Abstract
OBJECTIVE: To compare the Model for End-Stage Liver Disease (MELD) with the modified model including sodium (MELDNa) for predicting 180-day mortality in patients with alcoholic hepatitis (AH) and determine the subset in whom serum sodium may enhance 180-day mortality prediction.
PATIENTS AND METHODS: We examined 26 patients with AH enrolled in a prospective trial between June 1, 2004, and June 30, 2007, at Mayo Clinic. Logistic regression analysis was done to assess the effect of MELD and MELDNa scores on 180-day mortality. The C statistic was derived to compare MELD with MELDNa in patients with and without ascites.
RESULTS: MELD (odds ratio [OR], 1.22; 95% confidence interval [CI], 1.05-1.47; P=.007; C statistic, 0.81) and MELDNa (OR, 1.24; 95% CI, 1.05-1.56; P=.008; C statistic, 0.78) were significant predictors of 180-day mortality in patients with AH. A MELD score of 27.0 and a MELDNa score of 28.0 had sensitivity of 76.5% and 87.5% and specificity of 64.9% and 52.5%, respectively. In patients with AH and ascites, MELDNa (OR, 2.27; 95% CI, 1.22-36.68; P=.008; C statistic, 0.97) was a better predictor of 180-day mortality than MELD (OR, 1.37; 95% CI, 1.07-2.12; P=.006; C statistic, 0.90). A MELD score of 29.0 and a MELDNa score of 34.0 had sensitivity of 85.7% and 83.3% and specificity of 31.0% and 16.7%, respectively.
CONCLUSION: MELD and MELDNa were similar predictors of 180-day mortality; however, MELDNa was a better predictor of mortality than MELD in patients with ascites. Hyponatremia in patients with AH without ascites is not a predictor of mortality because it may have a dilutional basis secondary to excessive intake of low-osmolar alcohol.
Although the Model for End-Stage Liver Disease (MELD) and MELDNa, a modification including sodium, similarly predicted 180-day mortality, MELDNa was a better predictor of mortality in patients with ascites.
AH = alcoholic hepatitis; ALD = alcoholic liver disease; CI = confidence interval; MELD = Model for End-Stage Liver Disease; Na = sodium; OR = odds ratio
Excessive alcohol consumption is a major source of chronic liver disease in the Western world.1 Alcoholic liver disease (ALD) encompasses a broad spectrum of conditions, including fatty liver, alcoholic hepatitis (AH), and cirrhosis. Fatty liver is reversible with alcohol cessation, but patients with AH and cirrhosis have a potentially more severe prognosis. Determining mortality risk in patients with ALD is important to guide treatment decisions. In this regard, mortality risk in patients with AH can be predicted by various models, including the Model for End-Stage Liver Disease (MELD) score.2-7
Hyponatremia is a sign of excessive vasopressin secretion and is predictive of poor survival in patients with cirrhosis.8 MELDNa, a modified MELD score that includes serum sodium (Na), has been shown to improve prediction of death in patients with cirrhosis when compared with MELD alone. MELDNa has been proposed as an alternative to MELD in other clinical scenarios as well, including transjugular intrahepatic portosystemic shunt placement.9 Furthermore, studies have shown that a reduced serum Na concentration may have a greater effect on mortality in patients with a low MELD score.10,11 However, the role of MELDNa in predicting survival in patients with AH has not been studied. Although a predictor of mortality in patients with cirrhosis, hyponatremia can be present even without cirrhosis in those addicted to alcohol, occurring on a dilutional basis because they consume excessive alcoholic beverages that are low in osmolarity (potomania).12 Determining the group of patients in whom the MELDNa score might improve mortality prediction beyond that achieved by MELD alone is important for predicting patient prognosis and assessing need for pharmacological interventions.
We hypothesized that MELD and MELDNa would be significant predictors of 180-day mortality in patients with AH. We also hypothesized that MELDNa might enhance 180-day mortality prediction in patients with AH and ascites because ascites reflects excessive activation of the renin-angiotensin system and vasopressin production.13 To study these hypotheses, we examined a well-described cohort of patients with AH to (1) compare the ability of MELD and MELDNa to predict 180-day mortality, (2) define the MELD and MELDNa score cut point with optimal sensitivity and specificity to predict mortality, and (3) determine the subset of patients in whom hyponatremia may enhance 180-day mortality prediction.
PATIENTS AND METHODS
Case Ascertainment
We included all patients enrolled between June 1, 2004, and June 30, 2007, at Mayo Clinic in Rochester, MN, in a multicenter trial of etanercept in the treatment of AH.14 This ancillary study was approved by the Mayo Clinic Institutional Review Board. Alcoholic hepatitis was diagnosed on the basis of clinical criteria that included jaundice, hepatomegaly, leukocytosis, fever, and elevations in transaminase levels, as well as exclusion of other causes of hepatitis, including viral (negative tests for hepatitis B surface antigen and hepatitis C virus antibody), autoimmune (antinuclear antibody titer <1:40, negative tests for antimitochondrial antibody and anti–smooth muscle antibody), drugs, or metabolic disorders (normal ceruloplasmin levels), in the setting of substantial alcohol consumption. Substantial alcohol consumption was defined as more than 40 g/d for a minimum of 6 months within 3 months of study enrollment. If the diagnosis of AH was uncertain, liver biopsy for histologic evidence of AH was performed. Enrollment required patients to have moderate to severe disease as evidenced by a MELD score of 15 or more.14
Statistical Analyses
All data used for analysis were collected on the first day of enrollment in the study. Baseline characteristics were compared by using the χ2 test for categorical data and the Mann-Whitney U test for continuous data. The MELD and MELDNa scores were calculated in all groups according to the following formulas:
MELD = 9.57 loge [Creatinine (mg/dL)] + 3.78 loge [Bilirubin (mg/dL)] + 11.20 loge [International Normalized Ratio] + 6.43 (to convert creatinine values to μmol/L, multiply by 88.4; to convert bilirubin to μmol/L, multiply by 17.104)5
MELDNa = MELD – Na – [0.025 × MELD × (140 – Na)] + 140 (where the serum Na concentration is bound between 125 and 140 mmol/L; to convert serum Na values to mmol/L, multiply by 1)8
The primary outcome was patient death at 180 days. For patients who were lost to 180-day follow-up, the mortality status and date of death were obtained from the Social Security Death Index. Three patients did not have serum Na data, and these patients were excluded from the MELDNa analysis. The independent effect of MELD and MELDNa scores on mortality was determined in univariate analysis as well as bivariate logistic regression analysis after adjusting for age and sex. Given that creatinine is 1 of the 3 components of the MELD score, the independent effect of MELD or MELDNa scores after adjustment for renal function could not be assessed because of the collinearity between MELD and serum creatinine level. C statistics were used to quantitatively compare MELD with MELDNa to predict 180-day mortality in patients with AH. The independent effect of serum Na on predicting mortality was determined after adjusting for the MELD score at baseline. Cut points with the optimal sensitivity and specificity were generated by plotting receiver operating characteristic curves. Given that serum Na may have different implications in patients with ascites than in those without ascites, we further compared the predictive ability of serum Na and MELDNa in patients with AH and coexisting ascites vs patients with AH without coexisting ascites.
RESULTS
Study Cohort
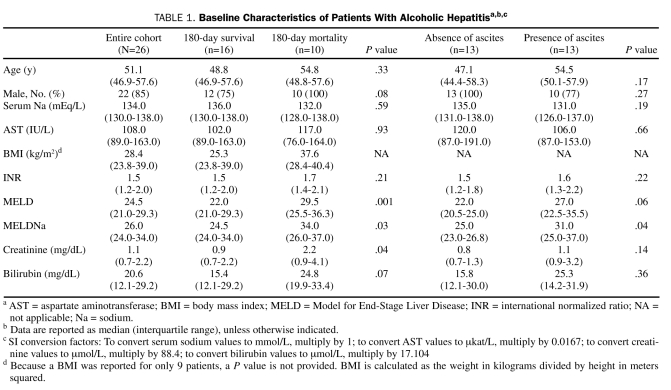
Overall, 26 patients with AH were analyzed. Table 1 shows the baseline characteristics of the total patient cohort, including both survivors and nonsurvivors. The median age was 51.1 years, and 85% of patients were male. The median MELD and MELDNa scores were 24.5 and 26.0, respectively.
TABLE 1.
Baseline Characteristics of Patients With Alcoholic Hepatitisa,b,c
Of the 26 patients with AH, 10 died within 6 months. The patients who died were older (median age, 54.8 years vs 48.8 years; P=.33) and all male (10 vs 0; P=.08). The P values were not significant. The median MELD score (29.5 vs 22.0; P=.001) and MELDNa score (34.0 vs 24.5; P=.03) were higher in patients who died within 6 months. The median creatinine level was also higher (2.2 mg/dL vs 0.85 mg/dL; P=.04) in patients who died within 6 months.
MELD and MELDNa Analysis
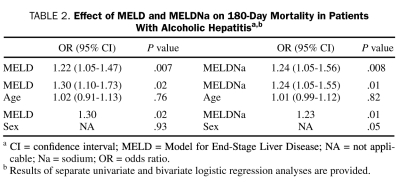
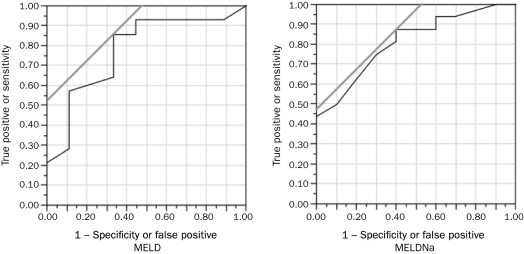
Both the MELD score (odds ratio [OR], 1.22 for every 1-point increase in MELD; 95% confidence interval [CI], 1.05-1.47; P=.007) and MELDNa score (OR, 1.24 for every 1-point increase in MELDNa; 95% CI, 1.05-1.56; P=.008) were significant predictors of 180-day mortality. The effect of MELD and MELDNa remained unchanged and statistically significant after adjustment for either age or sex (Table 2). The C statistic for 180-day mortality was similar: the C statistic was 0.81 for MELD and 0.78 for MELDNa (Figure 1). After adjustment for MELD, serum Na was not a significant predictor of mortality (P=.83). A MELD score of 27.0 and a MELDNa score of 28.0 had sensitivity of 76.5% and 87.5% and specificity of 64.9% and 52.5%, respectively, in predicting 180-day mortality (Figure 1).
TABLE 2.
Effect of MELD and MELDNa on 180-Day Mortality in Patients With Alcoholic Hepatitisa,b
FIGURE 1.
MELD (left) and MELDNa (right) as predictors of 180-day mortality in patients with alcoholic hepatitis. For MELD: ROC, 0.813; OR, 1.22; P=.007. For MELDNa: ROC, 0.778; OR, 1.24; P=.008. MELD = Model for End-Stage Liver Disease; Na = sodium; OR = odds ratio; ROC = receiver operating characteristics.
MELD and MELDNa in Patients With AH and Ascites
Because we hypothesized that MELDNa may play a greater role in predicting 180-day mortality in patients with ascites,11 we further separated the AH patients into 2 groups: those with ascites (n=13) and those without ascites (n=13). Overall, patients with ascites were older (median age, 54.5 years vs 47.1 years; P=.17), had a higher serum creatinine level (1.1 mg/dL vs 0.8 mg/dL; P=.14), and had a higher bilirubin level (25.3 mg/dL vs 15.8 mg/dL; P=.36). The P values were not significant. The median MELD score (27.0 vs 22.0; P=.06) and MELDNa score (31.0 vs 25.0; P=.04) were higher in patients with ascites.
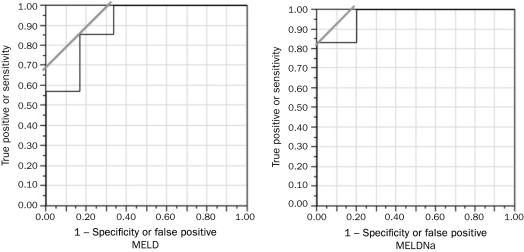
MELDNa achieved better predictability of 180-day mortality in the cohort of patients with AH and ascites (OR, 1.37 for every 1-point increase in MELD; 95% CI, 1.07-2.12; P=.006; OR, 2.27 for every 1-point increase in MELDNa; 95% CI, 1.22-36.68; P=.008). Bivariate logistic regression analysis was not possible because of the low number of events. For 180-day mortality, the C statistic was 0.90 for MELD and 0.97 for MELDNa (Figure 2). A MELD score of 29.0 and a MELDNa score of 34.0 had sensitivity of 85.7% and 83.3% and specificity of 31.0% and 16.7%, respectively (Figure 2).
FIGURE 2.
MELD (left) and MELDNa (right) as predictors of 180-day mortality in patients with alcoholic hepatitis and ascites. For MELD: ROC, 0.904; OR, 1.37; P=.006. For MELDNa: ROC, 0.967; OR, 2.28; P=.008. MELD = Model for End-Stage Liver Disease; Na = sodium; OR = odds ratio; ROC = receiver operating characteristics.
DISCUSSION
Prediction of mortality in AH is important for determining patient management approaches, including pharmacological intervention. In a well-characterized cohort of patients with AH, both MELD and MELDNa were positive predictors of 180-day mortality. In the subgroup of patients with ascites, MELDNa may be a stronger predictor of 180-day mortality than MELD. Therefore, use of MELDNa in patients with AH who have ascites may allow for more accurate prognostication and assessment of treatment options. This is an important subgroup because our prior studies showed that prognostic scores were most clinically useful in patients with AH who had ascites and/or encephalopathy.6
In addition to MELD and MELDNa, other models have been used to predict the severity of ALD. The Maddrey discriminant function (Discriminant Function = 4.6 × [Prolongation of Prothrombin Time in seconds + Bilirubin in mg/dL]) was developed in 1978 to predict risk of mortality in patients with AH and continues to be widely used in clinical practice.15 Indeed, a discriminant function of 32 or more has been suggested to predict benefit from corticosteroid treatment, and this cut point has also been used for pentoxifylline treatment.16 The Glasgow alcoholic hepatitis score is another recently developed model that takes age, white blood cell count, blood urea nitrogen concentration, prothrombin time ratio, and serum bilirubin concentration into consideration.17 Although this model is easier to use at the bedside, its superiority for predicting mortality as compared with other models requires further evaluation. The Lille model is a newer model that predicts survival in patients with AH treated with corticosteroids by using a combination of 6 variables (age, renal function, albumin, prothrombin time, bilirubin, 7-day change in bilirubin).18 A Lille score of more than 0.45 after 1 week of corticosteroid therapy predicts a corticosteroid nonresponder and indicates need for alternative therapies.
Ascites is the most common complication of cirrhosis and increases the chance of infection, renal failure, and death.19 Numerous studies have shown that ascites is associated with an increased mortality rate in patients with cirrhosis.20,21 To understand the connection between MELDNa as a mortality predictor in patients with AH and ascites, we need to consider the physiology behind hyponatremia in these patients. The development of hyponatremia in cirrhotic patients has been linked to activation of the vasopressin system. Hypersecretion of vasopressin in turn is thought to occur because of circulatory dysfunction that is common in patients with cirrhosis, a concept commonly referred to as the underfilling hypothesis.22 This excessive volume overload in the absence of increased uptake of Na causes a hypo-osmolar state and is reflected as hyponatremia. Because the degree of hyponatremia reflects the degree of circulatory dysfunction, hyponatremia predicts survival in patients with cirrhosis.
Hyponatremia may also be present in many patients with long-term alcohol intake independent of liver cirrhosis and excessive vasopressin secretion. Potomania is a term used to describe the excessive low-osmolar fluid consumed by those addicted to alcohol. Potomania occurs in those addicted to alcohol owing to limited dietary protein and salt intake that results in a reduction in excretion of urinary solutes.12 This promotes retention of water, leading to dilutional hyponatremia. Thus, potomania may account for hyponatremia in patients with long-term alcohol consumption and differs from the mechanism responsible for hyponatremia in cirrhotic patients. Our data suggest that, although hyponatremia in patients with ascites (which results from excessive vasopressin secretion and circulatory abnormalities) influences prognosis in patients with AH, hyponatremia from potomania does not. These issues may have emerging importance with the recent approval of vasopressin-2 receptor antagonists, which improve hyponatremia.23
Of note, the MELD and MELDNa cut point values of 27.0 and 28.0 in our patient cohort were somewhat higher than in previous studies. For example, a study done by Sheth et al24 showed a MELD cutoff score of more than 11.0 for predicting 30-day mortality; however, this study used the original MELD derivation, which computed a lower MELD for liver disease severity compared with the more widely used UNOS (United Network of Organ Sharing) modification of MELD. Other studies have determined a MELD cutoff score of more than 21.0 to be predictive of 90-day mortality.6 Furthermore, Lucey et al25 showed that a MELD score of 21.0 or more was associated with a 90-day mortality of 20%. However, it may be difficult to directly compare MELD cut point scores between studies because of differences in the severity of AH in different patient cohorts. Thus, although MELD is useful for prognostication in patients with AH, the precise cut point that best predicts mortality remains variable in different patient cohorts.
This study has some limitations, including the relatively small size of our patient cohort. Therefore, our MELD and MELDNa receiver operating characteristic curves could be overestimates of values that might be derived from a larger group of patients. Further multivariate models adjusting for other predictors of mortality were not possible. However, previous studies have shown that MELD and MELDNa are independent predictors of short-term mortality.4,8 Thus, studies in larger patient cohorts will be necessary to extend the data set in the future. In addition, moderate to severe AH was present in our patient cohort, although the overall mortality rate of close to 50% reflects a group of patients with severe AH. Nonetheless, differences in disease severity may be a reason why our MELD and MELDNa cutoff scores are higher than in previously published articles.10 Finally, we were unable to determine the effect of MELD independent of renal function in this limited data set. Given that creatinine is 1 of the 3 components of the MELD score, the 2 variables are collinear, and hence a model with both variables (MELD and serum creatinine level) cannot be interpreted. Because of multicollinearity, the 2 variables outcompete each other. Serum creatinine level is a poor marker of renal function in persons with end-stage liver disease and underestimates the degree of renal dysfunction. Serum Na adds to a model predicting short-term mortality by accounting for early renal dysfunction not captured by serum creatinine level. Hence, MELDNa has been proposed to be a better predictor of short-term mortality in persons with liver disease because it appears to capture the importance of renal function better than other objective parameters, in the absence of widely available measured glomerular filtration rate data.8 Similarly, in our study the effect of MELD and MELDNa in predicting mortality at 6 months in persons with AH persists, reflecting that a large part of the effect is driven by renal function that is captured by these objective scores.
CONCLUSION
This study showed that both MELD and MELDNa are accurate predictors of 180-day mortality in a well-characterized and prospectively collected cohort of patients with ALD. In patients with ascites and AH, MELDNa is a better predictor of 180-day mortality than MELD owing to hyponatremia occurring secondary to excessive vasopressin production. Further research into why MELDNa performs better in patients with ascites may help delineate mechanisms of Na dysfunction and circulatory imbalance in ALD, as well as better define indications for when to use MELDNa instead of MELD to predict mortality in these patients.
REFERENCES
- 1. Paula H, Asrani SK, Boetticher NC, Pedersen R, Shah VH, Kim WR. Alcoholic liver disease-related mortality in the United States: 1980-2003. Am J Gastroenterol. 2010;105(8):1782-1787 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Malinchoc M, Kamath P, Gordon FD, Peine CJ, Rank J, ter Borg PC. A model to predict poor survival in patients undergoing transjugular intrahepatic portosystemic shunts. Hepatology. 2000;31(4):864-871 [DOI] [PubMed] [Google Scholar]
- 3. Freeman RB, Jr, Wiesner RH, Harper A, et al. The new liver allocation system: moving toward evidence-based transplantation policy. Liver Transpl. 2002;8(9):851-858 [DOI] [PubMed] [Google Scholar]
- 4. Wiesner RH, McDiarmid SV, Kamath PS, et al. MELD and PELD: application of survival models to liver allocation. Liver Transpl. 2001;7(7):567-580 [DOI] [PubMed] [Google Scholar]
- 5. Kamath PS, Wiesner RH, Malinchoc M, et al. A model to predict survival in patients with end-stage liver disease. Hepatology. 2001;33(2):464-470 [DOI] [PubMed] [Google Scholar]
- 6. Dunn W, Jamil LH, Brown LS, et al. MELD accurately predicts mortality in patients with alcoholic hepatitis. Hepatology. 2005;41(2):353-358 [DOI] [PubMed] [Google Scholar]
- 7. Wiesner R, Edwards E, Freeman R, et al. United Network for Organ Sharing Liver Disease Severity Score Committee Model for End-stage Liver Disease (MELD) and allocation of donor livers. Gastroenterology. 2003;124(1):91-96 [DOI] [PubMed] [Google Scholar]
- 8. Kim WR, Biggins SW, Kremers WK, et al. Hyponatremia and mortality among patients on the liver-transplant waiting list. N Engl J Med. 2008;359(10):1018-1026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Guy J, Somsouk M, Shiboski S, Kerlan R, Inadomi JM, Biggins SW. New model for end stage liver disease improves prognostic capability after transjugular intrahepatic portosystemic shunt. Clin Gastroenterol Hepatol. 2009;7(11):1236-1240 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Heuman DM, Abou-Assi SG, Habib A, et al. Persistent ascites and low serum sodium identify patients with cirrhosis and low MELD scores who are at high risk for early death. Hepatology. 2004;40(4):802-810 [DOI] [PubMed] [Google Scholar]
- 11. Biggins SW, Kim WR, Terrault NA, et al. Evidence-based incorporation of serum sodium concentration into MELD. Gastroenterology. 2006;130(6):1652-1660 [DOI] [PubMed] [Google Scholar]
- 12. Liamis GL, Milionis HJ, Rizos EC, Siamopoulos KC, Elisaf MS. Mechanisms of hyponatraemia in alcohol patients. Alcohol Alcohol. 2000;35(6):612-616 [DOI] [PubMed] [Google Scholar]
- 13. Somsouk M, Guy J, Biggins SW, Vittinghoff E, Kohn MA, Inadomi JM. Ascites improves upon [corrected] serum sodium plus [corrected] model for end-stage liver disease (MELD) for predicting mortality in patients with advanced liver disease. Aliment Pharmacol Ther. 2009;30(7):741-748 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Boetticher NC, Peine CJ, Kwo P, et al. A randomized, double-blinded, placebo-controlled multicenter trial of etanercept in the treatment of alcoholic hepatitis. Gastroenterology. 2008;135(6):1953-1960 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Carithers RL, Jr, Herlong HF, Diehl AM, et al. Methylprednisolone therapy in patients with severe alcoholic hepatitis: a randomized multicenter trial. Ann Intern Med. 1989;110(9):685-690 [DOI] [PubMed] [Google Scholar]
- 16. Akriviadis E, Botla R, Briggs W, Han S, Reynolds T, Shakil O. Pentoxifylline improves short-term survival in severe acute alcoholic hepatitis: a double-blind, placebo-controlled trial. Gastroenterology. 2000;119:1637-1648 [DOI] [PubMed] [Google Scholar]
- 17. Forrest EH, Evans CD, Stewart S, et al. Analysis of factors predictive of mortality in alcoholic hepatitis and derivation and validation of the Glasgow alcoholic hepatitis score. Gut. 2005;54(8):1174-1179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Louvet A, Naveau S, Abdelnour M, et al. The Lille model: a new tool for therapeutic strategy in patients with severe alcoholic hepatitis treated with steroids. Hepatology. 2007;45(6):1348-1354 [DOI] [PubMed] [Google Scholar]
- 19. Gines P, Cardenas A, Arroyo V, Rodes J. Management of cirrhosis and ascites. N Engl J Med. 2004;350(16):1646-1654 [DOI] [PubMed] [Google Scholar]
- 20. Mackle IJ, Swann DG, Cook B. One year outcome of intensive care patients with decompensated alcoholic liver disease. Br J Anaesth. 2006;97(4):496-498 [DOI] [PubMed] [Google Scholar]
- 21. Pathak OK, Paudel R, Panta OB, Pant HP, Giri BR, Adhikari B. Retrospective study of the clinical profile and prognostic indicators in patients of alcoholic liver disease admitted to a tertiary care teaching hospital in Western Nepal. Saudi J Gastroenterol. 2009;15(3):171-175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Gines P, Guevara M. Hyponatremia in cirrhosis: pathogenesis, clinical significance, and management. Hepatology. 2008;48(3):1002-1010 [DOI] [PubMed] [Google Scholar]
- 23. Boyer TD. Tolvaptan and hyponatremia in a patient with cirrhosis. Hepatology. 2010;51(2):699-702 [DOI] [PubMed] [Google Scholar]
- 24. Sheth M, Riggs M, Patel T. Utility of the Mayo end-stage liver disease (MELD) score in assessing prognosis of patients with alcoholic hepatitis. BMC Gastroenterol. 2002;2:2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Lucey MR, Mathurin P, Morgan TR. Alcoholic hepatitis. N Engl J Med. 2009;360(26):2758-2769 [DOI] [PubMed] [Google Scholar]