Abstract
Celiac disease (CD) is a disorder of the small intestine caused by intolerance to wheat gluten and related proteins in barley and rye. CD4+ T cells play a central role in CD, recognizing and binding complexes of HLA-DQ2.5 bearing gluten peptides that have survived digestion and that are deamidated by tissue transglutaminase (TG2), propagating a cascade of inflammatory processes that damage and eventually destroy the villous tissue structures of the small intestine. Here we present data showing that recombinant DQ2.5-derived molecules bearing covalently tethered α2-gliadin-61-71 peptide have a remarkable ability to block antigen-specific T cell proliferation and inhibited pro-inflammatory cytokine secretion in human DQ2.5-restricted α2-gliadin specific T cell clones obtained from patients with celiac disease. The results from our in vitro studies suggest that HLA-DQ2.5 derived molecules could significantly inhibit and perhaps reverse the intestinal pathology caused by T cell mediated inflammation and the associated production of proinflammatory cytokines.
Keywords: Human, T cells, MHC, Antigen Presentation/Processing, celiac disease
Introduction
Celiac disease is an inflammatory disorder that has a complex genetic etiology involving both innate and adaptive immune responses (1–4). Celiac patients do not tolerate gluten, a protein found in wheat, rye and barley. At the heart of the problem are CD4+ T cells that recognize peptides deamidated by tissue transglutaminase (TG2), facilitating a cascade of inflammatory processes characterized by villus atrophy within the intestinal mucosa, enlarged hyperplastic crypts, and increased intestinal lymphocytes in the lamina propria and epithelium that damage and eventually destroy the villous architecture of the small intestine, interfering with the absorption of nutrients from food. The availability of accurate serologic tests have led to the realization that celiac disease is relatively common, affecting 1 of every 120-300 people in both Europe and North America, and it is estimated that subclinical forms of this disease are even more prevalent (5–7).
Activation of CD4+ T cells is a multi-step process initiated minimally by ligation of antigen-specific T cell receptor (TCR) and CD4 co-receptor with the MHC class II/peptide complex present on antigen presenting cells (APC) (signal 1), and co-stimulation through T cell surface molecules such as CD28 (signal 2). In studies focused on producing small single chain MHC mimetics that would retain primary signaling characteristics of the MHC class II heterodimer, we developed a unique class of molecules we termed Recombinant TCR Ligands (RTLs). These molecules were constructed by linking together the MHC class II beta-1 and alpha-1 domains, with and without covalently linked peptide antigen (8). Our earliest studies demonstrated that these compounds formed complexes with antigenic peptides that could be used to detect and inhibit rat encephalitogenic T cells and treat experimental autoimmune encephalomyelitis (EAE), an animal model of multiple sclerosis (9). Studies with CD4+, DR2-restricted T cell clones from human patients suffering from multiple sclerosis demonstrated that DR2-derived RTLs altered TCR zeta chain phosphorylation, induced a short-term sustained mobilization of calcium in T cells, and decreased ERK activity with concomitant induction of IL-10 production in an antigen-specific manner (10). Studies using the Lewis rat A1 hybridoma documented that T cell activation could be directly modulated in vitro by binding of multimeric RT1.B-derived RTLs, inducing early TCR signaling and a unique pattern of downstream activation (11). The ability of these compounds to tolerize antigen-specific T cell clones was recently expanded to include the construction of HLA-DP-derived RTLs with the ability to tolerize HLA-DP2-restricted beryllium-specific pathogenic T cells (12). The potential of these molecules for use in the treatment of other human diseases has been expanded from our studies in EAE (8–12) with tolerization data in animal models including collagen-induced arthritis (13) EAU (experimental autoimmune uveitis) (14), and neuroprotection data, modulating T cell trafficking, in the mid cerebral artery occlusion (MCAO) model of stroke (15). The broad applicability of this platform technology compelled us to explore its utility in celiac disease. DQ2.5-derived RTLs were designed, a purification scheme was developed, and DQ2.5-derived molecules were biochemically and biophysically characterized. DQ2.5-derived RTLs bearing covalently tethered gluten derived α2-gliadin-61-71 peptide showed a remarkable ability to block antigen-specific T cell proliferation and inhibited cytokine production in human DQ2 restricted α2-gliadin specific IEL T cell clones obtained from patients with celiac disease.
Results
Construction and characterization of DQ2-derived RTL800 and peptide tethered variants
We have constructed recombinant HLA-DQ2.5 (RTL800-series) using a strategy previously described for constructing other RTLs from MHC class II alleles (8–10, 12, 16). The primary sequence of “empty” rDQ2.5 (RTL800) aligned with the previously described HLA-DR2- and HLA-DP2-derived RTLs is shown in Figure 1. Ion exchange and size-exclusion FPLC were used to purify the molecules (Fig. 2A). The presence of the native conserved disulfide bond between DQB1*0201 C15 and C79 was demonstrated by a gel shift assay (Fig. 2A, inset). Similar to the progenitor DR2-derived RTLs, DQ2-derived RTLs tend to form oligomers of 15-22 molecules (Fig. 2A, and data not shown). In the studies described here we have used DQ2.5-derived RTL multimers, which as described below, retain potent biological activity. Mutation of DQA1*0501 Cysteine 44 to serine reduced the level of intermolecular disulfide cross-linked species in both the “empty” and peptide antigen coupled RTLs (compare reduced +β-ME vs -β-ME, Fig. 2A, inset). Authenticity of the purified proteins was further confirmed by N-terminal amino acid analysis (data not shown). Immunoblot analysis of the purified proteins with DQ2.5-specific mAb 2.12.E11 (Fig. 2B) and mAb Tu39 (data not shown) confirmed the identity of the compounds (Fig. 2B). Circular dichroism confirmed that the RTLs had highly ordered secondary structures (Fig. 2C), and comparison with the secondary structures of MHC class II molecules determined by x-ray crystallography provided strong evidence that the RTLs shared the beta-sheet platform/anti-parallel alpha-helix secondary structure common to all MHC class II antigen binding domains (Table I) (17–22).
Figure 1. Primary amino acid sequence alignment of human DQ2-, DR2- and DP2-derived RTLs.
“Empty” rDQ2.5 (RTL800) was derived from DQ2 (DQA1*0501/DQB1*0201), RTL302 from DR2 (DRA1*0101/DRB1*1501), and RTL600 from DP2 (DPA1*0103/DPB1*0201) primary sequences. Gaps in the sequences for optimal alignment (*) and the beta-1//alpha-1 junction (←|→) are shown. The conserved cysteines that form a disulfide bond are shaded yellow, and the cysteine residue present in DQ2-derived RTL800 that has been changed to Serine in RTL801 to prevent intermolecular disulfide bond formation and aggregation is underlined. Genes encoding RTL802 & RTL803, variants of 800 and 801, respectively, encode proteins that have the amino-terminal peptide sequence (MFPQPELPYPQPGSGSGSGSGSGSGSGS) instead of the starting methionine. These molecules bear antigenic α2-gliadin-61-71 (Q65E) “α-II” peptide and linker (the gliadin-derived sequence is underlined).
Figure 2. Characterization of human HLA-DQ2.5-derived RTL molecules.
(A) Size exclusion chromatography (superdex-75) of purified and refolded “empty” RTL800 and RTL801 (αC44S mutation), & gliadin-61-71-covalently tethered RTL802 and 803. Inset, Samples of purified RTLs were boiled for 5 minutes in Laemmli sample buffer ± the reducing agent s-mercaptoethanol (s-ME), and then analyzed by SDS-PAGE (12%). Non-reduced RTLs (-s-ME) have a smaller apparent molecular weight than reduced RTLs (+s-ME), indicating the presence of a disulfide bond. First and last lanes show the molecular weight standards albumin (66 kD), ovalbumin (45 D), carbonic anhydrase (31 kD) and soybean trypsin inhibitor (21.5 kD). Note that the molecules with the αC44S mutation (RTL801 & 803) have dramatically reduced levels of intermolecular disulfide linked aggregates compared to RTLs 800 & 802 with the wild-type sequence. (B) DQ2.5-derived RTLs are recognized by DQ2.5-specific mAb 2.12.E11. 5ug DQ2-derived RTL800 and 802, DR4-derived RTL363 as a negative control (40), or full-length recombinant DQ2/CLIP as a positive control were blotted in duplicate onto nitrocellulose membranes. Membranes were blocked with 10% FCS in PBS overnight and then incubated with anti-DQ mAbs SPV-L3 (undetermined epitope) (41), 2.12.E11 (DQB1*0201, 0202 and 0203- specific) (42) and SFR-20α5 (undetermined epitope) (43) for 1hr. Blots were washed 2X and then secondary antibodies goat-anti-rat HRP (SFR-20α5) and rabbit-anti-mouse HRP antibodies were used for the detection. IgG were used as the control. All three antibodies recognize full-length DQ2/CLIP, only mAb 2.12.E11 recognized DQ2-derived RTL800 and RTL802, and none of these antibodies could detect DR4-derived RTL363. Our data is consistant with SPV-L3 and SFR-20α5 recognizing the α2 or β2 domains of full-length DQ2 and mAb 2.12.E11 recognizing a unique epitope present in the DQ2.5-derived RTLs that minimally consists of β1-domain residues G45, E46, and F47 (50). (C) Circular dichroism measurements were performed at 25°C on an Aviv Model 215 CD spectrometer using 0.1 mm cells, at 0.5 nm intervals from 260 to 180 nm. Concentration values for each protein solution were determined by amino acid analysis. Buffer, 20 mM Tris, pH 8.5. Deconvolution and analysis of the secondary structure presented in Table I was performed using the variable selection method (51). Data are expressed as Delta-epsilon per mole per cm.
Table I.
Structural analysis of DQ2-derived RTLs
| Molecule | descriptiona | α-helix | β-sheet | β-turn | other | total | Reference |
|---|---|---|---|---|---|---|---|
| RTL800 | rDQ2 (WT) (DQA1*0501/DQB1*0201) | 0.25 | 0.30 | 0.19 | 0.26 | 1.0 | This study |
| RTL801 | rDQ2 (Cα44S) | 0.29 | 0.21 | 0.17 | 0.33 | 1.0 | This study |
| RTL802 | rDQ2 (WT)/gliadin 61-71(Q65E) | 0.27 | 0.27 | 0.19 | 0.27 | 1.0 | This study |
| RTL803 | rDQ2 (Cα44S)/gliadin 61-71(Q65E) | 0.25 | 0.28 | 0.19 | 0.28 | 1.0 | This study |
| RTL302 | DR2 (DRA1*0101/DRB1*1501) | 0.25 | 0.25 | 0.20 | 0.30 | 1.0 | (17) |
| RTL303 | 302/hu-MBP-85-99 | 0.26 | 0.24 | 0.19 | 0.31 | 1.0 | (17) |
| RTL600 | DP2 (DPA1*0103/DPB1*0201) | 0.16 | 0.23 | 0.25 | 0.36 | 1.0 | (12) |
| 1S9V | DQ2/gliadin-57-75 | 0.30 | 0.24 | 0.20 | 0.26 | 1.0 | (52) |
| 1BX2 | DR2/hu-MBP-86-99 | 0.32 | 0.37 | -- | 0.31 | 1.0 | (18) |
Derived from HLA-MHC class II beta-1 and alpha-1 domains as indicated. Secondary structure content derived from the deconvoluted spectra of the DQ2-derived RTLs presented in Fig. 2, and published studies, as indicated. Analysis of the secondary structure was performed using the variable selection method (51), and using CDNN software (version 2.0.3) authored by Gerald Bohm at the Insititut fur Biotechnology, Martin-Luther University, Halle-Wittenberg, Germany (53).
DQ2-derived RTL800 can discriminate between native and TG2-modified a2-gliadin peptides
Peptide binding studies were performed using α2-gliadin-59-78, a peptide that contains all three immunodominant epitopes present in the naturally occurring α2-gliadin-57-89 33-mer that remains intact following digestion with gastric, pancreatic and brush border membrane (BBM) proteases (Fig. 3) (1–4). “Empty” DQ2-derived RTL800 can clearly discriminate between the wild-type “QQ” vs the deamidated forms of the peptide, with the highest apparent affinity for the “EE” doubly deamidated peptide (Fig. 3). RTL800 remained stable for the duration of the experiments, as confirmed by commassie staining and western blot analysis (data not shown). RTL800 with captured rhodamine-labeled peptide migrates with a higher molecular weight than “empty” RTL800, and the appearance of multiple bands in the rhodamine panel suggests conformational heterogeneity for the population of RTL800 molecules loaded with peptide. Our interpretation is that the faint upper band may represent a proportion of RTL800 loaded with the “EE” peptide that is in a less compact “floppy” conformation, thus hindering its mobility on SDS-PAGE, consistent with previous studies using purified full-length MHC class II heterodimers (23, 24). Preliminary experiments using circular dichroism further suggest that the deamidated “EE” peptide is structurally distinct from the native peptide (data not shown) and these structural features strongly favor efficient and stable binding to DQ2-derived RTL800, supporting previous reported studies using full-length DQ2 (25).
Figure 3. Deamidated gliadin peptide binds specifically to HLA-DQ2.5-derived RTL800.
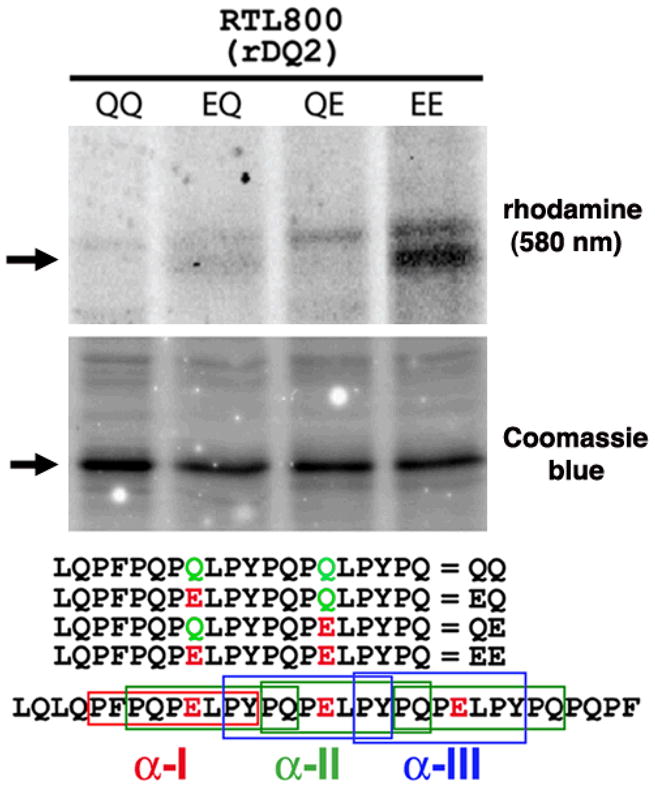
RTLs (20 pMol; ~500 ng) were mixed for 60h at 37 C with 4 nMol of rhodamine-labeled peptides in 100 mM Phosphate, pH 6.0, 0.01% sodium azide and 1 mM EDTA. The reactions were stopped by adding one volume (60 ul) of 1.5 M Tris, 0.1% SDS and 20% glycerol pH 8.8. Forty ul were analyzed by 18% SDS-PAGE and scanned at 580 nm to monitor binding of amino-terminal rhodamine labeled peptides (top panel). Equivalent amounts of RTLs were loaded in each lane, as indicated by staining with Coomassie Blue (middle panel). (Lower panel), the amino-terminal rhodamine-labeled peptides used in this binding study, aligned relative to their position within the immunodominant α2-gliadin-57-89 33-mer. Differences in the sequences of each peptide at key positions are indicated. Boxed regions of the α2-gliadin 33-mer correspond to the α-I (red), α-II (green), and α-III (blue) gliadin minimal epitopes, of which one, three and two copies of each, respectively, are found within the 33-mer peptide. Data are representative of three independent experiments.
HLA-DQ2 derived RTL802 blocked antigen specific T cell proliferation
Antigen specific T cell clones were cultured from small intestinal biopsies of patients with celiac disease. T cell proliferation and cytokine production were monitored in vitro as surrogate markers for T cell mediated inflammation hypothesized to perpetuate intestinal pathology, and we tested the ability of RTLs to modulate T cell proliferation and cytokine production, and determined the clonal specificity of this inhibition. Our strategy to design and produce DQ2.5-derived RTLs was based on our previous experience suggesting that these compounds could be used to specifically modulate the population(s) of cells involved in pathology, minimizing the impact on the rest of the immune repertoire. After optimizing our proliferation assay using HLA-DQ2.5 expressing EBV-transformed human B cells as antigen presenting cells (APCs), we compared the proliferation of patient-derived antigen-specific T clones exposed to 10 μg/ml of gluten or TG2-treated gluten, or 2 uM and 10 uM gluten-derived peptides p1274 (PQPELPYPQPQLPY), or p1729 (PQTQQPEQPFPQPQ). The proliferative response of clones TCC364.1.0.4 (specific for α2-gliadin 61-71(Q65E) “α-II” peptide found in p1274) (Fig. 4A) and TCC387.19 (specific for the “γ-VII” peptide p1729) (Fig. 4B) provided distinctive activation signals that allowed a critical assessment of peptide-specific inhibitory potential of the DQ2.5- derived RTL802 molecule bearing the covalently tethered α-II peptide. Three copies of the TCC364.1.0.14 α-II epitope, minimally consisting of the sequence PQPELPYPQ, are present within the naturally occurring immunodominant α2-gliadin-57-89 33-mer following deamidation by tissue transglutaminase (TG2) (Fig. 3, lower panel) (1–4). RTL802 carrying the covalently-tethered α-II epitope potently and specifically blocked proliferation of the cognate α-II-specific TCC364.1.0.14 (Fig. 4A). Importantly, RTL802 did not inhibit proliferation of the non-cognateγ-VII-specific TCC387.19 (Fig. 4B). RTL361, a recombinant DR4 molecule that carries a covalently tethered collagen peptide (AGFKGEQGPKGEP) was developed for treatment of arthritis and has demonstrated efficacy in protecting animals from collagen-induced arthritis (13). We used this molecule as a control in our studies. RTL361 did not inhibit proliferation of either clone (Fig. 4). Thus, inhibition of TCC364.1.0.14 by RTL802 required both the appropriate antigenic peptide and the appropriate HLA-DQ2.5-derived RTL platform
Figure 4. HLA-DQ2.5-derived RTL802 blocks antigen specific T cell proliferation.
Proliferation assays were performed using CD patient biopsy-derived antigen-specific T clones. TCC were pre-incubated with 4 or 8 uM of the study drug DQ2.5-derived RTL802, negative controls “empty” RTL800, DR4-derived RTL361, or buffer alone for 48 hours. (A) TCC364.1.0.14 proliferated in response to TG2-treated gluten (10 ug/ml) or the α-II p1274 (PQPELPYPQPQLPY) peptide (2 uM and 10 uM). (B) TCC387.19 proliferates in response to TG2-treated gluten (10 ug/ml) or the γ-VII p1729 (PQTQQPEQPFPQPQ) peptide (10 ug/ml). EBV-transformed human B cells expressing HLA-DQ2.5 loaded with antigens were used as APCs. The T:APC ratio was 1:1, with 60,000 T cells/well. Antigens used included 10 μg/ml of gluten, TG2-treated gluten, p1274 (PQPELPYPQPQLPY) or p1729 (PQTQQPEQPFPQPQ) gluten-derived peptides. Data from triplicate wells are presented as average CPM +/- SD. A representative example of three individual experiments is shown.
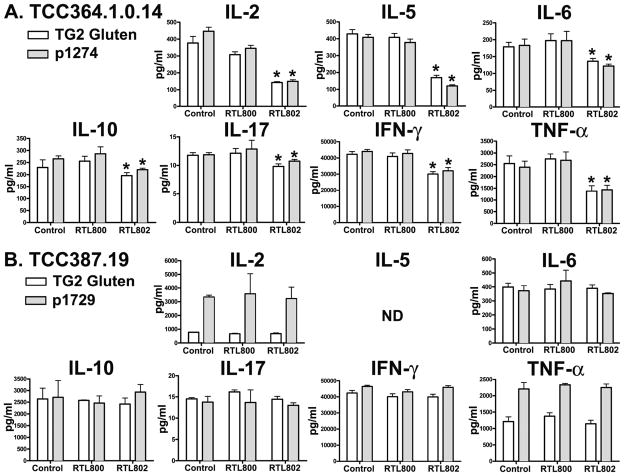
HLA-DQ2.5 derived RTL802 blocked antigen specific production of cytokines
Intestinal lymphocytes isolated from active lesions taken from intestinal biopsies of patients with CD show an extraordinarily complex cytokine profile. As well, long-term TCC from CD patient biopsies cultured in vitro maintain a complex cytokine profile. These TCCs showed DQ2.5-restricted proliferation (Fig. 4) and cytokine production (Fig. 5) to α2-gliadin peptides in culture upon restimulation with gliadin peptide. We have consistently documented that RTL therapy induces Ag-specific cytokine changes in T cell lines and clones isolated from both humans and rodents. Here we demonstrate for the first time that DQ2.5-derived RTL802 blocked Ag-specific cytokine production specifically in cognate T cells. As shown in Figure 5, both TCC364.1.0.14 {α-II p1274 (PQPELPYPQPQLPY) peptide-specific} and TCC 387.19 {γ- VII p1729 (PQTQQPEQPFPQPQ) peptide-specific} produced a wide range of cytokines upon exposure to EBV-transfected DQ2 expressing B cells loaded with TG2-treated gluten or cognate antigen, including IL-2, IL-5, IL-6, IL-10, IL-17, IFN-γ and TNF-α. Pretreatment of TCC364.1.0.14 with RTL802 (10 uM) bearing the cognate α-II p1274 peptide inhibited all secreted cytokines significantly, with the most dramatic reduction observed for IL-2 and IL-5 (~70% decrease), and TNF-α, either when TG2-treated gluten or when the p1274 peptide was used for stumulation (Fig. 5A). In contrast, RTL802 had no significant effect on cytokine secretion by TCC387.19 that recognizes the γ-VII p1729 peptide antigen. Moreover, “empty” DQ2-derived RTL800 had no significant effect on either T cell specificity (Fig. 5), nor did DR4-derived RTL363 (data not shown). These studies strongly support the idea that RTL802 can modulate the cognate pathogenic CD-associated CD4+ T cells.
Figure 5. HLA-DQ2.5-derived RTL802 significantly attenuates antigen-specific cytokine production.
Aliquots of supernatants harvested from the RTL proliferation assay at 48 hr after culture with APCs was used for cytokine analysis. A customized human Bio-Plex cytokine kit was used to detect IL-2, IL-5, IL-6, IL-10, IL-17, IFN-γ, and TNF-α. Each data point represented triplicate samples from each treatment group. Significance between the control and treatment groups was determined by Student’s t test. A p value of < 0.05 was considered statistically significant.
Discussion
Experiments presented in this manuscript describe, for the first time, the ability of recombinant single-chain HLA-DQ2.5-derived molecules to modulate the behavior of pathogenic CD4+ T cells isolated from the biopsies of CD patients. Soluble recombinant MHC class II molecules in various forms have been developed, including full length detergent solubilized MHC class II heterodimers (26, 27), various forms of the extracellular α1α2 and β1β2 heterodimeric domains (28–31), and recently the β1 and α1 domains genetically linked into a single polypeptide chain (8–10, 17, 32). RTL appears to be the smallest structure that retains the peptide binding/TCR recognition features of MHC class II molecules (33). While the ability of HLA-DR2- and HLA-DP2-derived RTLs to modulate T cell behavior in vitro (11, 32) and in vivo (12, 34–36) has been extensively studied, this is the first work to our knowledge that describes the applicability of RTL therapy toward HLA-DQ-mediated pathology.
We have constructed recombinant single-chain TCR ligands from HLA-DQ2.5 (RTL800-series) using the same logic as has been described for constructing other RTLs from MHC class II alleles, including human DR- and DP-derived (8, 10) (12), and murine IAs-, IAb and rat RT1.B-derived RTLs (8, 9, 16). What remains allele-dependent is whether or not the constructs form oligomers. In our previously described protein engineering studies of RTLs derived from HLA-DR2 (DRB1*1501/DRA*0101) (17), we found that DR2-derived molecules formed multimers, with approximately 10% of the molecules in the form of stable dimers and the remainder purified in the form of higher-order structures above 300,000 Daltons (17). HLA-DP2 molecules, however, are exclusively monomers (12). One part of the explanation for these observations is that the molecules contain self-binding motifs, with DR2 and DQ2 containing multiple self-binding motifs, whereas DP2 does not contain any [Burrows, manuscript in preparation]. While multimeric and monomeric RTLs retain biological activity (10, 34), we continue to work on methods with the goal of converting these multimeric complexes into monomers with retention of biological activity, as an FDA prerequisite toward eventual use as human therapeutics. In the case of DR2-derived RTLs, modification of five sites on the alpha-1 domain was sufficient for engineering monodisperse molecules that retained the ability to bind Ag-peptides, inhibit T cell proliferation in an Ag-specific manner and were able to treat experimental autoimmune encephalomyelitis (EAE) in vivo (37). While we have identified detergent conditions that will allow us to move forward into clinical trials with the DQ2.5-derived compounds (data not shown), we have not yet succeeded in engineering the DQ2-derived compounds to be monodisperse. This is an active area of investigation in our laboratory and in the studies described here we have used DQ2-derived RTL multimers, which as shown, retain potent biological activity. Our data presented here demonstrates that TG2-modified gliadin peptides bind to soluble HLA-DQ2.5-derived RTL molecules and that the covalently-tethered RTL802 version of this DQ2/peptide complex blocks Ag-specific T cell proliferation and cytokine production. As such, these recombinant HLA-DQ2/peptide molecules represent a powerful new approach toward a therapy for celiac disease.
Materials and Methods
Recombinant TCR ligands (RTLs)
General methods for the design, cloning and expression of RTLs have been described, and HLA-DP2 derived RTL600 was used as template in constructing recombinant HLA-DQ2 genes (8, 12, 17, 37). 9 pairs of oligo-primers specifically designed to modify the template were synthesized and used to generate “empty” rDQ2.5 (RTL800) (Figure 1). Further modifications to the RTL constructs included mutations at various positions toward the purpose of stabilizing monomeric versions of the constructs as described previously for HLA-DR2-derived compounds (37), and replacement of the cysteine at position 143 (HLA-DQ Cα44) with serine, generating RTL801. Genes encoding “empty” rDQ2.5 molecules RTL800 and 801 were modified by adding sequence encoding α2-gliadin-61-71 peptide (Q65E) (FPQPELPYPQP) with a linker (GSGSGSGSGSGSGSGS) to the 5′ end of the genes, encoding peptide-tethered RTL802 and RTL803, respectively. The genes were directionally ligated into pET21d(+) vector using NCoI and XhoI restriction enzymes (Novagen, Inc., Madison, WI) and transformed into Nova blue E. coli host (Novagen, Inc., Madison, WI) for positive colony selection and primary sequence of the constructs was confirmed by DNA sequencing. The corrected plasmid constructs were then transformed into E. coli strain BL21(DE3) expression host (Novagen, Inc., Madison, WI). Expression, purification and refolding of these proteins followed procedures described previously(35). In brief, BL21(DE3) cells containing the plasmid construct of interest were grown in 1-liter cultures to mid-logarithmic phase (OD600 = 0.6–0.8) in Luria-Bertani broth containing carbenicillin (50 mg/ml) at 37°C. Recombinant protein production was induced by addition of 0.5 mM isopropyl b-D-thiogalactoside (IPTG). After incubation for 3 h, the cells were harvested by centrifugation and stored at −80°C before processing. All subsequent manipulations of the cells were at 4°C. The cell pellets were resuspended in ice-cold PBS, pH 7.4, and sonicated for 4 × 20 secs with the cell suspension cooled in a salt/ice/water bath. The cell suspension was then centrifuged, the supernatant fraction poured off, and the cell pellet resuspended and washed three times in PBS, and then resuspended in 20 mM ethanolamine/6M urea, pH 10, for 4 h. After centrifugation, the supernatant containing the solubilized RTLs was collected and stored at 4°C until purification. RTLs were purified and concentrated by fast protein liquid chromatography (FPLC) ion-exchange chromatography using Source 30Q anion-exchange media (Pharmacia Biotech, Piscataway, NJ) in an XK26/20 column (Pharmacia Biotech) charged with buffer B (20mM Ethanolamine pH 10.0, 6M Urea, 2M NaCl) and then equilibrated with buffer A (equilibrated with buffer A (buffer B minus NaCl). Approx 100 ml lysate sample was loaded at 1.5–2.0 ml/min. Protein was eluted using a step gradient (105 ml 1%B, 75 ml 2%B, 120ml 3%B, 70ml 4%B), followed by a linear gradient (130 ml 4% to 100% B) and then cleared with 23 ml 100% B, with a flow rate of 5ml/min. Fractions containing RTLs were collected based on analysis of fractions by SDS-PAGE, pooled, and dialyzed extensively against buffer C (6M Urea, 20 mM Ethanolamine, pH 10, 200 mM NaCl). For purification to homogeneity a finish step using size-exclusion chromatography on Superdex 75 media (Pharmacia Biotech) in an HR16/50 column (Pharmacia Biotech) in buffer C was used. Fractions containing purified RTLs were pooled and diluted to 0.1 mg/ml. RTLs were refolded by extensive dialysis at 0.1 mg/ml against 20 mM Tris, pH 8.5. Protein was then concentrated to 1 mg/ml for short-term storage (4C) or snap-frozen in liquid N2 for long-term storage at −80C. The final yield of purified RTL800 and RTL802 (~90% pure as estimated by SDS-PAGE) varied between 15 and 30 mg/L of bacterial culture.
Circular Dichroism Measurements
Circular dichroism (CD) spectra were recorded on a JASCO J-500A spectropolarimeter with an IF-500 digital interface and thermostatically controlled quartz cells (Hellma, Mulheim, Germany) of 2-, 1-, 0.5-, 0.1-, and 0.05-mm path length depending on peptide and protein concentration. Data are presented as mean residue weight ellipticities. Calibration was regularly performed with (1)-10-camphorsulfonic acid (Sigma) to molar ellipticities of 7,780 and 216,160 degree cm2/dmol at 290.5 and 192.5 nm, respectively (38). In general, spectra were the average of 4–5 scans from 260 to 180 nm, recorded at a scanning rate of 5 nm/min with a 4-s time constant. Data were collected at 0.1-nm intervals. Spectra were averaged and smoothed using the built-in algorithms of the Jasco program, and buffer base lines were subtracted. Secondary structure was estimated with the program CONTIN (39).
Immunoblot analysis
Western and dot blot analysis (Figure 2) were performed to confirm integrity and identity of the RTL constructs. For dot blot analysis, 5ug DQ2.5-derived RTL800 and 802, DR4-derived RTL363 as a negative control (40), or full-length recombinant DQ2/CLIP as a positive control, were blotted in duplicate onto PVDF membranes. Membranes were blocked with 10% FCS in PBS overnight and then incubated with anti-DQ mAbs SPV-L3 (41), 2.12.E11 (DQB1*0201, 0202 and 0203-specific) (42) and SFR-20α5 (diluted in 1:1000) (43) for 1hr. Blots were washed 2X and then secondary antibodies goat-anti-rat HRP (SFR-20α5; diluted 1:5000) and rabbit-anti- mouse HRP antibodies (diluted in 1:5000) were used for detection. IgG was used as the control (Dot blot was a kind gift of Stig Tollefsen and Ludvig M. Sollid at the Centre for Immune Regulation, Institute of Immunology, University of Oslo and Rikshospitalet University Hospital, Oslo, Norway, Stig.Tollefsen@rr-research.no). All three antibodies recognize full-length DQ2/CLIP, only mAb 2.12.E11 recognized DQ2-derived RTL800 and RTL802, and none of these antibodies could detect DR4-derived RTL363. For Western blot analysis, SDS-PAGE separated proteins were transferred to PVDF membranes for 1h at RT. Membranes were blocked with 3% BSA in PBS/0.05% Tween 20 and probed with mAb Tu39-FITC (BD Biosciences, San Jose, CA). Tu39 is a mouse IgG2a antibody that recognizes an epitope conserved on all human HLA class II molecules (44, 45). PVDF membranes were scanned for FITC-cojugated Tu39 using a BioRad Molecular Imager FX.
Peptide binding assay
A peptide capture method was used to characterize peptide binding to the RTLs. In brief, 400 pmol (10 ug) of protein were mixed with 4 nmol of amino-terminal rhodamine-labeled peptides (Genscript, Piscataway, NJ) in a total volume of 60 ul buffer (100mM phosphate buffer at pH 6 containing 0.01% sodium azide, 1mM EDTA and 0.05% SDS) and incubated at 37 C for 60h. The reactions were stopped by adding one volume (60 ul) of 1.5 M Tris, 0.1% SDS and 20% glycerol pH 8.8 and placed on ice for 1 to 2 h. The capture mixture (40 ul containing 2.5 ug RTL) was analyzed by SDS-PAGE using 10–20% gradient Tris-tricine pre-cast peptide gels (Cat#345-0067, Bio-Rad Laboratories, Hercules, CA). Following separation, gels were scanned for rhodamine at 580 nm using a BioRad Molecular Imager FX to monitor peptide capture. Pre-stained molecular weight markers (Page-ruler prestained protein ladder (Cat#SM0671, Fermentas, Inc., Burlington, Ontario) were used to track location of captured peptides on gels.
RTL inhibition of proliferation assay
A T cell proliferation assay described previously (37, 46, 47) was used as an RTL function assay. (T cell proliferation assays were performed by Stig Tollefsen in Ludvig Sollid’s Laboratory at the Centre for Immune Regulation, Institute of Immunology, University of Oslo and Rikshospitalet University Hospital, Oslo, Norway.) In brief, 6×105 gliadin-specific T cells derived from patients with celiac disease were pre-incubated with 4 or 8 μM of DQ2.5-derived RTLs, DR4-derived RTL361 (negative control) or buffer alone for 48 hours and then washed twice with cultural medium to remove RTLs. Approximately 6×105 irradiated (7500 rad) EBV-transformed DQ2 expressing B cells (Clone, STEIINLIN, ECACC, Salisbury, GB) were pre- cultured with various gliadin antigens at 2 μM or 10 μM concentration for 20 hrs in U-bottom 96-well plate. Antigens tested included 10 μg/ml TG2-treated gluten, 2 μM and 10 μM gliadin peptide p1274 (PQPELPYPQPQLPY) which contains the minimal α-II peptide (PQPELPYPQ), and 10 μMγ-VII gliadin peptide p1729 (PQTQQPEQPFPQPQ) (48). Antigens used in the experiments were prepared as described previously (46, 49). Briefly, the 100 μM native gluten antigens were pre-incubated with 150 μg/ml human recombinant TG2 in 100 mM Tris (pH 7.4) with 2 mM CaCl2 for 2 h at 37°C. The synthetic peptides were prepared by solid-phase peptide synthesis on a robotic system (Syro MultiSynTech, Bochum, Germany) using Fmoc/O-t-butyl chemistry. Identity of the peptides was confirmed by MALDI-TOF mass spectrometry and purity was analyzed by reversed-phase HPLC. T cells were mixed with B cells in the presence of antigens (1:1 ratio) and incubated for 48 hrs. T cell proliferation was measured by the uptake of [3H]-thymidine (1 μCi [3H]-thymidine/well) that was added in the final 16 hr, and supernatants (50 μl) were collected before adding [3H] thymidine for cytokine analysis. Each treatment was performed in triplicate. Data is representative of three independent experiments.
Detection and quantification of cytokine production using Bio-Plex cytokine assays (Luminex)
Aliquots of supernatants harvested from the RTL proliferation assay at 48 hr after culture with APCs was used for cytokine analysis. A customized human Bio-Plex cytokine kit was used to detect IL-2, IL-5, IL-6, IL-10, IL-17, IFN-γ and TNF-α simultaneously, as described by the manufacturer’ protocol (http://www.bio-rad.com/BioPlexSystem/). Data was collected on a Luminex 200 (Bio- Rad, CA), and the data were analyzed using Bio-Plex Management software (Bio-Rad, CA). Each data point represented triplicate samples from each treatment group. Significance between the control and treatment groups was determined by Student’s t test. A p value of < 0.05 was considered statistically significant.
Acknowledgments
This work was supported by NIH Grants DK068881 and AI43960
The authors would like to thank Dr. Ludvig Sollid from the Institute of Immunology, University of Oslo, Oslo, Norway, for kindly providing the gliadin-specific T cell lines used in this study. The authors would also like to thank Dr. Stig Tollefsen from the Institute of Immunology for his expert work and advice in culturing the T cell lines and performing proliferation assays presented in this study.
Abbreviations in this paper
- Ag
antigen
- APC
antigen presenting cell
- CD
celiac disease
- HLA
histocompatibility leukocyte antigen
- MHC
major histocompatibility antigen
- RTL
Recombinant TCR Ligand
- TCR
T cell receptor
- TCC
T cell clone
- TCL
T cell line
Footnotes
Conflict of interest statement. Drs. Burrows, Offner & Vandenbark, and OHSU have a significant financial interest in Artielle ImmunoTherapeutics, Inc., a company that may have a commercial interest in the results of this research and technology. This potential conflict of interest has been reviewed and managed by the OHSU and VAMC Conflict of Interest in Research Committees.
References
- 1.Tuckova L, Novotna J, Novak P, Flegelova Z, Kveton T, Jelinkova L, Zidek Z, Man P, Tlaskalova-Hogenova H. Activation of macrophages by gliadin fragments: isolation and characterization of active peptide. Journal of Leukocyte Biology. 2002;71:625–631. [PubMed] [Google Scholar]
- 2.Maiuri L, Ciacci C, Ricciardelli I, Vacca L, Raia V, Auricchio S, Picard J, Osman M, Quaratino S, Londei M. Association between innate response to gliadin and activation of pathogenic T cells in coeliac disease. [see comment] Lancet. 2003;362:30–37. doi: 10.1016/S0140-6736(03)13803-2. [DOI] [PubMed] [Google Scholar]
- 3.Mamone G, Ferranti P, Rossi M, Roepstorff P, Fierro O, Malorni A, Addeo F. Identification of a peptide from alpha-gliadin resistant to digestive enzymes: implications for celiac disease. Journal of Chromatography B: Analytical Technologies in the Biomedical & Life Sciences. 2007;855:236–241. doi: 10.1016/j.jchromb.2007.05.009. [DOI] [PubMed] [Google Scholar]
- 4.Shan L, Molberg O, Parrot I, Hausch F, Filiz F, Gray GM, Sollid LM, Khosla C. Structural Basis for Gluten Intolerance in Celiac Sprue. Science. 2002;297:2275–2279. doi: 10.1126/science.1074129. [DOI] [PubMed] [Google Scholar]
- 5.Farrell RJ, MD, Kelly Ciaran P., MD Celiac Sprue. The New England Journal of Medicine. 2002;346:180–188. doi: 10.1056/NEJMra010852. [DOI] [PubMed] [Google Scholar]
- 6.Johnston SD, WR, McMillan SA, Sloan J, Love AH. Coeliac disease detected by screening is not silent simply unrecognized. QJM. 1998;91:853–860. doi: 10.1093/qjmed/91.12.853. [DOI] [PubMed] [Google Scholar]
- 7.Catassi C, FE, Ratsch IM, et al. The coeliac iceberg in Italy: a multicentre antigliadin antibodies screening for coeliac disease in schoolage subjects. Acta Paediatr Suppl. 1996;412:29–35. doi: 10.1111/j.1651-2227.1996.tb14244.x. [DOI] [PubMed] [Google Scholar]
- 8.Burrows GG, Chang JW, Bachinger HP, Bourdette DN, Offner H, Vandenbark AA. Design, engineering and production of functional single-chain T cell receptor ligands. Protein Eng. 1999;12:771–778. doi: 10.1093/protein/12.9.771. [DOI] [PubMed] [Google Scholar]
- 9.Burrows GG, Bebo BF, Jr, Adlard KL, Vandenbark AA, Offner H. Two-domain MHC class II molecules form stable complexes with myelin basic protein 69-89 peptide that detect and inhibit rat encephalitogenic T cells and treat experimental autoimmune encephalomyelitis. J Immunol. 1998;161:5987–5996. [PubMed] [Google Scholar]
- 10.Burrows GG, Chou YK, Wang C, Chang JW, Finn TP, Culbertson NE, Kim J, Bourdette DN, Lewinsohn DA, Lewinsohn DM, Ikeda M, Yoshioka T, Allen CN, Offner H, Vandenbark AA. Rudimentary TCR signaling triggers default IL-10 secretion by human Th1 cells. J Immunol. 2001;167:4386–4395. doi: 10.4049/jimmunol.167.8.4386. [DOI] [PubMed] [Google Scholar]
- 11.Wang C, Mooney JL, Meza-Romero R, Chou YK, Huan J, Vandenbark AA, Offner H, Burrows GG. Recombinant TCR ligand induces early TCR signaling and a unique pattern of downstream activation. J Immunol. 2003;171:1934–1940. doi: 10.4049/jimmunol.171.4.1934. [DOI] [PubMed] [Google Scholar]
- 12.Fontenot AP, Keizer TS, McCleskey M, Mack DG, Meza-Romero R, Huan J, Edwards DM, Chou YK, Vandenbark AA, Scott B, Burrows GG. Recombinant HLA-DP2 binds beryllium and tolerizes beryllium-specific pathogenic CD4+ T cells. J Immunol. 2006;177:3874–3883. doi: 10.4049/jimmunol.177.6.3874. [DOI] [PubMed] [Google Scholar]
- 13.Huan J, Kaler LJ, Mooney JL, Subramanian S, Hopke C, Vandenbark AA, Rosloniec EF, Burrows GG, Offner H. MHC class II derived recombinant T cell receptor ligands protect DBA/1LacJ mice from collagen-induced arthritis. J Immunol. 2008;180:1249–1257. doi: 10.4049/jimmunol.180.2.1249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Adamus G, Burrows GG, Vandenbark AA, Offner H. Treatment of autoimmune anterior uveitis with recombinant TCR ligands. Invest Ophthalmol Vis Sci. 2006;47:2555–2561. doi: 10.1167/iovs.05-1242. [DOI] [PubMed] [Google Scholar]
- 15.Sinha S, Subramanian S, Miller L, Proctor TM, Roberts C, Burrows GG, Vandenbark AA, Offner H. Cytokine switch and bystander suppression of autoimmune responses to multiple antigens in experimental autoimmune encephalomyelitis by a single recombinant T-cell receptor ligand. J Neurosci. 2009;29:3816–3823. doi: 10.1523/JNEUROSCI.5812-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Burrows GG, Adlard KL, Bebo BF, Jr, Chang JW, Tenditnyy K, Vandenbark AA, Offner H. Regulation of encephalitogenic T cells with recombinant TCR ligands. J Immunol. 2000;164:6366–6371. doi: 10.4049/jimmunol.164.12.6366. [DOI] [PubMed] [Google Scholar]
- 17.Chang JW, Mechling DE, Bachinger HP, Burrows GG. Design, engineering, and production of human recombinant t cell receptor ligands derived from human leukocyte antigen DR2. J Biol Chem. 2001;276:24170–24176. doi: 10.1074/jbc.M101808200. [DOI] [PubMed] [Google Scholar]
- 18.Smith KJ, Pyrdol J, Gauthier L, Wiley DC, Wucherpfennig KW. Crystal structure of HLA-DR2 (DRA*0101, DRB1*1501) complexed with a peptide from human myelin basic protein. J Exp Med. 1998;188:1511–1520. doi: 10.1084/jem.188.8.1511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Li Y, Li H, Martin R, Mariuzza RA. Structural basis for the binding of an immunodominant peptide from myelin basic protein in different registers by two HLA-DR2 proteins. J Mol Biol. 2000;304:177–188. doi: 10.1006/jmbi.2000.4198. [DOI] [PubMed] [Google Scholar]
- 20.Brown JH, Jardetzky TS, Gorga JC, Stern LJ, Urban RG, Strominger JL. Three dimensional structure of the human class II histocompatibility antigen HLA-DR1. Nature. 1993;364:33. doi: 10.1038/364033a0. [DOI] [PubMed] [Google Scholar]
- 21.Murthy VL, Stern LJ. The class II MHC protein HLA-DR1 in complex with an endogenous peptide: implications for the structural basis of the specificity of peptide binding. Structure. 1997;5:1385–1396. doi: 10.1016/s0969-2126(97)00288-8. [DOI] [PubMed] [Google Scholar]
- 22.Fremont DH, Hendrickson WA, Marrack P, Kappler J. Structures of an MHC class II molecule with covalently bound single peptides. Science. 1996;272:1001. doi: 10.1126/science.272.5264.1001. [DOI] [PubMed] [Google Scholar]
- 23.Sadegh-Nasseri S, McConnell HM. A kinetic intermediate in the reaction of an antigenic peptide and I-Ek. Nature. 1989;337:274–276. doi: 10.1038/337274a0. [DOI] [PubMed] [Google Scholar]
- 24.Sadegh-Nasseri S, Natarajan S, Chou CL, Hartman IZ, Narayan K, Kim A. Conformational heterogeneity of MHC class II: induced upon binding to different peptides is a key regulator in antigen presentation and epitope selection. Immunol Res. doi: 10.1007/s12026-009-8138-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Xia J, Sollid LM, Khosla C. Equilibrium and kinetic analysis of the unusual binding behavior of a highly immunogenic gluten peptide to HLA-DQ2. Biochemistry. 2005;44:4442–4449. doi: 10.1021/bi047747c. [DOI] [PubMed] [Google Scholar]
- 26.Nag B, Arimilli S, Mukku PV, Astafieva I. Functionally active recombinant alpha and beta chain-peptide complexes of human major histocompatibility class II molecules. J Biol Chem. 1996;271:10413–10418. doi: 10.1074/jbc.271.17.10413. [DOI] [PubMed] [Google Scholar]
- 27.Nag B, Kendrick T, Arimilli S, Yu SC, Sriram S. Soluble MHC II-peptide complexes induce antigen-specific apoptosis in T cells. Cell Immunol. 1996;170:25–33. doi: 10.1006/cimm.1996.0130. [DOI] [PubMed] [Google Scholar]
- 28.Arimilli S, Cardoso C, Mukku P, Baichwal V, Nag B. Refolding and reconstitution of functionally active complexes of human leukocyte antigen DR2 and myelin basic protein peptide from recombinant alpha and beta polypeptide chains. J Biol Chem. 1995;270:971–977. doi: 10.1074/jbc.270.2.971. [DOI] [PubMed] [Google Scholar]
- 29.Kozono H, White J, Clements J, Marrack P, Kappler J. Production of soluble MHC class II proteins with covalently bound single peptides. Nature. 1994;369:151–154. doi: 10.1038/369151a0. [DOI] [PubMed] [Google Scholar]
- 30.Appel H, Gauthier L, Pyrdol J, Wucherpfennig KW. Kinetics of T-cell receptor binding by bivalent HLA-DR. Peptide complexes that activate antigen-specific human T-cells. J Biol Chem. 2000;275:312–321. doi: 10.1074/jbc.275.1.312. [DOI] [PubMed] [Google Scholar]
- 31.Appel H, Seth NP, Gauthier L, Wucherpfennig KW. Anergy induction by dimeric TCR ligands. J Immunol. 2001;166:5279–5285. doi: 10.4049/jimmunol.166.8.5279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Huan J, Subramanian S, Jones R, Rich C, Link J, Mooney J, Bourdette DN, Vandenbark AA, Burrows GG, Offner H. Monomeric recombinant TCR ligand reduces relapse rate and severity of experimental autoimmune encephalomyelitis in SJL/J mice through cytokine switch. J Immunol. 2004;172:4556–4566. doi: 10.4049/jimmunol.172.7.4556. [DOI] [PubMed] [Google Scholar]
- 33.Burrows GG. Systemic Immunomodulation of Autoimmune Disease Using MHC-Derived Recombinant TCR Ligands. Curr Drug Targets Inflamm Allergy. 2005;4:185–193. doi: 10.2174/1568010053586363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Vandenbark AA, Rich C, Mooney J, Zamora A, Wang C, Huan J, Fugger L, Offner H, Jones R, Burrows GG. Recombinant TCR ligand induces tolerance to myelin oligodendrocyte glycoprotein 35-55 peptide and reverses clinical and histological signs of chronic experimental autoimmune encephalomyelitis in HLA-DR2 transgenic mice. J Immunol. 2003;171:127–133. doi: 10.4049/jimmunol.171.1.127. [DOI] [PubMed] [Google Scholar]
- 35.Rich C, Link JM, Zamora A, Jacobsen H, Meza-Romero R, Offner H, Jones R, Burrows GG, Fugger L, Vandenbark AA. Myelin oligodendrocyte glycoprotein-35-55 peptide induces severe chronic experimental autoimmune encephalomyelitis in HLA-DR2-transgenic mice. Eur J Immunol. 2004;34:1251–1261. doi: 10.1002/eji.200324354. [DOI] [PubMed] [Google Scholar]
- 36.Chou YK, Culbertson N, Rich C, LaTocha D, Buenafe AC, Huan J, Link J, Wands JM, Born WK, Offner H, Bourdette DN, Burrows GG, Vandenbark AA. T-cell hybridoma specific for myelin oligodendrocyte glycoprotein-35-55 peptide produced from HLA-DRB1*1501-transgenic mice. J Neurosci Res. 2004;77:670–680. doi: 10.1002/jnr.20201. [DOI] [PubMed] [Google Scholar]
- 37.Huan JY, Meza-Romero R, Mooney JL, Chou YK, Vandenbark AA, Bachinger HP, Burrows GG. Rationally designed mutations convert complexes of human recombinant T cell receptor ligands into monomers that retain biological activity. J Chem Tech & Biotech. 2005;80:2–12. doi: 10.1002/jctb.1086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chen GC, Yang JT. Some hydrodynamic and optical properties of polyribonucleotides. Biophys Chem. 1973;1:62–72. doi: 10.1016/0301-4622(73)80002-x. [DOI] [PubMed] [Google Scholar]
- 39.Provencher SW, Glockner J. Estimation of globular protein secondary structure from circular dichroism. Biochemistry. 1981;20:33–37. doi: 10.1021/bi00504a006. [DOI] [PubMed] [Google Scholar]
- 40.Huang YH, Colgrave ML, Daly NL, Keleshian A, Martinac B, Craik DJ. The biological activity of the prototypic cyclotide kalata b1 is modulated by the formation of multimeric pores. J Biol Chem. 2009;284:20699–20707. doi: 10.1074/jbc.M109.003384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Spits H, Keizer G, Borst J, Terhorst C, Hekman A, de Vries JE. Characterization of monoclonal antibodies against cell surface molecules associated with cytotoxic activity of natural and activated killer cells and cloned CTL lines. Hybridoma. 1983;2:423–437. doi: 10.1089/hyb.1983.2.423. [DOI] [PubMed] [Google Scholar]
- 42.Viken HD, Paulsen G, Sollid LM, Lundin KE, Tjonnfjord GE, Thorsby E, Gaudernack G. Characterization of an HLA-DQ2-specific monoclonal antibody. Influence of amino acid substitutions in DQ beta 1*0202. Hum Immunol. 1995;42:319–327. doi: 10.1016/0198-8859(94)00110-c. [DOI] [PubMed] [Google Scholar]
- 43.Johansen BH, Buus S, Vartdal F, Viken H, Eriksen JA, Thorsby E, Sollid LM. Binding of peptides to HLA-DQ molecules: peptide binding properties of the disease-associated HLA-DQ(alpha 1*0501, beta 1*0201) molecule. Int Immunol. 1994;6:453–461. doi: 10.1093/intimm/6.3.453. [DOI] [PubMed] [Google Scholar]
- 44.Ziegler A, Heinig J, Muller C, Gotz H, Thinnes FP, Uchanska-Ziegler B, Wernet P. Analysis by sequential immunoprecipitations of the specificities of the monoclonal antibodies TU22,34,35,36,37,39,43,58 and YD1/63.HLK directed against human HLA class II antigens. Immunobiology. 1986;171:77–92. doi: 10.1016/S0171-2985(86)80019-5. [DOI] [PubMed] [Google Scholar]
- 45.Viken HD, Thorsby E, Gaudernack G. Characterization and epitope mapping of four HLA class II reactive mouse monoclonal antibodies using transfected L cells and human cells transfected with mutants of DQB1*0302. Tissue Antigens. 1995;45:250–257. doi: 10.1111/j.1399-0039.1995.tb02448.x. [DOI] [PubMed] [Google Scholar]
- 46.Molberg O, McAdam SN, Sollid LM. Role of tissue transglutaminase in celiac disease. Journal of Pediatric Gastroenterology & Nutrition. 2000;30:232–240. doi: 10.1097/00005176-200003000-00005. [DOI] [PubMed] [Google Scholar]
- 47.Qiao SW, Bergseng E, Molberg O, Jung G, Fleckenstein B, Sollid LM. Refining the rules of gliadin T cell epitope binding to the disease-associated DQ2 molecule in celiac disease: importance of proline spacing and glutamine deamidation. Journal of Immunology. 2005;175:254–261. doi: 10.4049/jimmunol.175.1.254. [DOI] [PubMed] [Google Scholar]
- 48.Qiao SW, Piper J, Haraldsen G, Oynebraten I, Fleckenstein B, Molberg O, Khosla C, Sollid LM. Tissue Transglutaminase-Mediated Formation and Cleavage of Histamine-Gliadin Complexes: Biological Effects and Implications for Celiac Disease. J Immunol. 2005;174:1657–1663. doi: 10.4049/jimmunol.174.3.1657. [DOI] [PubMed] [Google Scholar]
- 49.Tollefsen S, Arentz-Hansen H, Fleckenstein B, Molberg O, Raki M, Kwok WW, Jung G, Lundin KE, Sollid LM. HLA-DQ2 and -DQ8 signatures of gluten T cell epitopes in celiac disease. Journal of Clinical Investigation. 2006;116:2226–2236. doi: 10.1172/JCI27620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Viken HD, Paulsen G, Sollid LM, Lundin KE, Tjonnfjord GE, Thorsby E, Gaudernack G. Characterization of an HLA-DQ2-specific monoclonal antibody. Influence of amino acid substitutions in DQ beta 1*0202. Human Immunology. 1995;42:319–327. doi: 10.1016/0198-8859(94)00110-c. [DOI] [PubMed] [Google Scholar]
- 51.Compton LA, Johnson WC., Jr Analysis of protein circular dichroism spectra for secondary structure using a simple matrix multiplication. Anal Biochem. 1986;155:155–167. doi: 10.1016/0003-2697(86)90241-1. [DOI] [PubMed] [Google Scholar]
- 52.Kim CY, Quarsten H, Bergseng E, Khosla C, Sollid LM. Structural basis for HLA-DQ2-mediated presentation of gluten epitopes in celiac disease. PNAS. 2004;101:4175–4179. doi: 10.1073/pnas.0306885101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Bohm G, Muhr R, Jaenicke R. Quantitative analysis of protein far UV circular dichroism spectra by neural networks. Protein Eng. 1992;5:191–195. doi: 10.1093/protein/5.3.191. [DOI] [PubMed] [Google Scholar]