Abstract
Background
Epidemiological data on myeloid malignancies are very rare in the literature due to a lack of registration by cancer registries until 2000. The Registry of Hematologic Malignancies of the Côte d’Or Department in France has, however, steadfastly registered data on cases occurring in the Department since 1980, resulting, to date, in a database of over 5,000 cases classified according to the ICD-O-3 classification, following the most recent World Health Organization classification criteria.
Design and Methods
Twenty-five years of data on myeloid malignancies, including acute myeloid leukemia, myeloproliferative neoplasms, myelodysplastic syndromes and myelodysplastic/myeloproliferative syndromes were analyzed. World population standardized incidence rates were calculated as were as observed and relative survival.
Results
Incidence rates per 100,000 inhabitants/year were 2.5 for acute myeloid leukemia, 1.3 for myelodysplastic syndromes, 3.2 for myeloproliferative neoplasms and 0.6 for myelodysplastic/myeloproliferative syndromes. It was found that the incidence rate of myelodysplastic syndromes increased significantly over the period. The median overall survival is 8.9 months for patients with acute myeloid leukemia, 33.8 months for patients with myelodysplastic syndromes, 91.7 months for those with myeloproliferative neoplasms and 26.6 months for patients with myelodysplastic/myeloproliferative syndromes. Observed and relative 20-year survival rates are, respectively, 12% and 13% in acute myeloid leukemia, 2% and 6% in myelodysplastic syndromes and 20% and 34% in myeloproliferative neoplasms.
Conclusions
These population-based data on myeloid malignancies are the first data collected over such a long period and provide interesting information for clinicians and public health authorities, particularly given the paucity of other long-term, population-based data from cancer registries.
Keywords: myeloid malignancies, incidence, survival, cancer registry
Introduction
Around 200 general cancer registries, currently active in the world, are used to collect new cases of hematologic malignancies according to the International Classification of Diseases - Oncology (ICD-O) criteria. Nevertheless, knowledge on these diseases is relatively recent, and is evolving quickly. Classifications have, therefore, been regularly modified, not always with unanimous acceptance. This has created difficulties for epidemiologists wishing to register data on different entities, and hematologic malignancies have usually been analyzed as broad categories such as “myeloid leukemia” or “unspecified leukemia” mixing both acute leukemia and other myeloid malignancies.1 Furthermore, because in the ICD-O-2 version, used until 1999, polycythemia vera, essential thrombocythemia, and all myelodysplastic syndromes (MDS) were not considered to be malignant, no data were recorded about these disorders in general cancer registries. As the ICD-O-3 version was developed, these diseases were recognized to be malignant and data for the 1998–2002 period, published in CIVC IX, distinguished myeloid leukemia, myeloproliferative disorders, now called myeloproliferative neoplasms (MPN), and MDS.2 The World Health Organization (WHO) classification of hematologic malignancies published in 2001 incorporated clinical, biological, pathological and prognostic aspects of the diseases and has been adopted worldwide.3 Furthermore, it was drawn up to show correspondence with the ICD-O-3 version, thus enabling the quality of data recorded about these diseases to be improved.
One of the major purposes of a specialized registry is to collect as detailed information as possible and to provide epidemiological data on well-defined subtypes of diseases. In hematology, they are very few such registries. The first specialized registry of hematologic malignancies in the world was established in 1980 in the Côte d’Or area, in the French region of Burgundy.4 The registration of cases has been uninterrupted since then, and thus over 25 years of epidemiological data concerning a population of around 500,000 inhabitants, which has been relatively stable over time, have been collected. Since the beginning, all subtypes of hematologic malignancies have been registered and classification modifications were implemented for each case. This allows the registry to provide detailed and accurate information on the incidence and survival of subjects with the major hematologic malignancies over an extended period of time.
Design and Methods
Population and cases
The Côte d’Or Department is a French administrative area of the Burgundy Region. The total population was 467,998 inhabitants in 1980 and 512,272 inhabitants in 2004 (+9.5%). All patients residing in the Department for more than 6 months in whom a new myeloid hematologic malignancy was diagnosed between January 1st, 1980 and December 31st, 2004 were registered after validation by a hematologist. In total, 5,086 cases were registered during this period. The ICD-O-3 version codes have been used since 2001. The various entities of MPN and MDS have consistently been registered since 1980. The ICD-O-2 codes for all cases diagnosed before 2001 were converted into their corresponding ICD-O-3 codes. Refractory anemia with an excess of blasts in transformation was reclassified as acute myeloid leukemia (AML) with multilineage dysplasia (9895/3).3 Therapy-related MDS (9987/3) and MDS/MPN were included in the therapy-related myeloid malignancies (9920/3) subgroup.5 Acute leukemias of ambiguous lineage were clustered within AML not otherwise specified (nos). Chronic myeloid leukemia, nos (CML) (9863/3) and CML, BCR-ABL-positive (9865/3) cases were clustered. Cases of acute leukemia arising after a MPN or MDS were excluded as they were not incident cases.
Registration
Items including administrative data, clinical and biological imaging at diagnosis and follow-up were registered. Registration rules of the International Association of Cancer Registries were followed. A dedicated computerized database approved by the French “Commission Nationale de l’Informatique et des Libertés” was used. The quality of the registry has been checked and validated through regular auditing by INSERM, the French Institute of Health Care Surveillance, the National Committee of Registries and the French National Cancer Institute, since 1989.
Statistics
Age- and sex-specific incidence rate calculations were performed using dataset estimates of the Côte d’Or population, supplied by the French National Institute of Statistics (INSEE). Rates were age-adjusted according to the world standard population as a standardization process. Incidence rates were calculated for each entity, per year and by 5-year periods of time. The date of diagnosis and vital status on 12/31/2008 were used to calculate observed survival by the Kaplan-Meier method. Relative survival was defined as the ratio of the observed survival to the expected survival. Survival data were analyzed by entity and by group of diseases using Stata 9 software. Comparisons between groups were performed using the log-rank test and two-sided P values less than 0.05 were considered statistically significant.
Results
Myeloid malignancies accounted for 30.5% (1549/5086) of all hematologic malignancies diagnosed in the Côte d’Or Department between 1980 and 2004. Of these myeloid malignancies, 30% (468/1549) were AML, 38% were (584/1549) MPN, 22% (345/1549) were MDS and 9.8% (152/1549) were MDS/MPN.
Incidence
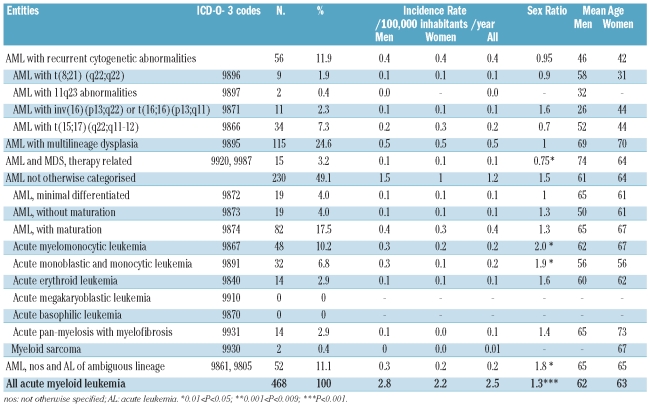
Analysis of the four major AML WHO categories showed that 48% were ‘not otherwise categorized’ (noc) while 25% were AML with multilineage dysplasia (Table 1). Within AML with recurrent cytogenetic abnormalities, the most frequent entity was AML with t(15;17) and within AML-noc, the majority of cases were AML with maturation and AML involving the monocytic lineage. The world standard population incidence rate of AML was not significantly different according to gender, being 2.8/100,000 inhabitants/year in men and 2.2/100,000 inhabitants/year in women although the sex ratio was biased at 1.3 (P<0.001). A reverse sex ratio was observed in therapy-related myeloid malignancies (0.7, P=0.02) and in AML with recurrent cytogenetic abnormalities (0.95), particularly in AML with t(15;17) (0.7). The median age of AML patients was 69.7 years while their mean age was 62 years (range, 0.2–98 years), being significantly lower in AML with recurrent cytogenetic abnormalities (44 years, P<0.001). The incidence of AML increased with age in all AML subgroups, particularly after the 55–59 years old age range. The incidence of overall AML was relatively stable over time, being 2.5/100 000 inhabitants/year in the period from 1980–1984 and 2.35/100,000 inhabitants/year 20 years later in the period from 2000–2004. The mean annual rate of variation was positive in men while it was negative in women (P=ns) (Online Supplementary Table S1). This trend was the same for all four subgroups of AML.
Table 1.
World-population-standardized incidence rate, sex ratio, mean age and urban/rural ratio of different entities of acute myeloid leukemia according to the 2001 WHO classification in the Côte d’Or Department of France (1980–2004).
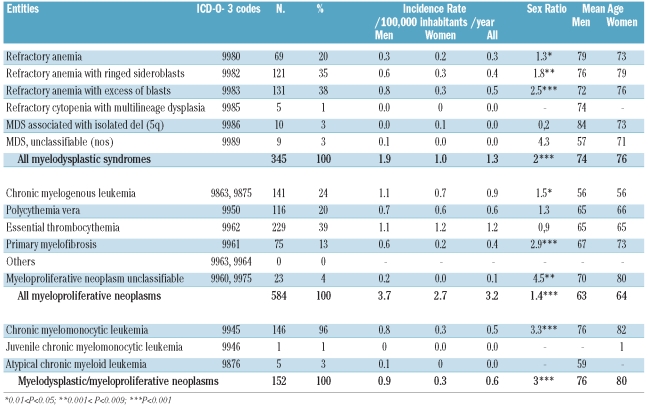
Following the introduction of the WHO classification, MDS became less frequent than MPN (1.35/100,000 inhabitants/year versus 3.2/100,000 inhabitants/year). The most frequent entity was refractory anemia with blasts, with an incidence rate of 0.55/100,000 inhabitants/year, followed by refractory anemia with ring sideroblasts with an incidence rate of 0.4/100,000 inhabitants/year. The male predominance was statistically significant for all entities except the 5q- syndrome which was more frequent in women (Table 2). MDS began to appear in people in their forties and the incidence rate increased markedly in subgroups older than 65–69 years, reaching 54/100,000 inhabitants/year for people older than 84. The incidence of MDS increased significantly throughout the period with a 2.4% mean annual increment rate (P<0.01) significantly partitioned as 2% in men (P<0.01) and 2.9% in women (P<0.01) (Online Supplementary Table S1).
Table 2.
World-population-standardized incidence rate, sex ratio, mean age and urban/rural ratio of different entities of myelodysplastic syndromes (MDS), myeloproliferative neoplasms (MPN), myelodysplastic/myeloproliferative neoplasms (MDS/MPN), according to the 2008 WHO classification in the Côte d’Or Department of France (1980–2004).
Within MPN, essential thrombocythemia was the most frequent entity with an incidence rate 1.2/100,000 inhabitants/year, followed by CML (0.9/100,000 inhabitants/year) and polycythemia vera (0.6/100,000 inhabitants/year) (Table 2). Essential thrombocythemia was also characterized by a reversed sex ratio (0.9), contrasting with other MPN in which the male predominance was marked. This gender-related difference was statistically significant for CML and chronic idiopathic myelofibrosis. The mean age of occurrence of CML was approximately 10 years lower than that for other MPN. With regard to age, incidence rates of MPN increased from 25–29 years onwards, although a slight decrease was observed after 80 years old. An increase in incidence throughout the period studied was noted in women with an annual rate of 2.3% per year (P<0.001), while there was a slight decrease in men (Online Supplementary Table S1).
By far the most common MDS/MPN was chronic myelomonocytic leukemia (96% of the cases). The incidence rate of chronic myelomonocytic leukemia was 0.52/100,000 inhabitants/year with a markedly significant predominance in males. The mean age at diagnosis was 78.5 years old and was higher in women than in men (Table 2). An increasing incidence rate was found in both sexes throughout the observation period, although this increase was not statistically significant.
Survival
In this large cohort, the median observed survival was 91.7 months for patients with MPN, 33.8 months for those with MDS, 26.6 months for patients with MDS/MPN and 8.9 months for those with AML (Online Supplementary Table S2). There were, however, considerable differences between subgroups and entities.
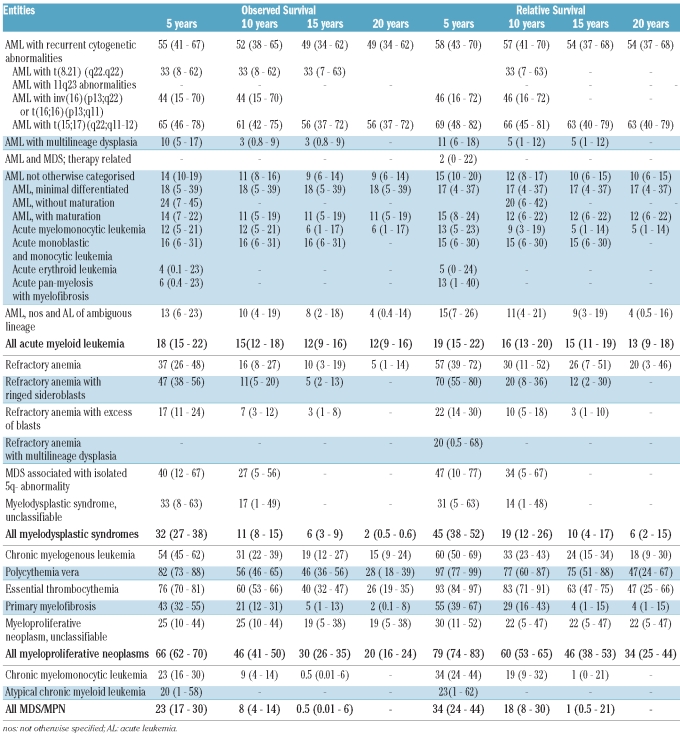
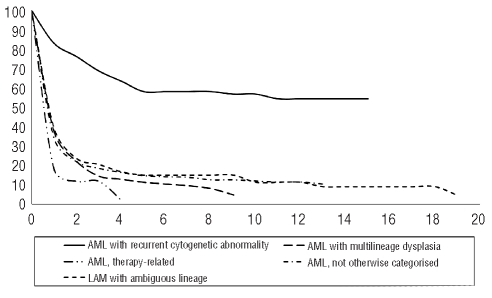
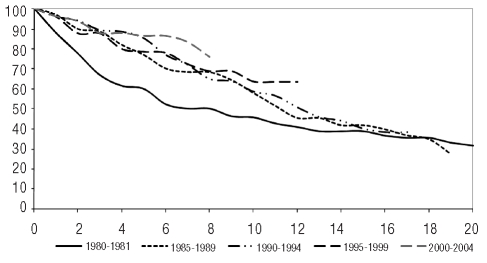
Within patients with AML, the significantly best median observed survival was in those with AML with recurrent cytogenetic abnormalities (126 months). For all entities, relative survival was quite similar to observed survival (Table 3). Analyzed according to subgroups, the best 20-year relative survival was observed in patients with AML with recurrent cytogenetic abnormalities (Figure 1) especially those with t(15;17) (63%). With regards to gender, there was no difference in AML, but the relative survival of patients with AML with recurrent cytogenetic abnormalities was better in men than in women (20-year relative survival: 64% in men and 45% in women; P=ns). Analysis by age showed that the 15-year relative survival was 32% in patients younger than 63 years old (median age) but only 1% in patients over 63 years old (P<0.001). Over the 25 years of data registration analyzed, relative survival increased significantly in AML but surprisingly a decrease was observed in the 2000–2004 period (Figure 2).
Table 3.
Observed and relative survival at 5, 10, 15 and 20 years in percent (95% confidence Interval) by group and by type of myeloid malignances, in the Côte d’Or Department of France between 1980 and 2004.
Figure 1.
Relative survival (%) of the four categories of acute myeloid leukemia, by 5-year periods of time, since 1980 in the Côte d’Or Department of France.
Figure 2.
Relative survival (%) of patients with acute myeloid leukemia, by 5-year periods of time in the Côte d’Or Department of France (1980–2004).
Among patients with MDS, the median observed survival of those with refractory anemia with ring sideroblasts was 54.8 months whereas that of patients with refractory anemia was 30 months. In MDS, there was a slight difference with a 10-year relative survival of 19%, a 15-year relative survival of 10% and a 20-year relative survival of 6% compared with observed survival rates of 11%, 6% and 2%, respectively. With regard to gender, the 20-year relative survival of patients with MDS was significantly better in women (11%) than in men (3%) (P<0.008). This held true for cases of refractory anemia (33% versus 4%) and refractory anemia with ring sideroblasts (15-year relative survival: 20% versus 1%). No increase of relative survival was observed along the registration period (Figure 3).
Figure 3.
Relative survival (%) of patients with myeloproliferative neoplasms, by periods of time, in the Côte d’Or French Department (1980–2004).
Among MPN, the median observed survival of patients with polycythemia vera and essential thrombocythemia was 141 months and 129 months, respectively. In MPN, the relative survival was 15% higher than the observed survival with relative survival rates of 60% at 10 years, 46% at 15 years and 34% at 20 years. In MPN, the 20-year relative survival was better for women (39%) than for men (30%) (P<0.001), this being true only for CML (25% versus 11%). The 20-year relative survival decreased significantly with age (41% before 64 years old versus 11% after 64 years old, P<0.008). An increase of relative survival was observed in MPN across all 5-year periods, with this being particularly marked in patients with CML (Online Supplementary Figure S1). The survival rate of patients with chronic myelomonocytic leukemia was quite low with a 15-year observed survival rate of 0.5% and a relative survival of 1% (Table 3). No difference was found according to gender and relative survival did not change across the 25-year period studied.
Discussion
Limited data are available on the epidemiological characteristics of hematologic disorders of the myeloid lineage. The registry we report upon here is the oldest of its kind in the world. It allows us to provide epidemiological data for one of the longest registration periods reported so far in hematology (1980–2004).
The incidence rate of AML observed was similar to the rates reported in South-East England and Sardinia.6,7 Differences from American Surveillance, Epidemiology and End Results (SEER) data could be due to the reference population used for the standardization process: we used the world population while SEER used the US population.8 A higher incidence of AML cases recently reported in Sweden was due to the inclusion in their series of secondary cases occurring after previous MDS or other hematologic malignancies.9 As such cases are not incident AML cases, they were not included in our analyses. The higher incidence of AML in men is widely shared, but surprisingly the rate in our women was higher than that in other countries.2,6,7 The proportion of AML with recurrent cytogenetic abnormalities in our series was slightly lower (11.9%) than in larger series from Germany or England, probably because karyotypic information was not always available for older cases. Nevertheless our data are similar to those from Denmark.9–11 In all of the above series, except that from Germany, the most frequent disease was AML with t(15;17), partially because of cases secondary to mitoxantrone treatment of breast cancer or linked to the body-mass index increase in western populations.12,13 Since 1980, we have observed a non-significant trend towards an increase in the incidence of AML in men.7,14,15
There are three potential reasons why the incidence rates of MDS from the USA and the South Thames area (UK) are higher than those in the Côte d’Or.6,16,17 First, we used positive criteria, such as karyotypic abnormality, abnormal progenitor in vitro culture or evolution to a more aggressive entity,18 to register cases of refractory anemia in the database in order to avoid over-inclusion of false cases. Secondly, the changes in classification led to a partition of around 30% of cases (refractory anemia with excess of blast in transformation became included in AML, chronic myelomonocytic leukemia as MDS/MPN diseases). In our series, this partition was applied to cases since 1980 which is frequently not the case in other series.6 The third possible explanation was the reference population used for the standardization process as mentioned for AML. A marked increase in incidence was found despite temporal variations,19 probably because of population aging and a higher number of diagnostic procedures now performed for MDS.
MPN appears to be the most prominent group of myeloid diseases.6,17 The incidence rate of polycythemia vera varies from 1.97/100,00 inhabitants/year in the Göteborg area, 1.1/100,00 inhabitants/year in the SEER population, 0.74/100,00 inhabitants/year in the South Thames area, to 0.61/100,00 inhabitants/year in our region. By contrast, the incidence of essential thrombocythemia is higher in the Côte d’Or, England and Sweden (incidence rates per 100,00 inhabitants/year: 1.2, 1.1 and 1.55, respectively) than in the USA (0.5/100,00 inhabitants/year) and Denmark (0.6/100,00 inhabitants/year).20 Detection of the JAK2 mutation and new diagnostic criteria from the WHO will probably cause changes in these rates.5,21 A significant increase in incidence was observed in women in our series, confirming results of the Copenhagen district series, but contrasting with those from Göteborg for the period from 1983–1999.20,22 The mean age at diagnosis in our series was close to that in both the Swedish and Danish populations but approximately 5 to 10 years younger than in patients from the South Thames area.6,22
Data on long-term survival, such as over 15 or 20 years, are quite rare in the literature, especially from population-based studies. In AML, the most remarkable finding of our series was the tapering of the survival increase observed in the 2000–2004 period. This observation had been hinted at on a European level by data from the EUROCARE database: increases in 5-year relative survival rates in AML had been reported for cases diagnosed in 1985–1989 and in 1995–1999, but a decrease was reported for cases diagnosed in 2000–2002.23–25 This scenario is difficult to explain as the quality of care probably improved and should have led to an increase in survival. Population aging might be responsible, since the incidence of AML increases with age, particularly for poor prognosis entities such as AML with multilineage dysplasia and therapy-related cases. Among MPN, the 5-year relative survival of patients with CML was much higher in our series than in the EURO-CARE 4 cohort. This was probably due to the inclusion of different types of chronic leukemia of myeloid tissue such as chronic myelomonocytic leukemia in the EUROCARE 4 series. Furthermore, the use of anti-tyrosine kinase-targeted therapy specific for BCR-ABL rearrangement in our area since the early 2000s could largely explain the observed increase in survival. The difference between long-term observed survival and relative survival reported here for cases of polycythemia vera and essential thrombocythemia indicates that patients more frequently die from another cause than from their hematologic myeloid disease, confirming the good prognosis of these disorders. Data on survival of MDS patients from population-based series are very rare. We report a 5-year observed survival of 32% and a relative survival of 45%, confirming the limited data from the USA and the South Thames area.6,16,17 The forms of MDS associated with best relative survival are refractory anemia with ring sideroblasts and refractory anemia, as reported in the South Thames series6 and in clinical series classified according to the International Prognostic Scoring System.26 Relative survival did not increase along the observation period, probably because of the lack of a specific therapeutic regimen available over that time period. This might very well change with the emergence of specific therapeutic approaches and the development of new drugs such as hypomethylating agents (azacitidine and decitabine) to tackle the observed increase in the incidence of these diseases due to the worldwide aging of the population.
Our population-based data highlight some useful points for clinicians and public health authorities such as the surprising decrease of the relative survival of AML in the most recent period and the increasing incidence of MDS concomitant with a lack of increase in relative survival in these disorders.
Footnotes
Funding: the Registry of Hematologic Malignancies of Côte d’Or was funded by the Institut de Veille Sanitaire (InVS) and by INSERM.
The online version of this article has a Supplementary Appendix.
Authorship and Disclosures
The information provided by the authors about contributions from persons listed as authors and in acknowledgments is available with the full text of this paper at www.haematologica.org.
Financial and other disclosures provided by the authors using the ICMJE (www.icmje.org) Uniform Format for Disclosure of Competing Interests are also available at www.haematologica.org.
References
- 1.Cancer incidence in five continents. IARC Sci Publ. 2002;VIII(155):1–781. [PubMed] [Google Scholar]
- 2.Cancer incidence in five continents. IARC Sci Publ. 2008;IX(160):1–837. [PubMed] [Google Scholar]
- 3.Jaffe ES, Harris N.L, Stein H, Vardiman JW, editors. Pathology and Genetics of Tumours of Haematopoietic and Lymphoid Tissues. Lyon: International Agency for Research on Cancer; 2001. [Google Scholar]
- 4.Carli PM, Milan C, Lange A, Devilliers E, Guy H, Faivre J. Haematopoietic malignancies in Cote d’Or (France): a population based study. Br J Cancer. 1986;53(6):811–5. doi: 10.1038/bjc.1986.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Swerdlow SH, Campo E, Harris NL, Jaffe ES, Pileri SA, Stein H, Thiele J, Vardiman JW, editors. WHO Classification of Tumours of Haematopoietic and Lymphoid Tissues. 4th ed. Lyon: International Agency for Research on Cancer; 2008. [Google Scholar]
- 6.Phekoo KJ, Richards MA, Moller H, Schey SA. The incidence and outcome of myeloid malignancies in 2,112 adult patients in southeast England. Haematologica. 2006;91(10):1400–4. [PubMed] [Google Scholar]
- 7.Broccia G, Deplano W, Dessalvi P, Giannico B, Luxi G, Chessa E, et al. Hematological malignancies in the island of Sardinia, 1974–1993: age and sex distributions and temporal changes in incidence. Hematol Oncol. 2004;22(3):91–109. doi: 10.1002/hon.733. [DOI] [PubMed] [Google Scholar]
- 8.Deschler B, Lubbert M. Acute myeloid leukemia: epidemiology and etiology. Cancer. 2006;107(9):2099–107. doi: 10.1002/cncr.22233. [DOI] [PubMed] [Google Scholar]
- 9.Sanderson RN, Johnson PR, Moorman AV, Roman E, Willett E, Taylor PR, et al. Population-based demographic study of karyotypes in 1709 patients with adult acute myeloid leukemia. Leukemia. 2006;20(3):444–50. doi: 10.1038/sj.leu.2404055. [DOI] [PubMed] [Google Scholar]
- 10.Bacher U, Kern W, Schnittger S, Hiddemann W, Haferlach T, Schoch C. Population-based age-specific incidences of cytogenetic subgroups of acute myeloid leukemia. Haematologica. 2005;90(11):1502–10. [PubMed] [Google Scholar]
- 11.Preiss BS, Kerndrup GB, Schmidt KG, Sorensen AG, Clausen NA, Gadeberg OV, et al. Cytogenetic findings in adult de novo acute myeloid leukaemia. A population-based study of 303/337 patients. Br J Haematol. 2003;123(2):219–34. doi: 10.1046/j.1365-2141.2003.04568.x. [DOI] [PubMed] [Google Scholar]
- 12.Estey E, Thall P, Kantarjian H, Pierce S, Kornblau S, Keating M. Association between increased body mass index and a diagnosis of acute promyelocytic leukemia in patients with acute myeloid leukemia. Leukemia. 1997;11(10):1661–4. doi: 10.1038/sj.leu.2400783. [DOI] [PubMed] [Google Scholar]
- 13.Carli PM, Sgro C, Parchin-Geneste N, Isambert N, Mugneret F, Girodon F, et al. Increase therapy-related leukemia secondary to breast cancer. Leukemia. 2000;14 (6):1014–7. doi: 10.1038/sj.leu.2401787. [DOI] [PubMed] [Google Scholar]
- 14.Derolf AR, Kristinsson SY, Andersson TM, Landgren O, Dickman PW, Bjorkholm M. Improved patient survival for acute myeloid leukemia: a population-based study of 9729 patients diagnosed in Sweden between 1973 and 2005. Blood. 2009;113(16):3666–72. doi: 10.1182/blood-2008-09-179341. [DOI] [PubMed] [Google Scholar]
- 15.Xie Y, Davies SM, Xiang Y, Robison LL, Ross JA. Trends in leukemia incidence and survival in the United States (1973–1998) Cancer. 2003;97(9):2229–35. doi: 10.1002/cncr.11316. [DOI] [PubMed] [Google Scholar]
- 16.Ma X, Does M, Raza A, Mayne ST. Myelodysplastic syndromes: incidence and survival in the United States. Cancer. 2007;109(8):1536–42. doi: 10.1002/cncr.22570. [DOI] [PubMed] [Google Scholar]
- 17.Rollison DE, Howlader N, Smith MT, Strom SS, Merritt WD, Ries LA, et al. Epidemiology of myelodysplastic syndromes and chronic myeloproliferative disorders in the United States, 2001–2004, using data from the NAACCR and SEER programs. Blood. 2008;112(1):45–52. doi: 10.1182/blood-2008-01-134858. [DOI] [PubMed] [Google Scholar]
- 18.Maynadie M, Verret C, Moskovtchenko P, Mugneret F, Petrella T, Caillot D, et al. Epidemiological characteristics of myelodysplastic syndrome in a well-defined French population. Br J Cancer. 1996;74(2):288–90. doi: 10.1038/bjc.1996.354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Germing U, Strupp C, Kundgen A, Bowen D, Aul C, Haas R, et al. No increase in age-specific incidence of myelodysplastic syndromes. Haematologica. 2004;89(8):905–10. [PubMed] [Google Scholar]
- 20.Jensen MK, de Nully Brown P, Nielsen OJ, Hasselbalch HC. Incidence, clinical features and outcome of essential thrombocythaemia in a well defined geographical area. Eur J Haematol. 2000;65(2):132–9. doi: 10.1034/j.1600-0609.2000.90236.x. [DOI] [PubMed] [Google Scholar]
- 21.Girodon F, Bonicelli G, Schaeffer C, Mounier M, Carillo S, Lafon I, et al. Significant increase in the apparent incidence of essential thrombocythemia related to new WHO diagnostic criteria: a population-based study. Haematologica. 2009;94(6):865–9. doi: 10.3324/haematol.2008.004234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Johansson P, Kutti J, Andreasson B, Safai-Kutti S, Vilen L, Wedel H, et al. Trends in the incidence of chronic Philadelphia chromosome negative (Ph-) myeloproliferative disorders in the city of Goteborg, Sweden, during 1983–99. J Intern Med. 2004;256 (2):161–5. doi: 10.1111/j.1365-2796.2004.01357.x. [DOI] [PubMed] [Google Scholar]
- 23.Carli PM, Coebergh JW, Verdecchia A. Variation in survival of adult patients with haematological malignancies in Europe since 1978. Eur J Cancer. 1998;34(14):2253–63. doi: 10.1016/s0959-8049(98)00339-6. [DOI] [PubMed] [Google Scholar]
- 24.Berrino F, De Angelis R, Sant M, Rosso S, Bielska-Lasota M, Coebergh JW, et al. Survival for eight major cancers and all cancers combined for European adults diagnosed in 1995–99: results of the EURO-CARE-4 study. Lancet Oncol. 2007;8(9):773–83. doi: 10.1016/S1470-2045(07)70245-0. [DOI] [PubMed] [Google Scholar]
- 25.Verdecchia A, Francisci S, Brenner H, Gatta G, Micheli A, Mangone L, et al. Recent cancer survival in Europe: a 2000–02 period analysis of EUROCARE-4 data. Lancet Oncol. 2007;8(9):784–96. doi: 10.1016/S1470-2045(07)70246-2. [DOI] [PubMed] [Google Scholar]
- 26.Doll DC, Taylor HM, Yesus YW, Caldwell CW, Anderson S, Madsen R, et al. Myelodysplastic syndrome: prospective evaluation of fifty-one patients using the Dutcher scoring system. Acta Haematol. 1989;81(2):86–90. doi: 10.1159/000205532. [DOI] [PubMed] [Google Scholar]