Google Scholar]
- 36.Mérida I, Avila-Flores A, Merino E. Diacylglycerol kinases: at the hub of cell signalling. Biochem J. 2008;409:1–18. doi: 10.1042/BJ20071040. [DOI] [PubMed] [Google Scholar]
- 37.Baldanzi G, Alchera E, Imarisio C, Gaggianesi M, Dal Ponte C, Nitti M, Domenicotti C, van Blitterswijk WJ, Albano E, Graziani A, et al. Negative regulation of diacylglycerol kinase theta mediates adenosine-dependent hepatocyte preconditioning. Cell Death Differ. 2010;17:1059–1068. doi: 10.1038/cdd.2009.210. [DOI] [PubMed] [Google Scholar]
- 38.Baeuerle PA, Henkel T. Function and activation of NF-kappa B in the immune system. Annu Rev Immunol. 1994;12:141–179. doi: 10.1146/annurev.iy.12.040194.001041. [DOI] [PubMed] [Google Scholar]
- 39.Funaki H, Shimizu K, Harada S, Tsuyama H, Fushida S, Tani T, Miwa K. Essential role for nuclear factor kappaB in ischemic preconditioning for ischemia-reperfusion injury of the mouse liver. Transplantation. 2002;74:551–556. doi: 10.1097/00007890-200208270-00021. [DOI] [PubMed] [Google Scholar]
- 40.Imada K, Leonard WJ. The Jak-STAT pathway. Mol Immunol. 2000;37:1–11. doi: 10.1016/s0161-5890(00)00018-3. [DOI] [PubMed] [Google Scholar]
- 41.Matsumoto T, O'Malley K, Efron PA, Burger C, McAuliffe PF, Scumpia PO, Uchida T, Tschoeke SK, Fujita S, Moldawer LL, et al. Interleukin-6 and STAT3 protect the liver from hepatic ischemia and reperfusion injury during ischemic preconditioning. Surgery. 2006;140:793–802. doi: 10.1016/j.surg.2006.04.010. [DOI] [PubMed] [Google Scholar]
- 42.Semenza GL. Hypoxia-inducible factor 1: oxygen homeostasis and disease pathophysiology. Trends Mol Med. 2001;7:345–350. doi: 10.1016/s1471-4914(01)02090-1. [DOI] [PubMed] [Google Scholar]
- 43.Alchera E, Tacchini L, Imarisio C, Dal Ponte C, De Ponti C, Gammella E, Cairo G, Albano E, Carini R. Adenosine-dependent activation of hypoxia-inducible factor-1 induces late preconditioning in liver cells. Hepatology. 2008;48:230–239. doi: 10.1002/hep.22249. [DOI] [PubMed] [Google Scholar]
- 44.Amador A, Grande L, Martí J, Deulofeu R, Miquel R, Solá A, Rodriguez-Laiz G, Ferrer J, Fondevila C, Charco R, et al. Ischemic pre-conditioning in deceased donor liver transplantation: a prospective randomized clinical trial. Am J Transplant. 2007;7:2180–2189. doi: 10.1111/j.1600-6143.2007.01914.x. [DOI] [PubMed] [Google Scholar]
- 45.Déry MA, Michaud MD, Richard DE. Hypoxia-inducible factor 1: regulation by hypoxic and non-hypoxic activators. Int J Biochem Cell Biol. 2005;37:535–540. doi: 10.1016/j.biocel.2004.08.012. [DOI] [PubMed] [Google Scholar]
- 46.Pagé EL, Robitaille GA, Pouysségur J, Richard DE. Induction of hypoxia-inducible factor-1alpha by transcriptional and translational mechanisms. J Biol Chem. 2002;277:48403–48409. doi: 10.1074/jbc.M209114200. [DOI] [PubMed] [Google Scholar]
- 47.Koti RS, Tsui J, Lobos E, Yang W, Seifalian AM, Davidson BR. Nitric oxide synthase distribution and expression with ischemic preconditioning of the rat liver. FASEB J. 2005;19:1155–1157. doi: 10.1096/fj.04-3220fje. [DOI] [PubMed] [Google Scholar]
- 48.Barrier A, Olaya N, Chiappini F, Roser F, Scatton O, Artus C, Franc B, Dudoit S, Flahault A, Debuire B, et al. Ischemic preconditioning modulates the expression of several genes, leading to the overproduction of IL-1Ra, iNOS, and Bcl-2 in a human model of liver ischemia-reperfusion. FASEB J. 2005;19:1617–1626. doi: 10.1096/fj.04-3445com. [DOI] [PubMed] [Google Scholar]
- 49.Chen W, Qiu JF, Zhang ZQ, Luo HF, Rosello-Catafau J, Wu ZY. Gene expression changes after hypoxic preconditioning in rat hepatocytes. Hepatobiliary Pancreat Dis Int. 2006;5:416–421. [PubMed] [Google Scholar]
- 50.Yuan GJ, Ma JC, Gong ZJ, Sun XM, Zheng SH, Li X. Modulation of liver oxidant-antioxidant system by ischemic preconditioning during ischemia/reperfusion injury in rats. World J Gastroenterol. 2005;11:1825–1828. doi: 10.3748/wjg.v11.i12.1825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Jaeschke H. Molecular mechanisms of hepatic ischemia-reperfusion injury and preconditioning. Am J Physiol Gastrointest Liver Physiol. 2003;284:G15–G26. doi: 10.1152/ajpgi.00342.2002. [DOI] [PubMed] [Google Scholar]
- 52.Carini R, Bellomo G, Benedetti A, Fulceri R, Gamberucci A, Parola M, Dianzani MU, Albano E. Alteration of Na+ homeostasis as a critical step in the development of irreversible hepatocyte injury after adenosine triphosphate depletion. Hepatology. 1995;21:1089–1098. [PubMed] [Google Scholar]
- 53.Carini R, Autelli R, Bellomo G, Dianzani MU, Albano E. Sodium-mediated cell swelling is associated with irreversible damage in isolated hepatocytes exposed to hypoxia or mitochondrial toxins. Biochem Biophys Res Commun. 1995;206:180–185. doi: 10.1006/bbrc.1995.1025. [DOI] [PubMed] [Google Scholar]
- 54.Carini R, De Cesaris MG, Bellomo G, Albano E. Intracellular Na+ accumulation and hepatocyte injury during cold storage. Transplantation. 1999;68:294–297. doi: 10.1097/00007890-199907270-00023. [DOI] [PubMed] [Google Scholar]
- 55.Carini R, De Cesaris MG, Splendore R, Bagnati M, Bellomo G, Albano E. Alterations of Na(+) homeostasis in hepatocyte reoxygenation injury. Biochim Biophys Acta. 2000;1500:297–305. doi: 10.1016/s0925-4439(99)00114-3. [DOI] [PubMed] [Google Scholar]
- 56.Carini R, de Cesaris MG, Bellomo G, Albano E. Role of Na+/Ca2+ exchanger in preventing Na+ overload and hepatocyte injury: opposite effects of extracellular and intracellular Ca2+ chelation. Biochem Biophys Res Commun. 1997;232:107–110. doi: 10.1006/bbrc.1997.6227. [DOI] [PubMed] [Google Scholar]
- 57.Carini R, Bellomo G, Grazia De Cesaris M, Albano E. Glycine protects against hepatocyte killing by KCN or hypoxia by preventing intracellular Na+ overload in the rat. Hepatology. 1997;26:107–112. doi: 10.1002/hep.510260114. [DOI] [PubMed] [Google Scholar]
- 58.Carini R, Autelli R, Bellomo G, Albano E. Alterations of cell volume regulation in the development of hepatocyte necrosis. Exp Cell Res. 1999;248:280–293. doi: 10.1006/excr.1999.4408. [DOI] [PubMed] [Google Scholar]
- 59.Carini R, De Cesaris MG, Spendore R, Albano E. Ethanol potentiates hypoxic liver injury: role of hepatocyte Na(+) overload. Biochim Biophys Acta. 2000;1502:508–514. doi: 10.1016/s0925-4439(00)00075-2. [DOI] [PubMed] [Google Scholar]
- 60.Carini R, De Cesaris MG, Splendore R, Domenicotti C, Nitti MP, Pronzato MA, Albano E. Mechanisms of hepatocyte protection against hypoxic injury by atrial natriuretic peptide. Hepatology. 2003;37:277–285. doi: 10.1053/jhep.2003.50033. [DOI] [PubMed] [Google Scholar]
- 61.Peralta C, Bartrons R, Riera L, Manzano A, Xaus C, Gelpí E, Roselló-Catafau J. Hepatic preconditioning preserves energy metabolism during sustained ischemia. Am J Physiol Gastrointest Liver Physiol. 2000;279:G163–G171. doi: 10.1152/ajpgi.2000.279.1.G163. [DOI] [PubMed] [Google Scholar]
- 62.Jassem W, Fuggle SV, Rela M, Koo DD, Heaton ND. The role of mitochondria in ischemia/reperfusion injury. Transplantation. 2002;73:493–499. doi: 10.1097/00007890-200202270-00001. [DOI] [PubMed] [Google Scholar]
- 63.Lee WY, Lee SM. Ischemic preconditioning protects post-ischemic oxidative damage to mitochondria in rat liver. Shock. 2005;24:370–375. doi: 10.1097/01.shk.0000175895.33415.cd. [DOI] [PubMed] [Google Scholar]
- 64.Glanemann M, Vollmar B, Nussler AK, Schaefer T, Neuhaus P, Menger MD. Ischemic preconditioning protects from hepatic ischemia/reperfusion-injury by preservation of microcirculation and mitochondrial redox-state. J Hepatol. 2003;38:59–66. doi: 10.1016/s0168-8278(02)00327-6. [DOI] [PubMed] [Google Scholar]
- 65.Koti RS, Seifalian AM, McBride AG, Yang W, Davidson BR. The relationship of hepatic tissue oxygenation with nitric oxide metabolism in ischemic preconditioning of the liver. FASEB J. 2002;16:1654–1656. doi: 10.1096/fj.01-1034fje. [DOI] [PubMed] [Google Scholar]
- 66.Cavalieri B, Perrelli MG, Aragno M, Mastrocola R, Corvetti G, Durazzo M, Poli G, Cutrìn JC. Ischemic preconditioning attenuates the oxidant-dependent mechanisms of reperfusion cell damage and death in rat liver. Liver Transpl. 2002;8:990–999. doi: 10.1053/jlts.2002.35549. [DOI] [PubMed] [Google Scholar]
- 67.Peralta C, Bulbena O, Xaus C, Prats N, Cutrin JC, Poli G, Gelpi E, Roselló-Catafau J. Ischemic preconditioning: a defense mechanism against the reactive oxygen species generated after hepatic ischemia reperfusion. Transplantation. 2002;73:1203–1211. doi: 10.1097/00007890-200204270-00004. [DOI] [PubMed] [Google Scholar]
- 68.Peralta C, Bulbena O, Bargalló R, Prats N, Gelpí E, Roselló-Catafau J. Strategies to modulate the deleterious effects of endothelin in hepatic ischemia-reperfusion. Transplantation. 2000;70:1761–1770. doi: 10.1097/00007890-200012270-00016. [DOI] [PubMed] [Google Scholar]
- 69.Serafín A, Roselló-Catafau J, Prats N, Gelpí E, Rodés J, Peralta C. Ischemic preconditioning affects interleukin release in fatty livers of rats undergoing ischemia/reperfusion. Hepatology. 2004;39:688–698. doi: 10.1002/hep.20089. [DOI] [PubMed] [Google Scholar]
- 70.Lappas CM, Day YJ, Marshall MA, Engelhard VH, Linden J. Adenosine A2A receptor activation reduces hepatic ischemia reperfusion injury by inhibiting CD1d-dependent NKT cell activation. J Exp Med. 2006;203:2639–2648. doi: 10.1084/jem.20061097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Brazil DP, Park J, Hemmings BA. PKB binding proteins. Getting in on the Akt. Cell. 2002;111:293–303. doi: 10.1016/s0092-8674(02)01083-8. [DOI] [PubMed] [Google Scholar]
- 72.Moncada S, Erusalimsky JD. Does nitric oxide modulate mitochondrial energy generation and apoptosis? Nat Rev Mol Cell Biol. 2002;3:214–220. doi: 10.1038/nrm762. [DOI] [PubMed] [Google Scholar]
- 73.Fausto N. Liver regeneration and repair: hepatocytes, progenitor cells, and stem cells. Hepatology. 2004;39:1477–1487. doi: 10.1002/hep.20214. [DOI] [PubMed] [Google Scholar]
- 74.Rehman H, Connor HD, Ramshesh VK, Theruvath TP, Mason RP, Wright GL, Lemasters JJ, Zhong Z. Ischemic preconditioning prevents free radical production and mitochondrial depolarization in small-for-size rat liver grafts. Transplantation. 2008;85:1322–1331. doi: 10.1097/TP.0b013e31816de302. [DOI] [PubMed] [Google Scholar]
- 75.Tsang A, Hausenloy DJ, Mocanu MM, Yellon DM. Postconditioning: a form of "modified reperfusion" protects the myocardium by activating the phosphatidylinositol 3-kinase-Akt pathway. Circ Res. 2004;95:230–232. doi: 10.1161/01.RES.0000138303.76488.fe. [DOI] [PubMed] [Google Scholar]
- 76.Yellon DM, Hausenloy DJ. Realizing the clinical potential of ischemic preconditioning and postconditioning. Nat Clin Pract Cardiovasc Med. 2005;2:568–575. doi: 10.1038/ncpcardio0346. [DOI] [PubMed] [Google Scholar]
- 77.Zhao ZQ, Vinten-Johansen J. Postconditioning: reduction of reperfusion-induced injury. Cardiovasc Res. 2006;70:200–211. doi: 10.1016/j.cardiores.2006.01.024. [DOI] [PubMed] [Google Scholar]
- 78.Sun K, Liu ZS, Sun Q. Role of mitochondria in cell apoptosis during hepatic ischemia-reperfusion injury and protective effect of ischemic postconditioning. World J Gastroenterol. 2004;10:1934–1938. doi: 10.3748/wjg.v10.i13.1934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Wang KX, Hu SY, Jiang XS, Zhu M, Jin B, Zhang GY, Chen B. Protective effects of ischaemic postconditioning on warm/cold ischaemic reperfusion injury in rat liver: a comparative study with ischaemic preconditioning. Chin Med J (Engl) 2008;121:2004–2009. [PubMed] [Google Scholar]
- 80.Wang N, Lu JG, He XL, Li N, Qiao Q, Yin JK, Ma QJ. Effects of ischemic postconditioning on reperfusion injury in rat liver grafts after orthotopic liver transplantation. Hepatol Res. 2009;39:382–390. doi: 10.1111/j.1872-034X.2008.00462.x. [DOI] [PubMed] [Google Scholar]
- 81.Dal Ponte C, Alchera E, Imarisio C, Albano E, Carini R. Stimulation of adenosine A2a receptors induces postconditioning in isolated hepatocytes. J Hepatol. 2008;48:S66. [Google Scholar]
- 82.Clavien PA, Selzner M, Rüdiger HA, Graf R, Kadry Z, Rousson V, Jochum W. A prospective randomized study in 100 consecutive patients undergoing major liver resection with versus without ischemic preconditioning. Ann Surg. 2003;238:843–850; discussion 851-852. doi: 10.1097/01.sla.0000098620.27623.7d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Azoulay D, Lucidi V, Andreani P, Maggi U, Sebagh M, Ichai P, Lemoine A, Adam R, Castaing D. Ischemic preconditioning for major liver resection under vascular exclusion of the liver preserving the caval flow: a randomized prospective study. J Am Coll Surg. 2006;202:203–211. doi: 10.1016/j.jamcollsurg.2005.10.021. [DOI] [PubMed] [Google Scholar]
- 84.Azoulay D, Del Gaudio M, Andreani P, Ichai P, Sebag M, Adam R, Scatton O, Min BY, Delvard V, Lemoine A, et al. Effects of 10 minutes of ischemic preconditioning of the cadaveric liver on the graft's preservation and function: the ying and the yang. Ann Surg. 2005;242:133–139. doi: 10.1097/01.sla.0000167848.96692.ad. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Cescon M, Grazi GL, Grassi A, Ravaioli M, Vetrone G, Ercolani G, Varotti G, D'Errico A, Ballardini G, Pinna AD. Effect of ischemic preconditioning in whole liver transplantation from deceased donors. A pilot study. Liver Transpl. 2006;12:628–635. doi: 10.1002/lt.20640. [DOI] [PubMed] [Google Scholar]
- 86.Koneru B, Shareef A, Dikdan G, Desai K, Klein KM, Peng B, Wachsberg RH, de la Torre AN, Debroy M, Fisher A, et al. The ischemic preconditioning paradox in deceased donor liver transplantation-evidence from a prospective randomized single blind clinical trial. Am J Transplant. 2007;7:2788–2796. doi: 10.1111/j.1600-6143.2007.02009.x. [DOI] [PubMed] [Google Scholar]
- 87.Jassem W, Fuggle SV, Cerundolo L, Heaton ND, Rela M. Ischemic preconditioning of cadaver donor livers protects allografts following transplantation. Transplantation. 2006;81:169–174. doi: 10.1097/01.tp.0000188640.05459.37. [DOI] [PubMed] [Google Scholar]
- 88.Franchello A, Gilbo N, David E, Ricchiuti A, Romagnoli R, Cerutti E, Salizzoni M. Ischemic preconditioning (IP) of the liver as a safe and protective technique against ischemia/reperfusion injury (IRI) Am J Transplant. 2009;9:1629–1639. doi: 10.1111/j.1600-6143.2009.02680.x. [DOI] [PubMed] [Google Scholar]
- 89.Lang JD Jr, Teng X, Chumley P, Crawford JH, Isbell TS, Chacko BK, Liu Y, Jhala N, Crowe DR, Smith AB, et al. Inhaled NO accelerates restoration of liver function in adults following orthotopic liver transplantation. J Clin Invest. 2007;117:2583–2591. doi: 10.1172/JCI31892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Beck-Schimmer B, Breitenstein S, Urech S, De Conno E, Wittlinger M, Puhan M, Jochum W, Spahn DR, Graf R, Clavien PA. A randomized controlled trial on pharmacological preconditioning in liver surgery using a volatile anesthetic. Ann Surg. 2008;248:909–918. doi: 10.1097/SLA.0b013e31818f3dda. [DOI] [PubMed] [Google Scholar]
- 91.Landoni G, Fochi O, Torri G. Cardiac protection by volatile anaesthetics: a review. Curr Vasc Pharmacol. 2008;6:108–111. doi: 10.2174/157016108783955284. [DOI] [PubMed] [Google Scholar]
- 92.Fiorucci S, Antonelli E, Tocchetti P, Morelli A. Treatment of portal hypertension with NCX-1000, a liver-specific NO donor. A review of its current status. Cardiovasc Drug Rev. 2004;22:135–146. doi: 10.1111/j.1527-3466.2004.tb00136.x. [DOI] [PubMed] [Google Scholar]
- 93.Gao ZG, Jacobson KA. Emerging adenosine receptor agonists. Expert Opin Emerg Drugs. 2007;12:479–492. doi: 10.1517/14728214.12.3.479. [DOI] [PubMed] [Google Scholar]