Abstract
PSYCHIATRISTS SHOULD REVISIT THE ISSUE OF HOW TO ASSESS, OBJECTIVELY AND reliably, patients' cognitive status. Cognitive disorders, like ADHD (attention deficit/ hyperactivity disorder), and the various dementias are increasing in importance, and perhaps in number. The modern study of schizophrenia has focused on cognition as an outcome predictor; even the mood disorders can be associated, over time, with cognitive impairment. And with so many perfectly good drug alternatives in every therapeutic category, medications might be differentiated by virtue of their comparative effects on cognition. The best reason, however, is this: in clinical practice, cognitive assessment is either gross and insensitive or arduous and inordinately expensive.
The formal mental status examination, properly executed, is an objective measure of a patient's cognitive status. So is the Mini-Mental State Exam (MMSE), the Clock Test, other brief paper-and-pencil tests, and cognitive rating scales like the ADAS-Cog. These are quick “bedside” tests, easy to administer and to score, and sensitive to cognitive impairment, at least when symptoms are overt and the process that caused them is well-established. At the other end of the spectrum, neuropsychological testing is also an objective measure. It is expensive and time-consuming to be sure but comprehensive and reasonably sensitive to mild cognitive dysfunction. But neuropsychological batteries are comprehensive only because they are long and arduous. They are not well suited for repeated administration, especially at frequent intervals, and they are only marginally sensitive to the subtle effects of psychotropic medications.
Websites Associated with Computerized Neurological Psychological Tests
| CANTAB | www.cantab.com |
| CDR | www.cdr.org.uk |
| FePsy | www.euronet.nl/users/fepsy |
| Cogtest | www.cogtest.com |
| CogState | www.cogstate.com |
| Vital Signs | www.cnsvs.com |
| Conners CPT | www.mhs.com |
| TOVA | www.addwarehouse.com |
| Psychologix | www.psychologix.com |
| BMAB | www.memorytesting.com |
| Wisconsin Card Sort Test | www.ormond.co.za/cardsort.htm |
It is important to revisit the issue of objective cognitive assessment because psychiatrists need a technology that is more sensitive and more comprehensive than the mental status examination or the MMSE and less expensive and quicker than formal neuropsychological testing. Psychiatrists ought to be able to test patients and generate accurate, reliable data in a medical clinic setting, even under the oppressive cost constraints of modern-day practice. They ought to test patients at frequent intervals to measure the effects of treatment and to track the course of a patient's mental illness as the years pass. They need objective measures for the diagnosis of ADHD and for the detection of pre-symptommatic dementia. And, simply from an economic point-of-view, it helps the bottom line to adopt a clinical procedure, especially one that increases efficiency and generates added value to patients at little cost to the provider.
Computerized Neurological Psychological Testing
Computerized neurocognitive testing (CNT) has been with us since the days of the Commodore microcomputer and the Apple 2e. The earliest test batteries were performance assessment batteries (PABs), used mostly in military and aerospace medicine. Another impetus for CNT development came from the World Health Organization during the 1980s. Test developers devised a number of PABs to evaluate neurocognitive impairment in industrial workers.
During the 1980s and early 90s, computerized testing was largely confined to these small outposts. Medicine, in general, and neuropsychology, in particular, took little heed of CNT. There was little cross-talk between clinical researchers and the study groups working on PABs. Perhaps clinicians were put off by the major disadvantages of CNT, which included the fact that many batteries were, and still are, relatively stunted in terms of their psychometric development; that the performance of an unsupervised subject sitting in front of a “console” may not always represent an optimal testing environment; and that computerized tests can generate a mass of seemingly precise data, whose clinical salience may be hard even for a seasoned neuropsychologist to interpret properly.
On the other hand, CNT has clear advantages compared to traditional paper-and-pencil testing. These include better standardization in administration and scoring, the ability to generate numerous alternative forms suitable for repeated testing, precise stimulus control, the ability to track various components of subjects' responses, increased cost efficiency in testing, and the ability to develop large and accurate databases.1 In fact, when traditional neuropsychological batteries are compared directly to a CNT, the two are likely to show, “in general comparable results…the patients tolerated the computerized scan well. In contrast to the traditional battery, which taxes patients' endurance, patients seemed to appreciate the brevity of the computerized scan. They did not have difficulties operating the computer and informally they appeared more relaxed being tested by a computer rather than a person.”2
Over the last 10 years, computerized testing in medicine and neuropsychology has become a well-developed if not fully mature endeavor. Clinicians, as well as researchers, seem to understand the intrinsic limitations of CNT just as they are beginning to appreciate its enormous potential. CNT has not yet made the leap from research application to routine clinical use, but it is definitely on the brink.
Computerized Assessment in Psychiatry
There have been several attempts to introduce computerized assessment to the practice of psychiatry. For diagnosis, the structured diagnostic systems that began with the DSM-III, and the subsequent development of semistructured interview techniques (like the Diagnostic Interview Schedule) lent themselves to a computerized format.3–6 Computers have been used for patient self reports regarding, for example, alcohol or drug use, about which people may be embarrassed to discuss in a personal interview.7 Published reports are unanimous in acknowledging the feasibility of the technology, its acceptability to patients, and the reliability of the data thus generated.8
More to the point, CNTs are increasingly used in clinical studies of psychiatric disorders9 and the effects of psychotropic drugs.10,11 These applications have the most relevance to future practice. They may even be relevant to psychotherapeutic practice. Because “cognitive behavior therapy (CBT) depends on adequate functioning in patients,” prospective patients were screened, in one study, with the CNT, the MicroCog. CBT was more likely to be successful in the patients with better cognitive abilities.12 Another group actually used a multimedia, interactive, computerized CBT program to treat patients with anxiety or depression. The authors thought that it worked.13
The earliest computerized neurocognitive test batteries (CNTBs) were used, almost exclusively, in research settings, and mainly by the pharmaceutical industry. One of the best-known batteries is the Cambridge Neuropsychological Test Automated Battery (CANTAB). The CANTAB has been used quite extensively in the testing of patients with dementia, Parkinson's disease, Korsakoff's syndrome, depression, schizophrenia, HIV, and in children with learning disabilities and autism.14 It has also been used to evaluate cognitive effects of various drugs.15
Following CANTAB, several alternative CNTBs have been developed specifically for drug development research. The Cognitive Drug Research Microcomputerised Assessment System (CDR), for example, consists of a number of core tests and a number of other tests that can be employed if the client so wishes. The time taken to perform the tests is sufficiently brief that it can be administered repeatedly on study days to identify the time course of effects following treatment. The system developer, Keith Wesnes, maintains that “properly developed automated test systems…are more sensitive to change in cognitive function than traditional nonautomated procedures.”16
Research applications of the CDR system include studies of cognitive impairment related to cardiopulmonary bypass,17 microwave radiation,18 dementia,19 hypertension,20 environmental toxins,21 and a variety of drugs and nutraceuticals.22–27 The CDR system may have some useful clinical applications. Compared to several nonautomated measures, it was the most sensitive in identifying patients with Alzheimer's disease and differentiating them from patients with Huntington's disease.28 It has been used to differentiate different conditions that cause dementia, to identify cognitive impairment in patients with early Parkinson's disease, to identify attentional impairments in stroke patients, and to identify patients with mild cognitive impairment by speed of information retrieval from episodic memory.29
As the study of cognition occupies a place of increasing importance in neuropsychiatric research, new CNTBs are proliferating. FePsy, otherwise known as the “Iron Psyche,” has been used mainly in studies of antiepileptic drugs.30–32 It is PC-based but requires the presence of an examiner to supervise the subject during the test. Computerized neuropsychological scanning was originally designed for functional neuroimaging studies, but was subsequently proposed as a clinically relevant scan of neurocognitive abilities. It has been used in at least one study of schizophrenic patients.2,33 Though said to be ready for clinical and research applications, it is only available on a Mac platform, which may limit its appeal. The 11 tests take about an hour to administer. Cogtest is bundled with a data-management system for clinical trials. It uses an interactive touchscreen interface. The program can only be run on a PC supplied by the developers, who are in the United Kingdom.
All of these batteries are reliable and sensitive to the very small changes in cognition that accompany treatment with antihistamines, sedative-hypnotics, antidepressants, anti-epileptics, and antipsychotic drugs. In fact, CNTs, like CANTAB and CDR, are beginning to venture beyond research into the clinical arena. The fact that they all tend to rely on tests that are idiosyncratic—that is, unique to the CNTB and not used anywhere else—limits their appeal to clinicians, who tend to prefer established and familiar tests.
Nevertheless, research-oriented CNTBs have clearly demonstrated that computers can generate sound data quickly and efficiently. They have been limited to special applications, to be sure, but they have, in so doing, proven their relevance to areas of clinical practice. They have proven the value of the technology. The next question is: How is that technology evolving in the clinic?
Computerized Neuropsychological Test Batteries Designed for Clinical Use
The first CNT developed for clinical applications was MicroCog.34 This PC-based battery of neuropsychological tests was so successful that it became the first commercially available and widely marketed CNTB. In its present form, the MicroCog includes measures of a number of abilities in five cognitive domains, which include attention/mental control, memory, reasoning/calculation, spatial processing, and reaction time. There are 18 subtests in the standard administration, which takes approximately an hour. A short form (12 subtests) takes about half an hour.
The MicroCog is an excellent test battery that we have used in the clinic for many years. The report it generates, though, is somewhat turgid and difficult to interpret. Some of the tests in the MicroCog battery are traditional neurocognitive measures but most address constructs different from those of traditional neuropsychological tests. This makes it somewhat difficult to relate test performance on MicroCog to conventional methods of neuropsychological functioning.
Despite its modest price and its availability through one of the largest test publishers, MicroCog has not been used much in clinical practice or in research. There are only a few citations to be found in Medline. Elwood35 contends that it provides an accurate, cost-effective screen for early dementia among elderly subjects living in the community and that it can even distinguish dementia from depression. He points out, however, that its ability to detect cognitive decline in patients who are not elderly or to discriminate dementia from other mental disorders has not been established. MicroCog has also been used to measure cognitive impairment related to drug abuse.36,37
The CogState battery was developed as a dementia screening instrument and for concussion management. It is interesting because it is a neurocognitive test battery in the form of a card game, similar to the game “Concentration.” The display is a green baize field with playing cards face down or face up in different arrays. The subject plays a series of games that are graded in difficulty and that measure, in progression, a number of different cognitive domains, including reaction time, attention, and memory. The construction of this battery is such that there are no “ceiling” effects. That is, it is never possible to obtain a perfect score. For that reason, it is sensitive to cognitive decline even in gifted individuals who might attain perfect scores on other tests.
CogState is an engaging test and the graphics are impressive. However, it requires an active Internet connection to generate a report. The subject's data is uploaded and analyzed. Then a report is generated and e-mailed back to the provider.
CNS Vital Signs is a hybrid product that uses conventional cognitive tests, but is designed for serial administration and intrasubject comparison, like all of the PABs. The seven tests (verbal and visual memory, finger tapping, coding, the Stroop, shifting attention, and continuous performance) are as familiar as any test in neuropsychology, but every keystroke is recorded with millisecond accuracy.
Vital Signs consists of seven tests and reports results in five domains: memory, psychomotor speed, information processing speed, attention, and cognitive flexibility. The test is self-administered, and a normal fourth-grader can follow the instructions. It takes about half an hour to administer. Vital Signs generates unique profiles for patients with brain injuries, early dementia, and ADD, and is sensitive to the effects of psychostimulants, benzodiazepines, antidepressants, and mood-stabilizing drugs.11,38 On CNS Vital Signs, normals perform better than patients with unipolar depression, who, in turn, perform better than patients with bipolar disorder.39 It also appears to differentiate between mild cognitive impairment and dementia.40
Targeted Neuropsychological Tests
An alternative to computerizing a battery of tests covering multiple domains is to develop a program that has just one test or that has several tests that speak to a single domain. This approach has a distinct advantage: an individual tester can choose a test that is pertinent to the particular area in which he or she specializes. Clinics where a large number of ADHD patients are seen, for example, will usually have one of the computerized tests of sustained attention available. Geriatric clinics may be interested in a dementia screening battery or a fitness-to-drive test. Some neuropsychologists use CNTs to complement conventional batteries.
The number of individual computerized tests that is available at any one time is constantly changing. Some tests developed for the Mac never succeeded commercially, because most clinicians use PCs. Some tests developed for DOS never made the transition to Windows. Some were marketed for a while, and then were dropped by the publisher. In general, the tests that are currently available cover these areas: attention (ADHD), memory, and executive function.
Tests of vigilance or sustained attention are the most popular computerized tests because they are useful for evaluating ADHD, a cognitive disorder that afflicts perhaps five percent of the population. No one has ever maintained that a computerized test is sufficient for establishing the diagnosis of attention deficit disorder, but one can argue that it is inappropriate to make the diagnosis of ADHD without using at least one such test.
The continuous performance test is a venerable test of vigilance or sustained attention. Versions of the CPT have been used in research with brain injured patients, epileptics, and ADHD children for 40 years. It is an easy test to computerize; in fact, the only way one can administer the test, these days, is on a computer. Several free-standing CPTs are commercially available at this time. The most popular are the Conners CPT and the TOVA (Tests of Variables of Attention).
CPTs are usually marketed for ADHD evaluation. As screening instruments, their sensitivity and specificity is difficult to measure, since there is no “gold standard” for the diagnosis of ADD. They are clearly useful as adjuncts to clinical diagnosis, as their popularity attests. But they are not really diagnostic instruments. Like most of the components of PABs, the CPT only measures the subjects' performance at a point in time. Serial administration, for example, on and off an ADD medication, is more meaningful than a single-test administration.
There are also computerized tests of memory (e.g., Psychologix, the Brockway Memory Assessment Battery (BMAB), and of executive control functions. The classic neuropsychological tests of executive function are the Wisconsin Card Sort Test,41 Halstead Categories,42 and the Stroop Test (there are several computerized Stroops). Neuropsychologists employ these tests to complement conventional batteries, or for special research applications.
CNTs in Psychiatry Practice
CNTs are capable of improving the accuracy and the efficiency of neurocognitive evaluation. As screening batteries, they are as good as, or better than, conventional neuropsychological tests. They are not diagnostic instruments; they are very sensitive to mild cognitive dysfunction, but they are not very specific. They simply generate data that require the physician to undertake a differential diagnosis.
Practically, CNT requires a small room with a table for the computer and the printer and two chairs (some patients need supervision or help - usually, a family member can do that). An active Internet connection may or not be necessary, depending on the requirements of the software. Patients can be scheduled for test sessions, and psychiatrists can bill for testing using existing ICD-9 codes.
The authors developed a CNTB and, our data indicate the usefulness of computerized testing for various purposes, including the following:
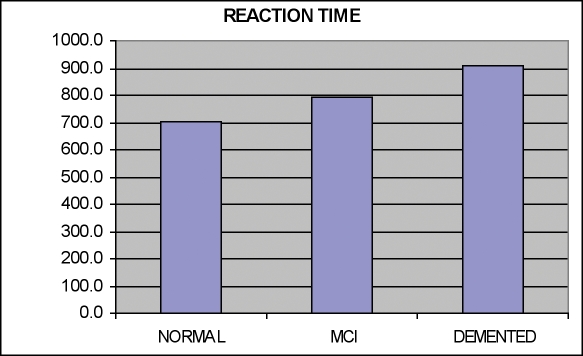
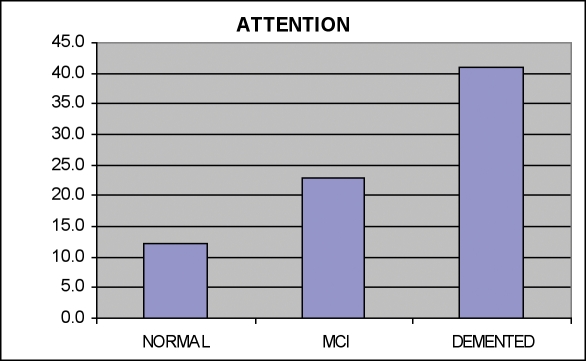
Mild cognitive impairment and dementia. It is increasingly important to detect the earliest stages of dementing conditions. CNTBs are ideally suited for large-scale dementia screening. Figures 1 and 2 indicate data from the CNS Vital Signs battery in 137 normal controls (mean age 60), 21 patients with mild cognitive impairment (age 57), and 25 patients with dementia (age 68).
Figure 1.
Reaction Times in Normals; Patients with MCI and Dementia
Figure 2.
Attentional Errors in Normals; Patients with MCI and Dementia
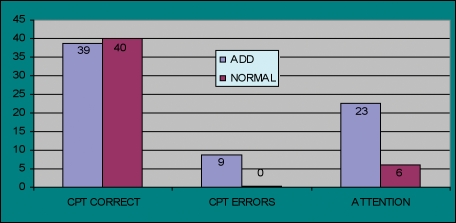
Attention deficit disorder. The Vital Signs battery was administered to ADD patients age 7 to 18 in the drug-free condition (n=111) compared to 80 normal children and adolescents. The ADD patients showed impairment in attention, reaction time, psychomotor speed, attention and cognitive flexibility. These deficits were normalized in patients on therapeutic doses of psychostimulants. The exception was the domain of memory, where significant deficits persisted, in spite of successful treatment (Figure 3). Controls make fewer errors, and have lower attention domain scores (lower is better).
Figure 3.
CPT Scores: Children with ADD and Normal Controls
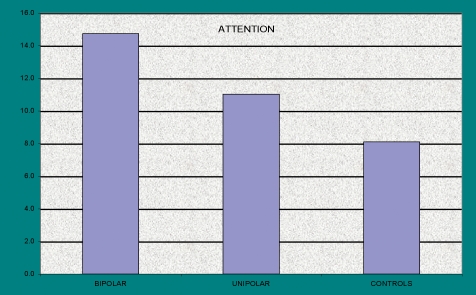
Depression and bipolar disorder. Bipolar depression is usually associated with complaints of cognitive dysfunction: poor memory, inattention, problems with planning, initiation and perseverance. While it is true that all forms of depression can be associated with cognitive complaints, they are thought to occur more commonly, and with greater severity, in patients with bipolar depression, compared to unipolar depressives.
We tested this widely-held belief in a study of patients with bipolar depression (n=64), compared to an age/gender/race matched comparison group of unipolar depressives (n=68) and to normal controls (n=363) (Age 18–60) (Figure 4). All patients were clinically stable on therapeutic regimes and free of significant co-morbidity. The results indicated that normal subjects performed significantly better than patients, and that unipolar depressives performed better than bipolars, on tests of attention, reaction time, psychomotor speed and cognitive flexibility. The differences in tests of visual and verbal memory obeyed the same pattern, but were not significant.
Figure 4.
Attention in Patients with Unipolar and Bipolar Depression compared to Normal Controls
Medication Evaluation
Antidepressant drugs, even the modern drugs, are known to affect cognition. The effects vary, depending on treatment-related and patient-related variables; they may be positive or negative. Because antidepressants are often prescribed to patients over the long-term, and because they are often prescribed to patients who are cognitively vulnerable (e.g., children, the elderly), it would be useful to have a way to track cognitive performance in patients taking antidepressants.
Table 1 shows data from a cross sectional study of 299 adult patients with major depression, followed in an outpatient neuropsychiatry clinic. All had responded favorably to antidepressant treatment and were on stable therapeutic doses.
Table 1.
Two-Hundred ninety-nine patients on antidepressants
| Medication | N | Age |
|---|---|---|
| Citalopram | 32.0 | 39.4 |
| Sertraline | 51.0 | 32.5 |
| Bupropion | 54.0 | 36.2 |
| Trazodone | 35.0 | 38.3 |
| Fluoxetine | 40.0 | 38.7 |
| Paroxetine | 40.0 | 34.3 |
| Venlafaxine | 470 | 37.6 |
| Untreated | 22.0 | 34.7 |
| Normals | 393.0 | 36.1 |
All patients were administered the CNS Vital Signs battery under optimal clinical conditions. Their results were compared to those of 22 matched controls who were depressed, but on no medication at all, and 393 normal subjects, matched for age, race, and gender.
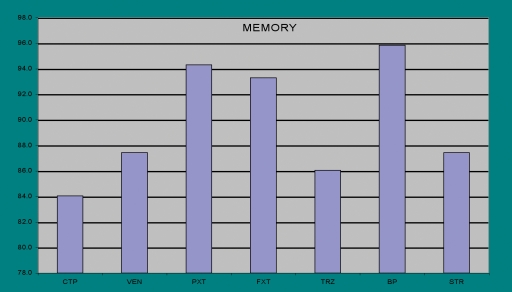
The results were analyzed parametrically, where trends were apparent, and non-parametrically, where highly significant results were attained. The test battery generated 31 cognitive measures. Bupropion scored best on 21 measures and second best on 7. Venlafaxine scored best on 4 and second best on 4. Citalopram and sertraline were superior to the other SSRIs and trazodone scored lowest on most measures. The positive cognitive effects of bupropion were most apparent on tests of memory, reaction time and information processing (Figure 5).
Figure 5.
Medication Effects on Memory
The noradrenergic antidepressant, bupropion, was clearly superior to other modern antidepressants in terms of its neurocognitive effects. The clinical salience of this effect is likely to be felt more strongly by patients who are cognitively impaired, or who rely on mental sharpness for their livelihood.
Which CNT to Choose?
The CNT that is best for your practice depends on the kind of practice you have and for what you want to use the test. Psychiatrists who specialize in ADD may simply elect to use one of the free-standing CPTs. General psychiatrists would probably prefer a CNTB that covers a wider range of domains. Medication effects, for example, and early dementia and cognitive deterioration associated with mental illness are not reliably manifest in any single cognitive domain. If one is using a screening battery for these indications, one should use a broad-spectrum test battery that measures sustained and complex attention, visual and verbal memory, psychomotor speed, reaction time and information processing speed, and executive control functions. The only test batteries currently available that meet these specifications (more or less) are the ANAM, the NES3, CogState and CNS Vital Signs. Only the last two have reliable commercial support.
Price is an important issue for clinicians to consider and includes the initial investment in equipment and software and the ongoing cost of test administration. Some tests require an active internet connection and are scored over the net; reports are e-mailed to the provider's office. Most practitioners seem to prefer tests that do not require a hot connection and that print out reports immediately. The time requirement ranges from 15 minutes for targeted batteries to more than an hour for some of the more elaborate jobs.
CNT can increase the productivity of psychiatrists, psychologists, and other professionals. It may be in some circles controversial, and that is understandable. Like every technology, it has its limitations. Like every machine that threatens the livelihood of skilled workers, it is open to derogation. But CNT is no more likely to supplant neuropsychologists than CAT scans supplanted neurologists. CNT is a tool with the potential to increase productivity, efficiency, and knowledge.
Summary
Cognitive assessment is a necessary part of the psychiatric evaluation. It is indicated, obviously, for patients who present with cognitive complaints; the two most common cognitive disorders, in psychiatry, are ADD and early dementia. But patients with affective disorders, psychotic disorders, personality disorders, and substance-abuse disorders (past or present) are also at high risk to be cognitively impaired.
Cognitive assessment ought to be a routine part of every initial psychiatric evaluation. Ideally, patients with chronic mental disorder should be re-evaluated at yearly intervals; patients treated long-term with psychoactive drugs ought to be evaluated at baseline, and then regularly, for example at 6- to 12-month intervals. The low cost of computerized assessment puts this ideal within reach.
Sources of Support
This research was supported by North Carolina Neuropsychia-try, PA. No support was sought or received from outside sources, including pharmaceutical manufacturers.
Disclosure
Dr. Gualtieri is one of the developers of CNS Vital Signs, and a partner in CNS Vital Signs LLC.
References
- 1. Kane RL, Kay GG. Computerized assessment in neuropsychology: A review of tests and test batteries. Neuropsychol Rev. 1992;3:1–117. doi: 10.1007/BF01108787. [DOI] [PubMed] [Google Scholar]
- 2. Gur RC, Ragland JD, Moberg PJ, et al. Computerized neurocognitive scanning: I. Methodology and validation in healthy people. Neuropsychopharmacology. 2001;25:766–76. doi: 10.1016/S0893-133X(01)00278-0. [DOI] [PubMed] [Google Scholar]
- 3. Mathisen KS, Evans FJ, Meyers K. Evaluation of a computerized version of the Diagnostic Interview Schedule. Hosp Community Psychiatry. 1987;38:1311–5. doi: 10.1176/ps.38.12.1311. [DOI] [PubMed] [Google Scholar]
- 4. Chen HY, Luo HC, Phillips MR. Computerized psychiatric diagnoses based on euclidean distances: a Chinese example. Acta Psychiatrica Scandinavica. 1992;85:11–14. doi: 10.1111/j.1600-0447.1992.tb01434.x. [DOI] [PubMed] [Google Scholar]
- 5. Gray GV, Glazer WM. Psychiatric decision making in the 90s: The coming era of decision support. Behavioral Healthcare Tomorrow. 1994;3:47–54. [PubMed] [Google Scholar]
- 6.Hauan MJ.JADE: Computerization of a structured interview for childhood psychiatric diagnosis. Proceedings of the AMIA Symposium. Washington, DC,1999:276-80. [PMC free article] [PubMed]
- 7. Bendtsen P, Timpka T. Acceptability of computerized self-report of alcohol habits: A patient perspective. Alcohol. 1999;34:575–80. doi: 10.1093/alcalc/34.4.575. [DOI] [PubMed] [Google Scholar]
- 8. Weber B, Fritze J, Schneider B, et al. Computerized self-assessment in psychiatric in-patients: Acceptability, feasibility, and influence of computer attitude. Acta Psychiatrica Scandanavica. 1998;98:140–5. doi: 10.1111/j.1600-0447.1998.tb10056.x. [DOI] [PubMed] [Google Scholar]
- 9.Gualtieri CT, Johnson LG, Bendict KB.Psychometric and Clinical properties of a new, computerized neurocognitive screening battery. American Neuropsychiatric Association Annual Meeting. Florida: 2004.
- 10. Stip E, Lussier I, Lalonde P, Luyet A, Fabian J. [Atypical neuroleptics and selective attention] Encephale. 1999;25:260–4. [PubMed] [Google Scholar]
- 11.Gualtieri CT, Johnson LG, Benedict KB.Drug sensitivity of a computerized neurocognitive test battery. INS Annual Meeting, Baltimore MD: 2004.
- 12. Aharonovich E, Nunes E, Hasin D. Cognitive impairment, retention, and abstinence among cocaine abusers in cognitive-behavioral treatment. Drug Alcohol Depend. 2003;71:207–11. doi: 10.1016/s0376-8716(03)00092-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Proudfoot J, Goldberg D, Mann A, et al. Computerized, interactive, multimedia cognitive-behavioural program for anxiety and depression in general practice. Psychological Med. 2003;33:217–27. doi: 10.1017/s0033291702007225. [DOI] [PubMed] [Google Scholar]
- 14. Robbins TW, James M, Owen AM, et al. A study of performance on tests from the CANTAB battery sensitive to frontal lobe dysfunction in a large sample of normal volunteers: Implications for theories of executive functioning and cognitive aging.Cambridge Neuropsychological Test Automated Battery. J Int Neuropsychol Soc. 1998;4:474–90. doi: 10.1017/s1355617798455073. [DOI] [PubMed] [Google Scholar]
- 15. Louis WJ, Mander AG, Dawson M, et al. Use of computerized neuropsychological tests (CANTAB) to assess cognitive effects of antihypertensive drugs in the elderly. Cambridge Neuropsychological Test Automated Battery. J Hypertension. 1999;17:1813–9. doi: 10.1097/00004872-199917121-00005. [DOI] [PubMed] [Google Scholar]
- 16. Wesnes K. Assessing cognitive function in clinical trials: Latest developments and future directions. Drug Discovery Today. 2002;7:29–35. doi: 10.1016/s1359-6446(01)02068-2. [DOI] [PubMed] [Google Scholar]
- 17. Fearn SJ, Pole R, Wesnes K, et al. Cerebral injury during cardiopulmonary bypass: emboli impair memory. J Thoracic Cardiovasc Surg. 2001;121:1150–60. doi: 10.1067/mtc.2001.114099. [DOI] [PubMed] [Google Scholar]
- 18. Preece AW, Iwi G, Davies-Smith A, et al. Effect of a 915-MHz simulated mobile phone signal on cognitive function in man. Int J Radiation Biol. 1999;75:447–56. doi: 10.1080/095530099140375. [DOI] [PubMed] [Google Scholar]
- 19. Walker MP, Ayre GA, Perry EK, et al. Quantification and characterization of fluctuating cognition in dementia with Lewy bodies and Alzheimer's disease. Dementia Geriatric Cognitive Disorders. 2000;11:327–35. doi: 10.1159/000017262. [DOI] [PubMed] [Google Scholar]
- 20. Harrington F, Saxby BK, McKeith IG, et al. Cognitive performance in hypertensive and normotensive older subjects. Hypertension. 2000;36:1079–82. doi: 10.1161/01.hyp.36.6.1079. [DOI] [PubMed] [Google Scholar]
- 21. Ritchie KA, Macdonald EB, Hammersley R, et al. A pilot study of the effect of low level exposure to mercury on the health of dental surgeons. Occupational Environmental Med. 1995;52:813–7. doi: 10.1136/oem.52.12.813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Ferris SH, Lucca U, Mohs R, et al. Objective psychometric tests in clinical trials of dementia drugs. Position paper from the International Working Group on Harmonization of Dementia Drug Guidelines. Alzheimer's Disease and Associated Disorders. 1997;11:34–8. [PubMed] [Google Scholar]
- 23. Parrott AC, Lees A, Garnham NJ, et al. Cognitive performance in recreational users of MDMA or 'ecstasy': Evidence for memory deficits. J Psychopharmacol. 1998;12:79–83. doi: 10.1177/026988119801200110. [DOI] [PubMed] [Google Scholar]
- 24. Beuzen JN, Taylor N, Wesnes K, Wood A. A comparison of the effects of olanzapine, haloperidol, and placebo on cognitive and psychomotor functions in healthy elderly volunteers. J Psychopharmacol. 1999;13:152–8. doi: 10.1177/026988119901300207. [DOI] [PubMed] [Google Scholar]
- 25. Neave N, Reid C, Scholey AB, et al. Dose-dependent effects of flumazenil on cognition, mood, and cardiorespiratory physiology in healthy volunteers. Br Dental J. 2000;189:668–74. doi: 10.1038/sj.bdj.4800860. [DOI] [PubMed] [Google Scholar]
- 26. O'Neill WM, Hanks GW, Simpson P, et al. The cognitive and psychomotor effects of morphine in healthy subjects: A randomized controlled trial of repeated (four) oral doses of dextropropoxyphene, morphine, lorazepam, and placebo. Pain. 2000;85:209–15. doi: 10.1016/s0304-3959(99)00274-2. [DOI] [PubMed] [Google Scholar]
- 27. Wesnes KA, Ward T, McGinty A, Petrini O. The memory enhancing effects of a Ginkgo biloba/Panax ginseng combination in healthy middle-aged volunteers. Psychopharmacology. 2000;152:353–61. doi: 10.1007/s002130000533. [DOI] [PubMed] [Google Scholar]
- 28. Mohr E, Walker D, Randolph C, et al. Utility of clinical trial batteries in the measurement of Alzheimer's and Huntington's dementia. Int Psychogeriatr. 1996;8:397–411. doi: 10.1017/s1041610296002761. [DOI] [PubMed] [Google Scholar]
- 29. Nicholl C, Lynch S, Kelly C, et al. The cognitive drug research assessment system in the evaluation of early dementia: is speed of the essence? Int J Geriatr Psychiatry. 1995;10:206. [Google Scholar]
- 30. Alpherts WCJ, Aldenkamp AP. Computerized neuropsychological assessment of cognitive functioning in children with epilepsy. Epilespsia. 1990;31(Suppl 4):S35–S40. doi: 10.1111/j.1528-1157.1990.tb05868.x. [DOI] [PubMed] [Google Scholar]
- 31. Vermeulen J, Aldenkamp AP, Alpherts WCJ. Memory complaints in epilepsy: Correlations with cognitive performance and neuroticism. Epilepsy Res. 1993;15:170. doi: 10.1016/0920-1211(93)90096-p. [DOI] [PubMed] [Google Scholar]
- 32. Aldenkamp AP, Mulder OG, Overweg J. Cognitive effects of Lamotrigine as first-line Add-on in patients with localization-related (partial) epilepsy. J Epilepsy. 1997;10:117–21. [Google Scholar]
- 33. Gur RC, Ragland JD, Moberg PJ, et al. Computerized neurocognitive scanning: II The profile of schizophrenia. Neuropsychopharmacology. 2001;25:777–88. doi: 10.1016/S0893-133X(01)00279-2. [DOI] [PubMed] [Google Scholar]
- 34. Powell J, Pickering A, Wyke M, Goggin T. The effects of anti-hypertensive medication on learning and memory. Br J Clin Pharmacol. 1993;35:105–13. doi: 10.1111/j.1365-2125.1993.tb05675.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Elwood RW. MicroCog: Assessment of cognitive functioning. Neuropsychol Rev. 2001;11:89–100. doi: 10.1023/a:1016671201211. [DOI] [PubMed] [Google Scholar]
- 36. Di Sclafani V, Tolou-Shams M, Price LJ, Fein G. Neuropsychological performance of individuals dependent on crack-cocaine or crack-cocaine and alcohol at 6 weeks and 6 months of abstinence. Drug Alcohol Dependence. 2002;66:161–71. doi: 10.1016/s0376-8716(01)00197-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Lopez SJ, Edwards LM, Floyd RK, et al. Note on comparability of MicroCog test forms. Perceptual Motor Skills. 2001;93:825–8. doi: 10.2466/pms.2001.93.3.825. [DOI] [PubMed] [Google Scholar]
- 38.Gualtieri CT, Johnson LG, Benedict KB. The comparative neurocognitive effects of seven antidepressents. New York, NY: APA Annual Meeting, 2004.
- 39.Gualtieri CT, Johnson LG, Benedict KB. Complex attention in unipolar and bipolar depression. Baltimore, MD: INS Annual Meeting, 2004.
- 40.Gualtieri CT, Johnson LG. A computerized cognitive screening battery that distinguishes mild cognitive impairment from dementia. Philadelphia, PA: Alzheimer's Association National Conference, 2004.
- 41. Tien AY, Eaton WW, Schlaepfer TE, et al. Exploratory factor analysis of MRI brain structure measures in schizophrenia. Schizophrenia Res. 1996;19:93–101. doi: 10.1016/0920-9964(96)88520-3. [DOI] [PubMed] [Google Scholar]
- 42. Berger SG, Chibnall JT, Gfeller JD. Construct validity of the computerized version of the Category Test. J Clin Psychol. 1997;53:723–6. doi: 10.1002/(sici)1097-4679(199711)53:7<723::aid-jclp9>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]