Abstract
Hypertension-induced renal injury is characterized by inflammation, fibrosis and proteinuria. Previous studies have demonstrated that N-acetyl-Ser-Asp-Lys-Pro (Ac-SDKP) inhibits renal damage following diabetes mellitus and anti-glomerular basement membrane nephritis. However, its effects on low renin hypertensive nephropathy are not known. Thus, we hypothesized that Ac-SDKP has renal protective effects on DOCA-salt hypertensive mice, decreasing inflammatory cell infiltration, matrix deposition and albuminuria. We uninephrectomized 16-week old C57BL/6J mice and treated them with either placebo, deoxycorticosterone acetate-salt (10mg/10gm body weight sub cutaneous) and 1% NaCl + 0.2% KCl in drinking water (DOCA-salt), or DOCA-salt + Ac-SDKP (800 μg/Kg/day) for 12 weeks. We measured blood pressure, urine albumin, glomerular matrix and renal collagen content, monocyte/macrophage infiltration and glomerular nephrin expression. Treatment with DOCA-salt significantly increased blood pressure (P<0.01), which remained unaltered by Ac-SDKP. Ac-SDKP decreased DOCA-salt-induced renal collagen deposition, glomerular matrix expansion and monocyte/macrophage infiltration. Moreover, DOCA-salt-induced increase in albuminuria was normalized by Ac-SDKP (controls, 10.8 ± 1.7; DOCA-salt, 41 ± 5; DOCA-salt + Ac-SDKP, 13 ± 3 μg/10gmBW/24hr; p< 0.001, DOCA-salt vs. DOCA-salt + Ac-SDKP). Loss of nephrin reportedly causes excess urinary protein excretion; therefore we determined whether Ac-SDKP inhibits proteinuria by restoring nephrin expression in the glomerulus of hypertensive mice. DOCA-salt significantly downregulated glomerular nephrin expression (controls, 37 ± 8; DOCA-salt, 10 ± 1.5 % of glomerular area; p < 0.01), which was partially reversed by Ac-SDKP (23 ± 4.0 % of glomerular area; p = 0.065, DOCA-salt vs. DOCA-salt + Ac-SDKP). We concluded that Ac-SDKP prevents hypertension-induced inflammatory cell infiltration, collagen deposition, nephrin downregulation and albuminuria, which could lead to renoprotection in hypertensive mice.
Keywords: hypertension, Ac-SDKP, nephrin, macrophage, albuminuria
INTRODUCTION
Hypertension is a major risk factor for renal disease. However, the mechanisms underlying development and progression of hypertensive renal injury are not fully understood. Several recent reports demonstrate that inflammation and fibrosis play an important role in high blood pressure-induced renal damage[1–4]. Moreover, proteinuria is a manifestation of hypertensive renal disease, and also accounts for increased cardiovascular risk in hypertensive patients[4,5]. Although proteinuria has been attributed to various mechanisms, including functional and structural abnormalities of the glomeruli, vessels and tubules, its origin remains obscure[6,7]. Recent studies indicate that the glomerular slit pore protein, nephrin, plays an important role in trafficking of albumin across the glomerular barrier[8,9]. Nephrin is synthesized by podocytes, and its expression is reduced in nephropathy due to diabetes and hypertension[10,11]. Reducing proteinuria would retard the progression of disease and improve the prognosis for hypertensive patients.
Ac-SDKP is a tetrapeptide formed by hydrolysis of its precursor thymosine β4[12]. It is thought to be synthesized in bone marrow and mononuclear cells, and is found in various organs including the heart and kidneys[13,14]. Ac-SDKP is exclusively hydrolyzed by angiotensin converting enzyme (ACE); consequently, its plasma and tissue level increases significantly following ACE inhibition[15–18]. We previously reported that Ac-SDKP inhibits hypertension-induced cardiac remodeling[18]. Recent studies suggested that Ac-SDKP also inhibits glomerulosclerosis and renal damage in rat model of diabetes, anti-glomerular basement membrane nephritis, and 5/6 nephrectomy-induced hypertension (a model of high renin hypertension) [6,19–21]. However, the effect of Ac-SDKP on high blood pressure-induced renal structural damage and proteinuria in models of low renin hypertension is not well studied.
Therefore, we elected to investigate whether Ac-SDKP exerts renal protective effects in DOCA-salt hypertensive mice. DOCA-salt treatment induced microalbuminuria, renal collagen matrix expansion and inflammatory cell infiltration, all of which were inhibited by a 12-week course of Ac-SDKP. In addition, we ascertained that Ac-SDKP augmented nephrin expression in hypertensive mice. This study suggests that Ac-SDKP could be a useful novel therapeutic hormone to target renal inflammation and proteinuria in hypertensive kidney disease.
METHODS
Animals and experimental design
Sixteen-week-old male C57BL/6J mice (Jackson Laboratories) were divided into 3 groups: (1) control, (2) DOCA-salt and (3) DOCA-salt + Ac-SDKP (800μg/Kg/day). Ac-SDKP dosage was based on data generated from our laboratory showing that exogenous Ac-SDKP at doses of 400 and 800 μg/Kg/day significantly increased plasma Ac-SDKP levels and inhibited left ventricular inflammatory cell infiltration and fibrosis similar to the effects produced by captopril therapy[18]. Mice were anesthetized with sodium pentobarbital (50 mg/kg IP), and the left kidney was removed. A silicone rubber sheet (silicone:DOCA ratio of 3:1, at a dose of 10 mg DOCA per 10 gm body weight[22]) and osmotic minipumps (Alzet Model 2004; Alza, Palo Alto, CA) containing Ac-SDKP (Bachem Bubendorf) or vehicle were implanted subcutaneously. Mice receiving DOCA (Sigma) were given 1% NaCl and 0.2% KCl to drink. Control mice were also uninephrectomized, and access to tap water was provided. Treatment started immediately after surgery and continued for 12 weeks. The pumps and pellets were replaced every 4 weeks. Mice are given butorphanol (5.4 mg/kg, s.c.) 1 hour prior and 6 hours post-surgery. This study was approved by the Henry Ford Hospital Care of Experimental Animals Committee.
Measurement of systolic blood pressure
Systolic blood pressure (SBP) was measured by tail cuff according to the manufacturere’s instructions (BP-2000, Visitech Systems)[22,23]. Mice were trained for 1 week before measuring SBP. Each session included 3 sets of 10 measurements for each mouse; to include each set of measurements, the computer had to identify a BP in at least 6 of the 10 trials within the set.
Tissue processing
Mice were anesthetized with 50 mg/kg sodium pentobarbital and the remaining kidney was rapidly excised and weighed. Half of the kidney was placed in optimum cutting temperature (OCT) formulation of water soluble glycol and resin (Tissue-Tek, Sakura Finetek U.S.A.) and frozen on dry ice for immunohistochemical studies, and about 20 mg (wet weight) from the other half was frozen and used to measure hydroxyproline.
Measurement of renal collagen content
Collagen content of renal tissue was determined by hydroxyproline assay as described previously[24]. Briefly, tissue was dried, homogenized, and hydrolyzed with 6N HCl for 16 hours at 110°C. A standard curve of 0 to 5 μg hydroxyproline was used. Data were expressed as μg collagen/mg dry kidney wt, assuming that collagen contains an average of 13.5% hydroxyproline[25].
Measurement of glomerular matrix
The glomerular matrix components were stained with periodic acid–Schiff (PAS) according to a published protocol[26]. The dark pink color in the glomerulus was considered to represent the extracellular matrix. Twenty to twenty-five glomeruli in each section were imaged at 400X magnification (IX70; OlympusAmerica, NY). PAS staining was quantified in a blinded fashion by calculating the proportion of area occupied by pink staining within each glomerulus[27] using a computer-based image analyzer (Microsuite, Biological Imaging Software, Olympus America, NY). The glomerular matrix was expressed as the percentage of a glomerular area.
Identification of monocytes/macrophages (CD68 immunohistochemical staining)
Immunohistochemistry for monocytes/macrophages was performed using a rat anti-mouse CD68 antibody (MCA 1957; Serotec) on acetone-fixed cryosections as described previously[18]. Sections were incubated with the primary antibody (1:200), followed by a biotinylated anti-rat IgG (Vector Laboratories) secondary antibody. A peroxidase reaction was performed according to the manufacturer’s protocol. Sections were viewed using a Zeiss AxioSkop microscope, and digital images were taken using a SPOT RT camera (software version 3.3; Diagnostic Instruments, MI). A blinded observer counted the number of macrophages in twenty randomly selected non-overlapping fields per section under × 400 magnification and took the average. Cells with dark brown nuclei were counted and expressed as number of macrophages/mm2.
Measurement of urinary albumin
After 12 weeks of drug treatment, mice were acclimated to the metabolic cages specially designed for mice 48 hours prior to the 24-hours urine collection. Urinary albumin was measured with an enzyme-linked immunosorbent assay kit (Exocell) and expressed as micrograms albumin per10 gram body weight per 24 hours.
Detection of nephrin (immunofluorescent staining)
Acetone-fixed kidney sections were immunostained for nephrin by indirect immunofluorescence with an anti-nephrin antibody (Research Diagnostic Inc, RDI-PROGPN2; 1:100) overnight at 4°C, followed by incubation with an FITC-conjugated donkey anti-guinea pig IgG (RDI) antibody for 1 hour at room temperature. Fluorescence was captured by an inverted light microscope (IX70; Olympus America, NY) equipped with epifluorescence and a computer-based image analysis system. Immunofluorescence was quantified by calculating the proportion of area occupied by green staining within each glomerulus[27] using a computer-based image analyzer (Microsuite, Biological Imaging Software, Olympus America, NY). At least twenty randomly chosen glomeruli per section were analyzed by a blind observer. Care was taken not to resample the same glomeruli by moving the microscope stage clockwise. Immunostaining for nephrin was expressed as the percentage of glomerular area.
Statistical Analysis
Blood pressure data was analyzed by analysis of variance for repeated measures. All other variables were assesed by one-way analysis of variance. In both cases all pairwise comparisons were then examined. Significance was determined using Hochberg’s method for adjustment for multiple comparisons. For the urinary albumin analysis the data was transformed using a log transformation to stabalize the variance. Results are expressed as mean +/− SEM. All statistical analyses were performed by a biostatistician (Biostatistics department at henry ford Hospital)..
RESULTS
Effects of Ac-SDKP on systolic blood pressure
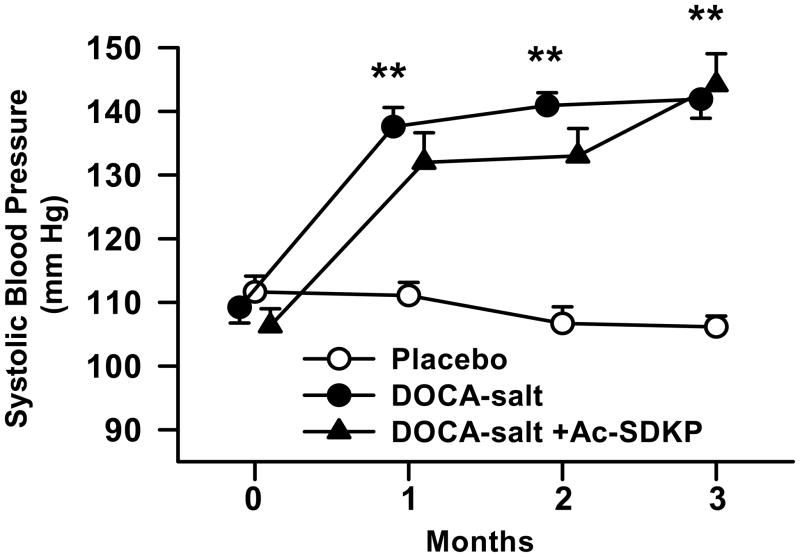
Treatment of mice with DOCA-salt significantly increased blood pressure over a two weeks period (controls, 111± 2; DOCA-salt, 138± 3 mmHg, p < 0.0001), and it remained elevated throughout the experiment. Ac-SDKP did not alter blood pressure in DOCA-salt treated mice (Figure 1).
Figure 1.
Systolic blood pressure in C57Bl6/J mice in response to DOCA-salt and Ac-SDKP. Baseline blood pressure was similar in all groups. DOCA-salt treated animals showed higher blood pressure, which remained unaltered by Ac-SDKP. **, p < 0.0001 placebo vs. DOCA-salt; p = NS, DOCA-salt vs. DOCA-salt + Ac-SDKP, n = 6–12 animals.
Effects of Ac-SDKP on renal monocytes/macrophage infiltration
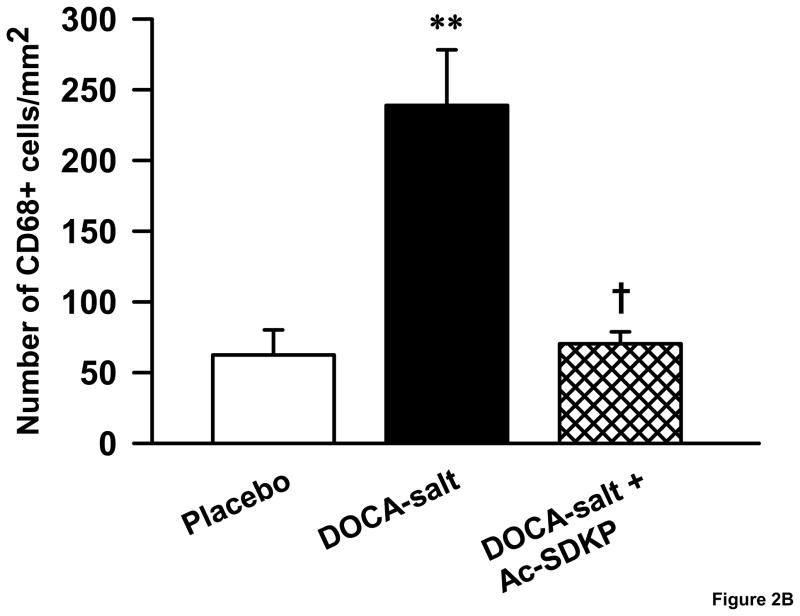
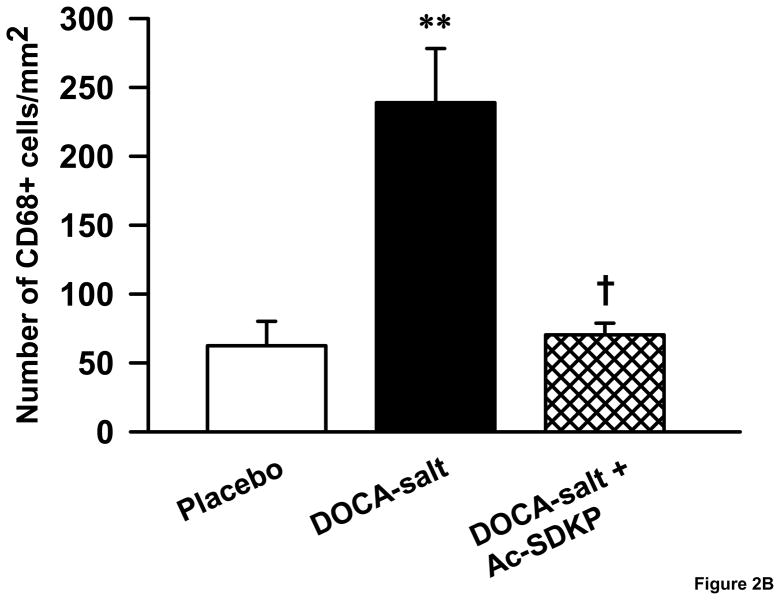
We identified monocytes/macrophages in kidney sections using an anti-CD68 antibody. DOCA-salt increased CD68-positive cells in the kidney 3.8 fold compared to controls (Figure 2A&B), which was significantly decreased by Ac-SDKP (number of monocytes/macrophages mm2: controls, 62 ± 17; DOCA-salt, 246± 39; DOCA-salt + Ac-SDKP, 70 ± 8; p <0.005, (DOCA-salt vs. DOCA-salt + Ac-SDKP).
Figure 2.
Effects of Ac-SDKP on renal macrophage infiltration in DOCA-salt hypertensive mice. A. Representative images of CD68 immunostained kidney sections. Arrow heads point to the monocytes/macrophages with brown staining nuclei. B. Bar graph showing the number of monocytes/macrophages in the control, DOCA-salt and DOCA-salt + Ac-SDKP treated animals. **, p < 0.005 placebo vs. DOCA-salt; †, p < 0.005, DOCA-salt vs. DOCA-salt + Ac-SDKP, n = 4–7 animals.
Effects of Ac-SDKP on renal collagen content and glomerular matrix expansion
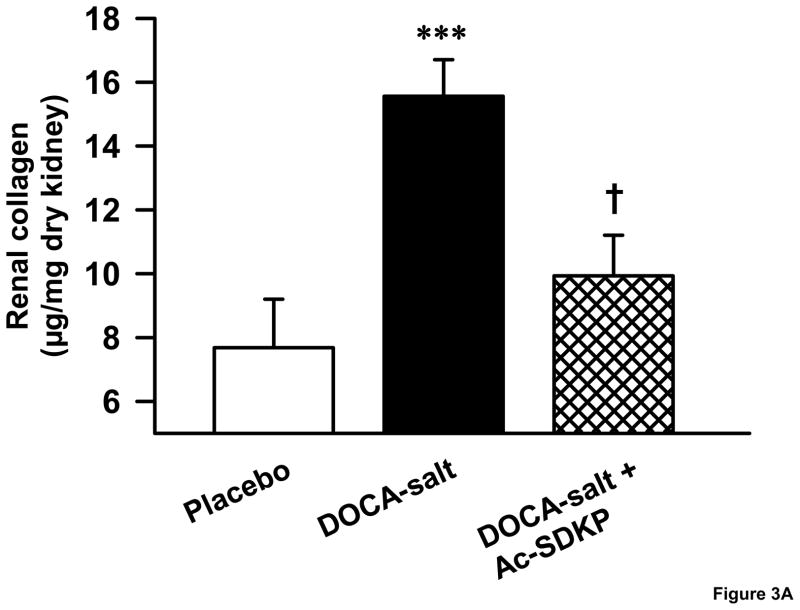
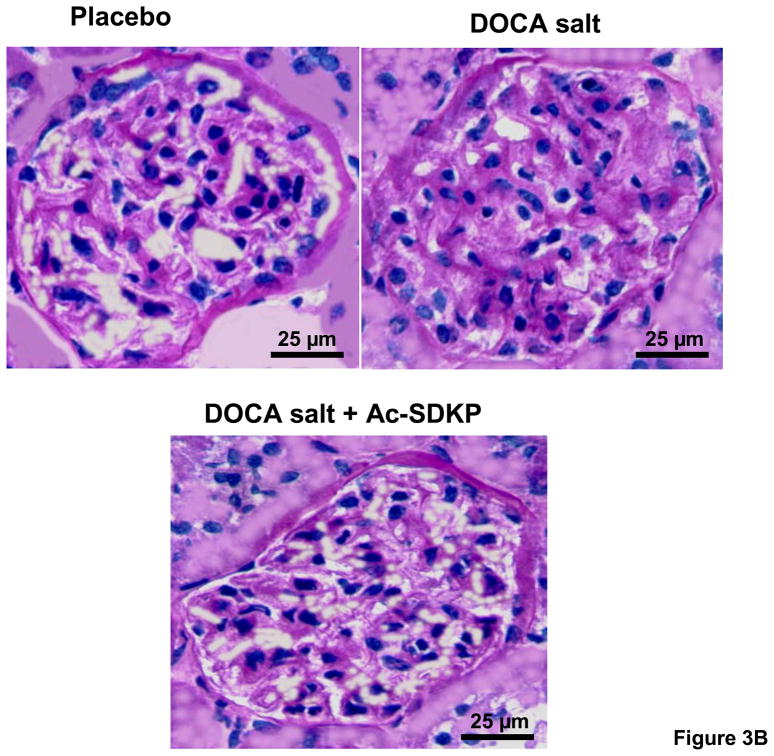
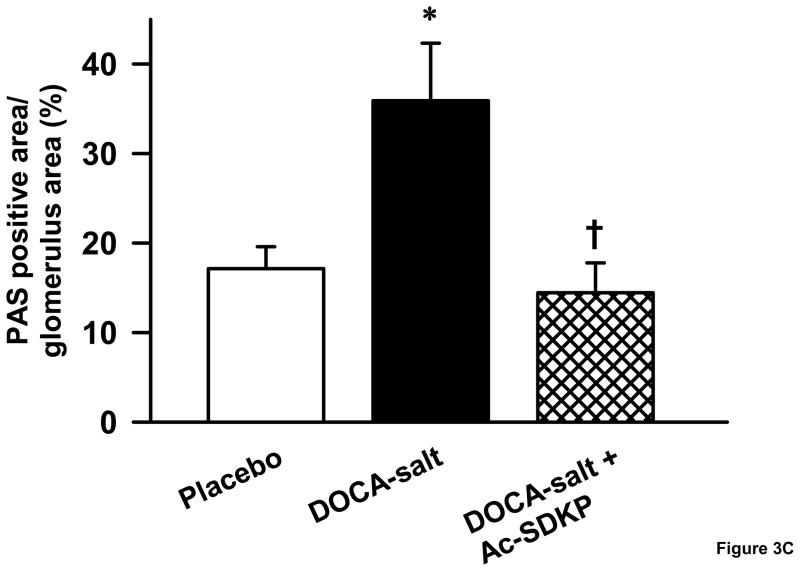
As hypertensive end organ damage is characterized by excess deposition of renal extracellular matrix, we tested whether Ac-SDKP inhibited this process in DOCA-salt hypertensive mice. Similar to our previous study on aldosterone salt hypertensive rats[28], DOCA-salt-treated mice had a significantly higher renal collagen content (Figure 3A), which was reduced by Ac-SDKP treatment (μg collagen/mg dry kidney-controls, 7.6 ± 1.5; DOCA-salt, 15.5 ± 1.1; DOCA-salt + Ac-SDKP, 9.9 ± 1.2; p <0.005, DOCA-salt vs. DOCA-salt + Ac-SDKP). Similarly, the area of PAS-positive matrix in the glomeruli of DOCA-salt-treated mice was significantly increased compared to the controls (Figure 3B and C). When DOCA-salt-treated mice were treated with Ac-SDKP, glomerular matrix expansion was completely prevented (controls, 17.1 ± 2.4; DOCA-salt, 35 ± 6.4; DOCA-salt + Ac-SDKP, 14.4 ± 3.3 % of glomerular area; p <0.05, DOCA-salt vs. DOCA-salt + Ac-SDKP).
Figure 3.
Effects of Ac-SDKP on renal matrix deposition as measured by hydroxyproline assay and PAS staining. A. Hydroxyproline assay showing the effects of DOCA-salt and Ac-SDKP on renal collagen content. DOCA-salt significantly increased renal collagen content (p < 0.005), which was prevented by Ac-SDKP (p < 0.01). B. Representative images of PAS-stained glomeruli from control, DOCA-salt and DOCA-salt + Ac-SDKP treated mice. C. Quantification of PAS-stained area in the glomeruli, which showed that DOCA-salt-induced glomerular matrix expansion was blocked by Ac-SDKP. ***, p < 0.05, placebo vs. DOCA-salt; †, p< 0.05 DOCA-salt vs. DOCA-salt + Ac-SDKP, n = 5–7 animals.
Effects of Ac-SDKP on albuminuria
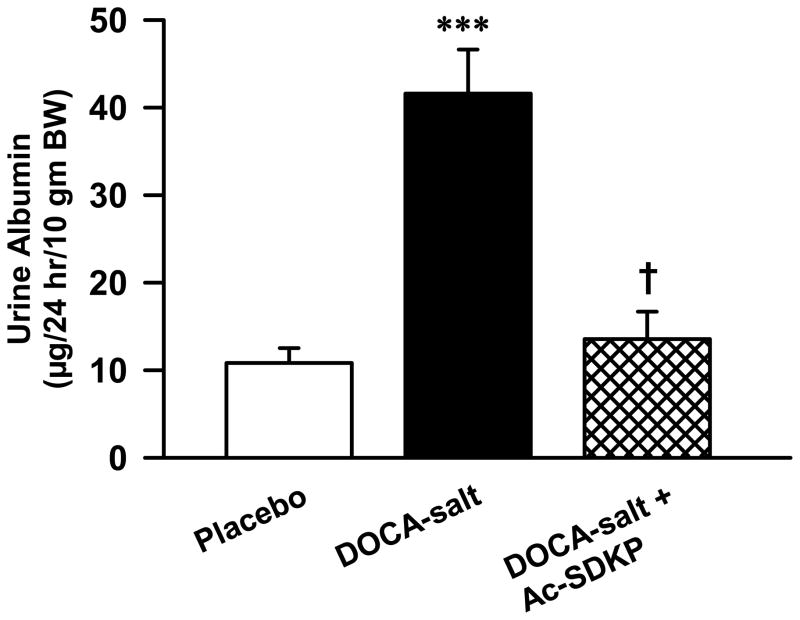
Albuminuria is a type of proteinuria characterized by high levels of albumin in the urine. Since proteinuria is a marker of hypertensive renal disease, we tested whether Ac-SDKP would inhibit albumin excretion in DOCA-salt hypertensive mice. At the end of 12 weeks, urine albumin levels significantly increased with DOCA-salt in C57Bl6J mice (Figure 4), which was prevented by Ac-SDKP (controls, 10.8 ± 1.7, DOCA-salt, 41 ± 5, DOCA-salt + Ac-SDKP, 13 ± 3 μg/10 gmBW/24hr; p < 0.001 DOCA-salt vs. DOCA-salt + Ac-SDKP).
Figure 4.
Effects of Ac-SDKP on urine albumin excretion in DOCA-salt-treated mice. Twenty four hour urine albumin excretion was measured 12-weeks after treatment with vehicle, DOCA-salt and DOCA-salt + Ac-SDKP. ***, p < 0.001 placebo vs. DOCA-salt; †, p < 0.001, DOCA-salt vs. DOCA-salt + Ac-SDKP; n = 4–8 animals.
Effects of Ac-SDKP on nephrin expression
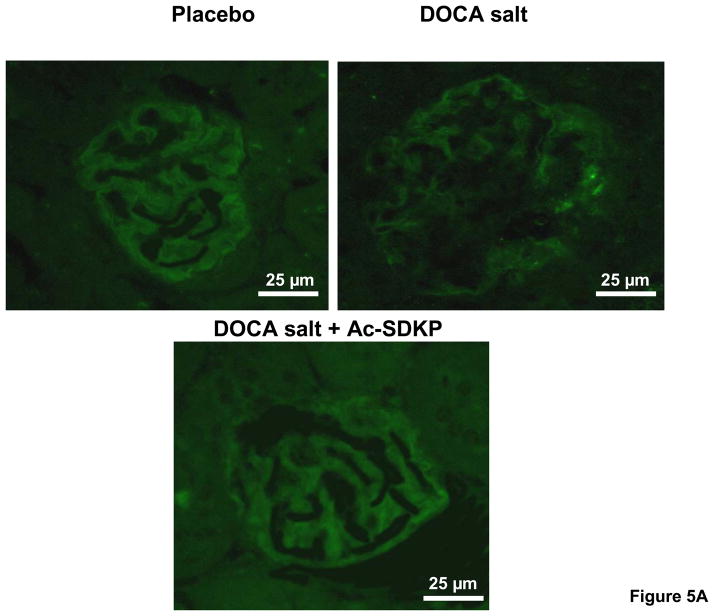
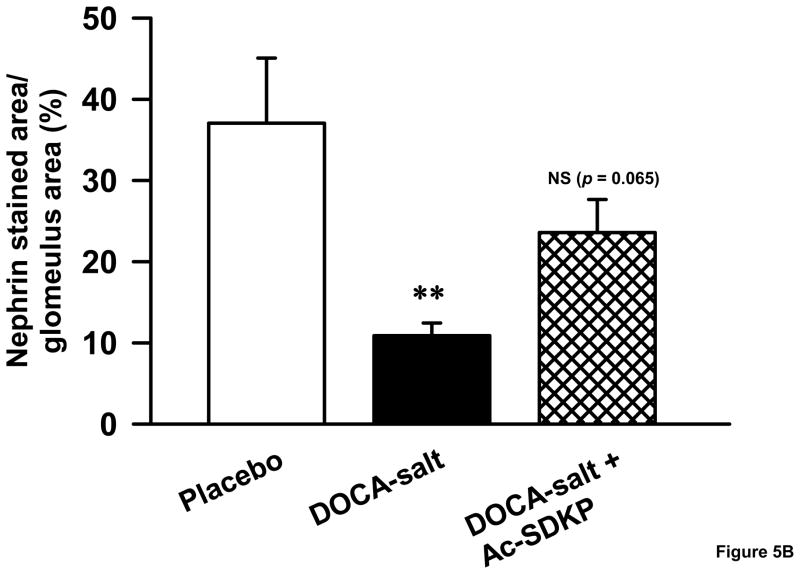
Since we noticed a significant difference in albuminuria between controls and DOCA-salt-treated animals, we investigated whether the glomerular slit pore protein, nephrin, was differentially expressed in these mice. We found that nephrin staining was significantly decreased in DOCA-salt-treated mice compared to the vehicle-treated group (Figure 5). When DOCA-salt-hypertensive animals were treated with Ac-SDKP, the proportion of glomerular area stained with nephrin tended to increase (controls, 37 ± 8; DOCA-salt, 10 ± 1.5; DOCA-salt + Ac-SDKP, 23 ± 4.0 % of glomerular area; p = 0.065, DOCA-salt vs. DOCA-salt + Ac-SDKP).
Figure 5.
Glomerular nephrin expression in uninephrectomized C57Bl6/J mice treated with and without DOCA-salt and Ac-SDKP. A. Representative immunofluorescent images of placebo, DOCA-salt and DOCA-salt + Ac-SDKP treated mice. B. Bar graph showing the effects of DOCA-salt and Ac-SDKP on nephrin staining. **, p < 0.05, placebo vs. DOCA-salt, †, p = 0.065, DOCA-salt vs. DOCA-salt + Ac-SDKP, n = 5–6 animals.
DISCUSSION
We and others previously demonstrated that Ac-SDKP has anti-fibrotic and anti-inflammatory properties. In a rat model of high circulating renin-angiotensin system components, 5/6 nephrectomy, we have demonstrated that Ac-SDKP attenuated albuminuria and renal fibrosis, and improves renal function. These effects were suggested to be related to decreased inflammation and increased nephrin protein. [21]However, its effects on a suppressed-renin model of hypertension-induced renal damage were not fully investigated. The major findings of this study are that Ac-SDKP, without changing systolic hypertension, prevents DOCA-salt-induced renal matrix expansion, macrophage infiltration, and nephrin downregulation. These changes are accompanied by reduced urine albumin excretion in hypertensive mice.
High blood pressure, DOCA-salt and Ac-SDKP
DOCA-salt induces severe hypertension in rats[29], while its effects on mice depend on the strain[30,31]. Accordingly, we noted that DOCA-salt-treated C57Bl6/J mice developed moderate hypertension. Long standing hypertension often triggers an inflammatory and fibrotic response in the glomeruli and tubules, which is not reversed by mere control of blood pressure[32]. In fact, ACE inhibitor ramipril prevented DOCA-salt-induced glomerular sclerosis and proteinuria without lowering blood pressure[30]. Here, we found that Ac-SDKP counteracted the pro-inflammatory and pro-fibrotic effects of DOCA-salt without having any effect on systolic blood pressure. This agrees with our earlier report that Ac-SDKP reversed renal and cardiac fibrosis in rats with hypertension without altering the mean arterial pressure[21,33]. These data support the notion that the renal protective effects of Ac-SDKP are not related to lowering of systemic blood pressure.
Macrophages, DOCA-salt and Ac-SDKP
Treatment of animals with mineralocorticoids and salt is known to induce a renal inflammatory response[34,35]. A recent study showed that DOCA-salt-induced renal inflammation is characterized by leukocyte infiltration, production of excess inflammatory cytokines and generation of reactive oxygen species (ROS)[36]. In agreement with these reports, we observed that DOCA-salt induced infiltration of monocyte/macrophage to the kidney. The process of leukocyte infiltration and kidney inflammation is not only apparent in DOCA-salt models, but also in other cases of hypertensive renal disease such as coarctation of the aorta[37]. We previously noted that Ac-SDKP inhibited inflammatory cell infiltration in the rat heart following myocardial infarction and 2K1C hypertension[38][39] or kidney following 5/6 nephrectomy[21]. Here, we noted that Ac-SDKP decreased infiltrating monocytes/macrophages in the kidney of DOCA-salt hypertensive mice. Collectively, this suggests that Ac-SDKP could be a potential anti-inflammatory agent to treat hypertensive end-organ damage.
Renal extracellular matrix, DOCA-salt and Ac-SDKP
Tubuloglomerular sclerosis and cardiac fibrosis are commonly observed in DOCA-salt and other hypertensive models[23,28,31,40]. This involves expression of various cytokines and pro-fibrotic agents, including renal transforming growth factor (TGF)-β1s[41,42]. Furthermore, administration of DOCA-salt induces oxidative stress by increasing the formation of superoxide and ROS, thereby contributing to end organ damage and reactive fibrosis[31,43]. These deleterious effects of DOCA-salt could also be mediated by excess infiltrating macrophages, which are known to produce various cytokines and ROS[44]. We and others previously reported that Ac-SDKP inhibits TGF-β signal transduction, collagen synthesis and deposition both in vitro and in vivo[11,15,45–47]. In this study, we found that Ac-SDKP also inhibited DOCA-salt-induced macrophage infiltration in the kidney. These effects of Ac-SDKP could be partly responsible for reducing renal matrix in DOCA-salt-treated animals by decreasing oxidative stress, which could be caused by excess macrophage infiltration in the kidney.
Nephrin expression, albuminuria and Ac-SDKP
Measurement of urinary albumin excretion is a major parameter for monitoring progression of hypertensive nephropathy. Though pathogenesis of proteinuria in hypertension has not been fully delineated, recent studies implicate the glomerular slit pore protein, nephrin, as playing an important role in albumin trafficking across the glomerular barrier[8]. Glomerular nephrin expression correlates inversely with urine protein levels. For example, nephrin expression is reported to be reduced in type II diabetic subjects with overt nephropathy and in spontaneously hypertensive rats (SHR) exhibiting proteinuria[10,11,27]. Here we report that induction of hypertension with DOCA-salt results in albuminuria with an accompanying decrease in nephrin staining in the glomeruli.
Although long-standing hypertension induces proteinuria, mere reduction of blood pressure does not correct albuminuria in hypertensive renal disease[27,32]. On the contrary, with equivalent blood pressure control, AT1 receptor blockers and ACE inhibitors effectively restore nephrin expression and prevent proteinuria in animal models of diabetes and hypertension[27]. In this study, we found that Ac-SDKP tended to increase nephrin staining in the kidneys of DOCA-salt hypertensive mice, with an accompanying decrease in albuminuria. This confirms our previous findings that Ac-SDKP protects nephrin protein in rats with 5/6 nephrectomy (high renin model)[21]. Collectively, this suggests that nephrin may be part of a complex system controlling protein loss in urine, and that upregulation of nephrin could be an important responsible for the anti-proteinuric effects of Ac-SDKP in hypertensive nephropathy.
Hypertensive renal disease, ACE inhibitor and Ac-SDKP
Hypertensive renal disease is characterized by inflammatory cell infiltration, oxidative stress, excess accumulation of extracellular matrix, decreased expression of slit pore protein nephrin, and proteinuria. ACE-inhibitors exert renal protective effects by counteracting these pathological changes in the kidney. ACE inhibitors alter not only the production of Ang II, but also other substances including bradykinin, Ang 1-7 and Ac-SDKP. Moreover, the renal protective effects of ACE-inhibitors are not always explained by lowering of blood pressure as even subpressor doses are found to be effective in decreasing hypertension-induced renal damage[30]. We previously found that infusion of captopril (100 mg/Kg/day) increased plasma Ac-SDKP levels by 5-fold, which was accompanied by a reduction in cardiac macrophage infiltration and fibrosis in hypertensive rats. Exogenous infusion of 400 and 800 μg/Kg/day of Ac-SDKP increased plasma Ac-SDKP levels by 4- and 10-fold, respectively, while both doses of Ac-SDKP mimicked the effects of captopril on the heart. Here and elsewhere[21], we noted that infusion of 800 μg/Kg/day of Ac-SDKP increased nephrin expression and lowered renal inflammation, glomerular matrix expansion and proteinuria, all of which were independent of systemic blood pressure. This underscores the potential role of Ac-SDKP as an additional strategy to ACE inhibition in hypertensive end organ-damage.
CONCLUSION
DOCA-salt hypertensive mice exhibited renal damage, including monocyte/macrophage infiltration, collagen deposition, and glomerular nephrin downregulation. These observations were accompanied by a rise in urinary protein excretion, most likely a result of kidney injury, secondary to renal inflammation and loss of nephrin. After Ac-SDKP treatment, urinary protein levels were markedly reduced in DOCA-salt-treated animals, indicating renal protection by Ac-SDKP.
Acknowledgments
This study was supported by an NIH grant to OA Carretero (HL028982) and NE Rhaleb (HL 071806).
Footnotes
Conflict of Interest: None
References
- 1.SAVOIA C, SCHIFFRIN EL. Inflammation in Hypertension. Curr Opin Nephrol Hypertens. 2006;15:152–158. doi: 10.1097/01.mnh.0000203189.57513.76. [DOI] [PubMed] [Google Scholar]
- 2.ZOCCALI C. Endothelial Dysfunction and the Kidney: Emerging Risk Factors for Renal Insufficiency and Cardiovascular Outcomes in Essential Hypertension. J Am Soc Nephrol. 2006;17:S61–S63. doi: 10.1681/ASN.2005121344. [DOI] [PubMed] [Google Scholar]
- 3.ZOCCALI C, MALLAMACI F, TRIPEPI G. Inflammation and Atherosclerosis in End-Stage Renal Disease. Blood Purif. 2003;21:29–36. doi: 10.1159/000067852. [DOI] [PubMed] [Google Scholar]
- 4.KELLY DJ, ZHANG Y, COX AJ, GILBERT RE. Combination therapy with tranilast and angiotensin-converting enzyme inhibition provides additional renoprotection in the remnant kidney model. Kidney Int. 2006;69:1954–1960. doi: 10.1038/sj.ki.5000376. [DOI] [PubMed] [Google Scholar]
- 5.TONELLI M, JOSE P, CURHAN G, SACKS F, BRAUNWALD E, PFEFFER M. Proteinuria, impaired kidney function, and adverse outcomes in people with coronary disease: analysis of a previously conducted randomised trial. Brit Med J. 2006;332:1426–1431. doi: 10.1136/bmj.38814.566019.2F. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.SHIBUYA K, KANASAKI K, ISONO M, SATO H, OMATA M, SUGIMOTO T, et al. N-acetyl-seryl-aspartyl-lysyl-proline prevents renal insufficiency and mesangial matrix expansion in diabetic db/db mice. Diabetes. 2005;54:838–845. doi: 10.2337/diabetes.54.3.838. [DOI] [PubMed] [Google Scholar]
- 7.KRIZ W, LEHIR M. Pathways to nephron loss starting from glomerular diseases—Insights from animal models. Kidney Int. 2005;67:404–419. doi: 10.1111/j.1523-1755.2005.67097.x. [DOI] [PubMed] [Google Scholar]
- 8.RUOTSALAINEN V, LJUNGBERG P, WARTIOVAARA J, LENKKERI U, KESTILÄ M, JALANKO H, et al. Nephrin is specifically located at the slit diaphragm of glomerular podocytes. Proc Natl Acad Sci USA. 1999;96:7962–7967. doi: 10.1073/pnas.96.14.7962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.KAWASHI H, KOIKE H, KURIHARA H, YAOITA E, ORIKASA M, SHIA MA, et al. Cloning of rat nephrin: Expression in developing glomeruli and in proteinuric states. Kidney Int. 2000;57:1949–1961. doi: 10.1046/j.1523-1755.2000.00044.x. [DOI] [PubMed] [Google Scholar]
- 10.BONNET F, COOPER ME, KAWACHI H, ALLEN TJ, BONER G, CAO Z. Irbesartan normalises the deficiency in glomerular nephrin expression in a model of diabetes and hypertension. Diabetologia. 2001;44:874–877. doi: 10.1007/s001250100546. [DOI] [PubMed] [Google Scholar]
- 11.PAGTALUNAN ME, MILLER PL, JUMPING-EAGLE S, NELSON RG, MYERS BD, RENNKE HG, et al. Podocyte loss and progressive glomerular injury in type II diabetes. J Clin Invest. 1997;99:342–348. doi: 10.1172/JCI119163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.CAVASIN MA, RHALEB N-E, YANG X-P, CARRETERO OA. Prolyl oligopeptidase is involved in release of the antifibrotic peptide Ac-SDKP. Hypertension. 2004;43:1140–1145. doi: 10.1161/01.HYP.0000126172.01673.84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.JUNOT C, NICOLET L, EZAN E, GONZALES M-F, MENARD J, AZIZI M. Effects of angiotensin-converting enzyme inhibition on plasma, urine, and tissue concentrations of hemoregulatory peptide Acetyl-Ser-Asp-Lys-Pro in rats. J Cardiovasc Pharmacol. 1999;291:982–987. [PubMed] [Google Scholar]
- 14.LIU J, ORMSBY A, OJA-TEBBE N, PAGANO PJ. Gene transfer of NAD(P)H oxidase inhibitor to the vascular adventitia attenuates medial smooth muscle hypertrophy. Circ Res. 2004;95:587–594. doi: 10.1161/01.RES.0000142317.88591.e6. [DOI] [PubMed] [Google Scholar]
- 15.POKHAREL S, VAN GEEL PP, SHARMA UC, CLEUTJENS JPM, BOHNEMEIER H, TIAN X-L, et al. Increased myocardial collagen content in transgenic rats overexpressing cardiac angiotensin-converting enzyme is related to enhanced breakdown of N-acetyl-Ser-Asp-Lys-Pro and increased phosphorylation of Smad2/3. Circulation. 2004;110:3129–3135. doi: 10.1161/01.CIR.0000147180.87553.79. [DOI] [PubMed] [Google Scholar]
- 16.AZIZI M, ROUSSEAU A, EZAN E, GUYENE T-T, MICHELET S, GROGNET J-M, et al. Acute angiotensin-converting enzyme inhibition increases the plasma level of the natural stem cell regulator N-acetyl-seryl-aspartyl-lysyl-proline. J Clin Invest. 1996;97:839–844. doi: 10.1172/JCI118484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.AZIZI M, EZAN E, NICOLET L, GROGNET JM, MENARD J. High plasma level of N-acetyl-seryl-aspartyl-lysyl-proline: a new marker of chronic angiotensin-converting enzyme inhibition. Hypertension. 1997;30:1015–1019. doi: 10.1161/01.hyp.30.5.1015. [DOI] [PubMed] [Google Scholar]
- 18.RASOUL S, CARRETERO OA, PENG H, CAVASIN MA, ZHUO J, SANCHEZ-MENDOZA A, et al. Antifibrotic effect of Ac-SDKP and angiotensin-converting enzyme inhibition in hypertension. J Hypertens. 2004;22:593–603. doi: 10.1097/00004872-200403000-00023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.TOJO A, WELCH WJ, BREMER V, KIMOTO M, KIMURA K, OMATA M, et al. Colocalization of demethylating enzymes and NOS and functional effects of methylarginines in rat kidney. Kidney Int. 1997;52:1593–1601. doi: 10.1038/ki.1997.490. [DOI] [PubMed] [Google Scholar]
- 20.GILBERT RE, WU LL, KELLY DJ, COX A, WILKINSON-BERKA JL, JOHNSTON CI, et al. Pathological expression of renin and angiotensin II in the renal tubule after subtotal nephrectomy. Implications for the pathogenesis of tubulointerstitial fibrosis. Am J Pathol. 1999;155:429–440. doi: 10.1016/S0002-9440(10)65139-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.LIAO T-D, YANG X-P, D’AMBROSIO M, ZHANG Y, RHALEB N-E, CARRETERO OA. N-Acetyl-Seryl-Aspartyl-Lysyl-Proline Attenuates Renal Injury and Dysfunction in Hypertensive Rats With Reduced Renal Mass. Hypertension. 2010;55:459–467. doi: 10.1161/HYPERTENSIONAHA.109.144568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.RHALEB N-E, PENG H, ALFIE M, SHESELY EG, CARRETERO OA. Effect of ACE inhibitor on DOCA-salt- and aortic coarctation-induced hypertension in mice. Do kinin B2 receptors play a role? Hypertension. 1999;33:329–334. doi: 10.1161/01.hyp.33.1.329. [DOI] [PubMed] [Google Scholar]
- 23.KREGE JH, HODGIN JB, HAGAMAN JR, SMITHIES O. A noninvasive computerized tail-cuff system for measuring blood pressure in mice. Hypertension. 1995;25:1111–1115. doi: 10.1161/01.hyp.25.5.1111. [DOI] [PubMed] [Google Scholar]
- 24.CLEUTJENS JP, VERLUYTEN MJ, SMITHS JF, DAEMEN MJ. Collagen remodeling after myocardial infarction in the rat heart. Am J Pathol. 1995;147:325–338. [PMC free article] [PubMed] [Google Scholar]
- 25.BROWN L, DUCE B, MIRIC G, SERNIA C. Reversal of cardiac fibrosis in deoxycorticosterone acetate-salt hypertensive rats by inhibition of the renin-angiotensin system. J Am Soc Nephrol. 1999;10:S143–S148. [PubMed] [Google Scholar]
- 26.MELHEM MF, CRAVEN PA, LIACHENKO J, DERUBERTIS FR. α-lipoic acid attenuates hyperglycemia and prevents glomerular mesangial matrix expansion in diabetes. J Am Soc Nephrol. 2002;13:108–116. doi: 10.1681/ASN.V131108. [DOI] [PubMed] [Google Scholar]
- 27.DAVIS BJ, CAO Z, DE GASPARO M, KAWACHI H, COOPER ME, ALLEN TJ. Disparate effects of angiotensin II antagonists and calcium channel blockers on albuminuria in experimental diabetes and hypertension: potential role of nephrin. J Hypertens. 2003;21:209–216. doi: 10.1097/00004872-200301000-00031. [DOI] [PubMed] [Google Scholar]
- 28.PENG H, CARRETERO OA, RAIJ L, YANG F, KAPKE A, RHALEB N-E. Antifibrotic effects of N-acetyl-seryl-aspartyl-lysyl-proline on the heart and kidney in aldosterone-salt hypertensive rats. Hypertension. 2001;37:794–800. doi: 10.1161/01.hyp.37.2.794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.NI W, LOOKINGLAND K, WATTS SW. Arterial 5-Hydroxytryptamine Transporter Function Is Impaired in Deoxycorticosterone Acetate and N? -Nitro-l-Arginine But Not Spontaneously Hypertensive Rats. Hypertension. 2006;48:134–140. doi: 10.1161/01.HYP.0000225754.15146.dd. [DOI] [PubMed] [Google Scholar]
- 30.PENG H, CARRETERO OA, ALFIE ME, MASURA JA, RHALEB N-E. Effects of angiotensin-converting enzyme inhibitor and angiotensin type 1 receptor antagonist in deoxycorticosterone acetate-salt hypertensive mice lacking Ren-2 gene. Hypertension. 2001;37:974–980. doi: 10.1161/01.hyp.37.3.974. [DOI] [PubMed] [Google Scholar]
- 31.LANDMESSER U, DIKALOV S, PRICE SR, MCCANN L, FUKAI T, HOLLAND SM, et al. Oxidation of tetrahydrobiopterin leads to uncoupling of endothelial cell nitric oxide synthase in hypertension. J Clin Invest. 2003;111:1201–1209. doi: 10.1172/JCI14172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.ANDERSON S, RENNKE HG, BRENNER BM. Therapeutic advantage of converting enzyme inhibitors in arresting progressive renal disease associated with systemic hypertension in the rat. J Clin Invest. 1986;77:1993–2000. doi: 10.1172/JCI112528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.PENG H, CARRETERO OA, BRIGSTOCK DR, OJA-TEBBE N, RHALEB NE. Ac-SDKP reverses cardiac fibrosis in rats with renovascular hypertension. Hypertension. 2003;42:1164–1170. doi: 10.1161/01.HYP.0000100423.24330.96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.BLASI ER, ROCHA R, RUDOLPH AE, BLOMME EAG, POLLY ML, MCMAHON EG. Aldosterone/salt reduces renal inflammation and fibrosis in hypertensive rats. Kidney Int. 2003;63:1791–1800. doi: 10.1046/j.1523-1755.2003.00929.x. [DOI] [PubMed] [Google Scholar]
- 35.HPETINSTALL RH, HILL GS. Steroid-induced hypertension in the rat. A study of the effects of renal artery constriction on hypertension caused by deoxycorticosterone. Lab Invest. 1967;16:751–767. [PubMed] [Google Scholar]
- 36.BESWICK RA, ZHANG H, MARABLE D, CATRAVAS JD, HILL WD, WEBB RC. Long-term antioxidant administration attenuates mineralocorticoid hypertension and renal inflammatory response. Hypertension. 2001;37:781–786. doi: 10.1161/01.hyp.37.2.781. [DOI] [PubMed] [Google Scholar]
- 37.RODRIGUEZ-ITURBE B, QUIROZ Y, KIM CH, VAZIRI ND. Hypertension Induced by Aortic Coarctation Above the Renal Arteries Is Associated With Immune Cell Infiltration of the Kidneys. Am J Hypertens. 2005;18:1449–1456. doi: 10.1016/j.amjhyper.2005.05.034. [DOI] [PubMed] [Google Scholar]
- 38.YANG F, YANG X-P, LIU Y-H, XU J, CINGOLANI O, RHALEB N-E, et al. Ac-SDKP reverses inflammation and fibrosis in rats with heart failure after myocardial infarction. Hypertension. 2004;43:229–236. doi: 10.1161/01.HYP.0000107777.91185.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.RHALEB N-E, PENG H, YANG X-P, LIU Y-H, MEHTA D, EZAN E, et al. Long-term effect of N-acetyl-seryl-aspartyl-lysyl-proline on left ventricular collagen deposition in rats with 2-kidney, 1-clip hypertension. Circulation. 2001;103:3136–3141. doi: 10.1161/01.cir.103.25.3136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.KIM S, OHTA K, HAMAGUCHI A, OMURA T, YUKIMURA T, MIURA K, et al. Role of angiotensin II in renal injury of deoxycorticosterone acetate-salt hypertensive rats. Hypertension. 1994;24:195–204. doi: 10.1161/01.hyp.24.2.195. [DOI] [PubMed] [Google Scholar]
- 41.OHTA K, KIM S, HAMAGUCHI A, YUKIMURA T, MIURA K, TAKAORI K, et al. Role of angiotensin II in extracellular matrix and transforming growth factor-beta 1 expression in hypertensive rats. Eur J Pharmacol. 1994;269:115–119. doi: 10.1016/0922-4106(94)90033-7. [DOI] [PubMed] [Google Scholar]
- 42.LIJNEN PJ, PETROV VV, FAGARD RH. Association between transforming growth factor-β and hypertension. Am J Hypertens. 2003;16:604–611. doi: 10.1016/s0895-7061(03)00847-1. [DOI] [PubMed] [Google Scholar]
- 43.MANNING RD, Jr, MENG S, TIAN N. Renal and vascular oxidative stress and salt-sensitivity of arterial pressure. Acta Physiol Scand. 2003;179:243–250. doi: 10.1046/j.0001-6772.2003.01204.x. [DOI] [PubMed] [Google Scholar]
- 44.ZUGHAIER SM, SHAFER WM, STEPHENS DS. Antimicrobial peptides and endotoxin inhibit cytokine and nitric oxide release but amplify respiratory burst response in human and murine macrophages. Cell Microbiol. 2005;7:1251–1262. doi: 10.1111/j.1462-5822.2005.00549.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.POKHAREL S, RASOUL S, ROKS AJM, VAN LEEUWEN REW, VAN LUYN MJA, DEELMAN LE, et al. N-acetyl-Ser-Asp-Lys-Pro inhibits phosphorylation of Smad2 in cardiac fibroblasts. Hypertension. 2002;40:155–161. doi: 10.1161/01.hyp.0000025880.56816.fa. [DOI] [PubMed] [Google Scholar]
- 46.KANASAKI K, KOYA D, SUGIMOTO T, ISONO M, KASHIWAGI A, HANEDA M. N-acetyl-seryl-aspartyl-lysyl-proline inhibits TGF-β mediated plasminogen activator inhibitor-1 expression via inhibition of Smad pathway in human mesangial cells. J Am Soc Nephrol. 2003;14:863–872. doi: 10.1097/01.asn.0000057544.95569.ec. [DOI] [PubMed] [Google Scholar]
- 47.RHALEB N-E, PENG H, HARDING P, TAYEH M, LAPOINTE MC, CARRETERO OA. Effect of N-acetyl-seryl-aspartyl-lysyl-proline on DNA and collagen synthesis in rat cardiac fibroblasts. Hypertension. 2001;37:827–832. doi: 10.1161/01.hyp.37.3.827. [DOI] [PMC free article] [PubMed] [Google Scholar]