Abstract
Stem cell niches are dynamic microenvironments that balance stem cell activity to maintain tissue homeostasis and repair throughout the lifetime of an organism. The development of strategies to monitor and perturb niche components has provided insight into the responsive nature of the niche and offers a framework to uncover how disruption of normal stem cell niche function may contribute to aging and disease onset and progression. Additional work in the identification of genetic factors that regulate the formation, activity, and size of stem cell niches will facilitate incorporation of the niche into stem cell-based therapies and regenerative medicine.
Stem cell niches are discrete and dynamic functional domains that influence stem cell behavior to govern tissue homeostasis under diverse physiological (development and aging) and pathological (injury and disease) conditions. The niche must be flexible in order to coordinate stem cell behavior with homeostasis and repair; however, the plasticity of a niche may be co-opted in cancer and chronic disease. Here, we review experimental data highlighting the relationships between stem cells and their niches, advances in imaging technologies that permit characterization of niches in vivo, and factors regulating niche involvement in tissue regeneration and cancer.
The Stem Cell Niche Hypothesis
In 1978, R. Schofield proposed that proliferative, hematopoietic cells derived from the spleen (spleen colony-forming cells, CFU-S) displayed decreased proliferative potential when compared to hematopoietic stem cells from the bone marrow because they were no longer in association with a complement of cells, a “niche,” which supports long-term stem cell activity (Schofield, 1978). This idea that specialized environments within tissues can preserve proliferative potential and block maturation of adult stem cells was the first description of the stem cell niche hypothesis. Implicit in this model is the prediction that removal of stem cells from the niche results in loss of stem cell identity, self-renewal capacity, and the onset of differentiation. As such, the niche would provide a mechanism to precisely balance the production of stem cells and progenitor cells to maintain tissue homeostasis. Therefore, a stem cell niche is not defined solely by the presence of stem cells but also by the ability to regulate stem cell behavior.
Characterization of somatic support cells that produce factors necessary for the maintenance of germline stem cells (GSCs) in C. elegans and Drosophila provided examples of discrete “niches” (Kiger et al., 2001; Kimble and White, 1981; Tulina and Matunis, 2001; Xie and Spradling, 2000) and, consequently, paradigms for the identification and characterization of stem cell niches in vertebrates. Development of functional assays to verify stem cell identity, characterize niche support cells, and technologies to visualize stem cell-niche cell interactions in vivo have enabled a better understanding of how stem cell niche dynamics are regulated in physiological and pathological processes.
Strategies to Identify Stem Cells and Putative Niches
Lineage Tracing
Lineage-tracing techniques and serial transplantation assays have confirmed the presence of stem cell populations in many tissues. Consequently, these methods have also aided in characterizing putative niches. In Drosophila, clonal analysis relies upon mitotic recombination to initiate marker expression in a random mitotic cell and all of its subsequent daughter cells (Harrison and Perrimon, 1993; Lee and Luo, 1999). This method has been used to identify a number of stem cell populations in tissues as diverse as the nervous system, gonads, and digestive tract (Figure 1) (Decotto and Spradling, 2005; Fox and Spradling, 2009; Gönczy and DiNardo, 1996; Margolis and Spradling, 1995; Micchelli and Perrimon, 2006; Nystul and Spradling, 2007; Ohlstein and Spradling, 2006; Singh et al., 2007).
Figure 1. Lineage Tracing Strategies Permit Identification of Stem Cell Location and Daughter Cell Fate.
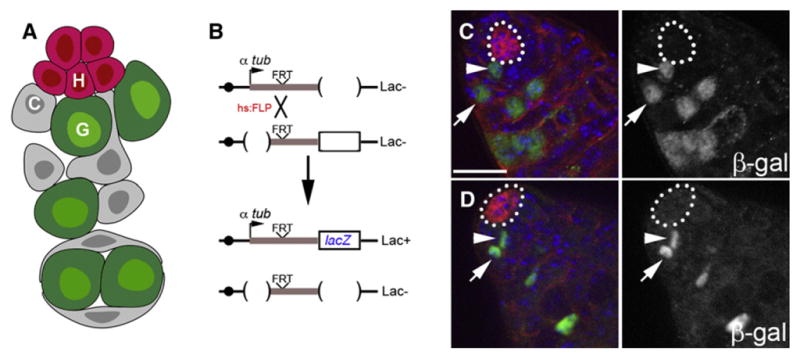
(A) Schematic of the stem cell niche in the Drosophila testis. Germline (G) and somatic cyst (C) stem cells are in direct physical contact with hub cells (H).
(B) Lineage tracing analysis utilizing an inducible recombination strategy that permanently labels a random mitotic cell and its daughter due to reconstitution of the tubulin promoter upstream of the lacZ gene.
(C) Labeled germline stem cell (green, arrowhead) adjacent to the hub (outline). Note its immediate daughter (arrow) is displaced from the hub.
(D) Labeled cyst stem cell (green, arrowhead) adjacent to the hub (outline). Note its immediate daughter (arrow) is displaced from the hub. Immunofluorescence images of testes stained for E-cadherin (red), β-galactosidase (green), and DAPI (blue). Lineage tracing performed as described in (Voog et al., 2008). Scale bar, 20 μm.
Within mammalian tissues, uptake and long-term retention of bromo-deoxyuridine (BrdU) or incorporation of fluorescently labeled histone H2B during DNA synthesis have been used as a marker for slowly cycling (“label-retaining”) putative stem cells. However, improved lineage-tracing strategies utilizing Cre re-combinase have facilitated locating stem/progenitor cell populations in vivo (Table 1), providing insight into ongoing debates regarding the nature of stem cell populations within tissues such as the digestive system and skin.
Table 1.
Assays Used to Determine Stem Cell Identity and Niche Components
| Organism | Tissue | Stem Cell Population | Stem Cell Functional Assay | Niche Support Cells/Components | Niche Cell Factors | Key References |
|---|---|---|---|---|---|---|
| C. elegans | gonad | germ line stem cell | spatial organization | distal tip cell | N | Kimble (1981) |
| D. melanogaster | ovary | germ line stem cell | lineage analysis | cap cells, terminal filament, escort stem cells | BMP | Margolis and Spradling (1995); Xie and Spradling (1998) |
| D. melanogaster | ovary | escort stem cell | lineage analysis | cap cells? | JAK-STAT | Decotto and Spradling (2005) |
| D. melanogaster | ovary | follicle stem cell | lineage analysis | basement membrane, cap cells, escort cells | HH, BMP | Margolis and Spradling (1995) |
| D. melanogaster | testis | germ line stem cell | lineage analysis | hub cells, somatic cyst stem cells | BMP, JAK-STAT | Kiger et al. (2001); Tulina and Matunis (2001) |
| D. melanogaster | testis | somatic cyst stem cell | lineage analysis | hub cells, germline stem cells | JAK-STAT | Gönczy and DiNardo, (1996); Leatherman and DiNardo (2008) |
| D. melanogaster | midgut | intestinal stem cell | lineage analysis | basement membrane, enterocytes? | N, Wg, JAK-Stat, Insulin | Micchelli and Perrimon (2006); Ohlstein and Spradling 2006) |
| D. melanogaster | hindgut | hindgut stem cell | lineage analysis | basement membrane? | Wg | Fox and Spradling (2009); Takashima et al. (2008) |
| M. musculus | blood | hematopoietic stem cell | single cell transplant | osteoblasts, osteoclasts, vascular, perivascular cells | Wnt, N, ANG1, OPN, CXCL12 | reviewed in Weissman et al. (2001); Wagers (2005); Garrett and Emerson (2009) |
| M. musculus | muscle | muscle stem cell | lineage analysis, single cell transplant | basement membrane, myofiber | N, Wnt, CXCL12 | (Conboy and Rando, 2002) |
| M. musculus | intestine | intestinal stem cell | lineage analysis, in vitro culture | vascular, fibroblasts, Paneth cells | Wnt, BMP, N | Barker et al. (2007) |
| M. musculus | hair follicle | hair follicle stem cell | lineage analysis | vascular, fibroblasts, dermis | Wnt, BMP | reviewed in Blanpain and Fuchs (2009) |
| M. musculus | epidermis | epidermal stem cell | lineage analysis, in vitro culture | basement membrane, dermis | N, Wnt, SHH | Clayton et al., (2007); Nowak et al. (2008) |
| M. musculus | sebaceous gland | sebaceous gland stem cell | lineage analysis | basement membrane, dermis | ? | Horsley et al. (2006) |
| M. musculus | testis | spermatogonial stem cell | lineage analysis, stem/progenitor transplant | vascular, interstitial cells, Sertoli cells | BMP, GDNF, FGF | Yoshida et al. (2007) |
| M. musculus | neural | neural stem cell | lineage analysis, in vitro culture | vascular, ependymal cells, astrocytes | Wnt, SHH, FGF, VEGF, N | Palmer et al. (2000) |
For example, intestinal stem cells in mammals had been proposed to reside at the +4 position (four cells above Paneth cells), based on the observation that these cells incorporated and retained BrdU (Potten et al., 1974). However, recent lineage-tracing analysis and in vitro culturing techniques provided convincing evidence that crypt base columnar cells (CBCs) that express leucine-rich repeat-containing G protein-coupled receptor 5 (Lgr5) behave as stem cells in intestinal crypts (Barker et al., 2007; Sato et al., 2009). In the murine small intestine, CBCs are intercalated between Paneth cells and are in direct contact with a basement membrane at the base of the intestinal crypt (Chang et al., 1974).
Using a Cre-inducible knockin allele of Lgr5, lineage tracing demonstrated that Lgr5+ cells were responsible for the maintenance of the entire villus and capable of long term (>12 month) self-renewal (Barker et al., 2007). In addition, single dissociated Lgr5+ crypt cells cultured in vitro generated cryptvillus organoid structures resembling intestinal epithelium and contained the appropriate differentiated cell types (Sato et al., 2009). Lgr5 is a Wnt target gene, and components of the Wnt signaling pathway are required for intestinal stem cell maintenance (Korinek et al., 1998). Mutations in APC or β-catenin are sufficient to induce colon carcinoma (Korinek et al., 1997), and deletion of Apc in Lgr5+ cells specifically led to transformation within days, suggesting that Lgr5+ CBC cells are a likely cell-of-origin of intestinal cancer (Barker et al., 2009). However, lineage-tracing analysis using a Cre-Bmi1 strategy supported the +4 position as another putative position for stem cells (Sangiorgi and Capecchi, 2008). Bmi1 and Lgr5 label cells at different locations within the intestinal crypts with distinct cellular morphologies; therefore, it is possible that these cell types may constitute overlapping stem cell populations.
It was long assumed that neighboring myofibroblasts acted as support cells within the crypts to provide a stromal niche for the intestinal stem cells. However, the ability of isolated stem cells to generate organized, crypt-like structures in vitro suggests that the stem cells are not absolutely dependent upon these fibro-blasts for maintenance (Sato et al., 2009). Given the proximity of Paneth cells to Lgr5+ CBC cells and the fact that they are a likely source of Wnt (Gregorieff et al., 2005), this cell type could easily act to support the adjacent stem cell population. If so, the ability of CBC cells to generate differentiated cells that then act as a niche component (Sato et al., 2009) would be similar to ability of somatic stem cells in the Drosophila testis, which give rise to differentiated cells that are an integral component of the testis niche (Voog et al., 2008). As Lgr5 appears to be a marker for epithelial stem cells in a number of tissues (Barker et al., 2010; Jaks et al., 2008), it will be interesting to determine whether cells that are functionally equivalent to Paneth cells exist within these niches.
Genetic labeling experiments have also aided in the identification of stem cell populations within the skin. The epidermis, hair follicles, and sebaceous glands are maintained by stem cell populations that reside in at least three distinct microenvironments: the basal layer of the interfollicular epidermis (IFE), the follicular bulge, and the base of the sebaceous gland. Epidermal stem cells in the IFE, which normally contribute to epidermal homeostasis, reside in nests near the basement membrane (Jones and Watt, 1993) and have been identified using clonal marking strategies (Clayton et al., 2007; Ghazizadeh and Taichman, 2005). The complete nature of the epidermal niche is not known, although the basement membrane likely provides positional information and proliferative cues (Lechler and Fuchs, 2005).
Stem cells residing in the bulge region of the outer root sheath of the hair follicle have been identified using long-term label retention (Cotsarelis et al., 1990; Tumbar et al., 2004) and lineage-tracing strategies (Jaks et al., 2008; Levy et al., 2005; Nowak et al., 2008; Zhang et al., 2009). These slow-cycling stem cells are specified early in development and are capable of contributing to the epidermis upon injury, as well as the sebaceous gland (Nowak et al., 2008). Based on molecular markers and proliferation capacity, bulge-derived stem cells are distinct from cells that reside in the hair germ (Greco et al., 2009), which are activated prior to each new hair cycle and are also capable of contributing to the bulge (Ito et al., 2004). Due to the dynamic nature of the stem cell niche in the hair follicle, both temporal (hair cycle stage or time after injury) and spatial information (location of stem cell population in relation to bulge or near wound edge) likely coordinate interactions between the distinct populations of stem cells in the bulge and hair germ (Greco et al., 2009). Dermal papilla (DP) cells are specialized, mesenchymal cells that lie at the base of the hair follicle and are marked by expression of the serine protease, Corin (Enshell-Seijffers et al., 2008). DP cells are capable of promoting hair follicle formation in skin epidermis in vitro (Jahoda et al., 1984) and clearly provide signals to activate the hair germ, as well as bulge stem cells (Greco et al., 2009); therefore, DP cells are likely a component of the hair follicle stem cell niche.
Single-Cell Transplantation
In addition to lineage tracing strategies, single-cell transplantation assays have confirmed stem cell identity and function in a number of tissues (Table 1). Isolation of prospective stem cells is accomplished using fluorescence-activated cell sorting (FACS) based upon expression of cell surface marker combinations and/or dye-exclusion properties, followed by transplantation of these cells into live tissues, typically manipulated so as to be devoid of endogenous stem cells. The fluid nature of the hematopoietic system has aided in the isolation of hematopoietic stem cells (HSC) that can be identified by numerous cell surface markers (Wilson et al., 2008) (reviewed in Wagers, 2005; Weissman et al., 2001). However, isolation of cells by FACS in conjunction with transplantation has also led to the identification of stem cells from solid tissues, including those from testis, muscle, breast, and prostate (Cerletti et al., 2008; Lawson et al., 2007; Leong et al., 2008; Shackleton et al., 2006; Sherwood et al., 2004; Shinohara et al., 2000; Stingl et al., 2006). Thus, optimized techniques for the isolation and characterization of putative stem cell populations have confirmed the multilineage differentiation capacity of stem cells at single-cell resolution, as well as shed light on the environmental influences that regulate their behavior.
Real-Time Imaging of Stem Cell-Niche Cell Interactions
Given the dynamic nature of the stem cell-niche relationship, the ability to continuously observe and analyze stem cell behavior in vivo, rather than at specific time points in fixed specimens, is essential for understanding the regulation of stem cell behavior by the niche (Rieger et al., 2009; Rieger and Schroeder, 2008) (reviewed in Schroeder, 2008). Advanced imaging strategies, enhanced fluorescent probes, and increased data analysis methods have given the field unprecedented access to observe the dynamics of stem cell behavior in a number of systems. For example, within the well-defined stem cell niche of the Drosophila gonad, time lapse imaging in explanted Drosophila testes have complemented observations that proper orientation of the mitotic spindle within GSCs is essential for asymmetric division of male GSCs (Cheng et al., 2008; Sheng et al., 2009; Yamashita et al., 2007). Asymmetric localization/inheritance of cell-fate determinants and niche support cells have also been visualized live in Drosophila ovaries and neuroblasts (Cabernard and Doe, 2009; Fichelson et al., 2009).
Much like lineage-tracing strategies, improved live imaging techniques have also provided direct evidence to support established hypotheses regarding the behavior of mammalian stem cells. Insight into cell types capable of influencing both adult hematopoietic stem/progenitor cells (HSPCs) and leukemic cells has been provided by elegant intravital microscopy studies using two-photon video imaging and high-resolution confocal optics (Lo Celso et al., 2009; Sipkins et al., 2005; Xie et al., 2009). Live imaging within the mouse calvarium indicated that HSPCs reside within perivascular sites near osteoblasts in close contact with endothelial vasculature. In these studies, HSPCs were observed to localize significantly closer to osteoblasts that constitutively express the PPR (parathyroid hormone/parathyroid hormone related peptide receptor), confirming that extrinsic factors are capable of regulating HSC/progenitor behavior (Arai et al., 2004; Calvi et al., 2003; Deneault et al., 2009; Essers et al., 2009; Zhang et al., 2003) (reviewed in Garrett and Emerson, 2009).
Within the murine testis, spermatogonia lie along the basement membrane of the seminiferous tubules and are in close contact with Sertoli cells (Tegelenbosch and de Rooij, 1993). Specific markers for spermatogonial stem cells (SSCs) do not currently exist, although a subset of undifferentiated spermatogonia (Aundiff) that express glial cell line-derived neurotrophic factor (GDNF) family receptor 1α and Nanos2 may be enriched for SSCs (Suzuki et al., 2009). In addition, Neurogenin3 (Ngn3+) has been used as a marker for (Aundiff) spermatogonia, which can be functionally identified via transplantation or colony forming assays (Nakagawa et al., 2007). Insights from time-lapse microscopy followed by three-dimensional reconstruction have provided an unprecedented view into putative SSC niches within the mouse testis. Here, Ngn3-GFP+ Aundiff spermatogonia preferentially localized to sites where vasculature lies close to adjacent seminiferous tubules (Yoshida et al., 2007). Signals from neighboring interstitial cells and vasculature likely influence SSCs within the Aundiff spermatogonial population, as differentiating spermatogonia migrate along the basement membrane away from localized regions of branching vessels (Yoshida et al., 2007). In addition, alterations in the vasculature due to surgical transplantation resulted in a relocalization of Aundiff, spermatogonia providing strong in vivo evidence that the putative SSC niche in the testis is remarkably flexible.
Vasculature has been implicated as contributing to the stem cell niche in other tissues, including the hematopoietic system (Kiel et al., 2005; Sugiyama et al., 2006) and regions of the hippocampus and lateral ventricles of the brain (Palmer et al., 2000; Shen et al., 2004, 2008; Tavazoie et al., 2008; Wurmser et al., 2004). Therefore, the vasculature may serve a conserved support role for stem cells throughout the body. In addition to providing key nutrients, the ability of the vasculature to provide circulating, systemic factors that regulate stem cells and/or the niche would provide a mechanism to coordinate stem cell activity in dynamic fashion in response to metabolic flux or other whole-organism changes, such as aging (Conboy et al., 2005; Mayack et al., 2010; Ryu et al., 2006).
Functional Characterization of the Niche: Ablation, Expansion, and Wound repair
Paramount to niche characterization are sensitive and specific tools to perturb and monitor stem cell activity and niche function to determine causal relationships. Experimental gain- and loss-of-function studies in cell types likely to influence stem cell behavior are necessary to rigorously define the niche. Two general classes of stem cell niches have been proposed based on the physical relationship of stem cells with neighboring niche components (Morrison and Spradling, 2008). Stromal niches can be thought of as discrete anatomical sites containing niche support cells that physically contact adjacent stem cells and influence stem cell behavior via close range signaling (e.g., Drosophila germline stem cell niches). In contrast, epithelial niches are depicted as those that lack specific niche support cells and typically consist of stem cells directly in contact with a basement membrane, as well as more mature cells of the lineage (e.g., mammalian muscle fiber). Monitoring niche size and function after genetic manipulation or after tissue injury suggest that both spatial and temporal restrictions regulate the activity and number of niches.
Stem Cell-Niche Relationships: Variation on a Theme
Laser ablation of the distal tip cell (DTC) in the C. elegans gonad resulted in loss of adjacent germline stem cells, indicating that the DTC is required for niche function (Kimble, 1981). Subsequent genetic analysis demonstrated that short range signaling via the Notch pathway from the DTC is a key molecular determinant of regulating stem cell behavior within this stromal niche (Austin and Kimble, 1987; Henderson et al., 1994). Interestingly, work in this model has revealed the presence of cells that can support germ line proliferation that are not normally found in contact with the stem cell population (McGovern et al., 2009). The presence of such a “latent niche” is quite provocative, as it presents an interesting model for investigating how distant niches could provide an environment conducive to stem cell maintenance and proliferation, such as in the case of metastasis.
In the Drosophila ovary, germline stem cells (GSCs) directly contact somatic cells, known as cap cells, which act as key support cells by secreting the self-renewal factor Decapentaplegic (Dpp) (Xie and Spradling, 1998, 2000). Notch signaling from the germ line to the soma influences the number of cap cells, and hyperactivation of Notch signaling leads to an increase in the number of cap cells with a concomitant expansion of GSCs (Song et al., 2007; Ward et al., 2006). In addition to directing the symmetric division of stem cells to maintain full occupancy of the niche (Xie and Spradling, 2000), the GSC niche is competent to direct the proliferation of nearby somatic stem cells that enter the niche upon depletion of endogenous GSCs. These data indicate that empty niches can regulate the behavior of “foreign” stem cells not normally found in that location (Kai and Spradling, 2003). Furthermore, GSCs that are unable to differentiate can outcompete normal GSCs for niche occupancy (Jin et al., 2008). Such findings underscore the flexibility of niche size and function and present an intriguing model for how stem cells that have accumulated mutations over time may evolve to predominate in their endogenous niche or thrive in distant niches in other tissues.
In the Drosophila testis, a cluster of 10–15 somatic support cells, known as the hub, contribute significantly to the stem cell niche in the testis (Kiger et al., 2001; Tulina and Matunis, 2001). Hub cells are specified during development (Boyle and DiNardo, 1995; Le Bras and Van Doren, 2006) and are maintained by an adjacent population of pluripotent somatic stem cells (Voog et al., 2008). Absence or depletion of GSCs during development leads to proliferation of the somatic stem cells, which appear to take on hub characteristics (Flatt et al., 2008; Gönczy and DiNardo, 1996), suggesting that the germ line may negatively regulate somatic stem cell proliferation, thereby influencing formation of the niche during development. Consistent with a role in hub maintenance, loss of the somatic stem cells led to subsequent loss of the hub and adjacent GSCs, providing indirect evidence that the hub is indeed necessary for promoting GSC maintenance (Voog et al., 2008). In addition, the adult testis niche is competent to support the dedifferentiation of spermatogonia into self-renewing stem cells to facilitate stem cell replacement, providing another paradigm for how niches may function in normal tissue homeostasis or endow differentiated cells with self-renewal capacity (Brawley and Matunis, 2004; Cheng et al., 2008; Sheng et al., 2009).
Gain and loss-of-function studies have indicated that mammalian hematopoietic stem/progenitor cells (HSPCs) receive inputs from multiple sources, including endosteal, endothelial, and advential cells, as well as adipocytes (Adams et al., 2006; Calvi et al., 2003; Essers et al., 2009; Fleming et al., 2008; Katayama et al., 2006; Naveiras et al., 2009; Stier et al., 2005; Sugiyama et al., 2006; Zhang et al., 2003). Directed expression of parathyroid hormone receptor (PTH) or deletion of BMPR1α specifically in osteoblasts led to an expansion of osteoblasts and a corresponding increase in putative HSCs, providing evidence that osteoblasts may be a crucial support cell within the HSC niche in the bone marrow (Calvi et al., 2003; Zhang et al., 2003). Consistent with this model, selective ablation of osteoblasts using a collagen α1 type I (Col1α1) promoter to express thymidine kinase resulted in a dramatic loss of bone marrow cellularity and loss of HPSCs (Visnjic et al., 2004). In contrast, biglycan-deficient mice that have reduced osteoblast populations showed no defects in hematopoiesis, HSC frequency, or function, indicating that fewer osteoblasts do not necessarily lead to reductions in HSCs (Kiel et al., 2007). As discussed above, the vasculature also contributes to the hematopoietic niche in the bone marrow, as putative HSCs clearly associate with sinusoidal endothelium, in addition to the endosteum (Kiel et al., 2005). Therefore, the hematopoietic niche includes several types of support cells that are involved in regulating HSC behavior; however, the degree to which each contributes under normal or pathological conditions or during transplantation is not clear (reviewed in Garrett and Emerson, 2009).
Niche Function during Tissue Regeneration
Radiation and cytotoxic therapies result in concomitant loss of hematopoietic, endothelial, and osteoblastic cells (Amsel and Dell, 1971; Shirota and Tavassoli, 1991). Recovery of HSPCs is dependent upon angiogenic and osteoblastic regeneration. Sinusoidal endothelial cells, which express VEGFR2/3 and are lost during radiation-induced injury, are capable of regeneration and necessary for the recruitment and maintenance of HSC pools (Hooper et al., 2009). Conditional deletion of VEGFR2 within endothelial cells resulted in inefficient sinusodial endothelial cell regeneration and hematopoietic recovery. Similarly, restoration and reversible expansion of the osteoblastic niche was observed after total body irradiation due, in part, to proliferation of osteoblasts with subsequent hematopoietic recovery (Dominici et al., 2009). Upon injury, megakaryocytes were found in close contact with osteoblasts and could facilitate osteoblast expansion/recovery through the production of TGF-β1 and PDGF-β (Dominici et al., 2009). In addition, genetic and in vitro studies have identified the chemokine CXCL12 (SDF-1), and its cognate receptor CXCR4, as important components that regulate neovascularization, mobilization of hematopoietic cells, and survival of megakaryocytes and osteoblast progenitors (Hodohara et al., 2000; Jin et al., 2006; Kortesidis et al., 2005). Conditional ablation of CXCL12 (and a host of other HSPC maintenance factors) from vascular and endosteal cells would be needed before definitive conclusions can be drawn regarding the dynamic nature of the hematopoietic niche. However, interactions between both vascular and endosteal niches are likely necessary for proper hematopoietic function and recovery.
Intestinal stem cells (ISCs) in the Drosophila midgut were originally identified using lineage-tracing strategies and are a prototypical example of stem cells that reside within an epithelial niche (Micchelli and Perrimon, 2006; Ohlstein and Spradling, 2006). ISCs reside along the basement membrane within clusters, or “nests,” of 2 to 3 basally located diploid cells, including daughter enteroblasts, that are interspersed between differentiated, polyploid enterocytes. Although all cells in the stem cell-containing nests are positive for the transcription factor Escargot, only the ISC directly contacts the basement membrane and stains positive for the Notch ligand Delta, whereas Notch signaling is activated exclusively in the daughter enteroblast (Micchelli and Perrimon, 2006; Ohlstein and Spradling, 2007).
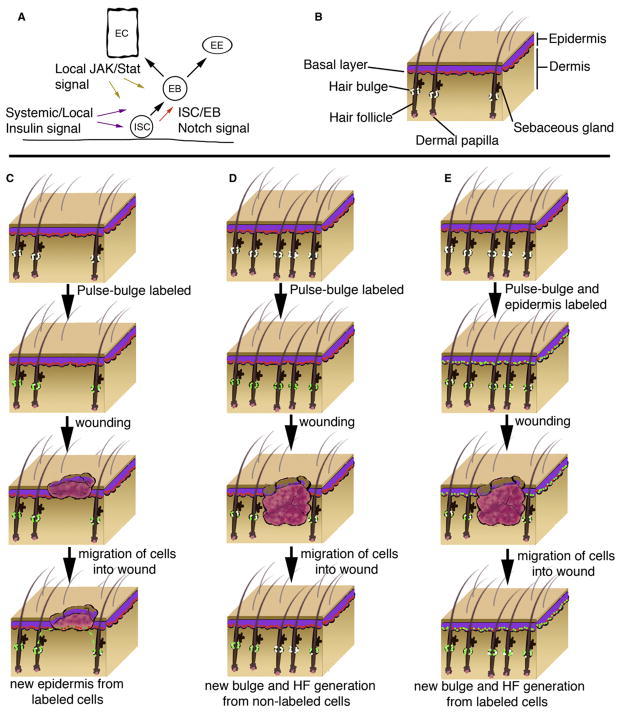
In response to intestinal injury, stress, or aging, ISC division rate increases (Amcheslavsky et al., 2009; Biteau et al., 2008; Choi et al., 2008; Jiang et al., 2009). Interestingly, facultative stem cells within the Drosophila hindgut also appear to be able to respond to damage to mediate tissue homeostasis (Fox and Spradling, 2009). Recent evidence indicated that differentiated enterocytes signal via the JAK-Stat pathway to stimulate proliferation of ISCs (Jiang et al., 2009; Beebe et al., 2010). Intestinal cell death due to apoptosis, stress, or infection was sufficient to induce the expression of the JAK-STAT ligands Upd/Upd2/Upd3, which stimulated both ISC division and enteroblast differentiation (Jiang et al., 2009). Therefore, signaling from lineage-related differentiated cells to initiate or accelerate stem cell division only when replacement cells are needed may serve as a niche-based mechanism to maintain tissue homeostasis and repair (Figure 2).
Figure 2. Models for Stem Cell Niche-Mediated Repair in Response to Injury.
(A) Local signals mediate stem cell response to injury in the Drosophila midgut. Notch signaling regulates intestinal stem cell (ISC) and enteroblast (EB) cell-fate decisions. In response to injury, localized JAK-STAT signaling from enterocytes (EC) promotes proliferation of ISCs and differentiation of EBs. EBs are capable of generating ECs or enteroendocrine (ee) cells. Systemic and localized insulin signaling also influences recovery.
(B) Schematic of mammalian epidermis. Epidermal stem cells reside in the basal layer (red) of the epidermis in contact with the basement membrane. Multipotent stem cells (white) reside in the bulge region of the outer root sheath of the hair follicle.
(C) Bulge stem cells can repair the epidermis upon injury. Lineage tracing strategy pulse-labels bulge stem cells (white) with an observable marker (green). Upon injury to epidermis, labeled bulge stem cells migrate and aid in transient repair of the epidermis by contributing to basal cell layer.
(D) Non-bulge stem cells contribute to de novo HF and bulge regeneration. Lineage tracing strategy pulse labels bulge stem cells; however, during wound repair, new hair follicles (HF) are generated that lack labeled bulge stem cells.
(E) Interfollicular epidermal cells contribute to de novo HF and bulge regeneration. Lineage tracing strategy pulse-labels bulge and epidermal stem cells. During wound repair, new bulge and HFs are generated that contain labeled cells.
Satellite cells (~5% of adult muscle nuclei) are mononuclear cells that reside adjacent to the muscle fiber and are enclosed by basal lamina (Mauro, 1961). Satellite cells represent a heterogeneous population of cells from which skeletal muscle precursors (SMPs) can be isolated based on expression of cell surface markers followed by FACS (Cerletti et al., 2008; Kuang et al., 2007; Sherwood et al., 2004). SMP identity and function has been confirmed based on transplantation assays, which demonstrate the ability of transplanted SMPs to contribute to and repair damaged muscle fibers and replenish the satellite cell pool (Cerletti et al., 2008; Collins et al., 2005; Sacco et al., 2008).
Notch signaling regulates mammalian satellite cell behavior postnatally and in response to injury (Conboy and Rando, 2002). Increases in expression of Delta in myofibers results in satellite cell/muscle stem cell proliferation. In older animals, satellite cells maintain expression of Notch; however, Delta expression is reduced (Conboy et al., 2003). Direct activation of Notch in injured muscle or exposure to young serum via parabiosis with a young animal is sufficient to enhance muscle regeneration (Conboy et al., 2003; Conboy et al., 2005), suggesting that the loss (or gain) of an age-specific systemic factor(s) is responsible for inefficient repair in aged animals. Additional inputs, including Wnt (Brack et al., 2007) and TGF-β (Carlson et al., 2008) signaling, may also regulate the ability of aged satellite cells to respond to Notch and participate in repair of injured muscle fibers. In mammalian muscle, satellite cells proliferate in response to myofiber ablation, suggesting that mature muscle fibers may act as a local niche to regulate satellite cell proliferation (Bischoff, 1990), similar to the way in which enterocytes regulate the proliferation of Drosophila ISCs (Figure 2). The prominent role for differentiated cells in regulating the behavior of nearby stem cells may prove to be prevalent in tissues for which stem cells are involved in acute repair, rather than continual tissue replacement.
In contrast to adult muscle regeneration, early developmental processes are recapitulated to guide tissue and niche regeneration of the epidermis and associated appendages. During normal skin homeostasis, bulge stem cells do not contribute to the epidermis. However, upon injury, bulge stem cells migrate, proliferate, and aid in epidermal regeneration transiently (Ito et al., 2005; Levy et al., 2007; Nowak et al., 2008). Similarly, during wound repair, de novo hair follicle regeneration has been observed that closely parallels embryonic hair development based on the expression profiles of molecular markers and involvement of Wnt signaling (Ito et al., 2007). Using a Cre-inducible Keratin15 labeling strategy, nascent hair follicles and newly generated bulge stem cells were observed to descend from the epidermis and/or upper region of the follicle (Figure 2). These data demonstrated that new stem cell niches could be generated during adulthood. The ability of resident stem cell populations in the epidermis and bulge to adjust fate decisions in the context of wounding suggests that these cells may be exposed to and molecularly competent to interpret alternative nonhomeostatic niche signals to orchestrate appropriate regeneration programs. Therefore, current models suggest that the niche can direct tissue repair via reiteration of developmental programs that specify or expand stem cells, such as Wnt-mediated repair of the epidermis, or distinct adult-specific genetic programs, such as positive feed-forward signals from differentiated cells, to stimulate stem cell proliferation. Regardless, the factors that regulate the transient generation and regression of facultative niches or alert endogenous niches to tissue damage may also be inappropriately utilized in the progression of some pathologies.
Niche Function in Disease Initiation and Progression
As discussed above, the dynamic relationship between stem cells and the niche may be most evident during tissue repair, as a consequence of injury. However, constitutive activation of wound healing programs, including accompanying inflammatory responses, likely lead to permanent changes in the niche that could lead to dysregulation of stem cell behavior and, ultimately, contribute to disease. However, a thorough assessment of the role of the niche in disease initiation and progression is dependent upon a clear understanding of the normal structural and molecular components that constitute each stem cell niche.
Aging
Although cell-autonomous changes clearly underlie altered stem cell behavior during aging, recent studies have demonstrated that a decline in niche function, including decreased production of local self-renewal factors, also contributes to reduced tissue homeostasis and repair in a number of systems (Boyle et al., 2007; Conboy et al., 2003; Hsu and Drummond-Barbosa, 2009; Pan et al., 2007; Ryu et al., 2006). In addition, changes in circulating, systemic factors also lead to decreased stem cell activity (Conboy et al., 2003; Hsu and Drummond-Barbosa, 2009; Mayack et al., 2010). However, restoration of niche function can counter aging-related changes in stem cell behavior (Boyle et al., 2007; Conboy et al., 2005; Mayack et al., 2010; Pan et al., 2007), indicating that strategies to rejuvenate or expand the niche may enhance stem cell-based therapies in regenerative medicine.
Aging may be perceived as systemic stress; however, the changes in stem cell-niche cell relationships in older animals could also be a consequence of chronic damage and wound repair. For example, wounding, infection, and aging all lead to an increase in ISC proliferation in the Drosophila midgut. During aging, normal stress response pathways are engaged; however, the ability of the daughter enteroblasts to differentiate appropriately into enterocytes or enteroendocrine cells, which is regulated by the Notch pathway, is disrupted (Biteau et al., 2008; Ohlstein and Spradling, 2007). The insulin-signaling pathway has been shown to regulate the proliferation of ISCs in response to intestinal damage (Amcheslavsky et al., 2009), as well as aging of the Drosophila ovarian niche, by modifying Notch function in support cells (Hsu and Drummond-Barbosa, 2009). Thus, it is possible that changes in the integration of systemic signals with local signaling pathways elicit different stem cell responses to normal stress and aging.
Cancer Initiation and Progression
Recent insights into mechanisms involved in regulating stem cell behavior can be directly applied to the study of cancer biology, including the role of the niche. Involvement of the local microenvironment in tumor initiation or metastasis has been postulated for over 100 years (Paget, 1889). Similar to the manner in which support cells, secreted factors, the extracellular matrix, and vasculature all contribute to the regulation of stem cell behavior, analogous components are easily identified in the tumor microenvironment. Irrespective of whether the tumor-initiating cell is a transformed tissue stem cell or a differentiated cell that has acquired the ability to self-renew, the local microenvironment could influence cancer initiation through a number of mechanisms.
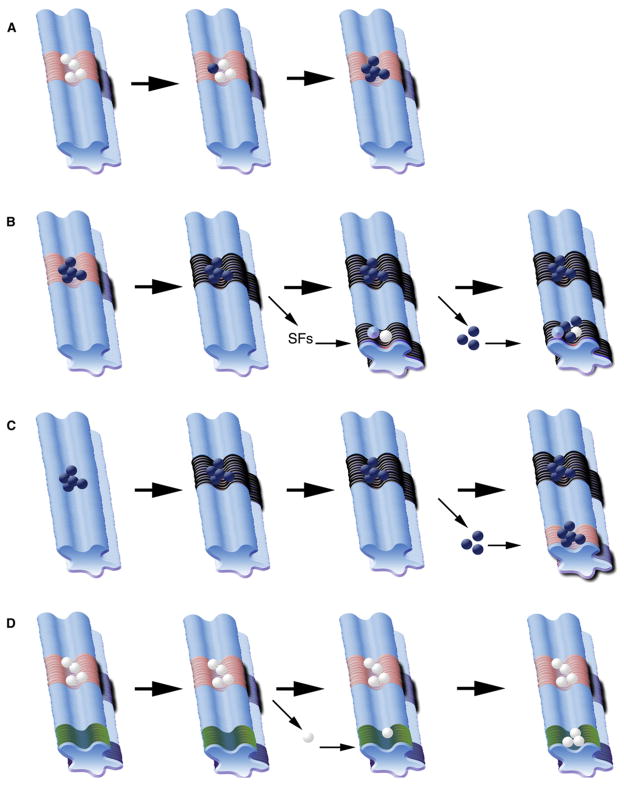
Experimental evidence has demonstrated that normal niches can support the reversion of differentiated cells back to a stem cell state (Brawley and Matunis, 2004; Cheng et al., 2008; Kai and Spradling, 2004; Sheng et al., 2009) and can become occupied by cells that outcompete normal stem cells for residence (Issigonis et al., 2009; Jin et al., 2008). In addition, niches can undergo changes that promote proliferation and maintenance of cancer cells (reviewed in Coussens and Werb, 2002; Tlsty and Coussens, 2006). “Premetastatic” niches can be generated by cancer cell proliferation or tumor-released factors that are capable of supporting the seeding and proliferation of migratory tumor-initiating cells in secondary and tertiary sites (reviewed in Wels et al., 2008). Lastly, wound healing or morphological damage can result in cells positioned near a signaling source that inappropriately directs proliferation (Kai and Spradling, 2003; McGovern et al., 2009) (Figure 3).
Figure 3. Role of the Niche in Cancer Progression.
(A) In an endogenous niche, stem cells (white) are in contact with niche support cells (pink region) near associated cells (light blue region). Cancer cells (navy blue) may outcompete stem cells for access to niche-derived signals, resulting in expansion of cancer cells and loss of endogenous stem cells.
(B) Cancer cells (navy blue) that occupy an endogenous niche (pink region) may transform or recruit surrounding support cells (black region). Distant premetastatic or malignant niches induced by signaling factors (SF) from primary tumor can recruit endogenous progenitor cells (white) or nonprogenitor cells (light blue) to aid in metastasis (navy blue spheres).
(C) Cancer cells may contribute to transformation of local environment (black region); metastatic cancer cells may home to distant endogenous niches (pink region).
(D) In a “latent niche” model, competent cells (white) from an endogenous niche (pink region) become physically displaced through developmental or injury induced mechanisms, and distinct signaling cells (green region) act as a niche to influence ectopic proliferation.
Basal cell carcinoma (BCC) is the most common cancer in the United States (800,000 cases/year). BCC can be recapitulated in transgenic human explants that overexpress Sonic Hedgehog in keratinocytes, and stroma associated with human BCC is critical for tumor progression (Fan et al., 1997; Van Scott and Reinertson, 1961). Within murine skin, signaling via the Bone Morphogenetic Protein (BMP) pathway is required for proper progenitor cell differentiation (Kobielak et al., 2003), and BCC cells express high levels of BMPs 2 and 4 (Sneddon et al., 2006). However, transcriptional profiling of human BCCs also demonstrated that the underlying tumor stroma expressed high levels of the BMP antagonist Gremlin1 de novo, which would block differentiation of the adjacent tumor cells (Sneddon et al., 2006). Isolation and culture of BCCs demonstrated that Gremlin1 suppresses BMP-mediated keratinocyte differentiation, suggesting that Gremlin1 expression promotes BCC proliferation in a localized, transformed niche (Sneddon et al., 2006).
Similarly, in invasive breast carcinomas, cancer-associated fibroblasts (CAFs) and the underlying stroma clearly promote tumor initiation and progression (Finak et al., 2008; Hu et al., 2008) (reviewed in Bissell, 2007). For example, expression of CXCL12 (SDF-1) by breast cancer-associated CAFs acts directly on the CXCR4 receptor expressed by the breast cancer cells and induces angiogenesis through the recruitment of endothelial progenitor cells (Orimo et al., 2005). CAFs phenotypically resemble myofibroblasts, cell types often associated with wound healing and tissue remodeling. While the origins of tumor-associated stroma and fibroblasts are not completely understood, genetic factors and a chronic inflammatory environment can contribute to the generation of a modified niche that supports tumor growth (Coppé et al., 2008; Rodier et al., 2009; Sneddon et al., 2006; Trimboli et al., 2009) (reviewed in Coussens and Werb, 2002; Tlsty and Coussens, 2006).
In addition to transformed niches that may influence a primary tumor, genetic approaches have identified niches induced by primary tumors located at distant sites within the body. Soluble factors secreted from primary tumors can induce “premetastatic” niches capable of recruiting cells that subsequently promote tumorigenesis at defined anatomical locations (reviewed in Wels et al., 2008). Factors such as VEGFA, TGFβ, and TNFα, which are secreted from primary tumors, can recruit support cells to and induce expression of inflammatory chemoattractants within tumor-specific premetastatic sites to facilitate the formation of secondary and tertiary tumors (Hiratsuka et al., 2006; Kaplan et al., 2005). Tumor-induced niches have also been shown to recruit normal progenitor cells and affect normal progenitor cell behavior in a leukemic xenograph mouse model. Intravital microscopy studies of Nalm-6 pre-B acute lymphoblastic leukemia (ALL) demonstrated that malignant cells metastasize to specific regions of the bone marrow that express CXCL12 (SDF-1) (Sipkins et al., 2005). In addition, leukemic cell proliferation resulted in the generation of malignant niches that downregulated CXCL12 (SDF-1) production and recruited normal hematopoietic progenitor (CD34+) cells. These malignant niches had high levels of stem cell factor (SCF) and negatively affected normal (CD34+) progenitor cell number and activity (Colmone et al., 2008).
The origin of a cancer cell and its internal transcriptional signature are important determinants of site-specific metastasis (Bos et al., 2009). Access to circulation and the ability to survive at distant locations also influence the metastatic potential of cancer cells (reviewed in Nguyen et al., 2009). To this end, certain cancer cell types appear to be capable of utilizing endogenous niches or aiding in the generation of malignant niches that can influence both normal and cancerous cells. Therefore, malignant niches may act as immune-evasive or antiapoptotic sanctuaries that harbor tumor-initiating cells throughout the course of standard therapeutic treatments.
Future Directions
Stem cell niches are physiologically dynamic domains that will continue to aid in both experimental and conceptual models of development, tissue maintenance, and disease. The stem cell niche hypothesis provided an initial framework within which to define cell types and factors responsible for regulating stem cell behavior, and advances in lineage-tracing techniques, single cell isolation and manipulation, and imaging technologies will continue to expand our knowledge of the nature of stem cell-niche cell interactions. A symbiotic relationship is present within the niche under homeostatic conditions; however, the involvement of the stem cell niche in the response to tissue injury and during aging and disease progression is less well understood. Additional work in the identification of genetic factors that regulate the formation, activity, and size of stem cell niches will be necessary in order to incorporate the niche into stem cell-based therapies and regenerative medicine. Furthermore, in cases where a modified niche accompanies disease progression, targeting the niche (niche ablation) could be considered an alternative, powerful therapeutic approach to accompany current drug regimes and treatments.
Acknowledgments
We would like to thank V. Greco, A. Wagers, and three anonymous reviewers for comments on the manuscript, and we apologize to those colleagues whose work could not be referenced directly due to space constraints. D.L.J. is funded by the ACS, NIH, CIRM, the Emerald Foundation, and the G. Harold and Leila Y. Mathers Charitable Foundation.
References
- Adams GB, Chabner KT, Alley IR, Olson DP, Szczepiorkowski ZM, Poznansky MC, Kos CH, Pollak MR, Brown EM, Scadden DT. Stem cell engraftment at the endosteal niche is specified by the calcium-sensing receptor. Nature. 2006;439:599–603. doi: 10.1038/nature04247. [DOI] [PubMed] [Google Scholar]
- Amcheslavsky A, Jiang J, Ip YT. Tissue damage-induced intestinal stem cell division in Drosophila. Cell Stem Cell. 2009;4:49–61. doi: 10.1016/j.stem.2008.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amsel S, Dell ES. Response of the preosteoblast and stem cell of rat bone marrow to a lethal dose of x-irradiation or cyclophosphamide. Cell Tissue Kinet. 1971;4:255–261. doi: 10.1111/j.1365-2184.1971.tb01536.x. [DOI] [PubMed] [Google Scholar]
- Arai F, Hirao A, Ohmura M, Sato H, Matsuoka S, Takubo K, Ito K, Koh GY, Suda T. Tie2/angiopoietin-1 signaling regulates hematopoietic stem cell quiescence in the bone marrow niche. Cell. 2004;118:149–161. doi: 10.1016/j.cell.2004.07.004. [DOI] [PubMed] [Google Scholar]
- Austin J, Kimble J. glp-1 is required in the germ line for regulation of the decision between mitosis and meiosis in C. elegans. Cell. 1987;51:589–599. doi: 10.1016/0092-8674(87)90128-0. [DOI] [PubMed] [Google Scholar]
- Barker N, van Es JH, Kuipers J, Kujala P, van den Born M, Cozijnsen M, Haegebarth A, Korving J, Begthel H, Peters PJ, Clevers H. Identification of stem cells in small intestine and colon by marker gene Lgr5. Nature. 2007;449:1003–1007. doi: 10.1038/nature06196. [DOI] [PubMed] [Google Scholar]
- Barker N, Ridgway RA, van Es JH, van de Wetering M, Begthel H, van den Born M, Danenberg E, Clarke AR, Sansom OJ, Clevers H. Crypt stem cells as the cells-of-origin of intestinal cancer. Nature. 2009;457:608–611. doi: 10.1038/nature07602. [DOI] [PubMed] [Google Scholar]
- Barker N, Huch M, Kujala P, van de Wetering M, Snippert H, van Es J, Sato T, Stange DE, Begthel H, van den Born M, et al. Lgr5+ve Stem Cells Drive Self-Renewal in the Stomach and Build Long-lived Gastric Units In Vitro. Cell Stem Cell. 2010;6:25–36. doi: 10.1016/j.stem.2009.11.013. [DOI] [PubMed] [Google Scholar]
- Beebe K, Lee WC, Micchelli CA. JAK-Stat signaling coordinates stem cell proliferation and multilineage differentiation in the Drosophila intestinal stem cell lineage. Dev Biol. 2010;338:28–37. doi: 10.1016/j.ydbio.2009.10.045. [DOI] [PubMed] [Google Scholar]
- Bischoff R. Interaction between satellite cells and skeletal muscle fibers. Development. 1990;109:943–952. doi: 10.1242/dev.109.4.943. [DOI] [PubMed] [Google Scholar]
- Bissell MJ. Modelling molecular mechanisms of breast cancer and invasion: lessons from the normal gland. Biochem Soc Trans. 2007;35:18–22. doi: 10.1042/BST0350018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biteau B, Hochmuth CE, Jasper H. JNK activity in somatic stem cells causes loss of tissue homeostasis in the aging Drosophila gut. Cell Stem Cell. 2008;3:442–455. doi: 10.1016/j.stem.2008.07.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanpain C, Fuchs E. Epidermal homeostasis: a balancing act of stem cells in the skin. Nat Rev Mol Cell Biol. 2009;10:207–217. doi: 10.1038/nrm2636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bos PD, Zhang XH, Nadal C, Shu W, Gomis RR, Nguyen DX, Minn AJ, van de Vijver MJ, Gerald WL, Foekens JA, Massagué J. Genes that mediate breast cancer metastasis to the brain. Nature. 2009;459:1005–1009. doi: 10.1038/nature08021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyle M, DiNardo S. Specification, migration and assembly of the somatic cells of the Drosophila gonad. Development. 1995;121:1815–1825. doi: 10.1242/dev.121.6.1815. [DOI] [PubMed] [Google Scholar]
- Boyle M, Wong C, Rocha M, Jones DL. Decline in self-renewal factors contributes to aging of the stem cell niche in the Drosophila testis. Cell Stem Cell. 2007;1:470–478. doi: 10.1016/j.stem.2007.08.002. [DOI] [PubMed] [Google Scholar]
- Brack AS, Conboy MJ, Roy S, Lee M, Kuo CJ, Keller C, Rando TA. Increased Wnt signaling during aging alters muscle stem cell fate and increases fibrosis. Science. 2007;317:807–810. doi: 10.1126/science.1144090. [DOI] [PubMed] [Google Scholar]
- Brawley C, Matunis E. Regeneration of male germline stem cells by spermatogonial dedifferentiation in vivo. Science. 2004;304:1331–1334. doi: 10.1126/science.1097676. [DOI] [PubMed] [Google Scholar]
- Cabernard C, Doe CQ. Apical/basal spindle orientation is required for neuroblast homeostasis and neuronal differentiation in Drosophila. Dev Cell. 2009;17:134–141. doi: 10.1016/j.devcel.2009.06.009. [DOI] [PubMed] [Google Scholar]
- Calvi LM, Adams GB, Weibrecht KW, Weber JM, Olson DP, Knight MC, Martin RP, Schipani E, Divieti P, Bringhurst FR, et al. Osteoblastic cells regulate the haematopoietic stem cell niche. Nature. 2003;425:841–846. doi: 10.1038/nature02040. [DOI] [PubMed] [Google Scholar]
- Carlson ME, Hsu M, Conboy IM. Imbalance between pSmad3 and Notch induces CDK inhibitors in old muscle stem cells. Nature. 2008;454:528–532. doi: 10.1038/nature07034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cerletti M, Jurga S, Witczak CA, Hirshman MF, Shadrach JL, Good-year LJ, Wagers AJ. Highly efficient, functional engraftment of skeletal muscle stem cells in dystrophic muscles. Cell. 2008;134:37–47. doi: 10.1016/j.cell.2008.05.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang LR, Chen TS, Huang KC. Electrolyte transport across the mouse small intestine. Proc Soc Exp Biol Med. 1974;145:1220–1224. doi: 10.3181/00379727-145-37985. [DOI] [PubMed] [Google Scholar]
- Cheng J, Türkel N, Hemati N, Fuller MT, Hunt AJ, Yamashita YM. Centrosome misorientation reduces stem cell division during ageing. Nature. 2008;456:599–604. doi: 10.1038/nature07386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi NH, Kim JG, Yang DJ, Kim YS, Yoo MA. Age-related changes in Drosophila midgut are associated with PVF2, a PDGF/VEGF-like growth factor. Aging Cell. 2008;7:318–334. doi: 10.1111/j.1474-9726.2008.00380.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clayton E, Doupé DP, Klein AM, Winton DJ, Simons BD, Jones PH. A single type of progenitor cell maintains normal epidermis. Nature. 2007;446:185–189. doi: 10.1038/nature05574. [DOI] [PubMed] [Google Scholar]
- Collins CA, Olsen I, Zammit PS, Heslop L, Petrie A, Partridge TA, Morgan JE. Stem cell function, self-renewal, and behavioral heterogeneity of cells from the adult muscle satellite cell niche. Cell. 2005;122:1–13. doi: 10.1016/j.cell.2005.05.010. [DOI] [PubMed] [Google Scholar]
- Colmone A, Amorim M, Pontier AL, Wang S, Jablonski E, Sipkins DA. Leukemic cells create bone marrow niches that disrupt the behavior of normal hematopoietic progenitor cells. Science. 2008;322:1861–1865. doi: 10.1126/science.1164390. [DOI] [PubMed] [Google Scholar]
- Conboy IM, Rando TA. The regulation of Notch signaling controls satellite cell activation and cell fate determination in postnatal myogenesis. Dev Cell. 2002;3:397–409. doi: 10.1016/s1534-5807(02)00254-x. [DOI] [PubMed] [Google Scholar]
- Conboy IM, Conboy MJ, Smythe GM, Rando TA. Notch-mediated restoration of regenerative potential to aged muscle. Science. 2003;302:1575–1577. doi: 10.1126/science.1087573. [DOI] [PubMed] [Google Scholar]
- Conboy IM, Conboy MJ, Wagers AJ, Girma ER, Weissman IL, Rando TA. Rejuvenation of aged progenitor cells by exposure to a young systemic environment. Nature. 2005;433:760–764. doi: 10.1038/nature03260. [DOI] [PubMed] [Google Scholar]
- Coppé JP, Patil CK, Rodier F, Sun Y, Muñoz DP, Goldstein J, Nelson PS, Desprez PY, Campisi J. Senescence-associated secretory phenotypes reveal cell-nonautonomous functions of oncogenic RAS and the p53 tumor suppressor. PLoS Biol. 2008;6:2853–2868. doi: 10.1371/journal.pbio.0060301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cotsarelis G, Sun TT, Lavker RM. Label-retaining cells reside in the bulge area of pilosebaceous unit: implications for follicular stem cells, hair cycle, and skin carcinogenesis. Cell. 1990;61:1329–1337. doi: 10.1016/0092-8674(90)90696-c. [DOI] [PubMed] [Google Scholar]
- Coussens LM, Werb Z. Inflammation and cancer. Nature. 2002;420:860–867. doi: 10.1038/nature01322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Decotto E, Spradling AC. The Drosophila ovarian and testis stem cell niches: similar somatic stem cells and signals. Dev Cell. 2005;9:501–510. doi: 10.1016/j.devcel.2005.08.012. [DOI] [PubMed] [Google Scholar]
- Deneault E, Cellot S, Faubert A, Laverdure JP, Fréchette M, Chagraoui J, Mayotte N, Sauvageau M, Ting SB, Sauvageau G. A functional screen to identify novel effectors of hematopoietic stem cell activity. Cell. 2009;137:369–379. doi: 10.1016/j.cell.2009.03.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dominici M, Rasini V, Bussolari R, Chen X, Hofmann TJ, Spano C, Bernabei D, Veronesi E, Bertoni F, Paolucci P, et al. Restoration and reversible expansion of the osteoblastic hematopoietic stem cell niche after marrow radioablation. Blood. 2009;114:2333–2343. doi: 10.1182/blood-2008-10-183459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enshell-Seijffers D, Lindon C, Morgan BA. The serine protease Corin is a novel modifier of the Agouti pathway. Development. 2008;135:217–225. doi: 10.1242/dev.011031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Essers MA, Offner S, Blanco-Bose WE, Waibler Z, Kalinke U, Duchosal MA, Trumpp A. IFNalpha activates dormant haematopoietic stem cells in vivo. Nature. 2009;458:904–908. doi: 10.1038/nature07815. [DOI] [PubMed] [Google Scholar]
- Fan H, Oro AE, Scott MP, Khavari PA. Induction of basal cell carcinoma features in transgenic human skin expressing Sonic Hedgehog. Nat Med. 1997;3:788–792. doi: 10.1038/nm0797-788. [DOI] [PubMed] [Google Scholar]
- Fichelson P, Moch C, Ivanovitch K, Martin C, Sidor CM, Lepesant JA, Bellaiche Y, Huynh JR. Live-imaging of single stem cells within their niche reveals that a U3snoRNP component segregates asymmetrically and is required for self-renewal in Drosophila. Nat Cell Biol. 2009;11:685–693. doi: 10.1038/ncb1874. [DOI] [PubMed] [Google Scholar]
- Finak G, Bertos N, Pepin F, Sadekova S, Souleimanova M, Zhao H, Chen H, Omeroglu G, Meterissian S, Omeroglu A, et al. Stromal gene expression predicts clinical outcome in breast cancer. Nat Med. 2008;14:518–527. doi: 10.1038/nm1764. [DOI] [PubMed] [Google Scholar]
- Flatt T, Min KJ, D’Alterio C, Villa-Cuesta E, Cumbers J, Lehmann R, Jones DL, Tatar M. Drosophila germ-line modulation of insulin signaling and lifespan. Proc Natl Acad Sci USA. 2008;105:6368–6373. doi: 10.1073/pnas.0709128105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleming HE, Janzen V, Lo Celso C, Guo J, Leahy KM, Kronenberg HM, Scadden DT. Wnt signaling in the niche enforces hematopoietic stem cell quiescence and is necessary to preserve self-renewal in vivo. Cell Stem Cell. 2008;2:274–283. doi: 10.1016/j.stem.2008.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox DT, Spradling AC. The Drosophila hindgut lacks constitutively active adult stem cells but proliferates in response to tissue damage. Cell Stem Cell. 2009;5:290–297. doi: 10.1016/j.stem.2009.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garrett RW, Emerson SG. Bone and blood vessels: the hard and the soft of hematopoietic stem cell niches. Cell Stem Cell. 2009;4:503–506. doi: 10.1016/j.stem.2009.05.011. [DOI] [PubMed] [Google Scholar]
- Ghazizadeh S, Taichman LB. Organization of stem cells and their progeny in human epidermis. J Invest Dermatol. 2005;124:367–372. doi: 10.1111/j.0022-202X.2004.23599.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gönczy P, DiNardo S. The germ line regulates somatic cyst cell proliferation and fate during Drosophila spermatogenesis. Development. 1996;122:2437–2447. doi: 10.1242/dev.122.8.2437. [DOI] [PubMed] [Google Scholar]
- Greco V, Chen T, Rendl M, Schober M, Pasolli HA, Stokes N, Dela Cruz-Racelis J, Fuchs E. A two-step mechanism for stem cell activation during hair regeneration. Cell Stem Cell. 2009;4:155–169. doi: 10.1016/j.stem.2008.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gregorieff A, Pinto D, Begthel H, Destrée O, Kielman M, Clevers H. Expression pattern of Wnt signaling components in the adult intestine. Gastroenterology. 2005;129:626–638. doi: 10.1016/j.gastro.2005.06.007. [DOI] [PubMed] [Google Scholar]
- Harrison DA, Perrimon N. Simple and efficient generation of marked clones in Drosophila. Curr Biol. 1993;3:424–433. doi: 10.1016/0960-9822(93)90349-s. [DOI] [PubMed] [Google Scholar]
- Henderson ST, Gao D, Lambie EJ, Kimble J. lag-2 may encode a signaling ligand for the GLP-1 and LIN-12 receptors of C. elegans. Development. 1994;120:2913–2924. doi: 10.1242/dev.120.10.2913. [DOI] [PubMed] [Google Scholar]
- Hiratsuka S, Watanabe A, Aburatani H, Maru Y. Tumour-mediated upregulation of chemoattractants and recruitment of myeloid cells predetermines lung metastasis. Nat Cell Biol. 2006;8:1369–1375. doi: 10.1038/ncb1507. [DOI] [PubMed] [Google Scholar]
- Hodohara K, Fujii N, Yamamoto N, Kaushansky K. Stromal cell-derived factor-1 (SDF-1) acts together with thrombopoietin to enhance the development of megakaryocytic progenitor cells (CFU-MK) Blood. 2000;95:769–775. [PubMed] [Google Scholar]
- Hooper AT, Butler JM, Nolan DJ, Kranz A, Iida K, Kobayashi M, Kopp HG, Shido K, Petit I, Yanger K, et al. Engraftment and reconstitution of hematopoiesis is dependent on VEGFR2-mediated regeneration of sinusoidal endothelial cells. Cell Stem Cell. 2009;4:263–274. doi: 10.1016/j.stem.2009.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horsley V, O’Carroll D, Tooze R, Ohinata Y, Saitou M, Obukhanych T, Nussenzweig M, Tarakhovsky A, Fuchs E. Blimp1 defines a progenitor population that governs cellular input to the sebaceous gland. Cell. 2006;126:597–609. doi: 10.1016/j.cell.2006.06.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu HJ, Drummond-Barbosa D. Insulin levels control female germline stem cell maintenance via the niche in Drosophila. Proc Natl Acad Sci USA. 2009;106:1117–1121. doi: 10.1073/pnas.0809144106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu M, Yao J, Carroll DK, Weremowicz S, Chen H, Carrasco D, Richardson A, Violette S, Nikolskaya T, Nikolsky Y, et al. Regulation of in situ to invasive breast carcinoma transition. Cancer Cell. 2008;13:394–406. doi: 10.1016/j.ccr.2008.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Issigonis M, Tulina N, de Cuevas M, Brawley C, Sandler L, Matunis E. JAK-STAT signal inhibition regulates competition in the Drosophila testis stem cell niche. Science. 2009;326:153–156. doi: 10.1126/science.1176817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito M, Kizawa K, Hamada K, Cotsarelis G. Hair follicle stem cells in the lower bulge form the secondary germ, a biochemically distinct but functionally equivalent progenitor cell population, at the termination of catagen. Differentiation. 2004;72:548–557. doi: 10.1111/j.1432-0436.2004.07209008.x. [DOI] [PubMed] [Google Scholar]
- Ito M, Liu Y, Yang Z, Nguyen J, Liang F, Morris RJ, Cotsarelis G. Stem cells in the hair follicle bulge contribute to wound repair but not to homeostasis of the epidermis. Nat Med. 2005;11:1351–1354. doi: 10.1038/nm1328. [DOI] [PubMed] [Google Scholar]
- Ito M, Yang Z, Andl T, Cui C, Kim N, Millar SE, Cotsarelis G. Wnt-dependent de novo hair follicle regeneration in adult mouse skin after wounding. Nature. 2007;447:316–320. doi: 10.1038/nature05766. [DOI] [PubMed] [Google Scholar]
- Jahoda CA, Horne KA, Oliver RF. Induction of hair growth by implantation of cultured dermal papilla cells. Nature. 1984;311:560–562. doi: 10.1038/311560a0. [DOI] [PubMed] [Google Scholar]
- Jaks V, Barker N, Kasper M, van Es JH, Snippert HJ, Clevers H, Toftgård R. Lgr5 marks cycling, yet long-lived, hair follicle stem cells. Nat Genet. 2008;40:1291–1299. doi: 10.1038/ng.239. [DOI] [PubMed] [Google Scholar]
- Jiang H, Patel PH, Kohlmaier A, Grenley MO, McEwen DG, Edgar BA. Cytokine/Jak/Stat signaling mediates regeneration and homeostasis in the Drosophila midgut. Cell. 2009;137:1343–1355. doi: 10.1016/j.cell.2009.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin DK, Shido K, Kopp HG, Petit I, Shmelkov SV, Young LM, Hooper AT, Amano H, Avecilla ST, Heissig B, et al. Cytokine-mediated deployment of SDF-1 induces revascularization through recruitment of CXCR4+ hemangiocytes. Nat Med. 2006;12:557–567. doi: 10.1038/nm1400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin Z, Kirilly D, Weng C, Kawase E, Song X, Smith S, Schwartz J, Xie T. Differentiation-defective stem cells outcompete normal stem cells for niche occupancy in the Drosophila ovary. Cell Stem Cell. 2008;2:39–49. doi: 10.1016/j.stem.2007.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones PH, Watt FM. Separation of human epidermal stem cells from transit amplifying cells on the basis of differences in integrin function and expression. Cell. 1993;73:713–724. doi: 10.1016/0092-8674(93)90251-k. [DOI] [PubMed] [Google Scholar]
- Kai T, Spradling A. An empty Drosophila stem cell niche reactivates the proliferation of ectopic cells. Proc Natl Acad Sci USA. 2003;100:4633–4638. doi: 10.1073/pnas.0830856100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kai T, Spradling A. Differentiating germ cells can revert into functional stem cells in Drosophila melanogaster ovaries. Nature. 2004;428:564–569. doi: 10.1038/nature02436. [DOI] [PubMed] [Google Scholar]
- Kaplan RN, Riba RD, Zacharoulis S, Bramley AH, Vincent L, Costa C, MacDonald DD, Jin DK, Shido K, Kerns SA, et al. VEGFR1-positive haematopoietic bone marrow progenitors initiate the premetastatic niche. Nature. 2005;438:820–827. doi: 10.1038/nature04186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katayama Y, Battista M, Kao WM, Hidalgo A, Peired AJ, Thomas SA, Frenette PS. Signals from the sympathetic nervous system regulate hematopoietic stem cell egress from bone marrow. Cell. 2006;124:407–421. doi: 10.1016/j.cell.2005.10.041. [DOI] [PubMed] [Google Scholar]
- Kiel MJ, Yilmaz OH, Iwashita T, Yilmaz OH, Terhorst C, Morrison SJ. SLAM family receptors distinguish hematopoietic stem and progenitor cells and reveal endothelial niches for stem cells. Cell. 2005;121:1109–1121. doi: 10.1016/j.cell.2005.05.026. [DOI] [PubMed] [Google Scholar]
- Kiel MJ, Radice GL, Morrison SJ. Lack of evidence that hematopoietic stem cells depend on N-cadherin-mediated adhesion to osteoblasts for their maintenance. Cell Stem Cell. 2007;1:204–217. doi: 10.1016/j.stem.2007.06.001. [DOI] [PubMed] [Google Scholar]
- Kiger AA, Jones DL, Schulz C, Rogers MB, Fuller MT. Stem cell self-renewal specified by JAK-STAT activation in response to a support cell cue. Science. 2001;294:2542–2545. doi: 10.1126/science.1066707. [DOI] [PubMed] [Google Scholar]
- Kimble J. Alterations in cell lineage following laser ablation of cells in the somatic gonad of Caenorhabditis elegans. Dev Biol. 1981;87:286–300. doi: 10.1016/0012-1606(81)90152-4. [DOI] [PubMed] [Google Scholar]
- Kimble JE, White JG. On the control of germ cell development in Caenorhabditis elegans. Dev Biol. 1981;81:208–219. doi: 10.1016/0012-1606(81)90284-0. [DOI] [PubMed] [Google Scholar]
- Kobielak K, Pasolli HA, Alonso L, Polak L, Fuchs E. Defining BMP functions in the hair follicle by conditional ablation of BMP receptor IA. J Cell Biol. 2003;163:609–623. doi: 10.1083/jcb.200309042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korinek V, Barker N, Morin PJ, van Wichen D, de Weger R, Kinzler KW, Vogelstein B, Clevers H. Constitutive transcriptional activation by a beta-catenin-Tcf complex in APC−/− colon carcinoma. Science. 1997;275:1784–1787. doi: 10.1126/science.275.5307.1784. [DOI] [PubMed] [Google Scholar]
- Korinek V, Barker N, Moerer P, van Donselaar E, Huls G, Peters PJ, Clevers H. Depletion of epithelial stem-cell compartments in the small intestine of mice lacking Tcf-4. Nat Genet. 1998;19:379–383. doi: 10.1038/1270. [DOI] [PubMed] [Google Scholar]
- Kortesidis A, Zannettino A, Isenmann S, Shi S, Lapidot T, Gronthos S. Stromal-derived factor-1 promotes the growth, survival, and development of human bone marrow stromal stem cells. Blood. 2005;105:3793–3801. doi: 10.1182/blood-2004-11-4349. [DOI] [PubMed] [Google Scholar]
- Kuang S, Kuroda K, Le Grand F, Rudnicki MA. Asymmetric self-renewal and commitment of satellite stem cells in muscle. Cell. 2007;129:999–1010. doi: 10.1016/j.cell.2007.03.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawson DA, Xin L, Lukacs RU, Cheng D, Witte ON. Isolation and functional characterization of murine prostate stem cells. Proc Natl Acad Sci USA. 2007;104:181–186. doi: 10.1073/pnas.0609684104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Bras S, Van Doren M. Development of the male germline stem cell niche in Drosophila. Dev Biol. 2006;294:92–103. doi: 10.1016/j.ydbio.2006.02.030. [DOI] [PubMed] [Google Scholar]
- Leatherman JL, Dinardo S. Zfh-1 controls somatic stem cell self-renewal in the Drosophila testis and nonautonomously influences germline stem cell self-renewal. Cell Stem Cell. 2008;3:44–54. doi: 10.1016/j.stem.2008.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lechler T, Fuchs E. Asymmetric cell divisions promote stratification and differentiation of mammalian skin. Nature. 2005;437:275–280. doi: 10.1038/nature03922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee T, Luo L. Mosaic analysis with a repressible cell marker for studies of gene function in neuronal morphogenesis. Neuron. 1999;22:451–461. doi: 10.1016/s0896-6273(00)80701-1. [DOI] [PubMed] [Google Scholar]
- Leong KG, Wang BE, Johnson L, Gao WQ. Generation of a prostate from a single adult stem cell. Nature. 2008;456:804–808. doi: 10.1038/nature07427. [DOI] [PubMed] [Google Scholar]
- Levy V, Lindon C, Harfe BD, Morgan BA. Distinct stem cell populations regenerate the follicle and interfollicular epidermis. Dev Cell. 2005;9:855–861. doi: 10.1016/j.devcel.2005.11.003. [DOI] [PubMed] [Google Scholar]
- Levy V, Lindon C, Zheng Y, Harfe BD, Morgan BA. Epidermal stem cells arise from the hair follicle after wounding. FASEB J. 2007;21:1358–1366. doi: 10.1096/fj.06-6926com. [DOI] [PubMed] [Google Scholar]
- Lo Celso C, Fleming HE, Wu JW, Zhao CX, Miake-Lye S, Fujisaki J, Côté D, Rowe DW, Lin CP, Scadden DT. Live-animal tracking of individual haematopoietic stem/progenitor cells in their niche. Nature. 2009;457:92–96. doi: 10.1038/nature07434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Margolis J, Spradling A. Identification and behavior of epithelial stem cells in the Drosophila ovary. Development. 1995;121:3797–3807. doi: 10.1242/dev.121.11.3797. [DOI] [PubMed] [Google Scholar]
- Mauro A. Satellite cells of muscle skeletal fibers. J Biophys Biochem Cytol. 1961;9:493–495. doi: 10.1083/jcb.9.2.493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayack SR, Shadrach JL, Kim FS, Wagers AJ. Rejuvenation of aged hematopoietic stem cells through local and systemic signals. Nature. 2010 doi: 10.1038/nature08749. in press. [DOI] [PubMed] [Google Scholar]
- McGovern M, Voutev R, Maciejowski J, Corsi AK, Hubbard EJ. A “latent niche” mechanism for tumor initiation. Proc Natl Acad Sci USA. 2009;106:11617–11622. doi: 10.1073/pnas.0903768106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Micchelli CA, Perrimon N. Evidence that stem cells reside in the adult Drosophila midgut epithelium. Nature. 2006;439:475–479. doi: 10.1038/nature04371. [DOI] [PubMed] [Google Scholar]
- Morrison SJ, Spradling AC. Stem cells and niches: mechanisms that promote stem cell maintenance throughout life. Cell. 2008;132:598–611. doi: 10.1016/j.cell.2008.01.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakagawa T, Nabeshima Y, Yoshida S. Functional identification of the actual and potential stem cell compartments in mouse spermatogenesis. Dev Cell. 2007;12:195–206. doi: 10.1016/j.devcel.2007.01.002. [DOI] [PubMed] [Google Scholar]
- Naveiras O, Nardi V, Wenzel PL, Hauschka PV, Fahey F, Daley GQ. Bone-marrow adipocytes as negative regulators of the haematopoietic microenvironment. Nature. 2009;460:259–263. doi: 10.1038/nature08099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen DX, Bos PD, Massagué J. Metastasis: from dissemination to organ-specific colonization. Nat Rev Cancer. 2009;9:274–284. doi: 10.1038/nrc2622. [DOI] [PubMed] [Google Scholar]
- Nowak JA, Polak L, Pasolli HA, Fuchs E. Hair follicle stem cells are specified and function in early skin morphogenesis. Cell Stem Cell. 2008;3:33–43. doi: 10.1016/j.stem.2008.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nystul T, Spradling A. An epithelial niche in the Drosophila ovary undergoes long-range stem cell replacement. Cell Stem Cell. 2007;1:277–285. doi: 10.1016/j.stem.2007.07.009. [DOI] [PubMed] [Google Scholar]
- Ohlstein B, Spradling A. The adult Drosophila posterior midgut is maintained by pluripotent stem cells. Nature. 2006;439:470–474. doi: 10.1038/nature04333. [DOI] [PubMed] [Google Scholar]
- Ohlstein B, Spradling A. Multipotent Drosophila intestinal stem cells specify daughter cell fates by differential notch signaling. Science. 2007;315:988–992. doi: 10.1126/science.1136606. [DOI] [PubMed] [Google Scholar]
- Orimo A, Gupta PB, Sgroi DC, Arenzana-Seisdedos F, Delaunay T, Naeem R, Carey VJ, Richardson AL, Weinberg RA. Stromal fibroblasts present in invasive human breast carcinomas promote tumor growth and angiogenesis through elevated SDF-1/CXCL12 secretion. Cell. 2005;121:335–348. doi: 10.1016/j.cell.2005.02.034. [DOI] [PubMed] [Google Scholar]
- Paget S. The distribution of secondary growths in cancer of the breast. Lancet. 1889;1:571–573. [PubMed] [Google Scholar]
- Palmer TD, Willhoite AR, Gage FH. Vascular niche for adult hippocampal neurogenesis. J Comp Neurol. 2000;425:479–494. doi: 10.1002/1096-9861(20001002)425:4<479::aid-cne2>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- Pan L, Chen S, Weng C, Call G, Zhu D, Tang H, Zhang N, Xie T. Stem cell aging is controlled both intrinsically and extrinsically in the Drosophila ovary. Cell Stem Cell. 2007;1:458–469. doi: 10.1016/j.stem.2007.09.010. [DOI] [PubMed] [Google Scholar]
- Potten CS, Kovacs L, Hamilton E. Continuous labelling studies on mouse skin and intestine. Cell Tissue Kinet. 1974;7:271–283. doi: 10.1111/j.1365-2184.1974.tb00907.x. [DOI] [PubMed] [Google Scholar]
- Rieger MA, Schroeder T. Exploring hematopoiesis at single cell resolution. Cells Tissues Organs. 2008;188:139–149. doi: 10.1159/000114540. [DOI] [PubMed] [Google Scholar]
- Rieger MA, Hoppe PS, Smejkal BM, Eitelhuber AC, Schroeder T. Hematopoietic cytokines can instruct lineage choice. Science. 2009;325:217–218. doi: 10.1126/science.1171461. [DOI] [PubMed] [Google Scholar]
- Rodier F, Coppé JP, Patil CK, Hoeijmakers WA, Muñoz DP, Raza SR, Freund A, Campeau E, Davalos AR, Campisi J. Persistent DNA damage signalling triggers senescence-associated inflammatory cytokine secretion. Nat Cell Biol. 2009;11:973–979. doi: 10.1038/ncb1909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryu BY, Orwig KE, Oatley JM, Avarbock MR, Brinster RL. Effects of aging and niche microenvironment on spermatogonial stem cell self-renewal. Stem Cells. 2006;24:1505–1511. doi: 10.1634/stemcells.2005-0580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sacco A, Doyonnas R, Kraft P, Vitorovic S, Blau HM. Self-renewal and expansion of single transplanted muscle stem cells. Nature. 2008;456:502–506. doi: 10.1038/nature07384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sangiorgi E, Capecchi MR. Bmi1 is expressed in vivo in intestinal stem cells. Nat Genet. 2008;40:915–920. doi: 10.1038/ng.165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato T, Vries RG, Snippert HJ, van de Wetering M, Barker N, Stange DE, van Es JH, Abo A, Kujala P, Peters PJ, Clevers H. Single Lgr5 stem cells build cryptvillus structures in vitro without a mesenchymal niche. Nature. 2009;459:262–265. doi: 10.1038/nature07935. [DOI] [PubMed] [Google Scholar]
- Schofield R. The relationship between the spleen colony-forming cell and the haemopoietic stem cell. Blood Cells. 1978;4:7–25. [PubMed] [Google Scholar]
- Schroeder T. Imaging stem-cell-driven regeneration in mammals. Nature. 2008;453:345–351. doi: 10.1038/nature07043. [DOI] [PubMed] [Google Scholar]
- Shackleton M, Vaillant F, Simpson KJ, Stingl J, Smyth GK, Asselin-Labat ML, Wu L, Lindeman GJ, Visvader JE. Generation of a functional mammary gland from a single stem cell. Nature. 2006;439:84–88. doi: 10.1038/nature04372. [DOI] [PubMed] [Google Scholar]
- Shen Q, Goderie SK, Jin L, Karanth N, Sun Y, Abramova N, Vincent P, Pumiglia K, Temple S. Endothelial cells stimulate self-renewal and expand neurogenesis of neural stem cells. Science. 2004;304:1338–1340. doi: 10.1126/science.1095505. [DOI] [PubMed] [Google Scholar]
- Shen Q, Wang Y, Kokovay E, Lin G, Chuang SM, Goderie SK, Roysam B, Temple S. Adult SVZ stem cells lie in a vascular niche: a quantitative analysis of niche cell-cell interactions. Cell Stem Cell. 2008;3:289–300. doi: 10.1016/j.stem.2008.07.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheng XR, Brawley CM, Matunis EL. Dedifferentiating spermatogonia outcompete somatic stem cells for niche occupancy in the Drosophila testis. Cell Stem Cell. 2009;5:191–203. doi: 10.1016/j.stem.2009.05.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherwood RI, Christensen JL, Conboy IM, Conboy MJ, Rando TA, Weissman IL, Wagers AJ. Isolation of adult mouse myogenic progenitors: functional heterogeneity of cells within and engrafting skeletal muscle. Cell. 2004;119:543–554. doi: 10.1016/j.cell.2004.10.021. [DOI] [PubMed] [Google Scholar]
- Shinohara T, Orwig KE, Avarbock MR, Brinster RL. Spermatogonial stem cell enrichment by multiparameter selection of mouse testis cells. Proc Natl Acad Sci USA. 2000;97:8346–8351. doi: 10.1073/pnas.97.15.8346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shirota T, Tavassoli M. Cyclophosphamide-induced alterations of bone marrow endothelium: implications in homing of marrow cells after transplantation. Exp Hematol. 1991;19:369–373. [PubMed] [Google Scholar]
- Singh SR, Liu W, Hou SX. The adult Drosophila malpighian tubules are maintained by multipotent stem cells. Cell Stem Cell. 2007;1:191–203. doi: 10.1016/j.stem.2007.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sipkins DA, Wei X, Wu JW, Runnels JM, Côté D, Means TK, Luster AD, Scadden DT, Lin CP. In vivo imaging of specialized bone marrow endothelial microdomains for tumour engraftment. Nature. 2005;435:969–973. doi: 10.1038/nature03703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sneddon JB, Zhen HH, Montgomery K, van de Rijn M, Tward AD, West R, Gladstone H, Chang HY, Morganroth GS, Oro AE, Brown PO. Bone morphogenetic protein antagonist gremlin 1 is widely expressed by cancer-associated stromal cells and can promote tumor cell proliferation. Proc Natl Acad Sci USA. 2006;103:14842–14847. doi: 10.1073/pnas.0606857103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song X, Call GB, Kirilly D, Xie T. Notch signaling controls germline stem cell niche formation in the Drosophila ovary. Development. 2007;134:1071–1080. doi: 10.1242/dev.003392. [DOI] [PubMed] [Google Scholar]
- Stier S, Ko Y, Forkert R, Lutz C, Neuhaus T, Grünewald E, Cheng T, Dombkowski D, Calvi LM, Rittling SR, Scadden DT. Osteopontin is a hematopoietic stem cell niche component that negatively regulates stem cell pool size. J Exp Med. 2005;201:1781–1791. doi: 10.1084/jem.20041992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stingl J, Eirew P, Ricketson I, Shackleton M, Vaillant F, Choi D, Li HI, Eaves CJ. Purification and unique properties of mammary epithelial stem cells. Nature. 2006;439:993–997. doi: 10.1038/nature04496. [DOI] [PubMed] [Google Scholar]
- Sugiyama T, Kohara H, Noda M, Nagasawa T. Maintenance of the hematopoietic stem cell pool by CXCL12-CXCR4 chemokine signaling in bone marrow stromal cell niches. Immunity. 2006;25:977–988. doi: 10.1016/j.immuni.2006.10.016. [DOI] [PubMed] [Google Scholar]
- Suzuki H, Sada A, Yoshida S, Saga Y. The heterogeneity of spermatogonia is revealed by their topology and expression of marker proteins including the germ cell-specific proteins Nanos2 and Nanos3. Dev Biol. 2009;336:222–231. doi: 10.1016/j.ydbio.2009.10.002. [DOI] [PubMed] [Google Scholar]
- Takashima S, Mkrtchyan M, Younossi-Hartenstein A, Merriam JR, Hartenstein V. The behavior of Drosophila adult hindgut stem cells is controlled by Wnt and Hh signalling. Nature. 2008;454:651–655. doi: 10.1038/nature07156. [DOI] [PubMed] [Google Scholar]
- Tavazoie M, Van der Veken L, Silva-Vargas V, Louissaint M, Colonna L, Zaidi B, Garcia-Verdugo JM, Doetsch F. A specialized vascular niche for adult neural stem cells. Cell Stem Cell. 2008;3:279–288. doi: 10.1016/j.stem.2008.07.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tegelenbosch RA, de Rooij DG. A quantitative study of spermatogonial multiplication and stem cell renewal in the C3H/101 F1 hybrid mouse. Mutat Res. 1993;290:193–200. doi: 10.1016/0027-5107(93)90159-d. [DOI] [PubMed] [Google Scholar]
- Tlsty TD, Coussens LM. Tumor stroma and regulation of cancer development. Annu Rev Pathol. 2006;1:119–150. doi: 10.1146/annurev.pathol.1.110304.100224. [DOI] [PubMed] [Google Scholar]
- Trimboli AJ, Cantemir-Stone CZ, Li F, Wallace JA, Merchant A, Creasap N, Thompson JC, Caserta E, Wang H, Chong JL, et al. Pten in stromal fibroblasts suppresses mammary epithelial tumours. Nature. 2009;461:1084–1091. doi: 10.1038/nature08486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tulina N, Matunis E. Control of stem cell self-renewal in Drosophila spermatogenesis by JAK-STAT signaling. Science. 2001;294:2546–2549. doi: 10.1126/science.1066700. [DOI] [PubMed] [Google Scholar]
- Tumbar T, Guasch G, Greco V, Blanpain C, Lowry WE, Rendl M, Fuchs E. Defining the epithelial stem cell niche in skin. Science. 2004;303:359–363. doi: 10.1126/science.1092436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Scott EJ, Reinertson RP. The modulating influence of stromal environment on epithelial cells studied in human autotransplants. J Invest Dermatol. 1961;36:109–131. [PubMed] [Google Scholar]
- Visnjic D, Kalajzic Z, Rowe DW, Katavic V, Lorenzo J, Aguila HL. Hematopoiesis is severely altered in mice with an induced osteoblast deficiency. Blood. 2004;103:3258–3264. doi: 10.1182/blood-2003-11-4011. [DOI] [PubMed] [Google Scholar]
- Voog J, D’Alterio C, Jones DL. Multipotent somatic stem cells contribute to the stem cell niche in the Drosophila testis. Nature. 2008;454:1132–1136. doi: 10.1038/nature07173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagers AJ. Stem cell grand SLAM. Cell. 2005;121:967–970. doi: 10.1016/j.cell.2005.06.017. [DOI] [PubMed] [Google Scholar]
- Ward EJ, Shcherbata HR, Reynolds SH, Fischer KA, Hatfield SD, Ruohola-Baker H. Stem cells signal to the niche through the Notch pathway in the Drosophila ovary. Curr Biol. 2006;16:2352–2358. doi: 10.1016/j.cub.2006.10.022. [DOI] [PubMed] [Google Scholar]
- Weissman IL, Anderson DJ, Gage F. Stem and progenitor cells: origins, phenotypes, lineage commitments, and transdifferentiations. Annu Rev Cell Dev Biol. 2001;17:387–403. doi: 10.1146/annurev.cellbio.17.1.387. [DOI] [PubMed] [Google Scholar]
- Wels J, Kaplan RN, Rafii S, Lyden D. Migratory neighbors and distant invaders: tumor-associated niche cells. Genes Dev. 2008;22:559–574. doi: 10.1101/gad.1636908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson A, Laurenti E, Oser G, van der Wath RC, Blanco-Bose W, Jaworski M, Offner S, Dunant CF, Eshkind L, Bockamp E, et al. Hematopoietic stem cells reversibly switch from dormancy to self-renewal during homeostasis and repair. Cell. 2008;135:1118–1129. doi: 10.1016/j.cell.2008.10.048. [DOI] [PubMed] [Google Scholar]
- Wurmser AE, Nakashima K, Summers RG, Toni N, D’Amour KA, Lie DC, Gage FH. Cell fusion-independent differentiation of neural stem cells to the endothelial lineage. Nature. 2004;430:350–356. doi: 10.1038/nature02604. [DOI] [PubMed] [Google Scholar]
- Xie T, Spradling AC. decapentaplegic is essential for the maintenance and division of germline stem cells in the Drosophila ovary. Cell. 1998;94:251–260. doi: 10.1016/s0092-8674(00)81424-5. [DOI] [PubMed] [Google Scholar]
- Xie T, Spradling AC. A niche maintaining germ line stem cells in the Drosophila ovary. Science. 2000;290:328–330. doi: 10.1126/science.290.5490.328. [DOI] [PubMed] [Google Scholar]
- Xie Y, Yin T, Wiegraebe W, He XC, Miller D, Stark D, Perko K, Alexander R, Schwartz J, Grindley JC, et al. Detection of functional haematopoietic stem cell niche using real-time imaging. Nature. 2009;457:97–101. doi: 10.1038/nature07639. [DOI] [PubMed] [Google Scholar]
- Yamashita YM, Mahowald AP, Perlin JR, Fuller MT. Asymmetric inheritance of mother versus daughter centrosome in stem cell division. Science. 2007;315:518–521. doi: 10.1126/science.1134910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida S, Sukeno M, Nabeshima Y. A vasculature-associated niche for undifferentiated spermatogonia in the mouse testis. Science. 2007;317:1722–1726. doi: 10.1126/science.1144885. [DOI] [PubMed] [Google Scholar]
- Zhang J, Niu C, Ye L, Huang H, He X, Tong WG, Ross J, Haug J, Johnson T, Feng JQ, et al. Identification of the haematopoietic stem cell niche and control of the niche size. Nature. 2003;425:836–841. doi: 10.1038/nature02041. [DOI] [PubMed] [Google Scholar]
- Zhang YV, Cheong J, Ciapurin N, McDermitt DJ, Tumbar T. Distinct self-renewal and differentiation phases in the niche of infrequently dividing hair follicle stem cells. Cell Stem Cell. 2009;5:267–278. doi: 10.1016/j.stem.2009.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]