Abstract
In conditions characterized by energetic constraints, such as in periods of low food availability, some trade-offs between reproduction and self-maintenance may be necessary; even year-round breeders may then be forced to exhibit some reproductive seasonality. Prior research has largely focused on female reproduction and physiology, and few studies have evaluated the impact of environmental factors on males. Here we assessed the effects of season and ambient temperatures on fecal glucocorticoid (fGC) and testosterone (fT) levels in male baboons in Amboseli, Kenya. The Amboseli basin is a highly challenging, semi-arid tropical habitat that is characterized by strongly seasonal patterns of rainfall and by high ambient temperatures. We previously reported that female baboons were impacted by these challenging environmental conditions. We ask here whether male baboons in the same environment and groups as females exhibit similar physiological effects. We found that after accounting for male age and individual variability, males exhibited higher fGC levels and lower fT levels during the dry season than during the wet season. Furthermore, fT but not fGC levels were lower in months of high average daily maximum temperatures, suggesting a direct impact of heat on testes. Our results demonstrate that male baboons, like females, experience ecological stress that alters their reproductive physiology. The impact of the environment on male reproduction deserves more attention both in its own right and because alteration in male physiology may contribute to the reduction in female fertility observed in challenging environments.
Keywords: dry season, food availability, heat stress, fecal hormones, male baboons
Environmental factors can have a dramatic impact on physiology and reproduction (Bronson, 1995). As in many other taxa, some primate species have adapted to seasonal variations in rainfall and food availability by exhibiting strict reproductive seasonality (e.g. tufted capuchin monkeys: Lynch et al., 2002; ring-tailed lemurs: Gould and Ziegler, 2007; Barbary macaques: Menard and Vallet, 1993; northern muriquis: Strier et al., 2001; golden lion tamarins: Bales et al. 2006; Verreaux’s sifaka: Brockmann et al. 1998; see also Janson and Verdolin, 2005). For some of these species, females are only receptive for a few hours (ring-tailed lemurs: Gould and Ziegler, 2007) to a few days (Verreaux’s sifaka: Brockmann et al. 1998) of a short breeding period concentrated in a few months of the year. During the less favorable season, reproductive processes shut down; females stop cycling and males have regressed gonads and low testosterone levels (Brockman et al., 1998; Lynch et al., 2002; Bales et al., 2006; Gould and Ziegler, 2007). Most other primate species reproduce at least occasionally in all months of the year in the wild but still exhibit relatively strong seasonal variability in conceptions and births (e.g. long-tailed macaques: van Schaik and van Noordwijk, 1985; hanuman langurs: Koenig et al., 1997; see Janson and Verdolin, 2005, Table 11.2). At the other extreme from the strictly seasonal breeders are a few species that breed at appreciable frequencies in all months of the year, exhibiting only the slightest seasonal variability. They include examples of new world monkeys (e.g. capuchins, howlers and spider monkeys: Fedigan and Rose, 1995; Strier et al., 2001), old world monkeys (e.g. yellow baboons: Alberts et al., 2005; geladas: Dunbar and Dunbar, 1975; pig-tailed macaques: Oi, 1996), great apes (chimpanzees: Goodall, 1983; gorillas: Watts, 1998) and humans (Ellison et al., 2005) (see also Janson and Verdolin, 2005, Table 11.2 for review).
Seasonal peaks in conception and birth often result from seasonal variability in energetic constraints (review in Bronson, 1995, see also Bailey et al., 1992). Available energy resulting from food consumption first must satisfy requirements associated with an individual’s survival such as cellular maintenance, thermoregulation and foraging for food. Growth, reproduction and non-foraging behavior are given lower priority. In periods of low food availability, available energy may be insufficient for both self-maintenance and reproduction, and some trade-offs may be necessary (Hau, 2001; Whitten and Turner, 2009). Ellison and colleagues found that during periods of low food availability women of a population living in Central Africa, the Lese, experience impaired reproductive function such as decrease in the frequency of ovulation, lower estrogen and progesterone levels, and longer intervals between menstruations (Ellison et al., 1986; Bailey et al., 1992; Bentley et al., 1998). These alterations in reproductive pattern were associated with a decrease in body weight in the Lese women. Attainment of menarche and termination of post-partum amenorrhea, two other important transitions leading to successful reproduction in humans, are delayed when resources are limited (Worthman et al., 1993; Riley, 1994; see also review by Bronson, 1995, and Cameron, 1996). Effects of food availability on reproduction have also been documented in some nonhuman primates (e.g. Hanuman langur: Koenig et al., 1997; Long-tailed macaques: van Schaik and van Noordwijk, 1985, Orangutans: Knott, 2005; see also chapters in Brockman and van Schaik, 2005).
The impact of energetic constraints may be further exacerbated by adverse ambient temperatures due to the cost of thermoregulation. High ambient temperatures affect female reproductive function by slowing sexual maturation, decreasing ovulation frequency, and increasing embryo mortality (Bronson, 1989). Low ambient temperatures can also affect primate reproduction, particularly at high latitudes or altitudes, e.g. in chacma baboons at high latitudes by increasing their inter-birth intervals (Hill et al., 2000) and increasing their cortisol levels (Weingrill et al., 2004), and in gelada monkeys at the high altitudes of the Simien mountains by reducing their birth rate (Ohsawa and Dunbar, 1984).
Effects of both ambient temperature and food availability on reproduction are, in part, mediated by steroid hormones. In the presence of a stressor, the hypothalamic-pituitary-adrenal (HPA) axis is activated, and glucocorticoids are secreted by the adrenals. Glucocorticoids activate processes necessary for the mobilization of energy and inhibit processes non essential for immediate survival such as growth, reproduction and immune function. Glucocorticoids decrease the secretion of several hormones of the hypothalamic-pituitary-gonadal (HPG) axis such as gonadotropin-releasing hormone (GnRH) and luteinizing hormone (LH), leading to a decrease in essential reproductive steroid hormones such as estrogens and testosterone (Collu et al., 1984). However, when the increase in glucocorticoids arises from the demands of mating, the reproductive system sometimes exhibits insensitivity to the action of glucocorticoids. An individual may then exhibit both elevated glucocorticoid and testosterone levels (Lynch et al. 2002; review by Bercovitch and Ziegler, 2002).
In contrast to the large body of information on the impact of environmental factors on female reproduction, few studies have evaluated the impact of environmental factors on male reproductive physiology in species that exhibit only minimal seasonal variability in reproduction, and almost all have been conducted in humans (see review by Bribiescas, 2001). Results from the studies in men have produced less clear results than for women. Under moderate energetic stress, male testosterone levels were not impacted while under similar conditions female reproductive hormones were strongly affected (Garrel et al., 1984; Bentley et al., 1993; Ellison and Panter-Brick, 1996). These authors suggested that the relative insensitivity of male testosterone levels to energy status may reflect the lower investment in reproduction by males as compared to females.
Under very extreme conditions, however, several studies have suggested that male testosterone levels are associated with energy status. For example, experimental studies found that testosterone levels declined after complete fasting both in male rhesus monkeys (Wahab et al., 2008) and in men (Cameron et al., 1993). Similar results were found for Indian men that were naturally experiencing severe malnutrition (Smith et al., 1975) and for adolescent and adult wild baboons during an extreme drought (Sapolsky, 1986). In addition, experimental exposure of testes to temperatures at or above body temperature results in a reduction in sperm quantity and quality in a variety of animal species (Kandeel and Swerdloff, 1988; Liu, 2005; Schwalm et al., 2007, see also review by Hansen, 2009). The effect of high temperatures on testosterone levels has only rarely been examined and to our knowledge, only in farm animals and rodents (Magal et al., 1981; Minton et al., 1981; Larsson et al., 1983; review by Gwazdauskas, 1985). These studies found that exposure to 35°C for long period of time (>4 days) led to a decrease in testosterone levels.
Taken together, the limited available evidence suggests that perhaps males of relatively non-seasonal primate species experience environmental impact on reproductive physiology only under very extreme acute or chronic environmental conditions, i.e. only under the equivalent of pharmacological rather than physiological levels of challenge. Here we capitalize on a long-term study of wild baboons in Amboseli, Kenya, to examine this proposition by evaluating the effects of a highly seasonal and challenging habitat on male physiology. The Amboseli basin is a semi-arid tropical habitat that is characterized by high ambient temperatures and low annual rainfall that is highly seasonal (Alberts et al., 2005; Gesquiere et al., 2008). Like humans, baboons in the tropics reproduce throughout the year and exhibit very little seasonality (Altmann, 1980; Bercovitch and Harding, 1993; also see Janson and Verdolin 2005 Table 11.2). Rates of reproduction in the Amboseli study groups are similar to those reported for other baboon populations, and the Amboseli population is at or slightly above replacement value over the several decades of data collection. However, previous findings from our group demonstrate that reproduction in Amboseli baboons is affected by environmental conditions. Over several decades of study, baboons in Amboseli exhibit a slight seasonal birth peak from August to October, corresponding to an increase in conceptions during the wetter season (Alberts et al., 2005). In addition, we recently demonstrated that females had elevated glucocorticoid levels during the dry season and during the hotter months (Gesquiere et al., 2008). Taken together, these studies demonstrate that environmental conditions experienced regularly in Amboseli are stressful for female baboons and result in alteration of their reproduction.
Male baboons live year-round in the same groups as females. Adult males are double the body size and are socially dominant to all females, readily securing priority of access to food and water resources, and perhaps buffering them from environmental effects on physiology. We ask here whether male baboons exhibit similar environmentally-induced physiological effects that have been previously found for females in the same environment and social groups. To answer this question, we evaluated the effect of the dry season and periods of extreme heat on males’ physiology by measuring fecal glucocorticoids (fGC) and testosterone (fT) concentrations. An absence of changes in both fGC and fT levels would indicate that males are more resistant to environmental challenges than females because of physiological or behavioral buffering. In contrast, an increase in fGC and a decrease in fT during the dry season and the hotter months would suggest that male baboons, like females, experience ecological stress that alters their reproductive physiology. This finding would suggest that changes in male as well as female physiology may contribute to the modest seasonal variability in birthrates.
METHODS
Study population
The subjects in the present study were 114 adult male members of five social groups of wild, non-provisioned baboons in the Amboseli basin, Kenya. Individual life-history data have been collected in the study population for almost four decades (e.g. Altmann and Muruthi, 1988; Altmann et al., 2002; Alberts et al., 2005; see www.princeton.edu/~baboon for a complete bibliography and the Amboseli Baboon Research Project, ABRP, data collection Monitoring Guide). Ages of the subjects were based on known birth dates for males born in study groups, or were estimated based on coat condition, degree of scarring, body carriage and canine tooth condition for immigrant males when they first joined the study population (see Alberts and Altmann, 1995 for details). Since late 1999, physiological data have been obtained from known individuals through non-invasive fecal hormone analysis.
Amboseli weather data
The Amboseli basin in Kenya (2°40′S, 37°15′E, 1100m altitude), is a semi-arid short-grass savannah ecosystem located in an ancient lake basin NW of the base of Mount Kilimanjaro. The environment is characterized by a predictable five-month-long dry season, starting in June that is devoid of rain and during which availability of food and drinking water progressively declines. This dry season is followed by a seven-month wetter period in which rainfall is highly and unpredictably variable from month to month. Rainfall is also highly variable across years (ranging from 150mm to 550mm per year, with a mean value of 348mm/year, see Altmann et al., 2002; Alberts et al., 2005), and drought conditions occur when the normal dry season is unpredictably extended at either end by failure of normal rains.
Although air temperature is less variable across months and years than is rainfall, high temperatures and, therefore, the risk of thermal stress is nonetheless greater in some months, and temperature has increased appreciably over the past several decades (Altmann et al., 2002).
Daily records of rainfall and of minimum and maximum temperature (Tmin and Tmax, respectively) were obtained using a rain gauge and min-max thermometer, placed in the shade, at the research field camp that is within 2-17 km of the ranges of the various baboon groups (Altmann et al., 2002). Analyses were done at the level of a month, using total rainfall and average daily Tmax for each month of the study.
Across the nine years of the present study (January 2000 - May 2008), the mean monthly Tmax was 33.1°C (28.9°C to 37.9°C) and daily Tmax values sometimes exceeded 40°C. Thus, maximum daily air temperatures in the shade were often close to and sometimes exceeded baboons’ normal core body temperature of 38°C (Funkhouser et al., 1967). Furthermore, environmental temperature that baboons experience (termed ‘perceived environmental temperature’ by Hill et al., 2004) will often be considerably above these shade values, depending on microhabitat, sun exposure, humidity, and behavior.
Hormone data
Fecal sample collection, storage, and extraction were as described previously (Khan et al., 2002; Lynch et al., 2003). In brief, immediately after collection of freshly deposited fecal samples from known individuals, these samples were mixed and placed in 95% ethanol, and stored in a charcoal refrigerator (~20-25°C) for no longer than two weeks. After being shipped to the University of Nairobi the samples were freeze-dried, then sifted to remove the vegetative matter, and stored at −20°C. After transport to Princeton University, 0.2 g of fecal powder was extracted into 2 ml 90% methanol using a multipulse vortexer for 30 minutes. Following extraction, samples were further purified using a prepped Oasis cartridge (Waters, Milford, MA) and stored at −20°C. The samples were then assayed for glucocorticoids (fGC) and testosterone (fT) by radioimmunoassay (Gesquiere et al., 2005; Gesquiere et al., 2008; Beehner et al., 2009; full laboratory protocols also available at www.princeton.edu/~baboon). The primary antibody in the Corticosterone kit for rats and mice (ICN Diagnostics, Costa Mesa, CA) cross-reacts with major cortisol metabolites present in baboon feces (Wasser et al., 2000). Inter-assay coefficients of variation were 13.6% and 10.7% (n=49), respectively for a low and high control. Intra-assay coefficients of variation were below 6% for both the low and high control (any duplicate above 15% was re-assayed). fT concentrations were determined using the Equate 125I Testosterone RIA kit (SolidPhase, Portland, ME) in samples collected from January 2000 through July 2004. Because the Equate kit was then discontinued, it was necessary to validate a new T RIA using the Diagnostics Systems Laboratories (DSL) 125I Testosterone kit (Beckman Coulter, Webster, TX). Results for parallelism, accuracy, and precision with the Equate and DSL T kit are published respectively in Lynch et al. (2003) and Beehner et al. (2009). In addition, we ran a subset of our samples previously assayed with the Equate kit with the DSL kit, in order to confirm that the two kits give comparable T concentrations. Our results showed a strong correlation between the T values obtained by the two different kits (R 2=0.906, n=124, p<0.001), but the T levels obtained with the DSL kit were higher than those with the Equate kit. Using the empirically derived linear regression equation (TDSL=1.9676*TEquate+16.9926), we transformed the T levels obtained with the Equate kit so that samples analyzed with both methods could be included in the same analysis. The hormone results are expressed as ng/g dry feces.
For this study we used all the fGC and fT data for males eight years and older obtained from January 2000 through May 2008. We had a total of 3885 fecal samples from 114 males. Because fecal samples were collected ad libitum, sample numbers were variable across males and months (N=0-8 samples/male/month; some males were not sampled in particular months). Individuals were never sampled more than once per day. For any month with multiple values for a male, the mean of his values for that month was used for analysis. As a result, we had a total of 2182 monthly values across the 114 males, with an average of 19 (range 1 to 67) months per male.
Statistical analysis
To evaluate the effect of rainfall and temperature on fGC and fT levels, a General Linear Mixed Model (GLMM) was constructed for each hormone, fGC and fT, using SPSS 17.0. In each case, baboon identity was entered into the model as a random factor to control for the unequal and unevenly distributed sampling among males. fGC and fT levels were log transformed to approach normality. The predictor variables entered in each model were two categorical environmental variables: season and temperature (see details for both variables below). We also included one continuous variable, age, in each GLMM, as concentrations of these steroids have been shown to vary with age (Sapolsky and Altmann, 1991; Altmann et al., in press).
After evaluating the full model, we focused first on the effects of season and then on the effects of temperature by analyzing the residual values of each hormone, fGC and fT, that were obtained from the GLMM. Residual values were obtained by running the model described above excluding the environmental variable of interest but including subject, age, and the other environmental variable. For example, to examine the effect of season on fGC, the residual fGC values were calculated by entering temperature and age as predictor variables in the GLMM and baboon identity as a random factor. Non-parametric tests were then performed using the residuals of this model as the response variable and season as the predictor variable, with the statistical threshold set at p<0.05.
Predictor Variables: age
For each fecal sample we determined a male’s age on the date the sample was collected. Then, for each male we calculated his monthly mean age at sample collection for each calendar month.
Predictor Variables: environmental factors
The ‘season’ variable divides months into two categories, the ‘dry season’ or ‘wet season’. ‘Dry season’ corresponded to the five-month-long dry season from June through October while the ‘wet season’ included the other seven months from November through May (see Alberts et al., 2005; Gesquiere et al., 2008).
The ‘temperature’ variable divides months into two categories, ‘cool’ or ‘hot’. Using the daily record of maximum temperatures (Tmax) over the 101 study months, we calculated a monthly mean Tmax for each month and considered a month as being ‘hot’ when its mean Tmax was above the 3rd quartile Tmax (>34.4°C), otherwise the month was considered ‘cool’ (see also Gesquiere et al., 2008).
RESULTS
Factors contributing to variation in fGC
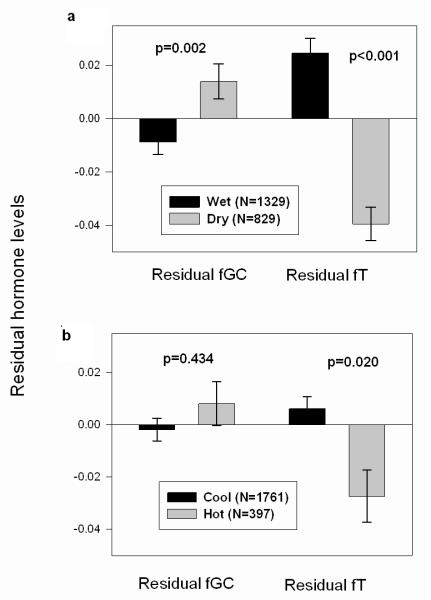
Season significantly predicted fGC concentrations in the overall GLMM, whereas temperature and male age did not (Table 1). In our analysis of residuals, adult male fGC concentrations were significantly higher during the dry season than the wet season (Mann-Whitney U: Z=−3.142, p=0.002; Fig. 1a), but did not vary with air temperature (Mann-Whitney U: Z=−0.783, p=0.434; Fig. 1b).
Table 1.
Results of GLMM assessing the effect of environmental predictors on fGC and fT concentrations while accounting for age, and with male identity entered as a random factor.
| Variables | Numerator df |
Denominator df |
F | Sig. | b |
|---|---|---|---|---|---|
| Dependant variable: log fGC | |||||
| Intercept | 1 | 403.028 | 4408.16 | <0.001 | |
| Age | 1 | 440.887 | 0.105 | 0.746 | 0.0007 |
| Wet/Dry season | 1 | 2093.096 | 9.085 | 0.003 | −0.0261 |
| Cool/Hot month | 1 | 2105.621 | 1.116 | 0.291 | −0.0116 |
| Dependant variable: log fT | |||||
| Intercept | 1 | 581.988 | 3835.386 | <0.001 | |
| Age | 1 | 1911.627 | 238.209 | <0.001 | −0.0457 |
| Wet/Dry season | 1 | 2051.124 | 61.714 | <0.001 | 0.0749 |
| Cool/Hot month | 1 | 2055.028 | 10.778 | 0.001 | 0.0396 |
Significant results appear in bold typeface.
Figure 1.
Differences in fGC and fT concentrations in (a) wet vs. dry se ason and (b) cool vs. hot months. The y axis represents the residual fGC or fT values obtained from the GLMM, which included all the predictors listed above in Table 1 except for the variable of interest (see Methods). Each value represents the mean ± SE across male monthly averages. N represents the number of monthly averages. Statistical significance was determined using the Mann Whitney U.
Factors contributing to variation in fT
Both season and temperature significantly predicted fT concentrations in the overall GLMM (Table 1). In addition, fT levels declined with age of subject (b=−0.0457, p<0.001). Our analysis of residuals showed that adult male fT concentrations were significantly lower during the dry season than the wet season (Mann-Whitney U: Z=−7.927, p<0.001; Fig. 1a) and were significantly lower during the hotter months than in cooler months (Mann-Whitney U: Z=−2.320, p=0.020; Fig. 1b).
DISCUSSION
The physiology of male baboons was clearly impacted by life under the challenging environmental conditions in Amboseli, as previously reported for females (Beehner et al., 2006; Gesquiere et al., 2008). Both fGC and fT levels exhibited differences between dry and wet seasons; residual fGC levels were 2.6 times higher during the dry season, and residual fT levels were 2.6 times lower during the dry season. High ambient temperatures were associated with residual fT levels that were 5.4 times lower than in cooler months. This effect was apparently independent of the HPA axis as fGC did not significantly increase in hotter months.
Seasonality
Male fGC levels were higher during the dry season, when food availability is lower, suggesting that Amboseli baboons seasonally experience energetic stress. As the dry season progresses, baboons in Amboseli switch their diet from easily processed food sources such as grass blades, grass seedheads and shrub/forb leaves to lower profitability fallback foods such as grass corms, which require considerably longer processing time (Post, 1981; Altmann, 1998; 2009; Alberts et al., 2005). The baboons also increase their energetic expenditure, spending more time foraging and less time resting as both food and water become more scarce and patchy (Post, 1981; Alberts et al., 2005; Gesquiere et al., 2008). Both reduction in food intake and increase in energetic expenditure contribute to a reduction in the baboons’ energy balance in the dry season. In addition, during the dry season or drought in Amboseli, the baboons’ body condition also appears to deteriorate (SCA and JA personal observation and unpublished data), consistent with the physiological findings presented here that male nutritional status is affected during the dry season. When energy reserves are limited, necessary trade-offs occur between energy allocated to self-maintenance and energy allocated to reproduction (Hau, 2001; Whitten and Turner, 2009). Previous studies on wild nonhuman primates have reported effects of energetic constraints on glucocorticoids in males under extreme conditions such as drought (e.g. Saplolsky, 1986 for olive baboons) but not generally otherwise (e.g. Bergman et al., 2005 for chacma baboons; but see Muller & Wrangham, 2004 for high urinary cortisol when both fruit availability was low and aggression levels were high in wild chimpanzees).
Our findings that male baboons had lower levels of the reproductive hormone fT during the dry season are striking. Although energetic impact on male reproductive physiology has not often been investigated, the limited available research on men and male nonhuman primates has reported impact only under extremely severe conditions (see introduction). A decrease in T levels may result in part from the inhibitory action of GC on the HPG axis but peptide hormones such as leptin and insulin may also be involved (reviewed by Schneider, 2004). Peptide hormones cannot be evaluated from fecal sources, and urine samples cannot be obtained from the study population, precluding assessment of those contributors.
Because T has an important role in spermatogenesis, decreases in fT levels such as those observed during the dry season in male baboons might impact male fertility. For example, seasonal changes in testosterone were correlated with changes in sperm quantity and quality in coyotes (Minter and DeLiberto, 2008). However, T may impact behavior more than sperm production in year-round breeding primates such as baboons or humans. T affects male reproductive behavior (such as courtship and aggressive behavior; see review by Wingfield et al., 1990), and even small reductions in fT levels may lead to a reduction in male mating effort and may thereby have consequences for male baboon reproduction.
Ambient temperature
Ambient temperatures also affected male baboon reproductive physiology; fT levels were lower in hotter months. This effect appears to be independent of glucocorticoids, as fGC levels were not higher in hotter months. High ambient temperatures can directly impact testes without activation of the HPA axis as occurs in cryptorchidism, in which the undescended testes are chronically exposed to the higher temperatures within the body. Experimental exposure of testes to elevated temperatures affects spermatogenesis and reduces sperm quality (Kandeel and Swerdloff, 1988; Liu, 2005; Schwalm et al., 2007). However, T levels are only rarely measured in studies of high temperatures. Although several studies have investigated the effect of high temperatures in farm animals and rodents (Magal et al., 1981; Minton et al., 1981; Larsson et al., 1983; see also review by Hansen 2009), we know of no studies of nonhuman primates and only one report focusing on humans (Dabbs, 1990). That study showed that T levels in men are lower during spring and summer than in other months. Ambient heat may also act indirectly as a stressor through the elevation of hormones other than GC, such as the peptide hormone, prolactin (Krulic et al., 1974; Ronchi et al., 2001). Experimental hyperprolactinemia has been reported to decrease T secretion in rats (Huang et al., 1999).
The absence of an increase in fGC in male baboons in hotter months was somewhat surprising as GC has been reported to increase in mammals experimentally exposed to acute ambient temperatures (reviewed in Johnson and Vanjonack, 1975; Gwazdauskas, 1985). However, chronic heat exposure induces a transient rise of GC followed by a return to pre-stress levels through negative feedback, in spite of continued heat treatment. The absence of persistent GC elevation in chronic heat stress is thought to be a thermoregulatory protective mechanism that prevents further metabolic heat production in a hot environment (review in Marai et al., 2002). Male baboons in Amboseli are generally exposed to very hot ambient temperatures for several consecutive weeks; the lack of fGC increase in the male baboons during hotter months may be explained by a return to pre-stress levels through negative feedback mechanisms. If so, why did we find an effect of heat on fGC for baboon females (Gesquiere et al., 2008)? Could male baboons be less sensitive to thermal stress than females? Gender differences in reaction to heat stress have been shown in humans (Frye and Kamon, 1981). Men have lower surface area relative to body mass than women, which results in men experiencing reduced heat gain from the environment on hot days (Falk, 1998; Anderson et al., 2000). In addition, men sweat more than women do (Mehnert et al., 2002; Hazelhurst and Claassen, 2006). Similar gender differences in thermoregulation may occur in adult baboons as males are approximately double the body size of females (Altmann et al., 1993; Altmann and Alberts, 2005), and adult male baboons thus have much lower surface area relative to body mass than adult females. An alternative and not mutually exclusive explanation is that male baboons may be able to spend more time resting or feeding in the shade than the females if they have lower energetic constraints from their much higher dominance status and feeding priority.
Human reproductive ecology traditionally focuses on variation in female reproductive function in response to environmental challenges. The impact of the environment on male reproduction has often been neglected, in part due to the lower metabolic cost of reproduction in males. This sex difference in costs of reproduction forms the cornerstone of the socioecological model of animal societies, which posits that female distributions are driven by food availability, and male distributions are driven by female availability (e.g. Emlen and Oring, 1977). However, our study suggests that in baboons, environmental challenges impact male physiology. Moreover, this effect is not a simple one driven by the presence of cycling females as has been shown for rhesus macaques (Vandenbergh and Drickamer, 1974). In Amboseli multiple fertile females (in the ovulatory phase of the cycle; Gesquiere et al., 2007) are generally available in each social group in all calendar months, albeit at somewhat lower levels during some months. For example, during the years of present study, approximately four fertile females per group were present in the dry season and an average of six during the wet season. Nonetheless, more subtle indirect environmental effects through each sex on the reproduction of the other despite year-round reproduction remain an intriguing possibility. Studies of environmental impacts on male reproduction in humans and other primates provide a potentially rich area for future research, both for a more complete understanding of male physiology itself and for explaining the reduced fertility observed in extreme environments, which may not be solely due to reduction in female fertility.
ACKNOWLEDGEMENTS
Supported by NSF IOB-0322613, NSF IOB-0322781, NSF BCS-0323553, NSF BCS-0323596, R03 MH65294, NIA P30AG024361, and the Chicago Zoological Society. Thanks to the Office of the President, Republic of Kenya, the Kenya Wildlife Services, its Amboseli staff and Wardens, the Institute of Primate Research, the National Museums of Kenya, the Department of Veterinary Anatomy and Physiology of the University of Nairobi, and the members of the Amboseli-Longido pastoralist communities. Particular thanks go to the Amboseli field team who contributed to sample and data collection (R.S. Mututua, S. Sayialel, and J.K. Warutere). Thanks to T. Wango, V.K. Oudu and C. Simao for the fecal samples processing and analyses. Thanks to N. Learn and to C. Markham for their input on data analysis or for providing comments on earlier drafts. All protocols were non-invasive, and adhered to the laws and guidelines of Kenya (Kenya Research Permit MOEST 13/001/C351 Vol. II) and were approved by the Animal Care and Use Committee at Princeton University (IACUC 1689, 9 November 2007).
Supported by NSF IOB-0322613, NSF IOB-0322781, NSF BCS-0323553, NSF BCS-0323596, R03 MH65294, NIA P30AG024361, and the Chicago Zoological Society.
LITERATURE CITED
- Alberts SC, Altmann J. Balancing costs and opportunities: dispersal in male baboons. Am Nat. 1995;145:279–306. [Google Scholar]
- Alberts SC, Hollister-Smith J, Mututua RS, Sayialel SN, Muruthi PM, Warutere JK, Altmann J. Seasonality and long-term change in a savanna environment. In: Brockman DK, van Schaik CP, editors. Seasonality in primates: studies of living and extinct human and non-human primates. Cambridge University Press; Cambridge; New York: 2005. pp. 157–195. [Google Scholar]
- Altmann J. Baboon mothers and infants. Harvard University Press; Cambridge, Massachusetts: 1980. [Google Scholar]
- Altmann J, Alberts SC. Growth rates in a wild primate population: ecological influences and maternal effects. Behav Ecol Sociobiol. 2005;57:490–501. [Google Scholar]
- Altmann J, Alberts SC, Altmann SA, Roy SB. Dramatic change in local climate patterns in the Amboseli basin, Kenya. Afr J Ecol. 2002;40:248–251. [Google Scholar]
- Altmann J, Gesquiere LR, Galbani J, Onyango PO, Alberts SC. The life history context of reproductive aging in a wild primate model. Ann NY Acad Sci. doi: 10.1111/j.1749-6632.2010.05531.x. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Altmann J, Muruthi P. Differences in daily life between semiprovisioned and wild-feeding baboons. Am J Primatol. 1988;15:213–221. doi: 10.1002/ajp.1350150304. [DOI] [PubMed] [Google Scholar]
- Altmann J, Schoeller D, Altmann SA, Muruthi P, Sapolsky RM. Body size and fatness of free living baboons reflect food availability and activity levels. Am J Primatol. 1993;30:149–161. doi: 10.1002/ajp.1350300207. [DOI] [PubMed] [Google Scholar]
- Altmann SA. Foraging for survival: yearling baboons in Africa. University of Chicago Press; Chicago: 1998. [Google Scholar]
- Altmann SA. Fallback foods, eclectic omnivores, and the packaging problem. Am J Phys Anthropol. 2009;140:615–619. doi: 10.1002/ajpa.21097. [DOI] [PubMed] [Google Scholar]
- Anderson SJ, Griesemer BA, Johnson MD, Martin TJ, McLain LG, Rowland TW, Small E. Climatic heat stress and the exercising child and adolescent. Pediatrics. 2000;106:158–159. [Google Scholar]
- Bailey RC, Jenike MR, Ellison PT, Bentley GR, Harrigan AM, Peacock NR. The ecology of birth seasonality among agriculturalists in central Africa. J Biosoc Sci. 1992;24:393–412. doi: 10.1017/s0021932000019957. [DOI] [PubMed] [Google Scholar]
- Bales KL, French JA, McWilliams J, Lake RA, Dietz JM. Effects of social status, age, and season on androgen and cortisol levels in wild male golden lion tamarins (Leontopithecus rosalia) Horm Behav. 2006;49:88–95. doi: 10.1016/j.yhbeh.2005.05.006. [DOI] [PubMed] [Google Scholar]
- Beehner JC, Gesquiere LR, Seyfarth RM, Cheney DL, Alberts SC, Altmann J. Testosterone related to age and life-history stages in male baboons and geladas. Horm Behav. 2009;56:472–480. doi: 10.1016/j.yhbeh.2009.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beehner JC, Onderdonk DA, Alberts SC, Altmann J. The ecology of conception and pregnancy failure in wild baboons. Behav Ecol. 2006;17:741–750. [Google Scholar]
- Bentley GR, Harrigan AM, Campbell B, Ellison PT. Seasonal effects on salivary testosterone levels among Lese males of the Ituri Forest, Zaire. Am J Hum Biol. 1993;5:711–717. doi: 10.1002/ajhb.1310050614. [DOI] [PubMed] [Google Scholar]
- Bentley GR, Harrigan AM, Ellison PT. Dietary composition and ovarian function among Lese horticulturalist women of the Ituri Forest, Democratic Republic of Congo. Eur J Clin Nutr. 1998;52:261–270. doi: 10.1038/sj.ejcn.1600547. [DOI] [PubMed] [Google Scholar]
- Bercovitch FB, Harding RSO. Annual birth patterns of savanna baboons (Papio cynocephalus anubis) over a 10-year period at Gilgil, Kenya. Folia Primatol. 1993;61:115–122. doi: 10.1159/000156738. [DOI] [PubMed] [Google Scholar]
- Bercovitch FB, Ziegler TE. Current topics in primate socioendocrinology. Annu Rev Anthropol. 2002;31:45–67. [Google Scholar]
- Bergman TJ, Beehner JC, Cheney DL, Seyfarth RM, Whitten PL. Correlates of stress in free-ranging male chacma baboons, Papio hamadryas ursinus. Anim Behav. 2005;70:703–713. [Google Scholar]
- Bribiescas RG. Reproductive ecology and life history of the human male. Yearb Phys Anthropol. 2001;44:148–176. doi: 10.1002/ajpa.10025.abs. [DOI] [PubMed] [Google Scholar]
- Brockman DK, van Schaik CP. Seasonality in primates: studies of living and extinct human and non-human primates. Cambridge University Press; Cambridge; New York: 2005. [Google Scholar]
- Brockman DK, Whitten PL, Richard AF, Schneider A. Reproduction in free-ranging male Propithecus verreauxi: the hormonal correlates of mating and aggression. Am J Phys Anthropol. 1998;105:137–151. doi: 10.1002/(SICI)1096-8644(199802)105:2<137::AID-AJPA3>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- Bronson FH. Mammalian reproductive biology. The University of Chicago Press; Chicago: 1989. [Google Scholar]
- Bronson FH. Seasonal variation in human reproduction: environmental factors. Q Rev Biol. 1995;70:141–164. doi: 10.1086/418980. [DOI] [PubMed] [Google Scholar]
- Cameron JL. Nutritional determinants of puberty. Nutr Rev. 1996;54:S17–S22. doi: 10.1111/j.1753-4887.1996.tb03866.x. [DOI] [PubMed] [Google Scholar]
- Cameron JL, Helmreich DL, Schreihofer DA. Modulation of reproductive hormone secretion by nutritional intake: stress signals versus metabolic signals. Hum Reprod Suppl. 1993;2:162–167. doi: 10.1093/humrep/8.suppl_2.162. [DOI] [PubMed] [Google Scholar]
- Collu R, Gibb W, Ducharme JR. Effects of stress on the gonadal function. J Endocrinol Invest. 1984;7:529–537. doi: 10.1007/BF03348463. [DOI] [PubMed] [Google Scholar]
- Dabbs JM. Age and seasonal variation in serum testosterone concentration among men. Chronobiol Int. 1990;7:245–249. doi: 10.3109/07420529009056982. [DOI] [PubMed] [Google Scholar]
- Dunbar RIM, Dunbar P. Social dynamics of gelada baboons. Contrib Primatol. 1975;6:1–157. [PubMed] [Google Scholar]
- Ellison PT, Panter-Brick C. Salivary testosterone levels among Tamang and Kami males of central Nepal. Hum Biol. 1996;68:955–965. [PubMed] [Google Scholar]
- Ellison PT, Peacock NR, Lager C. Salivary progesterone and luteal function in two low-fertility populations of northeast Zaire. Hum Biol. 1986;58:473–483. [PubMed] [Google Scholar]
- Ellison PT, Valeggia CR, Sherry DS. Human birth seasonality. In: Brockman DK, van Schaik CP, editors. Seasonality in primates: studies of living and extinct human and non-human primates. Cambridge University Press; Cambridge; New York: 2005. pp. 379–399. [Google Scholar]
- Emlen ST, Oring LW. Ecology, sexual selection, and the evolution of mating systems. Science. 1977;197:215–223. doi: 10.1126/science.327542. [DOI] [PubMed] [Google Scholar]
- Falk B. Effects of thermal stress during rest and exercise in the paediatric population. Sports Med. 1998;25:221–240. doi: 10.2165/00007256-199825040-00002. [DOI] [PubMed] [Google Scholar]
- Fedigan LM, Rose LM. Interbirth interval variation in three sympatric species of neotropical monkey. Am J Primatol. 1995;37:9–24. doi: 10.1002/ajp.1350370103. [DOI] [PubMed] [Google Scholar]
- Frye AJ, Kamon E. Responses to dry heat of men and women with similar aerobic capacities. J Appl Physiol. 1981;50:65–70. doi: 10.1152/jappl.1981.50.1.65. [DOI] [PubMed] [Google Scholar]
- Funkhouser GE, Higgins EA, Adams T, Snow CC. The response of the savannah baboon (Papio cynocephalus) to thermal stress. Life Sci. 1967;6:1615–1620. doi: 10.1016/0024-3205(67)90171-3. [DOI] [PubMed] [Google Scholar]
- Garrel DR, Todd KS, Pugeat MM, Calloway DH. Hormonal changes in normal men under marginally negative energy balance. Am J Clin Nutr. 1984;39:930–936. doi: 10.1093/ajcn/39.6.930. [DOI] [PubMed] [Google Scholar]
- Gesquiere LR, Altmann J, Khan MZ, Couret J, Yu JC, Endres CS, Lynch JW, Ogola P, Fox EA, Alberts SC, Wango EO. Coming of age: steroid hormones of wild immature baboons (Papio cynocephalus) Am J Primatol. 2005;67:83–100. doi: 10.1002/ajp.20171. [DOI] [PubMed] [Google Scholar]
- Gesquiere LR, Khan M, Shek L, Wango TL, Wango EO, Alberts SC, Altmann J. Coping with a challenging environment: effects of seasonal variability and reproductive status on glucocorticoid concentrations of female baboons (Papio cynocephalus) Horm Behav. 2008;54:410–416. doi: 10.1016/j.yhbeh.2008.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gesquiere LR, Wango EO, Alberts SC, Altmann J. Mechanisms of sexual selection: sexual swellings and estrogen concentrations as fertility indicators and cues for male consort decisions in wild baboons. Horm Behav. 2007;51:114–125. doi: 10.1016/j.yhbeh.2006.08.010. [DOI] [PubMed] [Google Scholar]
- Goodall J. Population dymamics during a 15 year period in one community of free-living chimpanzees in the Gombe National Park, Tanzania. Z Tierpsychol. 1983;61:1–60. [Google Scholar]
- Gould L, Ziegler TE. Variation in fecal testosterone levels, inter-male aggression, dominance rank and age during mating and post-mating periods in wild adult male ring-tailed Lemurs (Lemur catta) Am J Primatol. 2007;69:1325–1339. doi: 10.1002/ajp.20438. [DOI] [PubMed] [Google Scholar]
- Gwazdauskas FC. Effects of climate on reproduction in cattle. J Dairy Sci. 1985;68:1568–1578. doi: 10.3168/jds.S0022-0302(85)80995-4. [DOI] [PubMed] [Google Scholar]
- Hansen PJ. Effects of heat stress on mammalian reproduction. Phil Trans R Soc B. 2009;364:3341–3350. doi: 10.1098/rstb.2009.0131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hau M. Timing of breeding in variable environments: tropical birds as model systems. Horm Behav. 2001;40:281–290. doi: 10.1006/hbeh.2001.1673. [DOI] [PubMed] [Google Scholar]
- Hazelhurst LT, Claassen N. Gender differences in the sweat response during spinning exercise. J Strength Cond Res. 2006;20:723–724. doi: 10.1519/18155.1. [DOI] [PubMed] [Google Scholar]
- Hill RA, Lycett JE, Dunbar RIM. Ecological and social determinants of birth intervals in baboons. Behav Ecol. 2000;11:560–564. [Google Scholar]
- Hill RA, Weingrill T, Barrett L, Henzi SP. Indices of environmental temperatures for primates in open habitats. Primates. 2004;45:7–13. doi: 10.1007/s10329-003-0054-8. [DOI] [PubMed] [Google Scholar]
- Huang WJ, Yeh J-Y, Tsai S-C, Lin H, Chiao Y-C, Chen J-J, Lu C-C, Hwang S-W, Wang S-W, Chang LS, Wang PS. Regulation of testosterone secretion by prolactin in male rats. J Cell Biochem. 1999;74:111–118. [PubMed] [Google Scholar]
- Janson C, Verdolin J. Seasonality of primate births in relation to climate. In: Brockman DK, van Schaik CP, editors. Seasonality in primates: studies of living and extinct human and non-human primates. Cambridge University Press; Cambridge; New York: 2005. pp. 307–350. [Google Scholar]
- Johnson HD, Vanjonack WJ. Effects of environmental and other stressors on blood hormone patterns in lactating animals. J Dairy Sci. 1975;59:1603–1617. doi: 10.3168/jds.S0022-0302(76)84413-X. [DOI] [PubMed] [Google Scholar]
- Kandeel FR, Swerdloff RS. Role of temperature in regulation of spermatogenesis and the use of heating as a method for contraception. Fertil Steril. 1988;49:1–23. doi: 10.1016/s0015-0282(16)59640-x. [DOI] [PubMed] [Google Scholar]
- Khan MZ, Altmann J, Isani SS, Yu J. A matter of time: evaluating the storage of fecal samples for steroid analysis. Gen Comp Endocrinol. 2002;128:57–64. doi: 10.1016/s0016-6480(02)00063-1. [DOI] [PubMed] [Google Scholar]
- Knott CD. Energetic responses to food availability in the great apes: implications for hominid evolution. In: Brockman DK, van Schaik CP, editors. Seasonality in primates: studies of living and extinct human and non-human primates. Cambridge University Press; Cambridge; New York: 2005. pp. 351–378. [Google Scholar]
- Koenig A, Borries C, Chalise MK, Winkler P. Ecology, nutrition, and timing of reproductive events in an Asian primate, the Hanuman langur (Presbytis entellus) J Zool. 1997;243:215–235. [Google Scholar]
- Krulich L, Hefco E, Illner P, read CB. Effects of acute stress on secretion of LH, FSH, prolactin and GH in normal male rat, with comments on their statistical evaluation. Neuroendocrinology. 1974;16:293–311. doi: 10.1159/000122576. [DOI] [PubMed] [Google Scholar]
- Larsson K, Einarsson S, Lundstrom K, Hakkarainen J. Endocrine effects of heat stress in boars. Acta Vet Scand. 1983;24:305–314. doi: 10.1186/BF03546734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu YX. Control of spermatogenesis in primate and prospect of male contraception. Arch Androl. 2005;51:77–92. doi: 10.1080/01485010490485768. [DOI] [PubMed] [Google Scholar]
- Lynch JW, Khan MZ, Altmann J, Njahira MN, Rubenstein N. Concentrations of four fecal steroids in wild baboons: short-term storage conditions and consequences for data interpretation. Gen Comp Endocrinol. 2003;132:264–271. doi: 10.1016/s0016-6480(03)00093-5. [DOI] [PubMed] [Google Scholar]
- Lynch JW, Ziegler TE, Strier KB. Individual and seasonal variation in fecal testosterone and cortisol levels of wild male tufted capuchin monkeys, Cebus apella nigritus. Horm Behav. 2002;41:275–287. doi: 10.1006/hbeh.2002.1772. [DOI] [PubMed] [Google Scholar]
- Magal E, Kaplanski J, Sodmoriah UA, Hirschmann N, Nir I. Role of the pineal gland in male rats chronically exposed to increased temperature. J Neural Transm. 1981;50:267–273. doi: 10.1007/BF01249147. [DOI] [PubMed] [Google Scholar]
- Marai IFM, Habeeb AAM, Gad AE. Rabbits’ productive, reproductive and physiological performance traits as affected by heat stress: a review. Livest Prod Sci. 2002;78:71–90. [Google Scholar]
- Mehnert P, Brode P, Griefahn B. Gender-related difference in sweat loss and its impact on exposure limits to heat stress. Int J Ind Ergonom. 2002;29:343–351. [Google Scholar]
- Menard N, D Vallet. Population dynamics of Macaca sylvanus in Algeria: an 8-year study. Am J Primatol. 1993;30:101–118. doi: 10.1002/ajp.1350300203. [DOI] [PubMed] [Google Scholar]
- Minter LJ, DeLiberto TJ. Seasonal variation in serum testosterone, testicular volume, and semen characteristics in the coyote (Canis latrans) Theriogenology. 2008;69:946–952. doi: 10.1016/j.theriogenology.2008.01.010. [DOI] [PubMed] [Google Scholar]
- Minton JE, Wettemann RP, Meyerhoeffer DC, Hintz RL, Turman EJ. Serum luteinizing hormone and testosterone in bulls during exposure to elevated ambient temperature. J Anim Sci. 1981;53:1551–1558. doi: 10.2527/jas1982.5361551x. [DOI] [PubMed] [Google Scholar]
- Muller MN, Wrangham RW. Dominance, cortisol and stress in wild chimpanzees (Pan troglodytes schweinfurthii) Behav Ecol Sociobiol. 2004;55:332–340. doi: 10.1007/s00265-020-02872-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohsawa H, Dunbar RIM. Variations in the demographic structure and dynamics of gelada baboon populations. Behav Ecol Sociobio. 1984;15:231–240. [Google Scholar]
- Oi T. Sexual behavior and mating system of the wild pig-tailed macaque in West Sumatra. In: Fa JE, Lindburg DG, editors. Evolution and ecology of macaque societies. Cambridge University Press; Cambridge; New York: 1996. pp. 342–368. [Google Scholar]
- Post DG. Activity patterns of yellow baboons (Papio cynocephalus) in the Amboseli National Park, Kenya. Anim Behav. 1981;29:357–374. [Google Scholar]
- Riley AP. Determinants of adolescent fertility and its consequences for maternal health, with special reference to rural Bangladesh. In: Campbell KL, Wood JW, editors. Human reproductive ecology - interactions of environment, fertility, and behavior. New York Academy of Sciences; New York: 1994. pp. 86–100. [DOI] [PubMed] [Google Scholar]
- Ronchi B, Stradaioli G, Verini-Supplizi A, Bernabucci U, Lacetera N, Accorsi PA, Nardone A, Seren E. Influence of heat stress or feed restriction on plasma progesterone, oestradiol-17 beta, LH, FSH, prolactin and cortisol in Holstein heifers. Livest Prod Sci. 2001;68:231–241. [Google Scholar]
- Sapolsky RM. Endocrine and behavioral correlates of drought in wild olive baboons (Papio anubis) Am J Primatol. 1986;11:217–227. doi: 10.1002/ajp.1350110303. [DOI] [PubMed] [Google Scholar]
- Sapolsky RM, Altmann J. Incidence of hypercortisolism and dexamethasone resistance increases with age among wild baboons. Biol Psychiat. 1991;30:1008–1016. doi: 10.1016/0006-3223(91)90121-2. [DOI] [PubMed] [Google Scholar]
- Van Schaik CP, van Noordwijk MA. Interannual variability in fruit abundance and the reproductive seasonality in Sumatran long-tailed macaques (Macaca fascicularis) J Zool. 1985;206:533–549. [Google Scholar]
- Schneider JE. Energy balance and reproduction. Physiol Behav. 2004;81:289–317. doi: 10.1016/j.physbeh.2004.02.007. [DOI] [PubMed] [Google Scholar]
- Schwalm A, Gauly M, Erhardt G, Bergmann M. Changes in testicular histology and sperm quality in llamas (Lama glama), following exposure to high ambient temperature. Theriogenology. 2007;67:1316–1323. doi: 10.1016/j.theriogenology.2007.02.005. [DOI] [PubMed] [Google Scholar]
- Smith SR, Chhetri MK, Johanson AJ, Radfar N, Migeon CJ. The pituitary-gonadal axis in men with protein-calorie malnutrition. J Clin Endocrinol Metab. 1975;41:60–69. doi: 10.1210/jcem-41-1-60. [DOI] [PubMed] [Google Scholar]
- Strier KB, Mendes SL, Santo RR. Timing of births in sympatric howler monkeys (Alouatta fusca clamitans) and northern muriquis (Brachyteles arachnoides hypoxanthus) Am J Primatol. 2001;55:87–100. doi: 10.1002/ajp.1042. [DOI] [PubMed] [Google Scholar]
- Vandenbergh JG, Drickamer LC. Reproductive coordination among free-ranging rhesus monkeys. Physiol Behav. 1974;13:373–376. doi: 10.1016/0031-9384(74)90090-0. [DOI] [PubMed] [Google Scholar]
- Wahab F, Aziz F, Irfan S, Zaman WU, Shahab M. Short-term fasting attenuates the response of the HPG axis to kisspeptin challenge in the adult male rhesus monkey (Macaca mulatta) Life Sci. 2008;83:633–637. doi: 10.1016/j.lfs.2008.09.001. [DOI] [PubMed] [Google Scholar]
- Wasser SK, Hunt KE, Brown JL, Cooper K, Crockett CM, Bechert U, Millspaugh JJ, Larson S, Monfort SL. A generalized fecal glucocorticoid assay for use in a diverse array of nondomestic mammalian and avian species. Gen Comp Endocrinol. 2000;120:260–275. doi: 10.1006/gcen.2000.7557. [DOI] [PubMed] [Google Scholar]
- Watts DP. Seasonality in the ecology and life histories of mountain gorillas (Gorilla gorilla beringei) Int J Primatol. 1998;19:929–948. [Google Scholar]
- Weingrill T, Gray DA, Barrett L, Henzi SP. Fecal cortisol levels in free-ranging female chacma baboons: relationship to dominance, reproductive state and environmental factors. Horm Behav. 2004;45:259–269. doi: 10.1016/j.yhbeh.2003.12.004. [DOI] [PubMed] [Google Scholar]
- Whitten PL, Turner TL. Endocrine mechanisms of primate life history trade-offs: growth and reproductive maturation in vervet monkeys. Am J Hum Biol. 2009;21:754–761. doi: 10.1002/ajhb.20939. [DOI] [PubMed] [Google Scholar]
- Wingfield JC, Hegner RE, Dufty AM, Jr, Ball GF. The “Challenge Hypothesis”: theoretical implications for patterns of testosterone secretion, mating systems and breeding strategies. Am Nat. 1990;136:829–846. [Google Scholar]
- Worthman CM, Jenkins CL, Stallings JF, Lai DN. Attenuation of nursing-related ovarian suppression and high fertility in well-nourished, intensively breast-feeding Amele women of lowland Papua New Guinea. J Biosoc Sci. 1993;25:425–443. doi: 10.1017/s0021932000021817. [DOI] [PubMed] [Google Scholar]