Abstract
Objective
Suicidal ideation is a medical emergency, especially when severe. Little research has been done on pharmacological interventions that could address this problem. Ketamine, an N-methyl-D-aspartate (NMDA) antagonist, has been reported to have antidepressant effects within hours. We examined the effects of a single dose of ketamine on suicidal ideation in subjects with treatment-resistant major depressive disorder (MDD).
Method
Thirty-three subjects with DSM-IV-diagnosed MDD received a single open-label infusion of ketamine (0.5 mg/kg) and rated at baseline, 40, 80, 120, and 230 minutes post-infusion with the Scale for Suicide Ideation (SSI), the Montgomery-Asberg Depression Rating Scale (MADRS), the Hamilton Depression Rating Scale (HDRS), and the Beck Depression Inventory (BDI).
Results
Suicidal ideation scores decreased significantly on the SSI as well as on the suicide subscales of other rating instruments within 40 minutes; these decreases remained significant through the first four hours post-infusion (p<.001). Ten subjects (30%) had a SSI score 4 at baseline, and all dropped below a score of 4 (nine by 40 minutes and one by 80 minutes). For those starting below a score of 4 on the SSI, only one reached a score of 4. Depression, anxiety, and hopelessness were significantly improved at all time points (p<.001).
Conclusion
Suicidal ideation in the context of MDD improved within 40 minutes of a ketamine infusion and remained improved for up to four hours post-infusion. Future studies with ketamine in suicidal ideation are warranted due to its potential impact on public health.
Keywords: antidepressant, glutamate, ketamine, major depressive disorder, NMDA, suicide
Introduction
Individuals with serious psychiatric disorders commit suicide in disproportionate numbers, and the vast majority of patients who display suicidal behavior have major depressive disorder (MDD). Indeed, severity of depression has been associated with more suicide attempts 1. In addition, anxiety and hopelessness seem to increase suicide risk in depressed patients 1,.2. Among 4027 enrollees in the Sequenced Treatment Alternatives to Relieve Depression (STAR*D) study, 16.5% reported previous suicide attempts 1. Controlling for age, gender, and depressive symptom severity, previous attempters had more current general medical conditions (p<.0001), more current alcohol and substance abuse (p<.001), more work hours missed in the past week (26.2% versus 18.2%, p<.0001), and more current suicidal ideation (61.3% versus 45.5%, p<.0001) than non-attempters. The results suggest that depression with suicidal behavior is a more severe form of the illness that requires more aggressive treatment.
Suicidal ideation or attempts in patients with MDD is a serious and emergent condition that requires immediate treatment. A recent study of patients who attempted suicide found that 74% said the decision-making period (i.e., the period between decision and attempt) was very short—about 10 minutes or less 3. While the identification of risk factors is central to suicide prevention, one crucial factor regards the timing of suicidal behavior. Studies have found that individuals with mood disorders are at greatest risk of death from any cause, including suicide, in the first two years after their diagnosis 4. More specifically, studies have consistently identified the emergency room, the inpatient unit, time after discharge, and time after starting an antidepressant as time points where individuals are particularly vulnerable to suicidal ideation 5–9. These circumstances offer the possibility to intervene quickly and decisively to prevent suicidal behavior.
Data from the National Hospital Ambulatory Medical Care Survey indicates that between 1992 and 2001, emergency department visits for suicide attempts and self-injury increased by 47%, from 0.8 to 1.5 visits per 1000 in the United States (US) population 10. The risk of suicide attempts occurring in inpatient units is also a major concern and is the second most common sentinel event—defined as an unexpected occurrence involving death or serious physical or psychological injury, or the risk thereof—reported to the Joint Commission on Accreditation of Healthcare Organizations (JCAHO) 6. Approximately 1,500 suicides occur in inpatient hospital units in the US each year, and a staggering one-third of these take place while the patient is on 15-minute checks 7, 8. The JCAHO emphasized the need for around-the-clock observation for inpatients at high risk for suicide. However, there are no systematic studies or best-practice recommendations for patients in this situation 6. Another area where intervention with suicidal patients may be possible is the time following discharge. In a three-month case-control study of mortality in 238 psychiatric patients, Hunt and colleagues found that 43% of suicides occurred within a month of discharge. The first week and the first day after discharge were periods of particularly high risk 5.
Various therapeutic interventions to reduce suicide risk in serious psychiatric disorders are effective in long-term suicide prevention—most notably lithium for the treatment of bipolar disorder 11 and, to a lesser extent, MDD 12, as well as the atypical antipsychotic clozapine in schizophrenia 13. Different types of psychotherapy have also been shown to effectively prevent suicidal behavior over the long-term 14–16. However, the acute pharmacological management of suicidal risk in MDD remains comparatively under-investigated 17.
Emergency tranquilization with benzodiazepines and/or antipsychotic drugs is often recommended for patients at high risk of suicide 18; indeed, the management of significant suicidal ideation in an emergency room setting is often done in the context of agitation. For example, a review by the American College of Emergency Physicians found no Class I studies for the emergency treatment of non-psychotic agitation 18. With regards to atypical antipsychotic drugs, a recent controlled pharmacological study found that risperidone significantly reduced suicidal ideation in patients with MDD compared to placebo. The effects of risperidone augmentation were apparent at two weeks post-treatment and were sustained for the rest of the eight-week study 19.
The evidence regarding suicide risk and antidepressants has often appeared conflicting, a finding usually attributable to their delayed onset of action. Jick and colleagues observed an increased risk of suicide during the first month of antidepressant treatment, particularly during the first nine days; individuals showed similar rates of risk regardless of the chemical class of their antidepressant 9. In contrast, other studies found that suicide attempt rates either did not change 20, or were significantly lower among patients who were treated with antidepressants (particularly selective serotonin reuptake inhibitors (SSRIs) and tricyclic antidepressants) compared to those not treated; this protective effect was seen across all adult age groups 21. Another concern is that in the US, this issue has been clouded by the Food and Drug Administration (FDA)’s black box warning concerning the use of SSRIs in children, adolescents, and young adults. Regardless of their impact on suicide risk, the slow onset of action of antidepressants has prompted consideration of the use of electroconvulsive therapy (ECT) for severely depressed patients, and as a treatment for suicidal ideation 22.
In terms of the neurobiology of suicide, the most robust finding has involved multiple dimensions of serotonergic dysfunction including reduced central serotonin turnover and a polymorphism of the serotonin transporter 23. Noradrenergic and dopaminergic system dysfunction has also been implicated in suicide. Comparatively, the direct role of the glutamatergic system in suicide has received little attention. However, two recent studies—the STAR*D and the Munich Antidepressant Response Signature projects—found a relationship between treatment-emergent suicidal ideation and the glutamate system, specifically identifying the involvement of the genes GRIA3 and GRIK2 24, 25. In postmortem studies, Nowak and colleagues reported that the proportion of high-affinity, glycine-displaceable [3H] CGP-39653 binding to glutamate receptors was reduced in patients who committed suicide compared with control subjects 26. However, another study found no difference in the actual number of N-methyl-D-aspartate (NMDA) receptors in nine brain regions of 22 suicide victims and age/sex matched controls compared using [3H]MK-801-binding characteristics 27.
Notably, we previously reported that the NMDA antagonist ketamine resulted in a rapid antidepressant effect within hours as compared to placebo in a group of patients with treatment-resistant MDD 28. In that study, we also noted that the MADRS suicide subscore improved rapidly (i.e., earlier than previously reported in other pharmacological studies). However, our previous study did not permit the inclusion of patients with significant suicidal ideation, thus minimizing the variance in suicide subscores.
Based on this previous study, we hypothesized that a single-intravenous infusion of ketamine would bring about a rapid and clinically significant improvement in suicidal ideation within 230 minutes of the infusion. In order to assess this issue as carefully as possible, inpatients with treatment-resistant MDD currently experiencing a major depressive episode were included regardless of severity of suicidal ideation. Furthermore, we used a sensitive and specific scale to measure this construct: the Scale for Suicide Ideation (SSI) 29.
Method
Thirty-three patients aged 18 to 65 years old participated in this inpatient study between October 2006 and January 2009. Participants fulfilled DSM-IV criteria for MDD and had no diagnosis of alcohol or substance abuse or dependence in the past 90 days, as determined by the Structured Clinical Interview for Diagnosis, DSM-IV (SCID) 30. All patients were in good health, and unmedicated for at least two weeks prior to the ketamine infusion, as determined by medical history, physical examination, routine blood labs, electrocardiogram, urinalysis, and urine toxicology. Patients received a complete description of the study, and written informed consent was obtained. The study was approved by the Combined Neuroscience Institutional Review Board of the National Institutes of Health (NIH). Each subject was assigned a Clinical Research Advocate (CRA) from the NIMH Subjects Protection Unit to monitor the consent process; in addition, CRAs monitored subjects during research participation from this initial consent and throughout study participation.
Patients underwent a single infusion of ketamine hydrochloride (0.5 mg/kg) infused over 40 minutes followed by a double-blind randomization to riluzole or placebo six hours post-infusion. Here we report only the results of the open-label ketamine phase (up to 230 minutes post-infusion). This time point was chosen because previous studies have consistently found that 88% of all responders to ketamine reach response criteria by 230 minutes 28, 31, 32. Ratings included the MADRS 33, the 17-item HDRS 34, the Beck Depression Inventory (BDI; 35), the Clinician Administered Dissociative States Scale (CADSS; 36), the Brief Psychiatric Rating Scale (BPRS; 37) and the SSI 29. Ratings were obtained at baseline (60 minutes prior to the infusion) and 40, 80, 120, and 230 minutes post-infusion. All items on each scale were used. High interrater reliability was obtained for both the MADRS (intraclass correlation coefficient = 0.88) and the SSI (intraclass correlation coefficient = 0.94).
Statistics
Linear mixed models were used to examine the course of outcome measures over time where time was a fixed within-subjects factor and a fixed intercept was included. Schwarz’s Bayesian criteria were used to determine the best fitting variance-covariance structure that was a first-order autoregressive model. A random intercept and random effect for subject did not add to the model, so they were not included. Bonferroni adjusted post-hoc tests were used to examine the change from baseline to each post-infusion time. Significance was evaluated at p<.05, two-tailed.
Previous studies have identified a cutoff score >3 as indicating significant suicidal ideation on the SSI; as a result, patients in this study were separated into groups with significant suicidal ideation (SSI>3) and without significant suicidal ideation (SSI<4) at baseline 38. Youden’s index—the optimal trade-off between sensitivity and specificity—was achieved for the SSI scale at a cutoff threshold of >3, a score that is both clinically significant and an appropriate method for detecting significant suicidal ideation 38. Kaplan-Maier survival analysis was used to examine the average amount of time for patients with significant baseline ideation to reach minimal ideation (SSI<4). For those without significant baseline ideation, survival analysis examined how long patients took to develop significant ideation (SSI>3). The demographic characteristics of patients with and without suicidal ideation at baseline were examined with Student’s t-tests for continuous measures and chi-square tests for categorical ones.
Results
Table 1 shows demographic data for the full sample as well as for patients with (n=10) and without (n=23) significant baseline SSI scores. Patients with greater suicidal ideation at baseline were significantly more likely to have higher MADRS and HDRS total scores, higher MADRS and HDRS suicide items, higher HDRS anxiety subscores, and higher rates of past suicidal ideation and attempts. Paired t-tests indicated that the SSI scores did not change significantly from screening to baseline (an average of 8.0 (SD=4.1) days) for the total sample (t=1.39, df=23, p=.18; mean change=−1.0 (SD=3.7)), the higher SSI at baseline group (t=0.70, df=4, p=.52; mean change=−2.4 (SD=7.6)), or the lower SSI at baseline group (t=1.58, df=18, p=.13; mean change=-0.7 (SD=1.9)), indicating that scores were relatively stable at baseline.
Table 1.
Demographic and Course of Illness Characteristics
| SSI > 3 (n=10) | SSI < 4 (n=23) | Total (N=33) | High vs. Low SSI | ||||
|---|---|---|---|---|---|---|---|
| MEAN | SD | MEAN | SD | MEAN | SD | p | |
| Age | 49.3 | 13.4 | 45.0 | 13.9 | 46.3 | 13.7 | 0.42 |
| Age of Onset | 21.6 | 10.4 | 20.7 | 12.3 | 20.9 | 11.7 | 0.85 |
| Length of Illness (Years) | 28.8 | 11.7 | 24.3 | 13.0 | 25.5 | 12.6 | 0.40 |
| Length of Current Episode (Months) | 92.0 | 123.4 | 100.7 | 148.3 | 98.5 | 140.3 | 0.88 |
| Episodes | 44.1 | 51.3 | 24.4 | 40.7 | 29.0 | 43.3 | 0.30 |
| Height (cm) | 174.3 | 9.8 | 174.2 | 11.7 | 174.2 | 11.0 | 0.98 |
| Weight (kg) | 90.8 | 19.0 | 95.7 | 28.2 | 94.2 | 25.6 | 0.62 |
| BMI | 29.8 | 5.3 | 31.2 | 7.9 | 30.8 | 7.1 | 0.61 |
| Clinical Scales (Baseline) | |||||||
| • Suicide | |||||||
| • SSI | 8.7 | 7.0 | 0.6 | 0.9 | 3.0 | 5.4 | <.001 |
| • HDRS Item | 2.3 | 0.8 | 0.5 | 0.7 | 1.1 | 1.1 | <.001 |
| • MADRS Item | 3.4 | 1.0 | 1.7 | 0.9 | 2.2 | 1.2 | <.001 |
| • Depression | |||||||
| • HDRS | 24.5 | 5.0 | 18.7 | 2.7 | 20.5 | 4.4 | <.001 |
| • MADRS | 36.8 | 4.5 | 31.6 | 3.1 | 33.2 | 4.3 | <.001 |
| • Anxiety (HDRS Subscale) | 7.2 | 2.3 | 5.9 | 1.2 | 6.3 | 1.7 | 0.04 |
| • BPRS | 36.7 | 5.8 | 35.4 | 5.4 | 35.8 | 5.5 | 0.54 |
| • CADSS | 7.9 | 12.7 | 3.5 | 5.2 | 4.8 | 8.3 | 0.16 |
| N | % | N | % | N | % | p | |
| Gender (Male) | 6 | 60 | 14 | 61 | 20 | 61 | 0.96 |
| Education (College Graduate) | 4 | 50 | 14 | 64 | 18 | 60 | 0.50 |
| Mood Disorder Family History | 8 | 100 | 20 | 87 | 28 | 90 | 0.28 |
| Hospitalized (Lifetime) | 7 | 70 | 10 | 43 | 17 | 52 | 0.16 |
| Suicide | |||||||
| • Ideation | |||||||
| • Lifetime | 9 | 90 | 11 | 48 | 20 | 61 | 0.02 |
| • Admission | 8 | 80 | 4 | 17 | 12 | 36 | 0.001 |
| • Attempt | |||||||
| • Self | 7 | 70 | 3 | 13 | 10 | 30 | 0.001 |
| • Family History | 4 | 40 | 8 | 35 | 12 | 36 | 0.77 |
Abbreviations: BMI: Body Mass Index; BPRS: Brief Psychiatric Rating Scale; CADSS: Clinician Administered Dissociative States Scale; HDRS: Hamilton Depression Rating Scale; MADRS: Montgomery-Asberg Depression Rating Scale; SSI: Scale for Suicide Ideation
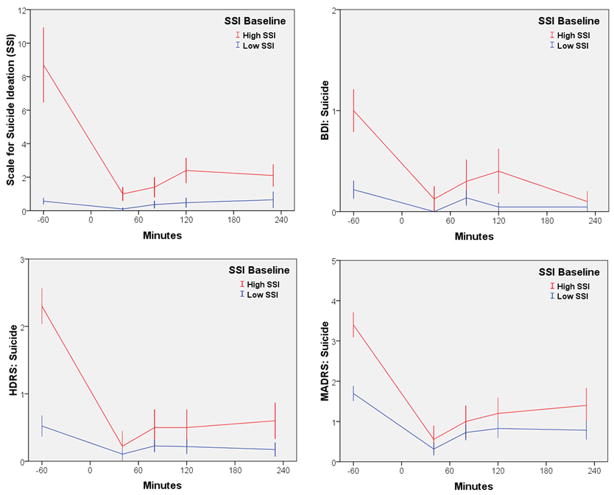
The linear mixed models with the full sample indicated significant improvement in all of the suicide scales following ketamine infusion (SSI: F=7.03, df= 4,97, p<.001; HDRS suicide item: F=17.25, df= 4,106, p<.001; MADRS suicide item: F=27.68, df= 4,110, p<.001; BDI suicide item: F=5.82, df= 4,103, p<.001) (See Figure 1). On each scale, scores were significantly lower at 40 minutes and remained significantly lower at 230 minutes. The effect for the full sample was very large at 40 minutes (d=1.05, 95% C.I.: 0.65–1.45), and moderate at 230 minutes (d=0.45, 95% C.I.: 0.12–0.77).
Figure 1.
Course of suicidal ideation over 230 minutes in patients with treatment-resistant MDD and with or without high suicidal ideation who received ketamine (n=33). High SSI was defined as >3. Low SSI was defined as <4. Abbreviations: BDI: Beck Depression Inventory; HDRS: Hamilton Depression Rating Scale; MADRS: Montgomery-Asberg Depression Rating Scale; SSI: Scale for Suicide Ideation.
In the subgroup with baseline SSI scores >3, the effect size was d=2.36 (95% C.I.: 1.56–3.16) at 40 minutes and d=1.27 (95% C.I.: 0.62–1.92) at 230 minutes. At an SSI cutoff of >4, the effect size was d=3.13 (95% C.I.: 1.97–4.30) at 40 minutes and d=1.84 (95% C.I.: 0.92–2.75) at 230 minutes. The same pattern was true for the depression and anxiety total scores (HDRS: F=29.57, df= 4,117, p<.001; MADRS: F=36.17, df= 4,117, p<.001; BDI: F=39.94, df= 4,112, p<.001; HDRS Anxiety: F=15.10, df= 4,115, p<.001; see Figure 2), as well as for the hopelessness subscale on the BDI (F=20.46, df= 4,104, p<.001).
Figure 2.
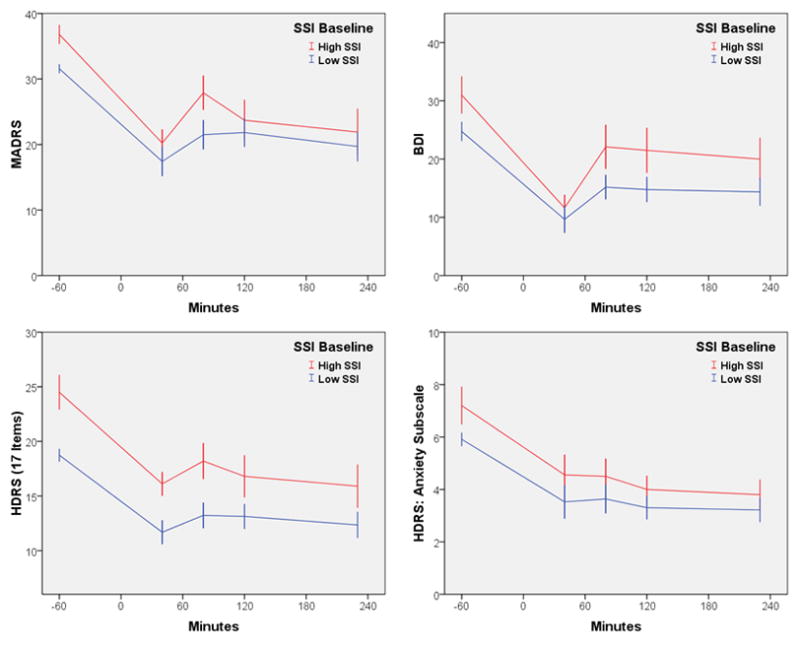
Course of depression and anxiety symptoms over 230 minutes in patients with treatment-resistant MDD and with or without high suicidal ideation who received ketamine (n=33). High SSI was defined as >3. Low SSI was defined as <4. Abbreviations: BDI: Beck Depression Inventory; HDRS: Hamilton Depression Rating Scale; MADRS: Montgomery-Asberg Depression Rating Scale; SSI: Scale for Suicide Ideation.
All 10 patients (100%) with higher baseline SSI scores went below an SSI score of 4 within the first hours after the infusion; nine of 10 (90%) went below a score of 4 within 40 minutes, and one within 80 minutes. The average time needed to achieve an SSI score less than 4 was 44 minutes (SE=4). Six (60%) of these patients reached SSI scores of zero during the first day, and five of these individuals achieved this reduction within 40 minutes; the remaining individual achieved this reduction within 80 minutes. For these patients, the average time to zero was 120 minutes (SE=29).
For patients with lower baseline SSI scores, only one (4%) went above a score of 3 on the first day after infusion. This patient took 80 minutes to reach 4 (from a baseline of 3). Furthermore, no serious adverse events occurred during the study. The adverse effects noted were comparable to those that occurred in our previous study, where mild perceptual disturbances were observed in most patients only in the first hour after infusion 28.
Discussion
Thirty-three subjects with treatment resistant DSM-IV-diagnosed MDD received a ketamine infusion as part of a clinical research protocol. Subjects with a SSI score of >3—indicating substantial suicidal ideation—improved significantly within 230 minutes, as assessed by not only SSI total scores but also MADRS, HDRS, and BDI suicide items. In fact, the mean time necessary for these individuals to achieve an SSI score lower than 4 was 44 minutes. The effect size associated with ketamine use was very large, regardless of the cutoff used on the SSI scale used (eg, >3 or >4) 39.
The rapidity of this improvement in suicidal ideation and the magnitude of the effect is especially notable given the treatment-resistant status of these patients; patients with more severe depression are at greater risk for more suicide attempts 1. This group of patients had, on average, been ill for 26 years, and their current major depressive episode was, on average, of eight years duration; furthermore, 61% of the patients had a lifetime history of suicidal ideation, and 30% had a previous suicide attempt. In addition, anxiety and hopelessness, which are well-recognized risk factors for suicide, also improved significantly and rapidly during the course of the study. Rapidly modifying these risk factors could also impact the short-term risk of suicidal behavior.
Several factors need to be considered in interpreting these data. Although the sample size was relatively small, four different scales showed comparable improvement in suicidal ideation and the effect sizes were very large. In addition, the drug was administered in an open-label fashion, which could have biased the reported response; however, it should be noted that the improvements in suicidal ideation and depressive symptoms observed in the present study over the course of 230 minutes closely paralleled the improvement of depressive symptoms seen in our previous controlled study 28. It is also possible that the results reflect a waxing and waning of suicidal ideation 40; however, we think this possibility is unlikely, because suicidal ideation in this treatment-resistant population of depressed patients was sustained over the course of eight days prior to ketamine infusion (i.e., it did not differ significantly from admission levels). Finally, it is important to note that the improvement in suicidal ideation observed here occurred in the context of severe depression. Whether ketamine rapidly reduces suicidal ideation in patients with a diagnosis other than MDD is unknown. In addition, the length of improvement remains unclear.
Suicide is one of the leading causes of death among young people worldwide, and particularly among those with psychiatric disorders. In the past decade, an increased urgency has accompanied efforts at suicide prevention, leading to the identification of additional risk factors for suicide, as well as improved preventive strategies 41. Suicidal ideation itself is a medical emergency that requires swift and careful treatment. Unfortunately, few currently available therapeutics can be used to immediately reduce suicidal ideation, often with tragic consequences. Relatedly, difficulties are also associated with treating individuals quickly enough to significantly reduce suicidal ideation; a key example of this is in the US military, where the stress of multiple deployments and the difficulties associated with successfully treating soldiers in the field have made suicide a particularly urgent issue 42.
It is clear that our preliminary results need to be interpreted with caution given the small group size and the open-label nature of the study. Nevertheless, the significant and often rapid response seen in some individuals who were refractory to many traditional antidepressants suggests that directly modulating the glutamatergic system may ultimately be effective in treating suicidal ideation. Due to their potentially significant impact on public health, further studies are needed to confirm these preliminary findings, and the continued examination of the role of the glutamatergic system in the pathophysiology and acute pharmacological treatment of suicidal ideation is clearly warranted.
Acknowledgments
This study was supported by the Intramural Research Program at the National Institute of Mental Health (NIMH), and the National Alliance for Research on Schizophrenia and Depression (NARSAD) (CZ).
Footnotes
Study ID Numbers: 040222; 04-M-0222; ClinicalTrials.gov Identifier: NCT00088699
Disclosure of Competing Interests and Financial Support: The author(s) declare that, except for income received from our primary employer, no financial support or compensation has been received from any individual or corporate entity over the past three years for research or professional service and there are no personal financial holdings that could be perceived as constituting a potential conflict of interest. Dr. Zarate is listed as a co-inventor on a patent for the use of ketamine in major depression. Dr. Zarate has assigned his patent rights on ketamine to the U.S. government. None of the other investigators in this study have a possible conflict of interest, financial or otherwise. Presented at: Society of Biological Psychiatry 64th Annual Scientific Convention & Meeting, Vancouver, British Columbia, Canada. May 14–16, 2009
References
- 1.Claassen CA, Trivedi MH, Rush AJ, et al. Clinical differences among depressed patients with and without a history of suicide attempts: findings from the STAR*D trial. J Affect Disord. 2007;97:77–84. doi: 10.1016/j.jad.2006.05.026. [DOI] [PubMed] [Google Scholar]
- 2.Diefenbach GJ, Woolley SB, Goethe JW. The association between self-reported anxiety symptoms and suicidality. J Nerv Ment Dis. 2009;197:92–97. doi: 10.1097/NMD.0b013e318196127c. [DOI] [PubMed] [Google Scholar]
- 3.Deisenhammer EA, Ing CM, Strauss R, Kemmler G, Hinterhuber H, Weiss EM. The duration of the suicidal process: how much time is left for intervention between consideration and accomplishment of a suicide attempt? J Clin Psychiatry. 2009;70:19–24. [PubMed] [Google Scholar]
- 4.Gausset MF, Casadebaig F, Guillaud-Bataille JM, Quemada N, Terra JL. Mortality of mentally ill patients. Review of the literature. Encephale. 1992;18:93–100. [PubMed] [Google Scholar]
- 5.Hunt IM, Kapur N, Webb R, et al. Suicide in recently discharged psychiatric patients: a case-control study. Psychol Med. 2009:443–449. doi: 10.1017/S0033291708003644. [DOI] [PubMed] [Google Scholar]
- 6.Janofsky JS. Reducing inpatient suicide risk: using human factors analysis to improve observation practices. J Am Acad Psychiatry Law. 2009;37:15–24. [PubMed] [Google Scholar]
- 7.Mills PD, DeRosier JM, Ballot BA, Shepherd M, Bagian JP. Inpatient suicide and suicide attempts in Veterans Affairs hospitals. Jt Comm J Qual Patient Saf. 2008;34:482–488. doi: 10.1016/s1553-7250(08)34061-6. [DOI] [PubMed] [Google Scholar]
- 8.Yeager KR, Saveanu R, Robers AR, et al. Measured response to identified suicide risk and violence: what you need to know about psychiatric patient safety. Brief Treat Crisis Interven. 2005;5:121–141. [Google Scholar]
- 9.Jick H, Kaye JA, Jick SS. Antidepressants and the risk of suicidal behaviors. JAMA. 2004;292:338–343. doi: 10.1001/jama.292.3.338. [DOI] [PubMed] [Google Scholar]
- 10.Larkin GL, Smith RP, Beautrais AL. Trends in US emergency department visits for suicide attempts, 1992–2001. Crisis. 2008;29:73–80. doi: 10.1027/0227-5910.29.2.73. [DOI] [PubMed] [Google Scholar]
- 11.Baldessarini RJ, Tondo L, Davis P, Pompili M, Goodwin FK, Hennen J. Decreased risk of suicides and suicide attempts during long-term lithium treatment: a meta-analytic review. Bipolar Disord. 2006;8:625–639. doi: 10.1111/j.1399-5618.2006.00344.x. [DOI] [PubMed] [Google Scholar]
- 12.Guzzetta F, Tondo L, Centorrino F, Baldessarini RJ. Lithium treatment reduces suicide risk in recurrent major depressive disorder. J Clin Psychiatry. 2007;68:380–383. doi: 10.4088/jcp.v68n0304. [DOI] [PubMed] [Google Scholar]
- 13.Meltzer HY, Alphs L, Green AI, et al. Clozapine treatment for suicidality in schizophrenia: International Suicide Prevention Trial (InterSePT) Arch Gen Psychiatry. 2003;60:82–91. doi: 10.1001/archpsyc.60.1.82. [DOI] [PubMed] [Google Scholar]
- 14.Linehan MM, Comtois KA, Murray AM, et al. Two-year randomized controlled trial and follow-up of dialectical behavior therapy vs therapy by experts for suicidal behaviors and borderline personality disorder. Arch Gen Psychiatry. 2006;63:757–766. doi: 10.1001/archpsyc.63.7.757. [DOI] [PubMed] [Google Scholar]
- 15.Fleischmann A, Bertolote JM, Wasserman D, et al. Effectiveness of brief intervention and contact for suicide attempters: a randomized controlled trial in five countries. Bull World Health Organ. 2008;86:703–709. doi: 10.2471/BLT.07.046995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Brown GK, Ten Have T, Henriques GR, Xie SX, Hollander JE, Beck AT. Cognitive therapy for the prevention of suicide attempts: a randomized controlled trial. JAMA. 2005;294:563–570. doi: 10.1001/jama.294.5.563. [DOI] [PubMed] [Google Scholar]
- 17.Ernst CL, Goldberg JF. Antisuicide properties of psychotropic drugs: a critical review. Harv Rev Psychiatry. 2004;12:14–41. doi: 10.1080/10673220490425924. [DOI] [PubMed] [Google Scholar]
- 18.Lukens TW, Wolf SJ, Edlow JA, et al. Clinical policy: critical issues in the diagnosis and management of the adult psychiatric patient in the emergency department. Ann Emerg Med. 2006;47:79–99. doi: 10.1016/j.annemergmed.2005.10.002. [DOI] [PubMed] [Google Scholar]
- 19.Reeves H, Batra S, May RS, Zhang R, Dahl DC, Li X. Efficacy of risperidone augmentation to antidepressants in the management of suicidality in major depressive disorder: a randomized, double-blind, placebo-controlled pilot study. J Clin Psychiatry. 2008;69:1228–1336. doi: 10.4088/jcp.v69n0805. [DOI] [PubMed] [Google Scholar]
- 20.Simon GE, Savarino J, Operskalski B, Wang PS. Suicide risk during antidepresant treatment. Am J Psychiatry. 2006;163:41–47. doi: 10.1176/appi.ajp.163.1.41. [DOI] [PubMed] [Google Scholar]
- 21.Gibbons RD, Brown CH, Hur K, Marcus SM, Bhaumik DK, Mann JJ. Relationship between antidepressants and suicide attempts: an analysis of the veterans health administration data sets. Am J Psychiatry. 2007;164:1044–1049. doi: 10.1176/ajp.2007.164.7.1044. [DOI] [PubMed] [Google Scholar]
- 22.Patel M, Patel S, Hardy DW, Benzies BJ, Tare V. Should electroconvulsive therapy be an early consideration for suicidal patients? J ECT. 2006;22:113–115. doi: 10.1097/00124509-200606000-00007. [DOI] [PubMed] [Google Scholar]
- 23.Currier D, Mann JJ. Stress, genes and the biology of suicidal behavior. Psychiatr Clin North Am. 2008;31:247–269. doi: 10.1016/j.psc.2008.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Laje G, Paddock S, Manji HK, et al. Genetic markers of suicidal ideation emerging during citalopram treatment of major depression. Am J Psychiatry. 2007;164:1530–1538. doi: 10.1176/appi.ajp.2007.06122018. [DOI] [PubMed] [Google Scholar]
- 25.Menke A, Lucae S, Kloiber S, et al. Genetic markers within glutamate receptors associated with antidepressant treatment-emergent suicidal ideation. Am J Psychiatry. 2008;165:917–918. doi: 10.1176/appi.ajp.2008.08020274. [DOI] [PubMed] [Google Scholar]
- 26.Nowak G, Ordway GA, Paul IA. Alterations in the N-methyl-D-aspartate (NMDA) receptor complex in the frontal cortex of suicide victims. Brain Res. 1995;675:157–164. doi: 10.1016/0006-8993(95)00057-w. [DOI] [PubMed] [Google Scholar]
- 27.Holemans S, De Paermentier F, Horton RW, Crompton MR, Katona CL, Maloteaux JM. NMDA glutamatergic receptors, labelled with [3H]MK-801, in brain samples from drug-free depressed suicides. Brain Res. 1993;616:138–143. doi: 10.1016/0006-8993(93)90202-x. [DOI] [PubMed] [Google Scholar]
- 28.Zarate CA, Jr, Singh JB, Carlson PJ, et al. A randomized trial of an N-methyl-D-aspartate antagonist in treatment-resistant major depression. Arch Gen Psychiatry. 2006;63(8):856–864. doi: 10.1001/archpsyc.63.8.856. [DOI] [PubMed] [Google Scholar]
- 29.Beck AT, Kovacs M, Weissman A. Assessment of suicidal intention: the Scale for Suicide Ideation. J Consult Clin Psychol. 1979;47:343–352. doi: 10.1037//0022-006x.47.2.343. [DOI] [PubMed] [Google Scholar]
- 30.First MB, Spitzer RL, Gibbon M, Williams JBW. Structured clinical interview for DSM-IV-TR axis disorders. New York: Biometrics Research Department, New York State Psychiatric Institute; 2001. [Google Scholar]
- 31.Phelps LE, Brutsche NE, Moral JR, Luckenbaugh DA, Manji HK, Zarate CA. Family history of alcohol dependence and initial antidepressant response to an N-methyl-D-aspartate antagonist. Biol Psychiatry. 2009;65:181–184. doi: 10.1016/j.biopsych.2008.09.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Salvadore G, Cornwell BR, Colon-Rosario V, et al. Increased anterior cingulate cortical activity in response to fearful faces: a neurophysiological biomarker that predicts rapid antidepressant response to ketamine. Biol Psychiatry. 2009;65:289–295. doi: 10.1016/j.biopsych.2008.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Montgomery SA, Asberg M. A new depression scale designed to be sensitive to change. Br J Psychiatry. 1979;134:382–389. doi: 10.1192/bjp.134.4.382. [DOI] [PubMed] [Google Scholar]
- 34.Hamilton M. A rating scale for depression. J Neurol Neurosurg Psychiatry. 1960;23:56–62. doi: 10.1136/jnnp.23.1.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Beck AT, Beamesderfer A. Assessment of depression: the depression inventory. Mod Probl Pharmacopsychiatry. 1974;7(0):151–169. doi: 10.1159/000395074. [DOI] [PubMed] [Google Scholar]
- 36.Bremner JD, Krystal JH, Putnam FW, et al. Measurement of dissociative states with the Clinician-Administered Dissociative States Scale (CADSS) J Trauma Stress. 1998;11(1):125–136. doi: 10.1023/A:1024465317902. [DOI] [PubMed] [Google Scholar]
- 37.Overall JE, Gorham DR. The Brief Psychiatric Rating Scale. Psychol Res. 1962;25:168–179. [Google Scholar]
- 38.Holi MM, Pelkonen M, Karlsson L, et al. Psychometric properties and clinical utility of the Scale for Suicidal Ideation (SSI) in adolescents. BMC Psychiatry. 2005;5:8. doi: 10.1186/1471-244X-5-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sokero TP, Melartin TK, Rystala HJ, Leskela US, Lestela-Mielonen MA, Isometsa ET. Suicidal ideation and attempts among psychiatric patients with major depressive disorder. J Clin Psychiatry. 2003;64:1094–1100. doi: 10.4088/jcp.v64n0916. [DOI] [PubMed] [Google Scholar]
- 40.Zisook S, Trivedi MH, Warden D, et al. Clinical correlates of the worsening or emergence of suicidal ideation during SSRI treatment of depression: An examination of citalopram in the STARD study. J Affect Disord. 2009 Feb 12; doi: 10.1016/j.jad.2009.01.002. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 41.U.S. Department of Health and Human Services. National Strategy for Suicide Prevention: Goals and Objectives for Action. Rockville, Maryland: U.S. Department of Health and Human Services; 2001. Report No.: SMA 3517. [PubMed] [Google Scholar]
- 42.Kuehn BM. Soldier suicide rates continue to rise. JAMA. 2009;301:1111–1113. doi: 10.1001/jama.2009.342. [DOI] [PubMed] [Google Scholar]