Abstract
hMena and the epithelial specific isoform hMena11a are actin cytoskeleton regulatory proteins belonging to the Ena/VASP family. EGF treatment of breast cancer cell lines upregulates hMena/hMena11a expression and phosphorylates hMena11a, suggesting cross-talk between the ErbB receptor family and hMena/hMena11a in breast cancer. The aim of this study was to determine whether the hMena/hMena11a overexpression cooperates with HER-2 signalling, thereby affecting the HER2 mitogenic activity in breast cancer. In a cohort of breast cancer tissue samples a significant correlation among hMena, HER2 overexpression, the proliferation index (high Ki67), and phosphorylated MAPK and AKT was found and among the molecular subtypes the highest frequency of hMena overexpressing tumors was found in the HER2 subtype. From a clinical viewpoint, concomitant overexpression of HER2 and hMena identifies a subgroup of breast cancer patients showing the worst prognosis, indicating that hMena overexpression adds prognostic information to HER2 overexpressing tumors. To identify a functional link between HER2 and hMena, we show here that HER2 transfection in MCF7 cells increased hMena/hMena11a expression and hMena11a phosphorylation. On the other hand, hMena/hMena11a knock-down reduced HER3, AKT and p44/42 MAPK phosphorylation and inhibited the EGF and NRG1-dependent HER2 phosphorylation and cell proliferation. Of functional significance, hMena/hMena11a knock-down reduced the mitogenic activity of EGF and NRG1. Collectively these data provide new insights into the relevance of hMena and hMena11a as downstream effectors of the ErbB receptor family which may represent a novel prognostic indicator in breast cancer progression, helping to stratify patients.
Introduction
Breast cancer is a heterogeneous disease and in recent years the introduction of new targeted therapeutic approaches highlights the need to stratify patients and consequently to identify accurate biomarkers to select the best therapeutic choice. The molecular classification of breast tumors has identified tumor subtypes, among which the overexpression of the ErbB family of receptors is confined to tumors with unfavourable prognosis [1]. The human ErbB receptor family comprises four tyrosine kinases members (EGFR, HER2, HER3 and HER4) and their deregulation has been correlated with cancer development and progression [1], [2]. Upon ligand binding ErbB receptors undergo homodimerization or heterodimerization and activate a complex signalling network that controls tumor cell proliferation as well as motility through different pathways that regulate rearrangements of the actin cytoskeleton [3]. HER2 functions as a common co-receptor recruited from EGFR or HER3 upon binding to their ligands [4] and HER2/HER3 heterodimer has been defined as the major oncogenic unit in HER2 positive breast cancer [5], [6]. The two main signalling pathways downstream from the ErbB receptors are the phosphatidylinositol 3′-kinase (PI3K) and the mitogen activated protein kinase (MAPK) [4]. PI3K activation results in the phosphorylation of PIP2 to yield PIP3 which in turn activates several downstream signalling molecules including AKT and regulates actin regulatory proteins such as cofillin and capping proteins [7], [8]. MAPK activation is responsible for the EGF dependent mitogenic effect in normal and transformed mammary epithelia [9] and its role in cell cycle progression is also sustained by cytoskeletal organization [10].
hMena (ENAH), the human ortholog of murine Mena [11], is a member of the Ena/VASP protein family which in mammalians includes Mena, VASP and Evl. Ena/VASP are key actin regulatory molecules that control cell shape, adhesion and migration [12], [13], processes that are frequently deregulated following neoplastic transformation. Ena/VASP proteins behave as homo and heterotetramers, and the functions of the three members are redundant in cells and in vivo. However, only Mena may be regulated by the tumor suppressor Tes, which binds the EVH1 domain of Mena and not VASP or EVL through an unconventional Lim-domain mediated interaction [14].
Recently, we have demonstrated that hMena overexpression represents an early marker of breast tumorigenesis, being undetectable in normal breast epithelium, and overexpressed in benign breast lesions with an increased risk of transformation and, in more than 70% of tumors, with HER2+, ER/PgR- and high Ki67 phenotype [15]. While Neuregulin-1 (NRG1) and EGF growth factors induce an increase of hMena expression, Herceptin treatment down-regulates hMena in breast cancer cell lines overexpressing HER2 [15], [16]. The hMena isoform hMena11a, recently characterized by our group, shows an additional 21 amino acids betweeen the F-actin binding and the coiled-coild domain in the EVH2 domain which possesses three putative phosphorylation sites [16]. This isoform undergoes phosphorylation upon long term stimulation with EGF, associated with p44/42 MAPK activation and with an increased proliferation rate in breast cancer cell lines [16]. hMena11a expression is associated with an epithelial phenotype [16], [17], [18] and identifies human pancreatic adenocarcinoma cell lines that are sensitive to the EGFR inhibitor Erlotinib [17]. Thus, we hypothesize that cross-talk takes place between the ErbB receptor family and hMena.
In the present work, through a combination of experimental and in vivo studies, we have demonstrated that hMena/hMena11a are downstream targets of HER2 activity and that they affect HER2 signalling. Moreover, hMena overexpression is a frequent event in the HER2 breast tumor subtype and significantly correlates with HER2 overexpressing tumors and an activated status of MAPK and AKT. On the contrary, depletion of hMena by specific RNA interference reduces the phosphorylation of HER3, p44/42 MAPK and AKT and inhibits both EGF and NRG1-mediated phosphorylation of HER2 and EGFR as well as cell proliferation. Of note in breast cancer patients, undetectable levels of hMena are more frequent in luminal breast tumors, and discriminate HER2 overexpressing tumors with downstream inactive pathways carried by patients that have a better prognosis. Conversely, the concomitant overexpression of HER2 and hMena identifies a subgroup of breast cancer patients with a worse prognosis indicating that hMena overexpression adds prognostic information in HER2 overexpressing tumors.
Results
hMena overexpression occurs more frequently in the HER2 subtype and correlates with Ki67, MAPK and AKT activity
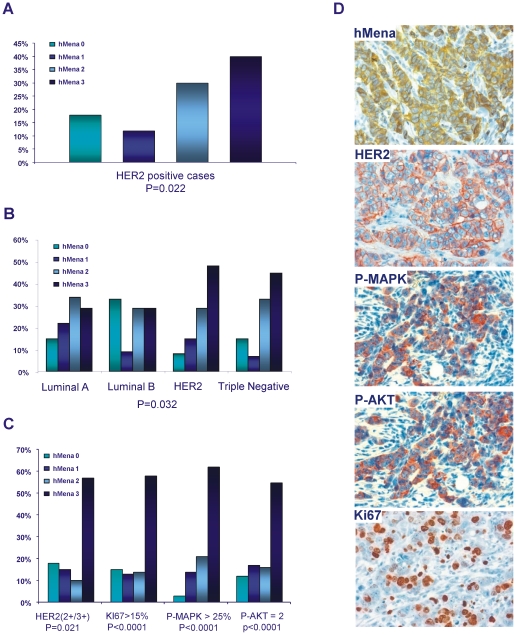
We have evaluated hMena expression in a series of 286 primary breast cancer (BC) classified in Luminal A (ERα/PR+ HER2-), Luminal B (ERα/PR+ HER2+), Triple Negative (ERα/PR/HER2−) and HER2 subtype (ERα/PR− HER2+), as indicated in Table 1. The immunohistochemical analysis demonstrated a significant linear trend (p = 0.022) between hMena and HER2 expression, independent of breast tumor subtype. Figure 1 panel A shows the frequency of HER2 positive tumors (2+ with gene amplification and 3+ score) with different hMena staining. Of interest, hMena is differently distributed within the four BC subtypes (p = 0.032). In fact, only 29% of the Luminal A and Luminal B subtypes overexpressed hMena with a score 3, whereas 48% and 44% of the HER2 and the Triple Negative subtypes, respectively showed hMena overexpression (Figure 1 panel B).
Table 1. Clinico-pathological characteristics of the patients.
| CHARACTERISTICS | No of cases | % |
| Number of patients | 286 | |
| Median age, y (range) 53 (28–86) | ||
| hMena | ||
| Negative (score 0/1) | 96 | 34 |
| Positive (score 2/3) | 190 | 66 |
| Histotype | ||
| Invasive ductal carcinoma | 246 | 86 |
| Invasive lobular carcinoma | 28 | 10 |
| Other carcinomas | 12 | 4 |
| Histologic grade | ||
| Grade 1 | 27 | 10 |
| Grade 2 | 146 | 54 |
| Grade 3 | 96 | 36 |
| Lymph node status | ||
| Negative | 154 | 54 |
| Positive | 132 | 46 |
| Tumor size | ||
| T1 | 171 | 60 |
| T2 | 80 | 29 |
| T3,4 | 28 | 11 |
| ER | ||
| Negative | 89 | 31 |
| Positive | 197 | 69 |
| PgR | ||
| Negative | 107 | 37 |
| Positive | 179 | 63 |
| Ki67 | ||
| Negative (≤15) | 164 | 57 |
| Positive (>15) | 122 | 43 |
| HER-2 | ||
| Negative | 204 | 71 |
| Positive | 82 | 29 |
| Molecular Subtypes | ||
| Luminal A | 177 | 62 |
| Luminal B | 34 | 12 |
| Triple Negative | 27 | 9 |
| HER2 subtype | 48 | 17 |
Figure 1. hMena is differently distributed among breast cancer subtypes and correlates with HER2 overexpression, MAPK, AKT activity and Ki67.
A. Relationship between hMena staining and HER2 positivity in human breast carcinomas. Histograms represent the percentage of the hMena staining (score 0; 1; 2; 3) in 82 HER2 positive breast cancer samples (2+ with gene amplification and 3+) (p = 0.02). B. hMena distribution within 286 human breast carcinomas grouped according to molecular subtypes. hMena expression (0, 1, 2, 3 scores) in Luminal A, Luminal B, HER2 subtypes and Triple Negative. The percentage of hMena-positive cases (score 3) is significantly higher in the HER2 subtype and Triple-Negative in comparison to Luminal A and Luminal B breast cancer (p = 0.032). C. Correlation between hMena expression and HER2, P-AKT, P-MAPK and Ki67 in a subgroup of 178 breast cancer patients. Percentage of HER2 positivity, high Ki67 proliferation index, elevated P-AKT and P-MAPK expression for each hMena score. The percentage of HER2 positivity (p = 0.016), P-AKT (p<0.001) and P-MAPK (p<0.001) overexpression, high Ki67 proliferation index (p = 0.016) increase significantly in relationship to hMena score (0 to 1 to 2 to 3). D. Representative case of an invasive ductal carcinoma with a strong hMena positivity (score 3+) displaying HER2 overexpression (score 3 by IHC), P-MAPK and P-AKT positivity, elevated Ki67 index. Magnification, 40x. Original scale 1 cm = 30micron (25 cm×33 cm).
To analyze downstream signalling pathways of the ErbB family by immunohistochemistry (IHC), the expression of the phospho-MAPK (P-MAPK) and phospho-AKT (P-AKT) were analyzed in 178 BC randomly selected from the entire series of 286 patients included in our study. The IHC findings, reported in Figure 1 panel C, clearly demonstrate a significant correlation between hMena (score 3), HER2 overexpression (p = 0.021) and the proliferation index as assessed by Ki-67 (p<0.0001), as we have already reported in a different cohort of patients [15]. A strong correlation among hMena overexpression and P-AKT and P-MAPK positivity (p<0.0001 in both cases) was found. Immunohistochemical analyses of serial sections of a representative invasive BC are reported in Figure 1 panel D.
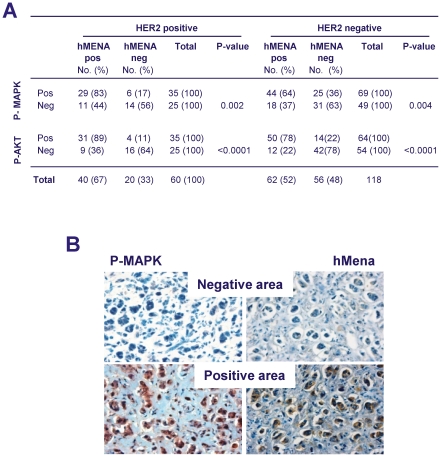
To evaluate the role of hMena overexpression in MAPK and AKT activation, we categorized in hMena negative (score 0/1) and hMena positive (score 2/3) 60 HER2 positive and 118 HER2 negative primary BC of 178 cases analyzed. As reported in Figure 2 panel A, only 17% and 11% HER2-positive/hMena-negative tumors expressed P-MAPK and P-AKT, whereas the majority of HER2-positive/hMena-positive tumors showed an activated status of both MAPK (83.1%) and AKT (89%). In HER2 negative tumors hMena expression has a minor contribution in MAPK and AKT activity, suggesting that hMena overexpression cooperates with HER2 in sustaining MAPK and AKT activity. Interestingly, as shown in a representative, although not paradigmatic case (Figure 2 panel B) in which P-MAPK displayed a heterogeneous immunostaining (40% tumor cells), hMena positivity was mainly found in the tumor area positive for P-MAPK and viceversa.
Figure 2. hMena overexpression correlates with the phosphorylated status of MAPK and AKT.
A. The table evidences that in 60 HER2 positive and 118 HER2 negative breast tumors the percentage of P-MAPK and P-AKT positive breast tumors is significantly higher in hMena positive (score 2/3) than in hMena negative breast tumors (score 0/1). B. A representative, although not a paradigmatic case, where P-MAPK displays a heterogeneous immunostaining (40% tumor cells). P-MAPK positivity is mostly confined in the tumor area (positive area) concomitantly showing a strong hMena (score 3) immunoreactivity.
HER2 overexpression and activity affect hMena/hMena11a overexpression in human breast cancer cell lines
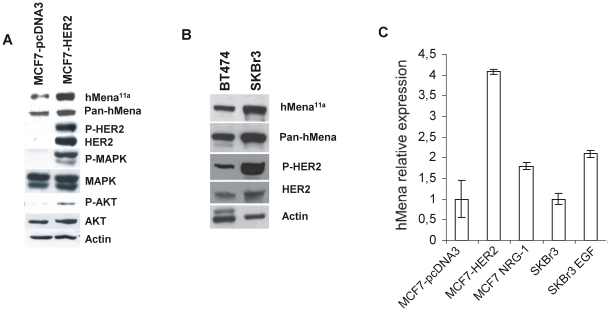
We previously reported that epithelial cancer cell lines express hMena and the epithelial specific hMena11a isoform [16]. To investigate the functional link between hMena/hMena11a and HER2, we stably transfected HER2 in MCF7, a luminal epithelial breast cancer cell line with low HER2 endogenous levels [19]. HER2 overexpression in MCF7 cells leads to the upregulation of hMena and hMena11a compared to control cells (MCF7-pCDNA3) as evaluated by pan-hMena and specific hMena11a antibodies (Figure 3 panel A). This was associated with a phosphorylation of the tyrosine Y1248 in HER2 receptor and the activation of MAPK and PI3K as documented by the amount of phosphorylated p44/42 MAPK and AKT (Figure 3 panel A). Phosphorylation at Y1248 of HER2 was also observed in BT474 and SKBr3 BC cells, naturally overexpressing HER2 and hMena/hMena11a (Figure 3 panel B). Real-time qRT-PCR experiments demonstrated a 4 fold upregulation of hMena mRNA in MCF7-HER2 cells with respect to MCF7-pcDNA3 control cells. Treatment for 24 h of MCF7-pcDNA3 and SKBr3 cells respectively with NRG1 (10 ng/ml) and EGF (100 ng/ml), factors that indirectly activate HER2 by heterodimerization with HER3 and EGFR respectively, determined an increase in hMena RNA (1.8 and 2.1 fold respectively) (Figure 3 panel C). These results show that the growth factor-mediated hMena protein upregulation reported previously [15], [16] is related to an increase in hMena mRNA and indicate that HER2 overexpression and activity contribute to hMena/hMena11a overexpression in BC cell lines.
Figure 3. HER2 overexpression and activity affect hMena/hMena11a expression in human breast cancer cell lines.
A. HER2 transfection affects hMena expression in MCF7 breast cancer cells. Western blot analysis of MCF7-pcDNA3 and MCF7-HER2 lysates (50 µg) with the indicated antibodies. Two different antibodies for hMena have been used: the pan-hMena antibody, recognizing hMena and hMena11a as a doublet of 88–90 kDa, and the anti-hMena11a specific antibody. Membranes were sequentially stripped and reprobed with the indicated total and phospho-specific antibodies. B. hMena/hMena11a expression in HER2 overexpressing breast cancer cell lines. Western blot analysis of BT474 and SKBr3 breast cancer cell line lysates (50 µg) with the indicated antibodies. As loading control, blots were probed with anti-Actin mAb (1 µg/ml). C. HER2 transfection, NRG and EGF treatment affect hMena expression at mRNA level. QRT-PCR analysis of hMena and housekeeping β-Actin mRNA levels performed on 10 ng of total RNA from the untreated MCF7-pcDNA3, HER2 transfected MCF7, or NRG1 treated MCF7 breast cancer cell line and SKBr3 cells either untreated or treated with EGF. Results are given as the ratio between hMena and β-Actin genes relative to the internal control.
HER2 overexpression affects hMena11a phosphorylation in breast cancer cells
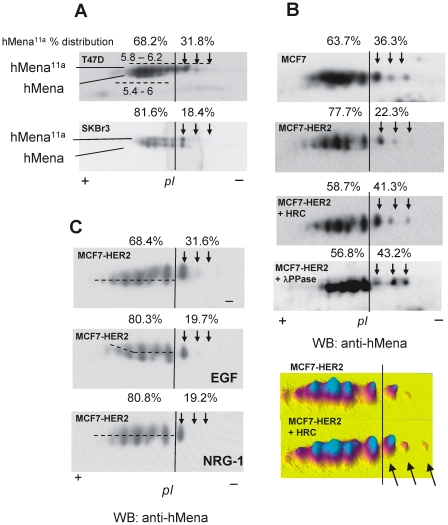
EGF treatment of BC cell lines promotes concomitant up-regulation of hMena and hMena11a, resulting in a higher fraction of phosphorylated hMena11a isoform. Moreover, hMena11a overexpression and phosphorylation lead to an increase in p44/42 mitogen-activated protein kinase (MAPK) activation and cell proliferation [16]. Thus we analyzed whether hMena11a phosphorylation is downstream to HER2 signalling. T47D and MCF7 cells, with low endogenous levels of HER2 [19], display only modest hMena11a phosphorylation as evaluated by 2D-E analysis (Figure 4, panels A and B). Conversely, SKBr3 cells, endogenously overexpressing highly phosphorylated HER2 (Figure 3 panel B) show high amounts of phosphorylated hMena11a (Figure 4 panel A). To better define hMena11a as a downstream target of HER2, the levels of hMena11a phosphorylation were evaluated in MCF7-HER2 cells. As depicted by the pattern of spots presented in Figure 4 panel B, a higher percentage (77.7% vs. 63.7%) of phosphorylated hMena11a was seen in the MCF7-HER2 with respect to control (MCF7) cells, as revealed by the more pronounced shift toward lower pH, which is significantly reduced by λ phosphatase treatment. Herceptin treatment down-regulates hMena in MCF7-HER2 cells (data not shown), confirming our previous results in endogenously HER2 overexpressing BC cell lines [15]. Thus, to determine if Herceptin treatment is able to inhibit HER2-dependent hMena11a phosphorylation, MCF7-HER2 cells were treated with Herceptin 25 µg/ml for 48 h, and the percentage of phosphorylated hMena11a was reduced (58.7% vs 77.7%) to a level similar to that observed after λ phosphatase treatment (Figure 4 panel B).
Figure 4. HER2 overexpression affects hMena11a phosphorylation in breast cancer cells.
A. Proteins from T47D and SKBr3 cell lines were resolved by 2D-electrophoresis on a pH 3–10 nonlinear range and 7.5% acrylamide SDS-PAGE. Proteins were then electrontransferred to nitrocellulose and hMena reactivity was revealed by Western blot. Protein spot trains were compared by using Progenesis PG240 v2006 software (Nonlinear dynamics, Newcastle, UK). The set of spots corresponding to hMena and hMena11a are indicated including the isoelectric point range (dashed line). The phosphorylation threshold (arrows and vertical bars) has been arbitrarily defined as described [13]. B. Lysates of MCF7 cells, either stably transfected with HER2 or with empty vector (pcDNA3) were resolved by 2D-electrophoresis as described above, showing that a higher fraction of hMena11a is constitutively phosphorylated in MCF7-HER2 cells. MCF7-HER2 cells treated with Herceptin for 48 h (MCF7-HER2+ HRC) show a fraction of phosphorylated hMena11a similar to the dephosphorylated MCF7-HER2 lysates. MCF7-HER2 cell lysates were also incubated without or with λ-phosphatase (λ-PPase) before 2D analysis. Arrows indicated hMena11a spots after phosphatase treatment. Below the densitometric analysis of the hMena spots revealed by Western blot in MCF7-HER2 cells treated without (- HRC) or with Herceptin for 48 h (+ HRC) are shown. Arrows indicated hMena11a spots that, after Herceptin treatment, resulted to be dephosphorylated. Pseudo-colours three-dimensional view has been obtained by using Progenesis PG240 v2006 software. C. Proteins from the MCF7-HER2 cell lines incubated with or without 100 ng/ml EGF or 10 ng/ml NRG1 for 24 h in serum starved medium, were resolved by 2D-electrophoresis and analyzed as described in panel A.
To evaluate whether hMena11a phosphorylation is further increased by ligand dependent HER2 activation, we treated MCF7-HER2 cells either with EGF or NRG1. After 24 h of EGF treatment, a higher amount of hMena11a was phosphorylated (80.2%) compared to untreated starved cells (68.3%) (Figure 4 panel C), suggesting that paracrine signals may affect hMena11a phosphorylation. NRG1 treatment induced a phosphorylation pattern of hMena11a (80.8%), similar to that observed with EGF treatment. Overall, these results show that hMena11a is downstream to either the EGFR/HER2 or HER2/HER3 signal transduction pathways. These findings were confirmed in the spontaneously HER2 overexpressing SKBr3 cells where treatment with both EGF and NRG1 slightly increased the phosphorylation level of the constitutively high phosphorylated hMena11a (data not shown).
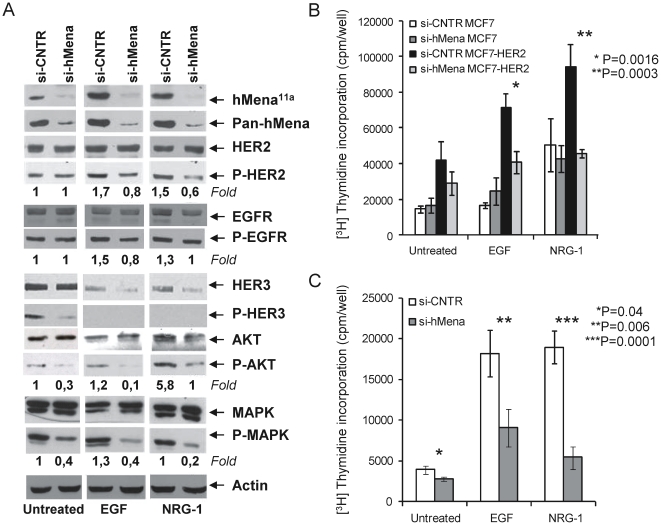
hMena knock-down inhibits ErbB family signalling and the EGF/NRG1-mediated mitogenic effect in MCF7-HER2 cells
To investigate whether hMena/hMena11a overexpression could affect HER2 expression and activation, the MCF7-HER2 cells were depleted for hMena/hMena11a by siRNA. hMena silencing in starved cells did not affect HER2 and EGFR expression and phosphorylation, but was associated with a reduction in phospho-HER3, phospho-AKT and phospho-p44/42 MAPK levels (Figure 5 panel A). On the other hand, hMena/hMena11a knock-down prevented EGF and NRG1 mediated HER2 and EGFR activation. In particular, hMena/hMena11a depleted cells (si-hMena) treated with EGF or NRG1 for 24 h showed a reduction of phospho-HER2 and phospho-EGFR levels with respect to control cells (Si-CNTR) (Figure 5 panel A). Phospho-HER3 was not detectable following EGF and NRG1 treatment, due to treatment induced down-regulation of the HER3 protein, more evident in the hMena/hMena11a depleted cells.
Figure 5. hMena knock-down affects HER2 signalling and inhibits the EGF/NRG1-mediated mitogenic effects in MCF7-HER2 cells.
A. Western blot analysis of MCF7-HER2 breast cancer cell line after 72 h transfection with control and hMena/hMena11a -specific siRNAs, untreated or treated with EGF (100 ng/ml) or NRG1 (10 ng/ml) for the last 24 h of transfection. hMena/hMena11a expression (evaluated by pan-hMena and hMena11a specific antibodies) and phosphorylation status of HER2, EGFR, HER3, AKT and MAPK (p44/42) from whole cell lysates were assessed. Membranes were sequentially stripped and reprobed with the indicated total and phospho-specific antibodies. Densitometric quantitation of anti-P-HER2, anti-P-AKT and anti-P-MAPK immunoreactivity was determined by Quantity One software (Biorad) and normalized in comparison with the Actin immunoreactivity. Densitometric quantitation of anti-P-HER3 immunoreactivity was not determined due to the fact that the total HER3 expression level is not unchanged following treatments. B–C. Silencing of hMena/hMena11a reduces EGF and NRG1-mediated cell proliferation of HER2 overexpressing MCF7-HER2 (B) and MDA-MB-361 (C) cell lines, but has no significant effect in MCF7 cells (B). Proliferation assays were conducted 72 h after the siRNA transfection by measuring [3H]thymidine incorporation as described in Materials and Methods. Si-CNTR: MCF7, MCF7-HER2 and MDA-MB-361 cells transfected with non-targeting siRNA; Si-hMena: MCF7, MCF7-HER2 and MDA-MB-361 cells transfected with specific hMena/hMena11a siRNA. Histograms represent the mean of three different experiments. Bars, Standard deviations. P, according to Student's t test (two tailed).
To explore the hypothesis that hMena/hMena11a might have a functional role in EGF and NRG1 mediated mitogenic effects, 3H-thymidine incorporation assays were performed. hMena/hMena11a knock-down induced a slight decrease in the proliferation rate of the silenced MCF7-HER2 cells with respect to the control in untreated conditions and abolished the proliferation activity mediated by EGF and NRG1 (Figure 5 panel B). Similar results were obtained in MDA-MB-361 cells naturally expressing high levels of HER2 and HER3 (Figure 5 panel C and Figure S1). On the contrary, in MCF7, cells with a low HER2 level, hMena/hMena11a knock-down (Figure S1) has no significant effect on the proliferation rate in untreated conditions or in EGF treated cells. A slight effect was observed in NRG1-mediated proliferation (Figure 5 panel B).
hMena overexpression adds prognostic information in BC patients with HER2 overexpressing tumors
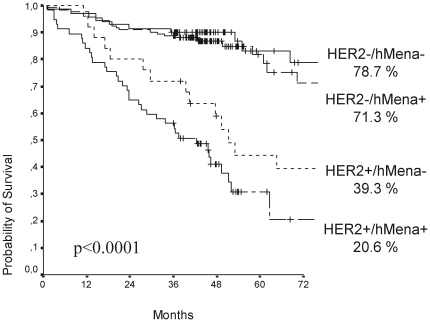
In view of the biochemical and functional data obtained, the prognostic impact of hMena overexpression on disease free survival (DFS) was further estimated in our series of 286 BC patients with a median follow-up of 45 months (1–188 months) in which 88 relapses were recorded.
When the whole series was analyzed by unadjusted Kaplan-Meier curves, hMena/HER2 covariates had different effect on DFS (Figure 6). In particular, in HER2 negative cases hMena positivity slightly reduced the DFS (78.7% BC patients bearing HER2-/hMena- primary tumor and 71.3% with HER2-/hMena+ tumors were disease free after 6 years follow up). Differently, among the HER2 positive cases, only 20.6% of the patients were disease free at a 6 year of follow-up when hMena was overexpressed with respect to 39.3% of patients with HER2+/hMena- tumors (p<0.0001). These data indicate that hMena overexpression adds prognostic information in breast cancer patients with HER2 overexpressing tumors.
Figure 6. hMena overexpression adds prognostic information in HER2 overexpressing tumors.
Kaplan-Meier estimates of disease-free survival (DFS) for hMena and HER2 status show that hMena/HER2 covariates have a different effect on DFS in relationship to the different phenotype combinations. In particular, the lower percentage of disease free patients at 6 year follow up was found in the group of HER2+/hMena+ tumors (p<0.0001).
Discussion
Previous experimental and in vivo data from our group suggested that the cytoskeleton regulatory protein hMena and its splice-variant deriving isoform, hMena11a, may couple ErbB family signalling to the actin cytoskeleton leading to cell proliferation in breast cancer [16]. HER2 positive breast cancers vary greatly in prognosis and response to therapy, underlying the need to identify new markers to further stratify HER2 positive tumors [20]. Here we present our attempts to provide clinical and experimental evidence that hMena/hMena11a are downstream targets and effectors of HER2 activity. The clinical data show that a link exists between hMena and HER2 overexpression, confirming our previous results [15], [16] and further evidences that, according to the molecular classification of breast tumors [21], hMena overexpression is more frequent in the HER2 subtype. On the other hand, the finding that hMena overexpression is correlated with the expression of phosphorylated MAPK and AKT and a high proliferation index in a cohort of primary breast tumors suggests a role for hMena in sustaining an activated status of HER2 pathways. Thus we attempted to delineate the mechanisms linking HER2 activity with hMena in breast cancer cell lines. Exogeneous overexpression of HER2 or stimulation by EGF and NRG1 of different breast cancer cell lines induce the up-regulation of hMena both at the mRNA and protein levels, which suggests a link between ErbB family activation and hMena. Apart from the hMena overexpression, HER2 activation promotes the phosphorylation of the epithelial specific hMena11a isoform [16]. This is indicated by the higher percentage of phosphorylated hMena11a observed in the MCF7-HER2 cells with respect to control and confirmed by the hMena11a dephosphorylation in the same cells treated with Herceptin. Although further studies are needed to clarify the role of hMena11a phosphorylation, we hypothesize it may alter the regulation of actin dynamics, since the 11a peptide is located in the EVH2 domain, the site of protein tetramerization and binding to the grown ends of actin filaments. Studies are in progress in order to define the residue and the kinase involved in phosphorylation. A recent work has described Evl-I, a splice variant derived isoform of Evl, that similarly to hMena11a shows a 21aa peptide included in the EVH2 domain which is phosphorylated by PKD1 and regulates lamellipodia formation and membrane ruffling [22]. In view of the ability of Ena/VASP proteins to form functional tetramers [23], we can hypohesize that the phosphorylation of the two members of Ena/VASP family may implicate a similar function, and a similar regulatory pathway. In line with our results on hMena11a phosphorylation, Janssens K and coauthors [22] report that Evl-I is strongly phosphorylated in the HER2 overexpressing SKBr3 breast cancer cell line, whereas a lower level of P-Evl-I expression is shown in MCF7 and T47D cells.
Growth factor treatment further increased the hMena11a phosphorylation in HER2 overexpressing cancer cells, suggesting that stroma mediated paracrine mechanisms not only induce hMena overexpression, but also sustain the phosphorylation of hMena11a. Members of HER family take part in a complex array of combinatorial interactions through the formation of homo and heterodimers between the different family members and not only the preferred heterodimerization partner HER2, but also EGFR and recently HER3 have been defined as important therapeutic targets in breast cancer [6]. Our data demonstrating that hMena/hMena11a expression as well as hMena11a phosphorylation are induced by EGF and NRG1, indicate that hMena/hMena11a are downstream to different receptor complexes (EGFR/HER2 and HER2/HER3), and might thus represent a relevant target for therapeutic regulation.
Functionally, we suggest a dynamic reciprocal cross-talk between ErbB family signalling and actin cytoskeleton regulators, as pointed out by the results that hMena/hMena11a knock-down reduces the phosphorylation of HER3, MAPK and AKT and abolishes the EGF and NRG1 mediated phosphorylation of HER2 and EGFR. The HER2/HER3 receptor pair form the most potent mitogenic receptor complex and HER2 overexpressing tumors frequently show phosphorylated HER3 [24]. Consistently, in our model the HER2 overexpressing MCF7 cells showed a ligand independent phosphorylated status of HER3 receptor. One could envisage that hMena/hMena11a are involved in the heterodimerization of HER2/HER3 as suggested by the reduction of HER3 phosphorylation observed in hMena/hMena11a knock down cells. This could also be the reason for the reduction of phosphorylated AKT and MAPK in untreated cells, since the HER2/HER3 complex is a potent activator of PI3K and MAPK signalling [25]. As already reported, growth factor stimulation determines a down-regulation of HER3 caused by the ligand-stimulated degradation of the receptor, that does not involve HER2 despite the dimerization of the two receptors [26]. This reduction at the protein level is more evident in hMena/hMena11a silenced cells, suggesting a role of hMena/hMena11a also in the post-transcriptional mechanisms regulating the HER3 receptor. The hypothesis that hMena/hMena11a play a role in the heterodimerization and activation of the ErbB family of receptors, an event sustained by actin cytoskeleton organization [27], is reinforced by the reduction of ligand-dependent phosphorylation of HER2 and EGFR in the hMena/hMena11a knock down cells.
hMena/hMena11a knock-down is accompanied by the inhibition of cell proliferation in HER2 overexpressing cells, inhibition which become significant in EGF and NRG1 treated cells, thus supporting the hypothesis that hMena/hMena11a are relevant mediators of the EGF and NRG1 mitogenic signals. Data presented here indicate the ability of hMena/hMena11a to sustain EGF and NRG1-mediated signalling responsible for a proliferative signature of breast cancer. The ErbB family is at the center of converging signals for cell proliferation and motility and it could be hypothesized that the regulation of hMena alternative splicing could contribute to this different cell behaviour. In line with this hypothesis, the recently described murine invasion isoform, Mena INV, sensitizes rat mammary tumor cells to EGF-dependent invasion and protrusion [28]. Moreover, in invasive tumor cells the 11a isoform is down-modulated with respect to the stationary ones [29], according with our previous data on the exclusive expression of hMena11a isoform in epithelial non invasive breast and pancreatic tumor cell lines [16], [17] and with the recent finding reporting the hMena11a expression under the control of epithelial specific splicing regulators [18]. This suggests that the availability of specific hMena isoform antibodies may represent a new tool in the clinical management of breast cancer.
The clinical data presented herein indicate that hMena cooperates with HER2 overexpression in breast cancer progression. In fact, HER2 overexpressing tumors lacking hMena overexpression frequently showed inactivated PI3K and MAPK pathways and the patients have a better prognosis with respect to those co-overexpressing HER2 and hMena. Overexpression of HER2 in the primary tumor represents one of the best prognostic indicators of breast cancer progression [30]. HER2 positive tumors were found to have elevated levels of phosphorylated HER3 [24], suggesting that the recruitment of HER3 contributes to malignant growth. On the other hand, co-overexpression of multiple ErbB family members is associated with decreased survival [31]. Although future studies are needed to clarify the role of hMena and its isoforms in breast cancer progression, our observation raised the possibility that hMena overexpression may contribute in mediating the activation of various ErbB receptors. As the inactivation of ErbB receptors is a new tool in cancer treatment, future studies could lead to the development of hMena overexpression as a marker of breast cancer progression.
Materials and Methods
Cell lines
The human breast carcinoma cell lines MCF7, BT474, SKBr3, MDA-MB-361, T47D and BT549 purchased from the ATCC (Rockville MD) were cultured in RPMI 1640 (Gibco, Invitrogen, Pisley, UK) supplemented with 10% fetal calf serum, glutamine, at 37°C in 5% CO2-95% air. The DAL cell line was obtained from the ascitic fluid of a breast cancer patient [32]. MCF7-HER2 and MCF7-pcDNA3 stable transfectants were obtained by selecting MCF7 cells transfected with HER2-pcDNA3.1 (kindly provided by Dr. Oreste Segatto), and with the empty vector respectively, using G418 500 µg/ml (Invitrogen) in complete culture medium. BT549 and DAL stable transfectants (shown in Figure S2) were obtained by selecting transfected hMena11a-pcDNA3.1 cells with G418. All cell lines were routinely morphologically checked by microscope, growth curve analysis by 3H-Thymidine incorporation assay and Mycoplasma detection (Roche, Monza, Italy).
Cell treatments
Cells were grown in 6 well plates to 50% confluence in RPMI supplemented with 10% fetal bovine serum. Eighteen hours after medium replacement with fresh medium containing 0.5% serum, cells were treated with recombinant human EGF 100 ng/ml (Promega Corporation, Madison, WI, USA), or NRG1 10 ng/ml (Promega Corporation) for 24 h.
Herceptin (Roche, Monza, Italy) for clinical and in vitro use was stored at 4°C and adjusted to the final concentration of 25 µg/ml with culture medium containing 10% serum. Exponentially growing cells were exposed to the treatment for 48 h. Treated and control cells were washed and processed according to the experiment to be done.
Western blot analysis
Cells were lysed for 30 min at 4°C in 10% glycerol, 0.1% SDS, 0.5% DOC, 1% NP-40 in PBS containing protease and phosphatase-inhibitors. Lysates were centrifuged and protein quantification of supernatants was determined using BCA Protein Assay Reagent (Pierce, Rockford, IL, USA). Lysates (50 µg) were resolved on 10% polyacrylamide gel and transferred to nitrocellulose membrane (GE-Healthcare, Little Chalfont, UK). For HER3 and Phospho-HER3 detection, 80 µg of lysates were used. Blots were blocked for 1 h with 3% skimmed milk in TBST and probed in 3% skimmed milk/TBST overnight at 4°C with the following antibodies: 10 µg/ml anti-hMena rabbit CKLK1 (pan-hMena) antibody [15]; 1 µg/ml anti-hMena11a rabbit antibody, that we developed against a portion of the peptide 11a (RDSPRKNQIVFDNRSYDS) by the use of the Primm service (Primm srl, Milan, Italy), showing a similar pattern of reactivity with the previously reported anti-Mena11a antibody [17] (characterization of the antibody is reported in the Figure S2); anti-Phospho-p44/42 MAPK (Thr202/Tyr204) mouse mAb, p44/42 MAPK rabbit antibody, Phospho-AKT (Ser473) antibody, AKT antibody and Phospho-HER3 were all from Cell Signaling Technology (Beverly, MA); anti-phospho-HER2 (Y1248) from Upstate Biotechnology (Lake Placid, NY); anti-Neu C18, anti-EGFR 1005 and anti-HER3 C17 were from Santa Cruz Biotechnology (Santa Cruz, CA); anti-EGFR (pY1068) phosphospecific antibody obtained from Biosource (Camarillo, CA). After 3 washes of 15 min each, blots were incubated with the appropriate secondary antibody conjugated with HRP for 1 h and then washed again three times. The protein signals were detected by ECL kit (GE-Healthcare). For actin signal, blots were reprobed with 1 µg/ml monoclonal anti-actin, mouse-ascites fluid clone AC-40 (Sigma Aldrich, Poole, UK). X-ray films were scanned by HP Scanjet 5470 and processed by Corel Photo Paint 12.
RNA extraction and real-time PCR
Five micrograms of total mRNA were extracted using Trizol reagent (Life Technologies Inc, Rockville, MD, USA) to obtain the relative cDNA by first strand cDNA synthesis kit (GE-Healthcare) according to the manufacturer's protocol. Real-time PCRs (RT-PCR) were run on an ABI Prism 7900 RT-PCR machine (Applied Biosystems) using the following cycling conditions: 50°C for 2 min, 95°C for 10 min, and 40 cycles at 95°C for 15 s each followed by termination at 60°C for 1 min. Each sample contained 10 ng template cDNA, 10 µl 2x Taqman Universal Master Mix (Applied Biosystems), 100 nmol/L of each primer, and 200 nmol/L probe in 20 µl volume. Amplification primers and probe (Assay ID Hs00430216) of Taqman gene expression assay (Applied Biosystem) were used for hMena. The β-Actin gene used as an endogenous control was amplified using the Taqman gene expression assay (Applied Biosystems). Data from triplicate samples were analyzed with SDS 2.1 software (Applied Biosystems) and relative hMena mRNA expression levels were calculated using the ΔΔC T method.
Two-Dimensional Electrophoresis (2DE)
Cells were washed extensively with PBS, pellets lyophilized and solubilized with 2DE solubilization buffer (9 M Urea, 10 mM Tris, 4% CHAPS, 65 mM DTT, 2% IPG buffer ampholine pH 3-10, protease inhibitor cocktail). Protein samples (250 µg) were applied to 7 cm IPGstrips pH 3-10NL (GE-Healthcare) by in-gel rehydration. Iso-electro Focusing (IEF) was performed with an IPGphor system (GE-Healthcare) following a standard protocol as described [33]. Strips were equilibrated in 50 mM pH 8.8 Tris-HCl buffer containing 6 M urea, 30% glycerol, 2% SDS and 2% DTT, followed by an incubation in the same buffer replacing DTT with 2.5% iodoacetamide. The strips were loaded on the top of 10% acrylamide SDS-PAGE gels for the second dimension separation. Proteins were electrontransferred onto nitrocellulose membranes and Western blot performed as described above. Images were acquired at high resolution and 2D immunoreactivity patterns analyzed using Progenesis PG240 v2005 software (Nonlinear dynamics, Newcastle, UK). Relative molecular mass (Mr) were estimated by comparison with Mr reference markers (Precision, Bio-Rad, Hercules, CA, USA) and isoelectric point (pI) values assigned to detected spots by calibration as described in the GE-Healthcare guidelines.
Phosphatase treatment
Lambda Protein Phosphatase (λ-PPase) (NewEngland BioLabs, Ipswich, MA, USA) treatment was performed as described [34] with modifications. In brief, pelleted cells (25×106) were lyophilized and resuspended in lysis buffer (1% w/v NP-40, 1% w/v SDS, 50 mM Tris pH 7.6, 150 mM NaCl, protease inhibitor cocktail). Sixty µl of lysate, corresponding to 600 µg of protein, were brought to a final volume of 600 µl with deionized water followed by the addition of 20 µl of 20 mM MnCl2 solution and 20 µl of λ-PPase buffer. For each addition, the solution was gently mixed. The mixture was divided into two aliquots, and 300 units of λ-PPase was added to one of the aliquots. After mixing, aliquots were incubated for 6 h at 30°C. Proteins were acetone precipitated at −20°C and used for 2DE analysis.
Small-interfering RNA treatment
Cells in exponential growth phase were plated in 6-well plates 3×105 cells/well. After 24 h cells were transfected with 100 nmol/L hMena-specific pooled siRNA duplexes (siENA SMART pool) or control non-specific siRNA (Dharmacon, Lafayette, CO) using Lipofectamine 2000 reagent (Invitrogen). After 72 h cells were analyzed by Western blot or, after 24 h, cells were serum starved for 18 h and then treated with 100 ng/ml EGF or 10 ng/ml NRG1 for additional 24 h for Western blot analysis or [3H]thymidine incorporation assay.
[3H]Thymidine Incorporation Assay
The day after siRNA transfection cells from each well were transferred to 4 wells of 48-well-plates and treated with growth factors as above described. For 3H-thymidine incorporation assay, 3H-thymidine (Perkin Elmer Life and Analytical Sciences, Boston, MA) was added at 5 µCi/mL for 4 h on the last day of treatment. Following medium removal, cells were washed twice with cold PBS, treated with 10% trichloroacetic acid for 30 min at 37°C, solubilized with 0.4 N NaOH and counted for incorporation of 3H on β liquid scintillation counter in 5 mL of scintillation fluid. Each experiment was done in quadruplicate and results were expressed as the means of at least three separate experiments.
Patients and Tissue Specimens
hMena was analyzed by immunohistochemistry in a series of 286 breast cancer patients ranging in age 28–86 years (median, 53 years) subjected to breast cancer surgery at the Regina Elena Cancer Institute (Rome, Italy) between 2000 and 2004. Follow-up data were obtained from hospital charts and by correspondence with the referring physicians. As shown in Table 1, the group included 246 (86%) invasive ductal carcinomas, 28 (10%) invasive lobular carcinomas, and 12 (4%) other histotypes. Among these, 171 (60%) were pT1, 80 (29%) pT2 and 28 (11%) pT3/4, 154 (54%) were node negative and 132 (46%) node positive, 27 (10%) G1, 146 (54%) G2 and 96 (34%) G3. Ki67 was found positive in 122 tumors (43%) and negative in 164 (57%), while hMena was overexpressed in 190 (66%) cases and negative in 96 (34%). Tumors were graded according to Bloom and Richardson and staged according to the Unione Internationale Contre le Cancer tumor-node-metastasis system criteria and histologically classified according to the World Health Organization [35]. Moreover, we studied hMena distribution according to the molecular subtypes identified by a few protein biomarkers, namely ERα, PgR, HER2. They were 177 (62%) Luminal A (LA, ERα/PgR positive and HER2 negative), 34 (12%) Luminal B (LB ERα/PgR positive and HER2 positive), 27 (9%) Triple Negatives (TN, ERα/PgR,HER2 negative) and 48 (17%) HER2 subtypes (HS, ERα/PgR negative, HER2 positive).
The study was reviewed and approved by the ethics committee of the Regina Elena National Cancer Institute, and written informed consent was obtained from all patients.
Immunohistochemistry and CISH
hMena immunostaining was performed by using a monoclonal antibody from BD Transduction (San Jose, CA, USA; 2.5 µg/ml). Anti estrogen (6F11 MoAb) and progesterone (1A6 MoAb) receptors were purchased from Novocastra (Menarini, Florence, Italy); HER-2 (A0485 pAb) and Ki67 (MIB-1 MoAb) antibodies from DAKO (Milan, Italy); phospho-p44/42 MAPK and phospho-AKT antibodies from Cell Signaling (SIAL, Rome, Italy). Immunostaining was revealed by a streptavidin-biotin enhanced immunoperoxidase technique (Super Sensitive MultiLink, Menarini) in an automated autostainer. Diaminobenzidine was used as chromogenic substrate.
The intensity of hMena cytoplasmic staining was scored from 0 to 3 as previously described [15]; Ki67 percentage, based on the median value of our series, was regarded as high if >15% of the cell nuclei were immunostained.
HER-2 immunostaining was performed following the manifacturer's protocol and protein overexpression was determined as defined in the HercepTest kit guide: score 0, 1+, 2+, 3+. All IHC 2+ tumors were analyzed with chromogenic in situ hybridization (CISH) as previously described [36] in order to determine the HER2 gene copy level. For statistical analysis, negative (HER2 IHC score 0, 1+ and 2+ cases lacking gene amplification) and positive (HER2 IHC score 2+ with gene amplification cases and score 3+) groups were created.
P-MAPK was considered positive when >25% of the neoplastic cells showed a distinct cytoplasmic staining. Breast cancers showing a distinct and intense cytoplasmic immunostaining for P-AKT were scored as strongly positive (2+), independent of the percentage of stained cells.
Evaluation of the immunohistochemical results was done independently and in blinded manner by two investigators (M.M., E.M.).
Statistical analysis
All experiments were repeated a minimum of three times. Data collected from [3H]thymidine incorporation assay were expressed as means ± standard deviation (SD). The data presented in some figures are from a representative experiment, which was qualitatively similar in the replicate experiments. Statistical significance was determined by Student's t test (two tailed) comparison between two groups of data sets. Asterisks indicate significant differences of experimental groups compared with the corresponding control condition (P<0.05, see figure legends). All the analysis were performed using GraphPad Prism 4, V4.03 software (GraphPad Inc., San Diego, CA). Change in the phosphorylation status was evaluated using Progenesis PG240 v.2006 software (Nonlinear dynamics) by optical density indicated as normalized spot volume. Normalization was done multiplying the total spot volume by the constant factor 100 which produces spot percentage volume.
The Chi-Square test and Fisher exact text, when appropriate were used to assess the relationship between hMena positivity and the other biological parameters (HER2, Ki-67, P-AKT, P-MAPK). The Chi-Square test for linear trend was also applied.
The disease-free survival (DFS) curves were estimated by the Kaplan-Meier product-limit method. The log-rank test was used to assess differences between subgroups. Significance was defined at the p<0.05 level. Statistical analysis for survival were performed with SPSS statistical software version 11.5 (SPSS inc., Chicago IL, USA).
Supporting Information
Western Blot analysis of MCF7 and MDA-MB-361 after 72 h transfection with control and hMena/hMena11a -specific siRNAs with pan-hMena antibody. As loading control blots were probed with anti-Actin antibody (1 µg/ml).
(TIF)
Characterization of anti-hMena11a antibody.
A. Western blot analysis with 1 µg/ml of anti-hMena11a antibody on lysates of SKBr3, expressing hMena11a, and BT549, negative for hMena11a expression, transfected with either control vector (pcDNA3) or hMena11a.
B–C. Western blot analysis of DAL cells transfected with either control vector (pcDNA3) or hMena11a with 1 µg/ml (B) or 0.1 µg/ml (C) of anti-hMena11a antibody. As loading control blots were probed with anti-Actin antibody (1 µg/ml).
(TIF)
Acknowledgments
The authors thank Dr. G. Zupi for her continuous support, Mrs. P.I. Franke and Mrs. M.V. Sarcone for the formal revision of the manuscript and for the secretarial assistance, Luisa Salvatori and Annalisa Onori for helpful suggestions and Giuliana Falasca and Novella Gualtieri for the technical contribution.
Footnotes
Competing Interests: The authors have declared that no competing interests exist.
Funding: Associazione Italiana per la Ricerca sul Cancro (AIRC)(P.N.,M.M.); Italian Ministry of Health Ricerca Finalizzata (P.N.); Italian Ministry of Health PIO-2006 RO strategici 5/07 (M.A.). Ludwig Fund Postdoctoral Fellowship (M.B.). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Yarden Y. The EGFR family and its ligands in human cancer. Signalling mechanisms and therapeutic opportunities. Eur J Cancer. 2001;4(Suppl):S3–8. doi: 10.1016/s0959-8049(01)00230-1. [DOI] [PubMed] [Google Scholar]
- 2.Nicholson RI, Gee JM, Harper ME. EGFR and cancer prognosis. Eur J Cancer. 2001;4(Suppl):S9–15. doi: 10.1016/s0959-8049(01)00231-3. [DOI] [PubMed] [Google Scholar]
- 3.Feldner JC, Brandt BH. Cancer cell motility–on the road from c-erbB-2 receptor steered signaling to actin reorganization. Exp Cell Res. 2002;272:93–108. doi: 10.1006/excr.2001.5385. [DOI] [PubMed] [Google Scholar]
- 4.Citri A, Yarden Y. EGF-ERBB signalling: towards the systems level. Nat Rev Mol Cell Biol. 2006;7:505–16. doi: 10.1038/nrm1962. [DOI] [PubMed] [Google Scholar]
- 5.Holbro T, Beerli RR, Maurer F, Koziczak M, Barbas CF, 3rd, et al. The ErbB2/ErbB3 heterodimer functions as an oncogenic unit: ErbB2 requires ErbB3 to drive breast tumor cell proliferation. Proc Natl Acad Sci U S A. 2003;100:8933–8. doi: 10.1073/pnas.1537685100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lee-Hoeflich ST, Crocker L, Yao E, Pham T, Munroe X, et al. A central role for HER3 in HER2-amplified breast cancer: implications for targeted therapy. Cancer Res. 2008;68:5878–87. doi: 10.1158/0008-5472.CAN-08-0380. [DOI] [PubMed] [Google Scholar]
- 7.Yin HL, Janmey PA. Phosphoinositide regulation of the actin cytoskeleton. Annu Rev Physiol. 2003;65:761–89. doi: 10.1146/annurev.physiol.65.092101.142517. [DOI] [PubMed] [Google Scholar]
- 8.Mouneimne G, Soon L, DesMarais V, Sidani M, Song X, et al. Phospholipase C and cofilin are required for carcinoma cell directionality in response to EGF stimulation. J Cell Biol. 2004;166:697–708. doi: 10.1083/jcb.200405156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Klapper LN, Kirschbaum MH, Sela M, Yarden Y. Biochemical and clinical implications of the ErbB/HER signaling network of growth factor receptors. Adv Cancer Res. 2000;77:25–79. [PubMed] [Google Scholar]
- 10.Margadant C, van Opstal A, Boonstra J. Focal adhesion signaling and actin stress fibers are dispensable for progression through the ongoing cell cycle. J Cell Sci. 2007;120:66–76. doi: 10.1242/jcs.03301. [DOI] [PubMed] [Google Scholar]
- 11.Di Modugno F, Bronzi G, Scanlan MJ, Del Bello D, Cascioli S, et al. Human Mena protein, a serex-defined antigen overexpressed in breast cancer eliciting both humoral and CD8+ T-cell immune response. Int J Cancer. 2004;109:909–18. doi: 10.1002/ijc.20094. [DOI] [PubMed] [Google Scholar]
- 12.Krause M, Dent EW, Bear JE, Loureiro JJ, Gertler FB. Ena/VASP proteins: regulators of the actin cytoskeleton and cell migration. Annu Rev Cell Dev Biol. 2003;19:541–64. doi: 10.1146/annurev.cellbio.19.050103.103356. [DOI] [PubMed] [Google Scholar]
- 13.Scott JA, Shewan AM, den Elzen NR, Loureiro JJ, Gertler FB, et al. Ena/VASP proteins can regulate distinct modes of actin organization at cadherin-adhesive contacts. Mol Biol Cell. 2006;17:1085–95. doi: 10.1091/mbc.E05-07-0644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Boëda B, Briggs DC, Higgins T, Garvalov BK, Faddena AJ, et al. Tes, a specific Mena interacting partner, breaks the rules for EVH1 binding. Mol Cell. 2007;28:1071–82. doi: 10.1016/j.molcel.2007.10.033. [DOI] [PubMed] [Google Scholar]
- 15.Di Modugno F, Mottolese M, Di Benedetto A, Conidi A, Novello F, et al. The cytoskeleton regulatory protein hMena (ENAH) is overexpressed in human benign breast lesions with high risk of transformation and human epidermal growth factor receptor-2-positive/hormonal receptor-negative tumors. Clin Cancer Res. 2006;12:1470–8. doi: 10.1158/1078-0432.CCR-05-2027. [DOI] [PubMed] [Google Scholar]
- 16.Di Modugno F, DeMonte L, Balsamo M, Bronzi G, Nicotra MR, et al. Molecular cloning of hMena (ENAH) and its splice variant hMena+11a: epidermal growth factor increases their expression and stimulates hMena+11a phosphorylation in breast cancer cell lines. Cancer Res. 2007;67:2657–65. doi: 10.1158/0008-5472.CAN-06-1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pino MS, Balsamo M, Di Modugno F, Mottolese M, Alessio M, et al. hMena+11a isoform serves as a marker of epithelial phenotype and sensitivity to EGFR inhibition in human pancreatic cancer cell lines. Clin Cancer Res. 2008;14:4943–50. doi: 10.1158/1078-0432.CCR-08-0436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Warzecha CC, Sato TK, Nabet B, Hogenesch JB, Carstens RP. ESRP1 and ESRP2 are epithelial cell-type-specific regulators of FGFR2 splicing. Mol Cell. 2009;33:591–601. doi: 10.1016/j.molcel.2009.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kenny PA, Lee GY, Myers CA, Neve RM, Semeiks JR, et al. The morphologies of breast cancer cell lines in three-dimensional assays correlate with their profiles of gene expression. Mol Oncol. 2007;1:84–96. doi: 10.1016/j.molonc.2007.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Staaf J, Ringnér M, Vallon-Christersson J, Jönsson G, Bendhal P, et al. Identification of subtypes in human epidermal growth factor receptor 2- positive breast cancer reveals a gene signature prognostic of outcome. J Clin Oncol. 2010;28:1813–20. doi: 10.1200/JCO.2009.22.8775. [DOI] [PubMed] [Google Scholar]
- 21.Perou CM, Sørlie T, Eisen MB, Rijnm Van De, Jeffery SS, et al. Molecular portraits of human breast tumours. Nature. 2000;406:747–52. doi: 10.1038/35021093. [DOI] [PubMed] [Google Scholar]
- 22.Janssens K, De Kimpe L, Balsamo M, Vandoninck S, Vandenheede JR, et al. Characterization of EVL-I as a protein kinase D substrate. Cell Signal. 2009;21:282–92. doi: 10.1016/j.cellsig.2008.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bachmann C, Fischer L, Walter U, Reinhard M. The EVH2 domain of the vasodilator-stimulated phosphoprotein mediates tetramerization, F-actin binding, and actin bundle formation. J Biol Chem. 1999;274:23549–57. doi: 10.1074/jbc.274.33.23549. [DOI] [PubMed] [Google Scholar]
- 24.Siegel PM, Ryan ED, Cardiff RD, Muller WJ. Elevated expression of activated forms of Neu/ErbB-2 and ErbB-3 are involved in the induction of mammary tumors in transgenic mice: implications for human breast cancer. EMBO J. 1999;18:2149–64. doi: 10.1093/emboj/18.8.2149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Baselga J, Swain SM. Novel anticancer targets: revisiting ERBB2 and discovering ERBB3. Nat Rev Cancer. 2009;9:463–75. doi: 10.1038/nrc2656. [DOI] [PubMed] [Google Scholar]
- 26.Cao Z, Wu X, Yen L, Sweeney C, Carraway KL., 3rd Neuregulin-induced ErbB3 downregulation is mediated by a protein stability cascade involving the E3 ubiquitin ligase Nrdp1. Mol Cell Biol. 2007;27:2180–8. doi: 10.1128/MCB.01245-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhao TT, Le Francois BG, Goss G, Ding K, Bradbury PA, et al. Lovastatin inhibits EGFR dimerization and AKT activation in squamous cell carcinoma cells: potential regulation by targeting rho proteins. Oncogene. 2010;29:4682–4692. doi: 10.1038/onc.2010.219. [DOI] [PubMed] [Google Scholar]
- 28.Philippar U, Roussos ET, Oser M, Yamaguchi H, Kim HD, et al. A Mena invasion isoform potentiates EGF-induced carcinoma cell invasion and metastasis. Dev Cell. 2008;15:813–28. doi: 10.1016/j.devcel.2008.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Goswami S, Philippar U, Sun D, Patsialou A, Avraham J, et al. Identification of invasion specific splice variants of the cytoskeletal protein Mena present in mammary tumor cells during invasion in vivo. Clin Exp Metastasis. 2009;26:153–9. doi: 10.1007/s10585-008-9225-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ménard S, Balsari A, Tagliabue E, Camerini T, Casalini P, et al. OMERO Group. Biology, prognosis and response to therapy of breast carcinomas according to HER2 score. Ann Oncol. 2008;19:1706–12. doi: 10.1093/annonc/mdn369. [DOI] [PubMed] [Google Scholar]
- 31.Wiseman SM, Makretsov N, Nielsen TO, Gilks B, Yorida E, et al. Coexpression of the type 1 growth factor receptor family members HER-1, HER-2, and HER-3 has a synergistic negative prognostic effect on breast carcinoma survival. Cancer. 2005;103:1770–7. doi: 10.1002/cncr.20970. [DOI] [PubMed] [Google Scholar]
- 32.Nisticò P, De Berardinis P, Morrone S, Alonzi T, Buono C, et al. Generation and characterization of two human alpha/beta T cell clones. Recognizing autologous breast tumor cells through an HLA- and TCR/CD3-independent pathway. J Clin Invest. 1994;94:1426–31. doi: 10.1172/JCI117479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Conti A, Ricchiuto P, Iannaccone S, Sferrazza B, Cattaneo A, et al. Pigment epithelium-derived factor is differentially expressed in peripheral neuropathies. Proteomics. 2005;5:4558–67. doi: 10.1002/pmic.200402088. [DOI] [PubMed] [Google Scholar]
- 34.Yamagata A, Kristensen DB, Takeda Y, Miyamoto Y, Okada K, et al. Mapping of phosphorylated proteins on two-dimensional polyacrylamide gels using protein phosphatase. Proteomics. 2002;2:1267–76. doi: 10.1002/1615-9861(200209)2:9<1267::AID-PROT1267>3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- 35.Tavassoli FA, Devilee P. In: Tumors of the Breast. Tavassoli FA, Devilee P, editors. Lyon, France: IARC Press; 2003. [Google Scholar]
- 36.Vocaturo A, Novelli F, Benevolo M, Piperno G, Marandino F, et al. Chromogenic in situ hybridization to detect HER-2/neu gene amplification in histological and ThinPrep-processed breast cancer fine-needle aspirates: a sensitive and practical method in the trastuzumab era. Oncologist. 2006;8:878–86. doi: 10.1634/theoncologist.11-8-878. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Western Blot analysis of MCF7 and MDA-MB-361 after 72 h transfection with control and hMena/hMena11a -specific siRNAs with pan-hMena antibody. As loading control blots were probed with anti-Actin antibody (1 µg/ml).
(TIF)
Characterization of anti-hMena11a antibody.
A. Western blot analysis with 1 µg/ml of anti-hMena11a antibody on lysates of SKBr3, expressing hMena11a, and BT549, negative for hMena11a expression, transfected with either control vector (pcDNA3) or hMena11a.
B–C. Western blot analysis of DAL cells transfected with either control vector (pcDNA3) or hMena11a with 1 µg/ml (B) or 0.1 µg/ml (C) of anti-hMena11a antibody. As loading control blots were probed with anti-Actin antibody (1 µg/ml).
(TIF)