Abstract
Background
Oral deferiprone was suggested to be more effective than subcutaneous desferrioxamine for removing heart iron. Oral once-daily chelator deferasirox has recently been made commercially available but its long-term efficacy on cardiac iron and function has not yet been established. Our study aimed to compare the effectiveness of deferasirox, deferiprone and desferrioxamine on myocardial and liver iron concentrations and bi-ventricular function in thalassemia major patients by means of quantitative magnetic resonance imaging.
Design and Methods
From the first 550 thalassemia subjects enrolled in the Myocardial Iron Overload in Thalassemia network, we retrospectively selected thalassemia major patients who had been receiving one chelator alone for longer than one year. We identified three groups of patients: 24 treated with deferasirox, 42 treated with deferiprone and 89 treated with desferrioxamine. Myocardial iron concentrations were measured by T2* multislice multiecho technique. Biventricular function parameters were quantitatively evaluated by cine images. Liver iron concentrations were measured by T2* multiecho technique.
Results
The global heart T2* value was significantly higher in the deferiprone (34±11ms) than in the deferasirox (21±12 ms) and the desferrioxamine groups (27±11 ms) (P=0.0001). We found higher left ventricular ejection fractions in the deferiprone and the desferrioxamine versus the deferasirox group (P=0.010). Liver iron concentration, measured as T2* signal, was significantly lower in the desferrioxamine versus the deferiprone and the deferasirox group (P=0.004).
Conclusions
The cohort of patients treated with oral deferiprone showed less myocardial iron burden and better global systolic ventricular function compared to the patients treated with oral deferasirox or subcutaneous desferrioxamine.
Keywords: thalassemia, iron chelation therapy, cardiac magnetic resonance imaging
Introduction
Heart failure mainly related to iron-induced toxicity remains the leading cause of morbidity and mortality in thalassemia major (TM) patients, although life-expectancy in this population has improved in the last years.1,2 Therefore, chelation strategies to prevent heart disease should have the highest priority.2
The oral chelating agent deferiprone was proved to be more effective than subcutaneous desferrioxamine for removing heart iron, measured by T2* MRI,3–5 and in improving survival.2,6–9 Also the novel oral once-daily iron chelator deferasirox, which should be able to improve compliance, is now commercially available. To date, no prospective randomized studies have been published on the cardiac efficacy of deferasirox. Some recent reports have suggested that deferasirox may be effective in increasing heart T2* signal in thalassemia major patients.10–12 However, these studies did not compare all three chelators (deferasirox, deferiprone and desferrioxamine) together. Conversely, a recently published observational study comparing four chelator regimens did not show a statistically significant improvement in cardiac iron load in thalassemia major patients treated with deferasirox,13 although this study suffered from a variable time period between MRI scans, and it did not take into account compliance or chelator dosage.
Magnetic resonance imaging (MRI) by the T2* technique allows highly reproducible and non-invasive quantifications of myocardial14–16 and liver 14,17,18 iron burden in the clinical arena, and has been contributing to improved survival in thalassemia patients.19 Moreover, MRI is the gold standard for quantifying biventricular function parameters.20
Therefore, the aim of our study was to compare retrospectively in a large cohort of patients with thalassemia major the effectiveness of the three available iron chelators (deferasirox, deferiprone and desferrioxamine) on myocardial and liver iron concentrations and on biventricular function measured by quantitative MRI.
Design and Methods
Study population
The MIOT (Myocardial Iron Overload in Thalassemia) project is a network involving six Italian Cardiac Magnetic Resonance (CMR) sites and 57 Italian thalassemia centers where CMR procedure is performed using homogeneous, standard and validated procedures.21 All centers are linked by a web-based network, configured to collect and share patients’ data.22 From the 550 thalassemia patients enrolled in the MIOT network, we retrospectively selected the 115 thalassemia major patients (73 males, age 9–56 years, mean age 31±9 years) receiving one chelator alone for longer than one year. All patients had been regularly transfused since early childhood, starting chelation treatment with desferrioxamine in the mid to late 1970s.
We identified three groups of patients: 24 patients treated with deferasirox, 42 patients treated with deferiprone and 89 patients treated with desferrioxamine. The mean administered dosages of the three chelators were: 1) deferasirox 26±6.3 mg/kg body weight per day (range 18–40 mg/kg body weight per day); 2) deferiprone 72±10 mg/kg body weight (range 50–86 mg/kg body weight per day), divided into three doses per day; 3) desferrioxamine 30±9 mg/kg body weight/infusion via subcutaneous route on 3–7 days per week (range 24–52 mg/kg body weight).
The study complied with the Declaration of Helsinki. All patients gave written informed consent to the protocol. The institutional review board approved this study.
Magnetic resonance imaging
At all six sites, CMR exams were performed by a 1.5 T scanner (GE Signa/Excite HD, Milwaukee, WI, USA) using previously reported cardiac-gated techniques.4,15,23
In brief, for the MIO measurements, a multislice multiecho T2* approach was used. Three parallel short-axis views (basal, medium and apical) of the left ventricle (LV) were acquired at nine increasing echo times (TE) (2.2–20.3 ms) in a single end-expiratory breath-hold. The transferability of multislice multiecho T2* within the MIOT network has been previously validated.21 For the measurement of liver iron overload, a T2* single breath-hold, 9-echo sequence (TEs 2.0–21 ms) of a transaxial slice was acquired.17
T2* image analysis was performed using a custom-written, previously validated software program (HIPPO MIOT®, IFC-CNR). The software was able to map the myocardial T2* distribution into a 16-segment LV model according to the American Heart Association (AHA)/American College of Cardiology (ACC) standardized myocardial segmentation.24 The intra-observer, inter-observer and inter-study variability of the proposed methodology had been previously assessed.15 The global T2* value was obtained by averaging segmental T2* values, and the T2* value in the mid-ventricular segment was obtained by averaging segmental T2* values in the mid-anterior septum and the mid-inferior septum. As previously shown,23,25 the developed procedure was able to correct for cardiac/visceral geometrical and susceptibility artefacts. T2* greater than 20 ms was considered as a conservative normal value for all 16 segments, for the mid-ventricular septum and for the entire heart.14,23
For the liver, the decay curve was extracted from a large region of interest of standard dimensions, chosen from a homogeneous area of liver parenchyma without blood vessels, and the value of T2* was calculated by fitting the decay curve model with a single exponential with a constant offset.16,17 We expressed the liver iron concentration (LIC) also in mg/g dry weight. We used the conversion from T2* to LIC, by using the formula, 0.202+25.4/T2* adapted from Wood et al.26
Steady-state free procession cine images were acquired during 8-second breath-holds in sequential 8-mm short-axis slices from the atrio-ventricular ring to the apex to assess biventricular function parameters quantitatively in a standard way,20 using MASS® software (Medis, Leiden, The Netherlands).
Statistical analysis
All data were analyzed using the SPSS version 12.0 statistical package. Summary data are presented as mean ± standard deviation. Comparisons among groups were made by one-way ANOVA for continuous values with normal distribution and Kruskal-Wallis test for continuous values with non-normal distribution (i.e. T2* data). Multiple comparisons were made by t test or Mann-Whitney test and P values were modified according to the Bonferroni correction. The χ2 test was used for non-continuous variables. Spearman’s test was used for correlation analysis. A two-tailed probability value of 0.05 was considered statistically significant.
Results
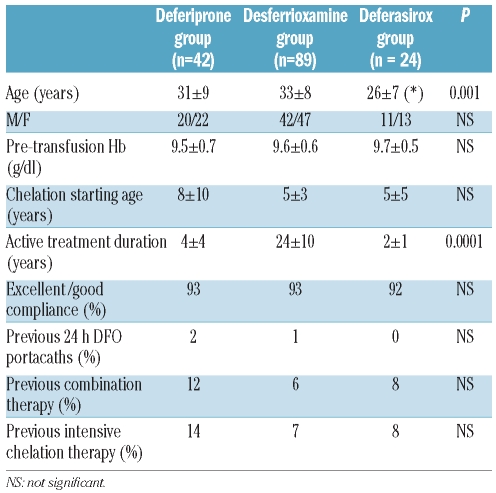
There was no difference between the three groups in gender, pre-transfusional Hb levels, age at the first chelation, compliance to the active chelation treatment or previous intensive chelation therapies (24 h infusions of desferrioxamine through portacaths and/or combination therapy with desferrioxamine and deferiprone). The deferasirox group was significantly younger than the deferiprone and the desferrioxamine groups (P=0.0001). Desferrioxamine treatment had been started significantly earlier (24±10 years, range 1–47 years) than the deferiprone treatment (4±4 years, range 1–18 years; P<0.0001) and the deferasirox treatment (2±1 years, range 1–5 years; P<0.0001). The basal mean serum ferritin levels in the 12 months before starting the active treatment at the time of MRI were significantly higher in the deferasirox group versus the desferrioxamine group (2,491±2,072 vs. 957±1,073; P=0.0003) while they were comparable with the deferiprone group (1,665±2,028; P=0.1); there was no significant difference in basal mean serum ferritin levels between the deferiprone group and the desferrioxamine group (P=0.1). The majority of the study patients were naïve to T2* MRI (85%). The 17 patients with a previous T2* MRI scan were homogenously distributed in the 3 groups (deferiprone 6 of 42 patients, desferrioxamine 7 of 89 patients, deferasirox 4 of 24 patients; P=NS). Out of the 17 patients with a previous T2* MRI, only 2 patients changed the chelation therapy between the two MRI scans. The 2 patients were switched from desferrioxamine to deferiprone due to an unsatisfactory compliance to the subcutaneous desferrioxamine administration. However, in both patients the study MRI and the previous MRI showed normal global heart T2* values. Baseline clinical findings for the three groups are reported in Table 1.
Table 1.
Clinically relevant findings in the three groups of chelation treatment.
Global and mid-ventricular septum heart T2* values were significantly higher in the deferiprone-group than the deferasirox and the desferrioxamine groups (P=0.0001) (Figure 1). Moreover, the number of segments with normal T2* values was significantly higher in the deferiprone and the desferrioxamine groups than in the deferasirox group (P=0.0001).
Figure 1.
Global heart T2* and the T2* in the mid-ventricular septum in the groups treated with deferiprone, desferrioxamine and deferasirox. There were significant differences among groups (P=0.0001). Post hoc analysis showed significantly higher global heart T2* and T2* in the mid-ventricular septum in the group treated with deferiprone versus the group treated with desferrioxamine and deferasirox. Each box shows the median, quartiles, and extreme values within the category.
We did not find a significant correlation between the log global heart T2* and the iron chelator dosage (deferiprone r=−0.13 P=0.45; desferrioxamine r=−0.19 P=0.09; deferasirox r=−0.098 P=0.762). The correlation between the log global heart T2* and the LIC (mg/g dw) was not significant in the deferiprone and deferasirox groups (r=0.279 P=0.07, r=−0.33 P=0.11, respectively). We found a mild and significant correlation between the log global heart T2* and the LIC (mg/g dw) in the desferrioxamine group (r=−0.354 P=0.001).
The deferiprone group showed significantly higher left ventricular ejection fraction than the desferrioxamine and deferasirox groups (P=0.010) (Figure 2). Similarly, the right ventricular ejection fraction was higher in the deferiprone group than in the desferrioxamine and deferasirox groups (P=0.042). Although none of the patients suffered from decompensated heart failure (HF) at the time of the scan, 5 patients showed the left ventricular ejection fraction (LVEF) less than 50% (3 patients treated with desferrioxamine and 2 patients treated with deferasirox), 5 patients treated with desferrioxamine showed the right ventricular ejection fraction (RVEF) less than 45%, and one patient treated with desferrioxamine showed the LVEF less than 50% and the RVEF less than 45%. There was no statistically significant difference in left and right end-diastolic volume indices and in left ventricular mass index between groups.
Figure 2.
Left ventricular ejection fraction in the groups treated with deferiprone, desferrioxamine and deferasirox. There were significant differences among groups (P=0.010). Post hoc analysis showed a significantly higher left ventricular ejection fraction in the group treated with deferiprone versus the group treated with deferasirox. Each box shows the median, quartiles, and extreme values within the category.
Liver iron concentrations (mg/g dw) were significantly lower in the desferrioxamine group than in the deferiprone and deferasirox groups (P=0.004) (Figure 3).
Figure 3.
Estimated MRI LIC (mg/g dw) in the groups treated with deferiprone, desferrioxamine and deferasirox. There were significant differences among groups (P=0.004). Post hoc analysis showed a significantly lower LIC in the group treated with desferrioxamine versus the group treated with deferiprone and the group treated with deferasirox. Each box shows the median, quartiles, and extreme values within the category.
Mean serum ferritin levels in the last year of the active treatment were significantly higher in the deferasirox-group (2,516±2,106 ng/mL) versus the desferrioxamine-group (987±915 ng/mL) (P=0.001) while they were comparable with the deferiprone group (1,493±1,651 ng/ml) (P=NS) (Figure 4). Comparisons between heart and liver iron and biventricular function parameters in the different groups by MRI are reported in Table 2.
Figure 4.
Mean serum ferritin in the groups treated with deferiprone, desferrioxamine and deferasirox. There were significant differences among groups (P=0.002). Post hoc analysis showed a significantly higher mean ferritin value in the group treated with deferasirox versus the group treated with desferrioxamine. Each box shows the median, quartiles, and extreme values within the category.
Table 2.
Comparison of MRI data (heart and liver iron, and biventricular function parameters) among the three different groups of chelation treatment.
Discussion
The availability of three iron chelators allows physicians to tailor the chelation regimens to the needs of different patients. Therefore, it is important to establish whether these drugs have a different effect on different organs. Although surrogate markers such as serum ferritin levels and liver iron concentration have been shown to be prognostic indicators in most of the thalassemia patients, they do not always correlate with the cardiac iron burden and function.13,4,25 MRI, a technique that provides highly reproducible measurements of myocardial14–16 and liver iron burden,17,18 and biventricular function parameters,20 is ideally suited for comparing different chelation treatments in thalassemia major patients.3–5 In particular, the method used in this study to quantify the myocardial iron burden assessed not only the T2* in the mid-ventricular septum, but also the global heart iron and the number of segments with normal T2* value. This assessment is not usually referred to in studies on thalassemia patients and it can be advantageous in that the distribution of iron in the heart is heterogeneous.15,27
Our large multicenter observational study showed that thalassemia major patients treated with deferiprone alone had significantly less global and segmental myocardial iron burden and significantly better global systolic ventricular function than patients treated with desferrioxamine and deferasirox alone for a minimum of 12 months at comparable levels of compliance (Figures 1 and 2). Moreover, none of the patients treated with deferiprone showed LVEF less than 50% and/or RVEF less than 45% that can be considered indicative of heart failure in thalassemia major patients.28 Conversely, the desferrioxamine group showed less liver iron burden than the patients treated with the other two chelators (Figure 3).
There was no significant difference in age at which chelation was started and the exposure to intensive chelation therapy among groups. All three groups showed high and comparable levels of compliance. In our outpatients population, compliance data were collected by the investigators of each thalassemia center and seem to be concordant with the levels of liver and cardiac iron burden. This datum could be justified by the organization of the national health system, by the free availability of MRI, and by the support of the voluntary associations and of the family. As expected, in our study population, desferrioxamine, which has been on the market for the past thirty years, had been started significantly earlier than the deferiprone and the deferasirox treatments.
Deferiprone and deferasirox groups showed comparable basal mean serum ferritin levels. Thus, we cannot attribute the greater efficacy of deferiprone in removing/preventing cardiac iron to a lower baseline iron burden.
Previous knowledge of cardiac iron burden seems not to have introduced any bias in our study population. In fact, a high percentage of the patients (85%) were naïve to T2* MRI and patients with a previous T2* MRI were homogenously distributed across the three groups. Moreover, of the patients with a previous T2* MRI, only 2 patients had changed the chelation therapy between the two MRI scans and these 2 patients had normal global heart T2* values in both scans; the chelation therapy had been changed due to patient compliance.
Previous retrospective3,4 and prospective randomized clinical trials5 have shown deferiprone to be more effective than subcutaneous desferrioxamine in preventing and removing myocardial iron burden and improving survival.2,6–9
In one recently published preliminary prospective study,10 deferasirox when given at a mean dosage of 32.6mg/kg/day for one year in thalassemia major patients with cardiac iron and normal/borderline left ventricular ejection fraction, led to negative cardiac and liver iron balance and maintained ejection fraction. One observational prospective, open-label, single-arm study11 suggested that deferasirox monotherapy, administered at the dose of 30–40 mg/kg/day for 18 months, maintained unchanged the ejection fraction in patients with normal/borderline left ventricular ejection fraction and was effective in patients with mild-to-moderate iron stores in removing cardiac iron; however, this monotherapy failed in patients with severe hepatic iron burdens. Another single-arm observational study showed significant improvement in cardiac T2* in heavily iron loaded thalassemia major patients receiving iron deferasirox over an 18-month period.12 All these studies were limited by the short period of treatment evaluation.
On the contrary, a recently published non-prospective and non-randomized observational study from Greece showed no statistically significant improvement in cardiac iron load in thalassemia major patients on deferasirox at any level of cardiac iron loading.13
In the three above mentioned prospective studies,10–12 as opposed to the Greek13 and to our study, deferasirox was given at higher doses (30 mg/kg/day with the possibility of increasing the dose to over 40 mg), possibly explaining the difference in results obtained. Moreover, out of the 24 patients in the deferasirox group, 13 patients had participated in clinical trials in the 12 months before the MRI. In this subgroup, the mean dosage of the deferasirox in the last 12 months before the scan was 30±7 mg/kg/day (range 20–40 mg/kg/day) and we cannot exclude a previous underdosing. Anyway, in our study population, we did not find significant correlation between the global heart T2* and the drug dosage of the three iron chelators.
With respect to the liver, in contrast to the lower myocardial iron overload in the deferiprone group, the desferrioxamine group showed significantly higher liver iron T2* values than the other two groups, and significantly lower mean serum ferritin levels in the last year of treatment than the deferasirox group. The significantly lower mean ferritin levels and LIC in the desferrioxamine group versus the deferasirox group could be influenced by the significantly lower basal serum ferritin levels in the desferrioxamine group versus the deferasirox group. The ability of desferrioxamine to significantly reduce body iron burden has been repeatedly demonstrated.29 However, in all the series reported, 65% of the patients treated with desferrioxamine alone were found to have a T2* in the mid-ventricular septum less than 20 ms and, therefore, less than an optimal target.30 These findings confirm a possible important difference in drug action: deferiprone seems to be a better agent for removing/preventing myocardial siderosis, but less effective in removing hepatocellular iron, while the opposite appears to be true for desferrioxamine.4,5,30 The data available in literature for the deferasirox seem to confirm a good action on the liver, but the data regarding the heart are so far preliminary and contradictory.
The limits of our study were that it was retrospective and non-randomized. Several biases could be present. First, the duration of the treatment was significantly shorter for deferasirox versus desferrioxamine and this drug could be effective on the heart only after a longer time of treatment, considering that cardiac iron removal is a slow process with a clearance rate of around 1.5% per month.11 Second, even though there was no significant difference in the low percentage of patients who had had previous combination therapy among the three groups, the higher percentage in the deferiprone group could have influenced the results. Third, the choice of a particular regime was based on clinical grounds and on evidence-based medicine. However, the choice based on evidence-based medicine would have avoided the preferential prescription of deferasirox in the patients with greater iron load and at high risk for cardiac complications. In fact, at the time of the patients’ enrolment, deferiprone had proved to be more effective than subcutaneous desferrioxamine for removing heart iron and in improving survival.5,6 Thus, deferasirox was prescribed for contradictions or non-optimal compliance to the other chelators, or in patients preferring a more friendly oral and once-daily chelator. Moreover, 13 patients started deferasirox because they were enrolled in clinical trials and the patients with a poor response or non-compliance with previously prescribed therapy were excluded.31
In conclusion, in patients treated for a minimum of 12 months at comparable levels of compliance, oral deferiprone showed less myocardial iron burden and better left global systolic ventricular function compared to the patients treated with oral deferasirox or subcutaneous desferrioxamine, suggesting greater efficacy in removing or preventing cardiac iron, with a concordant positive effect on heart function. Conversely, desferrioxamine appeared to be more effective in removing or preventing iron deposition in the liver. However, well designed, prospective and randomized comparative clinical trials are clearly necessary, as difficult as they may be to manage, in order to prescribe the most appropriate chelator (or a combination thereof) for the individual thalassemia major patient.
Appendix
We thank Dr. Cristina Salvatori (“G. Monasterio” Foundation and CNR Institute of Clinical Physiology, Pisa) for database development and management, Claudia Santarlasci (“G. Monasterio” Foundation and CNR Institute of Clinical Physiology, Pisa) for her skillful secretarial work and Alison Frank for her assistance in editing this manuscript. We would like to thank the following colleagues from the Italian thalassemia centers involved in the MIOT network for having enrolled the patients and collected the clinical data: V. De Sanctis (St Anna Hospital, Ferrara); C. Gerardi (“Ospedali Civili Riuniti” Sciacca Agrigento); A. Pietrangelo (University Hospital of Modena and Reggio Emilia, Modena); S. Grimaldi (Presidio Ospedaliero ASL 5, Crotone); G. Roccamo [Ospedale “Civile”, S. Agata di Militello (ME)]; A. Meo (Policlinico G. Martino, Messina); M. Rizzo (Ospedale “Sant’ Elia”, Caltanisetta); D. Maddaloni (Ospedale Engles Profili, Ancona). We also thank the following colleagues from the Italian MRI centers involved in the MIOT network for having acquired and collected MRI data: L. Natale (Policlinico Gemelli, Roma); G. Valeri (Ospedali Riuniti Umberto I-Lancisi-Salesi, Ancona). We also thank all patients for their cooperation.
Footnotes
Funding: The MIOT project received “no-profit support” from Chiesi, Bayer-Schering and GE Healthcare. It was also supported by the Italian Foundation “Leonardo Giambrone” and was undertaken on behalf of the Italian Society for Thalassemias and Hemoglobinopathies (SITE).
Authorship and Disclosures
The information provided by the authors about contributions from persons listed as authors and in acknowledgments is available with the full text of this paper at www.haematologica.org.
Financial and other disclosures provided by the authors using the ICMJE (www.icmje.org) Uniform Format for Disclosure of Competing Interests are also available at www.haematologica.org.
References
- 1.Borgna-Pignatti C, Rugolotto S, De Stefano P, Zhao H, Cappellini MD, Del Vecchio GC, et al. Survival and complications in patients with thalassemia major treated with transfusion and deferoxamine. Haematologica. 2004;89(10):1187–93. [PubMed] [Google Scholar]
- 2.Maggio A, Vitrano A, Capra M, Cuccia L, Gagliardotto F, Filosa A, et al. Improving survival with deferiprone treatment in patients with thalassemia major: a prospective multicenter randomised clinical trial under the auspices of the Italian Society for Thalassemia and Hemoglobinopathies. Blood Cells Mol Dis. 2009;42(3):247–51. doi: 10.1016/j.bcmd.2009.01.002. [DOI] [PubMed] [Google Scholar]
- 3.Anderson LJ, Wonke B, Prescott E, Holden S, Walker JM, Pennell DJ. Comparison of effects of oral deferiprone and subcutaneous desferrioxamine on myocardial iron concentrations and ventricular function in β-thalassaemia. Lancet. 2002;360(9332):516–20. doi: 10.1016/s0140-6736(02)09740-4. [DOI] [PubMed] [Google Scholar]
- 4.Pepe A, Lombardi M, Positano V, Cracolici E, Capra M, Malizia R, et al. Evaluation of the efficacy of oral deferiprone in β-thalassemia major by multislice multiecho T2*. Eur J Haematol. 2006;76(3):183–92. doi: 10.1111/j.1600-0609.2005.00587.x. [DOI] [PubMed] [Google Scholar]
- 5.Pennell DJ, Berdoukas V, Karagiorga M, Ladis V, Piga A, Aessopos A, et al. Randomized controlled trial of deferiprone or deferoxamine in β-thalassemia major patients with asymptomatic myocardial siderosis. Blood. 2006;107(9):3738–44. doi: 10.1182/blood-2005-07-2948. [DOI] [PubMed] [Google Scholar]
- 6.Borgna-Pignatti C, Cappellini MD, De Stefano P, Del Vecchio GC, Forni GL, Gamberini MR, et al. Cardiac morbidity and mortality in deferoxamine- or deferiprone-treated patients with thalassemia major. Blood. 2006;107(9):3733–7. doi: 10.1182/blood-2005-07-2933. [DOI] [PubMed] [Google Scholar]
- 7.Piga A, Gaglioti C, Fogliacco E, Tricta F. Comparative effects of deferiprone and deferoxamine on survival and cardiac disease in patients with thalassemia major: a retrospective analysis. Haematologica. 2003;88(5):489–96. [PubMed] [Google Scholar]
- 8.Ceci A, Baiardi P, Catapano M, Felisi M, Cianciulli P, De Sanctis V, et al. Risk factors for death in patients with β-thalassemia major: results of a case-control study. Haematologica. 2006;91(10):1420–1. [PubMed] [Google Scholar]
- 9.Telfer P, Coen PG, Christou S, Hadjigavriel M, Kolnakou A, Pangalou E, et al. Survival of medically treated thalassemia patients in Cyprus. Trends and risk factors over the period 1980–2004. Haematologica. 2006;91(9):1187–92. [PubMed] [Google Scholar]
- 10.Pennell D, Porter JB, Cappellini MD, El-Beshlawy A, Chan LL, Aydinok Y, et al. Efficacy of Deferasirox in reducing and preventing cardiac iron overload in β-thalassemia. Blood. 2010;115(12):2364–71. doi: 10.1182/blood-2009-04-217455. [DOI] [PubMed] [Google Scholar]
- 11.Wood JC, Kang B, Thompson AA, Giardina P, Harmatz P, Glynos T, et al. The effect of deferasirox on cardiac iron in thalassemia major: impact of total body iron stores. Blood. 2010;116(4):537–43. doi: 10.1182/blood-2009-11-250308. [DOI] [PubMed] [Google Scholar]
- 12.Pathare A, Taher A, Daar S. Deferasirox (Exjade(R)) significantly improves cardiac T2* in heavily iron-overloaded patients with β-thalassemia major. Ann Hematol. 2010;89(4):405–9. doi: 10.1007/s00277-009-0838-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Berdoukas V, Chouliaras G, Moraitis P, Zannikos K, Berdoussi E, Ladis V. The efficacy of iron chelator regimes in reducing cardiac and hepatic iron in patients with thalassaemia major: a clinical observational study. J Cardiovasc Magn Reson. 2009;11 (1):20. doi: 10.1186/1532-429X-11-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Anderson LJ, Holden S, Davis B, Prescott E, Charrier CC, Bunce NH, et al. Cardiovascular T2-star (T2*) magnetic resonance for the early diagnosis of myocardial iron overload. Eur Heart J. 2001;22 (23):2171–9. doi: 10.1053/euhj.2001.2822. [DOI] [PubMed] [Google Scholar]
- 15.Pepe A, Positano V, Santarelli F, Sorrentino F, Cracolici E, De Marchi D, et al. Multislice multiecho T2* cardiovascular magnetic resonance for detection of the heterogeneous distribution of myocardial iron overload. J Magn Reson Imaging. 2006;23(5):662–8. doi: 10.1002/jmri.20566. [DOI] [PubMed] [Google Scholar]
- 16.Westwood MA, Firmin DN, Gildo M, Renzo G, Stathis G, Markissia K, et al. Intercentre reproducibility of magnetic resonance T2* measurements of myocardial iron in thalassaemia. Int J Cardiovasc Imaging. 2005;21(5):531–8. doi: 10.1007/s10554-005-0651-2. [DOI] [PubMed] [Google Scholar]
- 17.Positano V, Salani B, Pepe A, Santarelli MF, De Marchi D, Ramazzotti A, et al. Improved T2* assessment in liver iron overload by magnetic resonance imaging. Magn Reson Imaging. 2009;27(2):188–97. doi: 10.1016/j.mri.2008.06.004. [DOI] [PubMed] [Google Scholar]
- 18.Noetzli LJ, Carson SM, Nord AS, Coates TD, Wood JC. Longitudinal analysis of heart and liver iron in thalassemia major. Blood. 2008;112(7):2973–8. doi: 10.1182/blood-2008-04-148767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Modell B, Khan M, Darlison M, Westwood MA, Ingram D, Pennell DJ. Improved survival of thalassaemia major in the UK and relation to T2* cardiovascular magnetic resonance. J Cardiovasc Magn Reson. 2008;10(1):42. doi: 10.1186/1532-429X-10-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sironi M, Lombardi M, Pepe A, De Marchi D. Study of heart function. In: S-VI, editor. MRI of Heart and Vessels Lombardi M, Bartolozzi C. 2005. [Google Scholar]
- 21.Ramazzotti A, Pepe A, Positano V, Rossi G, De Marchi D, Brizi MG, et al. Multicenter validation of the magnetic resonance T2* technique for segmental and global quantification of myocardial iron. J Magn Reson Imaging. 2009;30(1):62–8. doi: 10.1002/jmri.21781. [DOI] [PubMed] [Google Scholar]
- 22.Meloni A, Ramazzotti A, Positano V, Salvatori C, Mangione M, Marcheschi P, et al. Evaluation of a web-based network for reproducible T2* MRI assessment of iron overload in thalassemia. Int J Med Inform. 2009;78:503–12. doi: 10.1016/j.ijmedinf.2009.02.011. [DOI] [PubMed] [Google Scholar]
- 23.Positano V, Pepe A, Santarelli MF, Scattini B, De Marchi D, Ramazzotti A, et al. Standardized T2* map of normal human heart in vivo to correct T2* segmental artefacts. NMR Biomed. 2007;20:578–90. doi: 10.1002/nbm.1121. [DOI] [PubMed] [Google Scholar]
- 24.Cerqueira MD, Weissman NJ, Dilsizian V, Jacobs AK, Kaul S, Laskey WK, et al. Standardized myocardial segmentation and nomenclature for tomographic imaging of the heart: a statement for healthcare professionals from the Cardiac Imaging Committee of the Council on Clinical Cardiology of the American Heart Association. Circulation. 2002;105(4):539–42. doi: 10.1161/hc0402.102975. [DOI] [PubMed] [Google Scholar]
- 25.Aessopos A, Fragodimitri C, Karabatsos F, Hatziliami A, Yousef J, Giakoumis A, et al. Cardiac magnetic resonance imaging R2* assessments and analysis of historical parameters in patients with transfusion-dependent thalassemia. Haematologica. 2007;92(1):131–2. doi: 10.3324/haematol.10455. [DOI] [PubMed] [Google Scholar]
- 26.Wood JC, Enriquez C, Ghugre N, Tyzka JM, Carson S, Nelson MD, et al. MRI R2 and R2* mapping accurately estimates hepatic iron concentration in transfusion-dependent thalassemia and sickle cell disease patients. Blood. 2005;106(4 ):1460–5. doi: 10.1182/blood-2004-10-3982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Positano V, Pepe A, Santarelli MF, Ramazzotti A, Meloni A, De Marchi D, et al. Multislice multiecho T2* cardiac magnetic resonance for the detection of heterogeneous myocardial iron distribution in thalassaemia patients. NMR Biomed. 2009;22(7):707–15. doi: 10.1002/nbm.1382. [DOI] [PubMed] [Google Scholar]
- 28.Westwood MA, Anderson LJ, Maceira AM, Shah FT, Prescott E, Porter JB, et al. Normalized left ventricular volumes and function in thalassemia major patients with normal myocardial iron. J Magn Reson Imaging. 2007;25(6):1147–51. doi: 10.1002/jmri.20915. [DOI] [PubMed] [Google Scholar]
- 29.Roberts DJ, Rees D, Howard J, Hyde C, Alderson P, Brunskill S. Desferrioxamine mesylate for managing transfusional iron overload in people with transfusion-dependent thalassaemia. Cochrane Database Syst Rev. 2005(4):CD004450. doi: 10.1002/14651858.CD004450.pub2. [DOI] [PubMed] [Google Scholar]
- 30.Tanner MA, Galanello R, Dessi C, Westwood MA, Smith GC, Nair SV, et al. Myocardial iron loading in patients with thalassemia major on deferoxamine chelation. J Cardiovasc Magn Reson. 2006;8(3):543–7. doi: 10.1080/10976640600698155. [DOI] [PubMed] [Google Scholar]
- 31.Cappellini MD, Cohen A, Piga A, Bejaoui M, Perrotta S, Agaoglu L, et al. A phase 3 study of deferasirox (ICL670), a once-daily oral iron chelator, in patients with β-thalassemia. Blood. 2006;107(9):3455–62. doi: 10.1182/blood-2005-08-3430. [DOI] [PubMed] [Google Scholar]