Abstract
Background
Vascular α1- and α2-adrenergic receptors (ARs) mediate vasoconstriction and are major determinants of peripheral vascular tone. There is wide variability in vasoconstrictor sensitivity to α1- and α2AR-agonists among individuals. In previous studies this variability was not explained by identified α1- and α2-AR genetic variants. Thus, we hypothesized that adrenergic vasoconstrictor sensitivity is determined by shared constrictor mechanisms downstream of the individual receptors and that α1- and α2-AR-mediated vasoconstrictor sensitivity would therefore be correlated.
Methods
Dorsal hand vein responses to increasing doses of the α1-AR agonist phenylephrine (12 ng/min –12,000 ng/min) and the α2-AR agonist dexmedetomidine (0.01 ng/min – 100 ng/min) were measured in healthy subjects using a linear variable differential transformer. From individual dose-response curves we calculated the dose of phenylephrine and dexmedetomidine that produced 50% (ED50) of maximum venoconstriction (Emax) for each subject. We examined the correlation between phenylephrine and dexmedetomidine ED50 and Emax before and after adjustment for covariates (age, gender, ethnicity, BMI, blood pressure, heart rate, and baseline plasma norepinephrine concentrations).
Results
In 62 subjects (36 males, 34 African American, 28 Caucasians) the median ED50 for dexmedetomidine was 1.32 ng/min (IQR, 0.45–5.37 ng/min), and for phenylephrine 177.8 ng/min (IQR, 40.7– 436.5 ng/min). The Emax for phenylephrine was 90.8% (82.2–99.6%) and for dexmedetomidine 80.0% (64.7–95.2%). There was no correlation between individual sensitivities (ED50) to phenylephrine and dexmedetomidine, before and after adjustment for covariates (p>0.30).
Conclusions
Phenylephrine and dexmedetomidine both produce strong venoconstriction in the dorsal hand vein; however, there is no significant correlation between vascular sensitivity to an α1-AR and α2-AR agonist. These findings suggest independent regulation of vascular α1- and α2-AR-mediated responses.
Keywords: α1 adrenoceptors, α2 adrenoceptors, vasoconstriction
INTRODUCTION
Post-synaptic α1- and α2-adrenergic receptors (AR) are widely distributed in the peripheral vasculature [1, 2] and both mediate vasoconstriction [1, 2]. In humans, direct infusion of the α2-AR agonist, clonidine, into the brachial artery causes vasoconstriction [3, 4], and in the forearm vasculature of young healthy men post-synaptic α2-ARs contribute more than α1-ARs to basal vascular tone [5]. In the hand vein, we and others have shown that both α1-AR and α2-AR agonists cause pronounced vasoconstriction, with sensitivity among individuals varying several fold [6–8].
However, the factors contributing to this interindividual variability in vascular sensitivity are poorly understood. Pharmacogenetic studies have not identified α1- and α2-AR variants that affect response to agonists substantially. For example, variants in the gene (ADRA2B) encoding the α2B-AR, an important mediator of vasoconstriction [9], contribute only a small amount to variability in dorsal hand vein responses to the α2-AR agonist, dexmedetomidine [10–12]. Similarly, genetic variants of the α1-AR studied thus far do not explain the large interindividual variability in vascular response to the α1-AR agonist phenylephrine [8].
Since genetic variants of the respective receptors studied to date do not explain variability in α-AR-mediated vascular response, this variability may be determined by factors that are not directly dependent on the receptor, and are shared by α1- and α2-ARs such as factors that determine the drug concentration at the site of action, or factors that determine the response of pathways downstream of the receptor. If such factors contribute to variability in α1- and α2-ARs responses, then one would expect the sensitivities to vasoconstriction mediated by α1- and α2-ARs to be correlated. However, the possibility that α1-AR and α2-AR vascular sensitivity are co-regulated, has not been studied.
Therefore, to examine the hypothesis that α1- and α2-AR-mediated vasoconstrictor sensitivity are correlated we measured responses to the α1-AR agonist, phenylephrine, and to the highly selective α2-AR agonist, dexmedetomidine, in healthy subjects in vivo using the dorsal hand vein model.
METHODS
Subjects
The Institutional Review Board of Vanderbilt University Medical Center approved the study protocol, and subjects gave written informed consent. Male and female Caucasians and African-Americans were eligible for the study if they were 18 to 45 years of age and had no clinically significant abnormalities according to medical history, physical examination, and laboratory testing.
Sixty-two subjects were studied. Ethnicity, family history of hypertension, and exercise history were determined by self-report, and body mass index (BMI) was calculated. Subjects took no medications for at least 2 weeks, and abstained from alcohol and caffeine for at least 5 days before the study. Each subject received a diet containing 150 mmol/day of sodium, 70 mmol/day of potassium, and 600 mmol/day of calcium for the 5 days prior to each study day. Studies were performed in the morning after an overnight fast, in the same temperature-controlled room.
Measurement of Vascular Responses
Hand vein responses to the α2-AR agonist dexmedetomidine HCl (Abbott Laboratories, Chicago, IL, USA) [6] and the α1-AR agonist phenylephrine HCL (Elkins-Sinn, Cherry Hill, N.J., USA) [8] were measured on separate days. Venous responses were measured in a dorsal hand vein by use of a linear variable differential transformer (LVDT), (Schaevitz, model 100 MHR) as previously described [13, 14]. This instrument, when mounted on the hand, measures and records changes in the diameter of the vein.
Subjects rested on a comfortable bed and remained supine throughout the study. The subject’s arm was placed on a support sloping upward. A 23-gauge needle was inserted into a suitable dorsal hand vein, and an infusion of normal saline was administered for at least 30 minutes. Following three stable baseline measurements of hand vein diameter, drugs were infused into the vein over which the LVDT was mounted.
Phenylephrine (12 ng/min to 12,000 ng/min) or dexmedetomidine (0.01 ng/min to 100 ng/min) were administered in increasing doses with each dose infused for 7 minutes using a Harvard syringe pump and response recorded during the last 2 minutes of each infusion. The total flow rate into the vein was maintained constant by changing the rate of infusion of saline and drug. Heart rate was monitored continuously with a bedside cardiac monitor, and blood pressure was measured in the arm on the side opposite the side receiving the hand vein infusion using a semiautomated device (Dinamap MPS, Johnson and Johnson Medical, Tampa, Fla. USA). A blood sample for measurement of plasma norepinephrine concentration was obtained on the first study day at baseline before drug infusion.
Analysis of hand-vein response to dexmedetomidine and phenylephrine
Venoconstriction was expressed as the percentage reduction in vein diameter from maximal dilation, which was defined as the average of three stable baseline measurements of hand vein diameter. Measurements for the phenylephrine and dexmedetomidine hand vein responses were plotted as individual semi-logarithmic dose-response curves and analyzed using a sigmoid dose-response model (Prism 5.01 software). The dose that produced 50% (ED50) of maximum venoconstriction (Emax) was determined for each subject, and these values were converted to molar concentrations to compare sensitivity (ED50) to dexmedetomidine and phenylephrine for each subject.
Statistical analyses
Continuous parameters were expressed as median and interquartile range (IQR). ED50 values are presented in original units (ng/min), and in log transformed molar units [log femtomole/min (log fmol/min)] for the comparison of ED50 values for dexmedetomidine and phenylephrine.
ED50 values for dexmedetomidine and phenylephrine in the same individuals were compared using Wilcoxon signed rank test, as were the Emax values. The Kruskal-Wallis test was used to compare dexmedetomidine ED50 among quartiles of phenylephrine ED50 values.
Correlations between the ED50 for dexmedetomidine and phenylephrine, the Emax for dexmedetomidine and phenylephrine, and between plasma norepinephrine concentrations and the ED50 for dexmedetomidine and phenylephrine were calculated using Spearman’s test.
To evaluate the effects of covariates on the relationship between dexmedetomidine and phenylephrine ED50 values, we performed multiple linear regression analyses using log transformed dexmedetomidine ED50 as outcome and log transformed phenylephrine ED50 as the independent variable, adjusting for the covariates age, gender, ethnicity, BMI, mean arterial pressure, heart rate, and plasma norepinephrine concentrations. Similarly, we used multiple linear regression analysis to evaluate the effect of these covariates on phenylephrine log transformed ED50.
All tests were two-sided, and p-values < 0.05 were considered significant. Statistical analyses were performed with the statistical software SPSS v. 17 (SPSS Inc, Chicago, Il, USA).
RESULTS
Subjects
We studied 62 subjects [36 men (56.3%), 34 African Americans (53.1%), and 28 Caucasians (43.8%)] with a median age (IQR) of 25.0 years (22.0– 32.0), and BMI 25.3 kg/m2 (22.7– 28.3). Demographic and baseline characteristics are shown in Table 1.
Table 1.
Subject characteristics and baseline measurements [Number (percentage); Median (IQR)]
| Characteristic | Value |
|---|---|
| Men | 36 (56.3%) |
| Race: African Americans: Caucasians |
34 (53.1%): 28 (43.8%) |
| Family history of hypertension | 23 (35.9%) |
| Regular exercise, >4 times/week | 23 (35.9%) |
| Age (years) | 25.0 ( 22.0– 32.0) |
| BMI (kg/m2) | 25.3 (22.7– 28.3) |
| Systolic blood pressure (mm Hg) | 109.5 (102.8– 116.3) |
| Diastolic blood pressure (mm Hg) | 61.0 (56.0– 67.0) |
| Mean arterial pressure (mm Hg) | 78.3 (72.6–82.3) |
| Heart rate (beats per minute) | 60.0 (53.0– 66.0) |
| Plasma norepinephrine (pg/mL) | 156.0 ( 127.0– 202.0) |
Hand-vein responses dexmedetomidine and phenylephrine
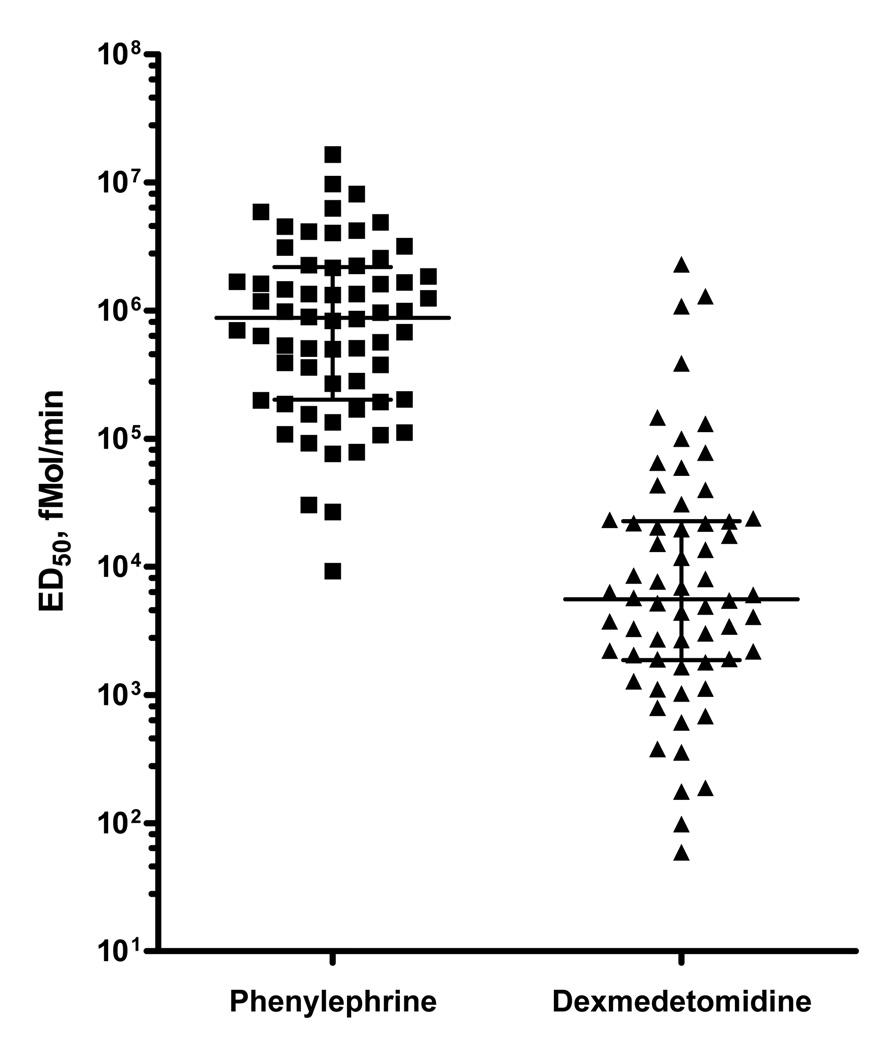
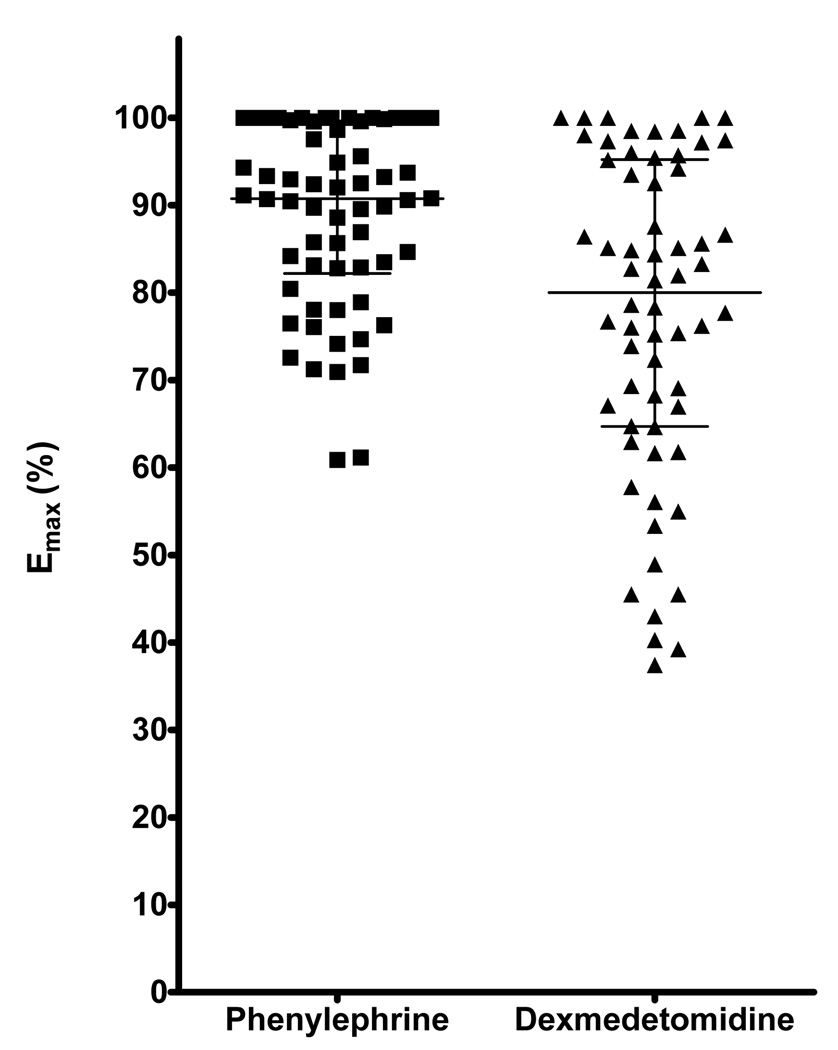
The median ED50 for dexmedetomidine was 1.32 ng/min (0.45–5.37 ng/min) [3.75 log fmol/min (3.27–4.36 log fmol/min)], and the ED50 for phenylephrine was 177.8 ng/min (40.7– 436.5 ng/min) [5.94 log fmol/min (5.31–6.34 log fmol/min)]. The ED50 in molar units was significantly smaller for dexmedetomidine than that for phenylephrine (p<0.001; Figure 1), suggesting greater vascular sensitivity to dexmedetomidine in the dorsal hand vein. The Emax for phenylephrine [90.8% (82.2–99.6%)] was significantly greater than that for dexmedetomidine [80.0% (64.7–95.2%)], (p<0.001; Table 2; Figure 2).
Figure 1. Hand vein sensitivity to dexmedetomidine and phenylephrine.
The horizontal line represents the median, the error bars represent the IQR
Data are presented in log fmole/min.
P<0.001 comparing the sensitivities to phenylephrine and dexmedetomidine
Table 2.
Hand vein responses to phenylephrine and dexmedetomidine
| Phenylephrine | Dexmedetomidine | P-value | |
|---|---|---|---|
| ED50 (ng/min) | 177.8 ng/min (40.7– 436.5) | 1.3 ng/min (0.45–5.37) | <0.001* |
| Emax (%) | 90.8 (82.2–99.6) | 80.0 (64.7–95.2) | <0.001 |
Data are expressed as median (IQR)
Emax – Maximal venoconstriction, expressed as the percentage reduction in vein diameter from maximal dilation
ED50 - The dose of phenylephrine and dexmedetomidine that produced 50% of maximum venoconstriction (Emax) for each subject
P-value for the comparison of ED50 of phenylephrine and dexmedetomidine in fmole/min (see also Figure 1)
Figure 2. Maximal effect (Emax) for dexmedetomidine and phenylephrine.
Emax – Maximal venoconstriction, expressed as the percentage reduction in vein diameter from maximal dilation
The horizontal line represents the median and the bars represent the IQR;
P<0.001 comparing Emax for dexmedetomidine and phenylephrine
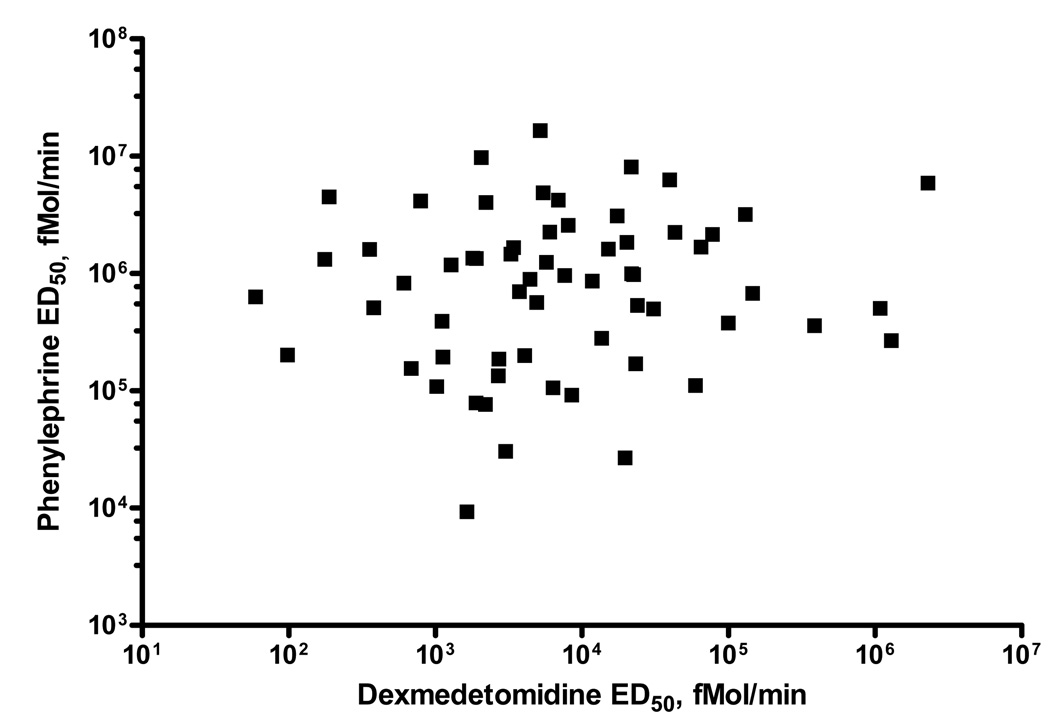
There was no correlation between individual sensitivities (ED50) to phenylephrine and dexmedetomidine in all subjects [r= 0.12 (p=0.34)], or when African Americans [r= 0.06 (p=0.74)] and Caucasians [r= 0.21 (p=0.29)] were analyzed separately (Table 3; Figure 3). Dexmedetomidine ED50 did not differ significantly among subjects grouped by quartiles of phenylephrine ED50 (p=0.22) (Table 4).
Table 3.
Correlation between measures of phenylephrine and dexmedetomidine vascular response
| ED50 Rho (p-value) |
Emax Rho (p-value) |
|
|---|---|---|
| All subjects (n=62) |
0.12 (p=0.38) | 0.15 (p=0.23) |
| African Americans (n= 34) |
0.06 (p=0.74) | 0.18 (p=0.32) |
| Caucasians (n=28) |
0.21 (p=0.29) | 0.17 (p=0.39) |
Emax – Maximal venoconstriction, expressed as the percentage reduction in vein diameter from maximal dilation
ED50 - The dose of phenylephrine and dexmedetomidine that produced 50% of maximum venoconstriction (Emax) for each subject
Figure 3. Relationship between log ED50 for phenylephrine and dexmedetomidine.
ED50 - The dose of phenylephrine and dexmedetomidine that produced 50% of maximum venoconstriction (Emax) for each subject
ED50 is expressed in fmol/min
Spearman rho for the correlation: r= 0.12 (p=0.34)
Table 4.
Comparison of dexmedetomidine ED50 in quartiles of phenylephrine ED50
| Phenylephrine ED50 quartile |
1st quartile (lowest quartile) |
2nd quartile | 3rd quartile | 4th quartile (highest quartile) |
P- value |
|---|---|---|---|---|---|
| Dexmedetomidine ED50 (ng/min, median, IQR) | 0.64 (0.30–1.89) | 3.23 (0.26–34.57) | 1.20 (0.43–5.08) | 1.63 (0.52–9.48) | 0.22 |
ED50 - The dose of phenylephrine and dexmedetomidine that produced 50% of maximum venoconstriction (Emax) for each subject
Similarly, there was no correlation between individual Emax values for phenylephrine and dexmedetomidine in all subjects [r= 0.15 (p=0.23)], or when African Americans [r= 0.18 (p=0.32)] and Caucasians [r= 0.17 (p=0.39)] were analyzed separately (Table 3).
Determinants of hand-vein responses to dexmedetomidine and phenylephrine
In univariate analysis, plasma norepinephrine concentrations and phenylephrine ED50 were significantly correlated [r=0.28 (p=0.037)], suggesting that higher plasma norepinephrine concentrations are associated with reduced phenylephrine sensitivity. In contrast, there was no significant correlation between plasma norepinephrine concentrations and dexmedetomidine ED50 [r=−0.01 (p=0.96)].
In multiple linear regression analyses, phenylephrine ED50 was not associated with dexmedetomidine ED50 (β=0.25, p= 0.31), and neither was any of the other covariates (all p-values > 0.15). With phenylephrine ED50 as the dependent variable, the association between phenylephrine ED50 and plasma norepinephrine was weakened after adjustment for covariants, and of borderline statistical significance (p=0.063).
DISCUSSION
The major new finding of this study is the lack of correlation between the vascular sensitivities (ED50) for dexmedetomidine and phenylephrine in vivo in humans. Therefore, our findings suggest that using the hand vein model with α1- and α2-AR-specific agonists provides independent information about α2-AR and α1-AR-mediated vasoconstrictor responses.
Interactions between the signaling pathways of α1-, α2- and β2-ARs were suggested by previous in vitro studies [15, 16]. Simultaneous activation of α2B-ARs and β2-ARs decreased the threshold concentration of epinephrine required for α2B-AR down-regulation, and this was associated with up-regulation of GRK3 expression [15]. Co-activation of α1a-ARs and β2-ARs resulted in a facilitatory interaction which led to increases in calcium influx from the extracellular compartment [16]. Thus, we tested the hypothesis that variability in downstream constrictor pathways or other factors that are shared by α1- and α2-ARs contribute to the interindividual differences in α1- and α2-AR-mediated vasoconstrictor responses in the human hand vein. Our findings suggest that the large variability among individuals in both α1- and α2-AR-mediated hand vein vasoconstrictor responses is not explained by interindividual differences in factors that are shared by both receptors.
We found that in the dose-range of agonists used in this study (selected previously to elicit maximal response in the hand vein without systemic hemodynamic effects [6, 8]), the human dorsal hand vein is more sensitive to dexmedetomidine than to phenylephrine, since a lower molar concentration was required to produce half the maximal constriction. Also, although both agonists resulted in pronounced venoconstriction (Emax >80%), phenylephrine resulted in a larger median maximal venoconstrictor effect than dexmedetomidine.
We found a negative correlation between resting plasma norepinephrine concentrations and vascular sensitivity to phenylephrine. This finding is consistent with down-regulation of vascular α1-ARs by norepinephrine. The modulating effect of endogenous non-selective adrenergic agonists such as norepinephrine, which activates both α1-ARs and α2-ARs in vivo, on hand vein responses has not been defined. We have previously shown that hand vein response to α1-AR -mediated venoconstriction is stable over time (and is thus used to produce background vasoconstriction in studies of hand vein vasodilation) [17]. These two observations - the previously reported lack of desensitization with short-term exposure to an exogenous agonist such as phenylephrine, and our current finding of a negative correlation between resting plasma norepinephrine concentrations and vascular α1-AR sensitivity - would be most consistent with near maximal α1-AR desensitization at baseline conditions in vivo.
However, plasma norepinephrine concentrations are affected by several factors, including BMI, gender and ethnicity [18], and after adjustment for these and other covariates, the association between resting plasma norepinephrine concentrations and vascular sensitivity to phenylephrine was weakned and of borderline significance. Thus, it is unclear if there is a direct relationship between phenylephrine sensitivity and norepinephrine concentrations, or if it is due to factors that affect norepinephrine concentrations. To determine the specific relationship between endogenous sympathetic tone and α1-AR-mediated local vascular response would require a study designed specifically for that purpose.
There are several methodological considerations regarding the measurement of vascular α1-AR and α2-AR responses to agonist in vivo. Systemic administration of either α1-AR or α2-AR agonists increases and decreases blood pressure, respectively, and thus results in the activation of homeostatic cardiovascular reflexes that would confound the measures of local vascular response. In particular, activation of central α2-ARs results in a decrease in sympathetic tone, a factor that would mask the direct vasoconstriction mediated by peripheral α2-ARs [4, 19]. Thus, although vascular α2-ARs are functional, it has been difficult to establish their importance in the vasculature relative to α1-ARs.
Accordingly, in order to define α1-AR and α2-AR vascular sensitivity it is necessary to minimize the effects on blood pressure and sympathetic activity that occur after systemic administration of agonist. Thus, we used the dorsal hand vein model [13, 14], that allows the direct infusion into the vessel studied of low doses of drugs that act on α1- and α2-ARs locally minimizing systemic effects [6, 13] and thus avoiding reflex cardiovascular responses that occur after systemic administration.
We defined α1-and α2-AR sensitivity using highly selective agonists, but cannot rule out the possibility of some non-selective α-AR activation. However, this is unlikely. First, phenylephrine and dexmedetomidine are both highly selective for their respective α-ARs, as compared to other α-ARs agonists for in vivo use [20, 21]. Second, non-selective α-AR activation by either agonist would be expected to increase the correlation between responses to dexmedetomidine and phenylephrine, whereas our study showed lack of correlation between responses.
The clinical implications of the current study are speculative. Since our findings suggest independent regulation of α1- and α2-AR- mediated vasoconstriction, dual blockade of both receptors peripherally could theoretically be used clinically to achieve an antihypertensive or vasodilating effect. However, while α1-AR antagonists are extensively used in the treatment of hypertension, α2-AR antagonists such as yohimbine cause an increase in blood pressure,[22] presumably because of the blockade of central pre-synaptic α2-ARs, resulting in increased sympathetic outflow. Currently, no selective, peripherally acting post-synaptic α2-AR antagonist is available for use in humans, precluding the use of α2-AR antagonists as potential antihypertensive agent [23].
In summary, we found that phenylephrine and dexmedetomidine both produce powerful venoconstriction in the dorsal hand vein in vivo, but that there was no correlation between α1- and α2-AR vascular sensitivity. The large variability among individuals in α1- and α2-AR vascular sensitivity is not explained by differences in shared pre- or post-receptor factors.
Acknowledgments
The study was supported by grants:
Supported by United States Public Health Service grants PO1 HL56693, and GM 5MO1-RR00095 and by the National Institutes of Health/ National Institute of General Medical Sciences Pharmacogenetics Research Network and Database (U01GM61374, pharmgkb.org) under grant U01 HL65962.
Abbreviations and symbols
- Emax
Maximal venoconstriction, expressed as the percentage reduction in vein diameter from maximal dilation
- ED50
The dose of agonist that produced 50% of maximum venoconstriction (Emax) for each subject
- AR
adrenergic receptors
- LVDT
linear variable differential transformer
- IQR
interquartile range
- fmol/min
femtomole/minute
- BMI
body mass index
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Presented in part as an oral presentation at the American Society for Clinical Pharmacology and Therapeutics conference, Washington DC, 2009 (Clin Pharmacol Ther. 2009; 85: S7)
Drs. Muszkat, Kurnik and Sofowora were recipients of a Merck Sharp & Dohme International Fellowship in Clinical Pharmacology
There are no conflicts of interests
Reference List
- 1.Docherty JR. Subtypes of functional alpha1 and alpha2 adrenoceptors. Eur J Pharmacol. 1998;361:1–15. doi: 10.1016/s0014-2999(98)00682-7. [DOI] [PubMed] [Google Scholar]
- 2.Guimaraes S, Moura D. Vacular adrenceptors: An update. Pharmacol Rev. 2001;53:319–356. [PubMed] [Google Scholar]
- 3.Kiowski W, Hulthuren UL, Ritz R, Buhler FR. Alpha 2 adrenoceptor-mediated vasoconstriction of arteries. Clin Pharmacol Ther. 1983;34:565–569. doi: 10.1038/clpt.1983.216. [DOI] [PubMed] [Google Scholar]
- 4.Talke PO, Lobo EP, Brown R, Richardson CA. Clonidine-induced vasoconstriction in awake volunteers. Anesth Analg. 2001;93(2):271–276. doi: 10.1097/00000539-200108000-00006. [DOI] [PubMed] [Google Scholar]
- 5.Dinenno FA, Eisenach JH, Dietz NM, Joyner MJ. Post-junctional alpha-adrenoceptors and basal limb vascular tone in healthy men. J Physiol. 2002;540(Pt 3):1103–1110. doi: 10.1113/jphysiol.2001.015297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Muszkat M, Sofowora GG, Wood AJ, Stein CM. Alpha2-adrenergic receptor-induced vascular constriction in blacks and whites. Hypertension. 2004;43:31–35. doi: 10.1161/01.HYP.0000103694.30164.C7. [DOI] [PubMed] [Google Scholar]
- 7.King D, Etzel JP, Chopra S, Smith J, Cadman PE, Rao F, Funk SD, Rana BK, Schork NJ, Insel PA, O'Connor DT. Human Response to α2-adrenergic Agonist Stimulation Studied in an Isolated Vascular Bed in Vivo: Biphasic Influence of Dose, Age, Gender, and Receptor Genotype*Human Response to α2-adrenergic Agonist Stimulation Studied in an Isolated Vascular Bed in Vivo: Biphasic Influence of Dose, Age, Gender, and Receptor Genotype. Clin Pharmacol Ther. 2005;77:388–403. doi: 10.1016/j.clpt.2005.02.008. [DOI] [PubMed] [Google Scholar]
- 8.Sofowora GG, Dishy V, Landau R, et al. Alpha1A-adrenergic receptor polymorphism and vascular response. Clin Pharmacol Ther. 2004;75:539–545. doi: 10.1016/j.clpt.2004.02.006. [DOI] [PubMed] [Google Scholar]
- 9.Link RE, Desai K, Hein L, Stevens ME, Chruscinski A, Bernstein D, Brash GS, Kobilka BK. Cradiovascular regulation in mice lacking alpha2-adrenergic receptor subtypes b and C. Science. 1996;273:803–805. doi: 10.1126/science.273.5276.803. [DOI] [PubMed] [Google Scholar]
- 10.Muszkat M, Sofowora GG, Xie HG, Wood AJJ, Stein CM. Alpha 2B – Adrenergic Receptor 301–303 Deletion Polymorphism and Vascular Alpha2 Adrenergic Receptor Response. Pharmacogenetics and Pharmacogenomics. 2005;15:23–28. doi: 10.1097/01213011-200501000-00004. [DOI] [PubMed] [Google Scholar]
- 11.Muszkat M, Kurnik D, Solus J, Sofowora GG, Xie HG, Jiang L, McMunn C, Ihrie P, Harris JR, Dawson EP, Williams SM, Wood AJJ, Stein CM. Variation in the Alpha 2B – Adrenergic Receptor Gene (ADRA2B) and its relationship to vascular response in vivo. Pharmacogenetics and Pharmacogenomics. 2005;15:407–414. doi: 10.1097/01213011-200506000-00006. [DOI] [PubMed] [Google Scholar]
- 12.Muszkat M, Kurnik D, Sofowora GG, Solus J, Xie HG, Harris PA, Williams SM, Wood AJJ, Stein CM. Desensitization of vascular response in vivo: Contribution of genetic variation in the alpha2B-adrenergic receptor subtype. J Hypertension. 2010;27 doi: 10.1097/HJH.0b013e328333d212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Aellig WH. Clinical pharmacology, physiology and pathophysiology of superficial veins--2. Br J Clin Pharmacol. 1994;38(4):289–305. doi: 10.1111/j.1365-2125.1994.tb04357.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Aellig WH. Clinical pharmacology, physiology and pathophysiology of superficial veins--1. Br J Clin Pharmacol. 1994;38(3):181–196. doi: 10.1111/j.1365-2125.1994.tb04341.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Desai AN, Standifer KM, Eikenburg DC. Simultaneous alpha2B- and beta2-adrenoceptor activation sensitizes the alpha2B-adrenoceptor for agonist-induced down-regulation. J Pharmacol Exp Ther. 2004;311:794–802. doi: 10.1124/jpet.104.069674. [DOI] [PubMed] [Google Scholar]
- 16.Copik AJ, Ma C, Kosaka A, Sahdeo S, Trane A, Ho H, Dietrich PS, Yu H, Ford AP, Button D, Milla ME. Facilitatory interplay in alpha 1a and beta 2 adrenoceptor function reveals a non-Gq signaling mode: implications for diversification of intracellular signal transduction. Mol Pharmacol. 2009;75(3):713–728. doi: 10.1124/mol.108.050765. [DOI] [PubMed] [Google Scholar]
- 17.Dishy V, Sofowora GG, Xie HG, Kim RB, Byrne DW, Stein CM, Wood AJ. The effect of common polymorphisms of the beta2-adrenergic receptor on agonist-mediated vascular desensitization. N Engl J Med. 2001;345:1030–1035. doi: 10.1056/NEJMoa010819. [DOI] [PubMed] [Google Scholar]
- 18.González-Trápaga JL, Nelesen RA, Dimsdale JE, Mills PJ, Kennedy B, Parmer RJ, Ziegler MG. Plasma epinephrine levels in hypertension and across gender and ethnicity. Life Sciences. 2000;66:2383–2392. doi: 10.1016/s0024-3205(00)00568-3. [DOI] [PubMed] [Google Scholar]
- 19.Bloor BC, Ward DS, Belleville JP, Maze M. Effects of intravenous dexmedetomidine in humans. II. Hemodynamic changes. Anesthesiology. 1992;77(6):1134–1142. doi: 10.1097/00000542-199212000-00014. [DOI] [PubMed] [Google Scholar]
- 20.Virtanen R, Savola JM, Saano V, Nyman L. Characterization of the selectivity, specificity and potency of medetomidine as an alpha 2-adrenoceptor agonist. Eur J Pharmacol. 1988 doi: 10.1016/0014-2999(88)90744-3. [DOI] [PubMed] [Google Scholar]
- 21.Hamasaki J, Tsuneyoshi I, Katai R, Hidaka T, Boyle WA, Kanmura Y. Dual α2-Adrenergic Agonist and α1-Adrenergic Antagonist Actions of Dexmedetomidine on Human Isolated Endothelium-Denuded Gastroepiploic Arteries. Anesth Analg. 2002;94:1434–1440. doi: 10.1097/00000539-200206000-00010. [DOI] [PubMed] [Google Scholar]
- 22.Etzel JP, Rana BK, Wen G, Parmer RJ, Schork NJ, O'Connor DT, Insel PA. Genetic variation at the human alpha2B-adrenergic receptor locus: role in blood pressure variation and yohimbine response. Hypertension. 2005 Jun;45(6):1207–1213. doi: 10.1161/01.HYP.0000166721.42734.49. [DOI] [PubMed] [Google Scholar]
- 23.Crassous PA, Flavahan S, Flavahan NA. Acute dilation to alpha(2)-adrenoceptor antagonists uncovers dual constriction and dilation mediated by arterial alpha(2)-adrenoceptors. Br J Pharmacol. 2009;158:1344–1355. doi: 10.1111/j.1476-5381.2009.00403.x. [DOI] [PMC free article] [PubMed] [Google Scholar]