Abstract
Background
Sorafenib is a multi-kinase inhibitor with activity against fms-like tyrosine kinase 3 with internal tandem duplication mutation and Raf kinase among others. A phase I dose escalation study of sorafenib was conducted in patients with advanced myelodysplastic syndrome and relapsed or refractory acute leukemias.
Design and Methods
Fifty patients received one of two different schedules; Schedule “A”: once or twice daily, five days per week, every week for a 21 day cycle, and Schedule “B”: once or twice daily, for 14 days every 21 days. Dose limiting toxicities were grade 3/4 hypertension, hyperbilirubinemia, and amylase elevation. The recommended phase II dose in hematologic malignancies is 400 mg twice daily for both schedules.
Results
Complete remissions or complete remissions with incomplete recovery of platelets were achieved in 5 (10%) patients (all with fms-like tyrosine kinase 3-internal tandem duplication). Significant reduction in bone marrow and/or peripheral blood blasts was seen in an additional 17 (34%) patients (all with fms-like tyrosine kinase 3-internal tandem duplication). Eleven of these responses (including 3 complete remissions/complete remissions with incomplete recovery) lasted for 2 cycles or beyond. In conclusion, sorafenib is active and well tolerated in acute myelogenous leukemia with fms-like tyrosine kinase 3 internal tandem duplication mutation.
Conclusions
Additional studies of sorafenib in patients with acute myelogenous leukemia, particularly those with fms-like tyrosine kinase 3 internal tandem duplication, are warranted, including sorafenib-based combinations. (ClinicalTrials.gov Identifier: NCT00217646)
Keywords: sorafenib, FLT3 mutation, leukemia
Introduction
Signaling through multiple membrane receptor kinases stimulated by growth factors or extra-cellular stimuli is integrated in the mitogen activated protein kinase (MAPK) pathway. Ras/Raf/MEK/ERK (MAPK) signaling cascade is one of the most studied signaling pathways in hematologic malignancies.1 Raf kinase is downstream of guanosine tri-phosphate (GTP) activated Ras and in turn activates mitogen activated protein kinase (MEK). MEK in turn activates external signal-regulated kinase (ERK) 1 and 2 (MAPK). MAPK signaling is constitutively activated in more than 50% of primary acute myeloid leukemia (AML) samples2 and is associated with poor outcome.3 Inhibition of MAPK signaling not only results in growth arrest of leukemia cells but also in activation of the apoptotic pathway through upregulation of pro-apoptotic proteins like Bim,4 reduced phosphorylation of anti-apoptotic Bcl-25 and enhanced proteasome mediated destruction of anti-apoptotic Mcl-1.6 Inhibition of MAPK pathway has been shown to impair growth and survival of primary samples with constitutive MAPK activation.7 Sorafenib is a multi-kinase inhibitor that inhibits several critical tyrosine kinases including vascular endothelial growth factor receptor (VEGFR-2), FMS like tyrosine kinase-3 (Flt-3), and c-Kit and Ret.8,9 Sorafenib inhibits activation of MAPK by inhibiting its upstream kinase Raf.
FLT3 kinase activity is necessary for stem and progenitor cell proliferation.10 Activating mutations of FLT3 kinase-internal tandem duplication (ITD) and point mutations (D835) are present in 20–30% of adult AML patients, and are associated with worse outcome, particularly in patients with cytogenetically normal AML.11,12 The MAPK pathway is activated downstream of mutant FLT3 signaling.13 Sorafenib directly targets mutant FLT3 kinase, inhibiting the growth and survival of mouse and human FLT3-ITD AML cells at low nanomolar concentrations with minimal or no effect against wild-type FLT3 or FLT3 D835.14,15 The potential dual inhibition of Raf and FLT3 kinases by sorafenib makes it an attractive agent for the treatment of acute myeloid leukemia. We report on the results of a phase I trial of sorafenib in patients with refractory acute leukemias or myelodysplastic syndromes, exploring two different dosing schedules.
Design and Methods
Eligibility
Patients with relapsed or refractory acute myeloid leukemia (AML), acute lymphoblastic leukemia (ALL), myelodysplastic syndromes (MDS), chronic myelomonocytic leukemia (CMML) or chronic myeloid leukemia in blastic phase (CML-BP) were eligible. Eligibility criteria also included: 1) Eastern Cooperative Oncology Group performance status ≤2; 2) age 18 years or older; 3) adequate organ function (total bilirubin ≤1.5 mg/dL; ALT (SGPT) ≤2.5 times the institutional upper limit of normal; creatinine ≤2.0 mg/dL, or creatinine clearance of 60 mL/min/1.73m2 or more for patients with creatinine levels over 2.0 mg/dL; 4) no chemotherapy except hydroxyurea within two weeks of study drug treatment (hydroxyurea should have been discontinued at least 24 hours prior to start of study drug); 5) absence of proliferative disease defined as absolute blast count over 20×109/L (except patients with FLT3 ITD who were allowed to take part regardless of blast count). Patients with uncontrolled hypertension (i.e. sustained systolic blood pressure ≥150 mmHg or diastolic ≥90 mmHg) were excluded. The Institutional Review Board (IRB) approved the protocol and the informed consent.
Treatment plan
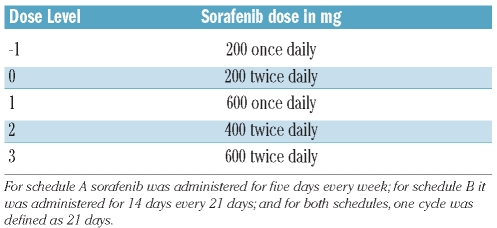
Sorafenib was supplied as 200 mg tablets for oral administration. Two different schedules of administration were investigated; Schedule “A”: once or twice daily, five days per week, every week for a 21 day cycle, and Schedule “B”: once or twice daily, for 14 days every 21 days. The starting dose for both schedules was 200 mg twice daily (BID) (i.e. dose level 0) (Table 1). One cycle of therapy was defined as 21 days for both schedules. Patients with persistent grade 2 or with any grade 3-4 drug-related toxicity had treatment interrupted until toxicity resolved to grade 1 or less, to be restarted at the next lower dose level without making up for missed doses. For patients who achieved a remission or normalized their counts, therapy could be interrupted for cytopenias (granulocytes < 1×109/L or platelets <50×109/L) with dose reduction upon resumption of therapy if recovery took over two weeks. Intrapatient dose escalation was permitted once the next dose level was considered safe. Treatment could continue for six months from the time of the first dose or until disease progression or unacceptable adverse event.
Table 1.
Dose levels of sorafenib.
Response evaluation
Response was evaluated according to the modified International Working Group (IWG) criteria.16 A complete response (CR) required disappearance of all signs and symptoms related to disease, peripheral blood counts with absolute neutrophil count 1×109/L or over and platelet count 100×109/L or over, and normal bone marrow morphology with no evidence of dysplasia and 5% blasts or under. Complete response with incomplete platelet recovery (CRp) was defined as a CR but with a platelet count of less than 100×109/L without platelet transfusion requirements. Partial response (PR) was defined as fulfilling the criteria for CR in the peripheral blood but with 6% to 25% blasts in the marrow or at least a 50% decrease in bone marrow blasts compared with pre-treatment values.
Translational studies
Peripheral blood mononuclear cell isolation: heparinized whole-blood (10 ml) collected at baseline (day 0, before sorafenib administration), day +1 and day +4, was subjected to RBC lysis in hypotonic buffer (0.15 M NH4Cl, 0.02 M Tris–HCl), and mononuclear cells were resuspended and washed once with PBS.
For evaluation of apoptosis in leukemic blasts, the mononuclear cells from patient blood samples were stained with APC-conjugated anti-CD34 or anti-CD33 antibodies and annexin-V-FLUOS, and the changes in cellular mitochondrial inner transmembrane potential were determined by staining with chloromethyl-X-rosamine (CMXRos, Invitrogen Co). The samples were analyzed by three-color flow cytometry as previously described.17, 18
For immunoblot analyses, rabbit polyclonal phospho-FLT3 (Tyr591) (Cell Signaling Technology, Beverly, MA, USA), rabbit polyclonal FLT3 (Santa Cruz Biotechnology, Santa Cruz, CA, USA), mouse monoclonal phospho-ERK (Thr202/Tyr204), horseradish peroxidase (HRP)-conjugated goat anti-rabbit and anti-mouse IgG secondary antibodies (Santa Cruz Biotechnology) were used. Antibody binding was visualized with the use of an enhanced chemiluminescence detection system (ECL-plus; Amersham Pharmacia Biotech). The semiquantitative immunoblotting data were generated by Scion Imaging software (Beta 4.03; Scion Corporation, Frederick, MD, USA).
Statistical analysis
Dose escalations were carried out in standard “3+3” design. Occurrence of dose limiting toxicity (DLT) in first cycle in any patient was cause for the addition of at least 3 additional patients at that dose level. All patients who received therapy on study were considered evaluable for toxicity. DLT was defined according to toxicity occurring during the first cycle. Non-hematologic DLT was defined as grade 3 or 4 toxicity (NCI common criteria, version 3.0) considered at least possibly related to sorafenib. Grade 3 or 4 nausea, vomiting and diarrhea were considered DLT only if uncontrolled by adequate therapy. Hematologic DLT was defined as grade 3 or higher pancytopenia with a hypocellular bone marrow and no marrow blasts lasting for six weeks or more after the start of a course.
Patients were enrolled into one of the two schedules using an alternating assignment strategy starting with Schedule A. Once enrollment in one dose level was completed and all patients were evaluated for toxicity and determined not to have DLT, the next cohort was enrolled in the alternative schedule. Patients who were removed from study before completion of one cycle of therapy in the absence of DLT (e.g. for disease progression) were replaced. The alternating assignment strategy was repeated until a maximally tolerated dose (MTD) was reached within a schedule after which all subsequent patients were enrolled into the schedule that remained open. MTD was defined as the highest dose at which fewer than 2 of 6 patients experienced DLT. Additional patients were enrolled at the MTD on each schedule to further define safety.
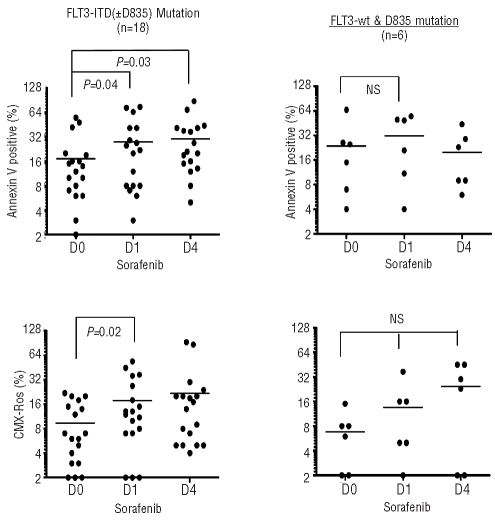
Changes in apoptosis (Annexin V binding), mitochondrial inner membrane potential (CMXRos staining) on days 1 and 4 compared to baseline in leukemia cells from patients with FLT3 ITD mutation ± D835 mutation versus FLT3 wild-type/D835 mutation alone were assessed by Student’s t-test for significance.
Results
Patients’ characteristics
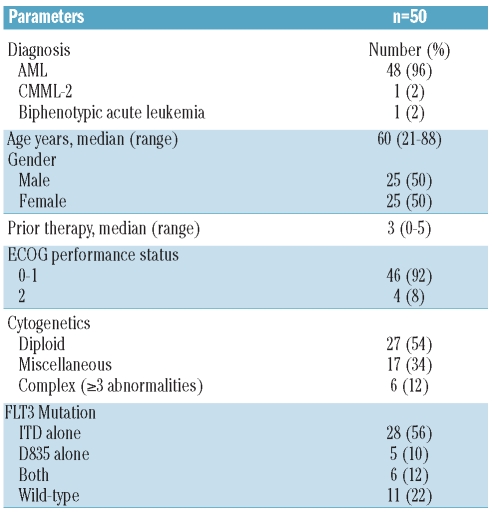
From August 2006 to December 2009, 50 patients were enrolled: 31 to Schedule A and 19 to Schedule B. More patients were enrolled to Schedule A as this schedule was favored first for expansion at MTD. The median age was 61 years (range 21-88 years). Forty-eight patients had a diagnosis of AML, one CMML-2, and one with biphenotypic leukemia (Table 2). Twelve (25%) of the 48 patients with AML had secondary AML and all but one (with CMML-2) had received prior therapies (median 3; range 0–5 prior therapies). Twenty-eight (56%) patients had FLT3-ITD only, 5 (10%) had D835 only, and 6 (12%) had both mutations. Three (6%) patients had received other FLT3 inhibitors (AC220=2, KW 2449=1) and 8 (16%) had prior stem cell transplant (SCT) (Table 2).
Table 2.
Characteristics of patients treated with sorafenib (n=50).
Patients received a total of 120 cycles of sorafenib. Five patients needed brief (one to 7 days) interruptions due to toxicities. Eight (16%) patients discontinued therapy before the end of the first cycle; 2 of them discontinued for DLT and 6 (12%) for progressive disease (5 in Schedule A and one in Schedule B) and were thus replaced. Twenty-five (81%) of 31 patients in Schedule A and 16 (84%) of 19 patients in Schedule B completed at least one cycle of study treatment. Twenty patients (40%) completed more than one cycle (2 in 10 patients, 3 in 3 patients, and 4 or more in 7 patients).
Toxicity
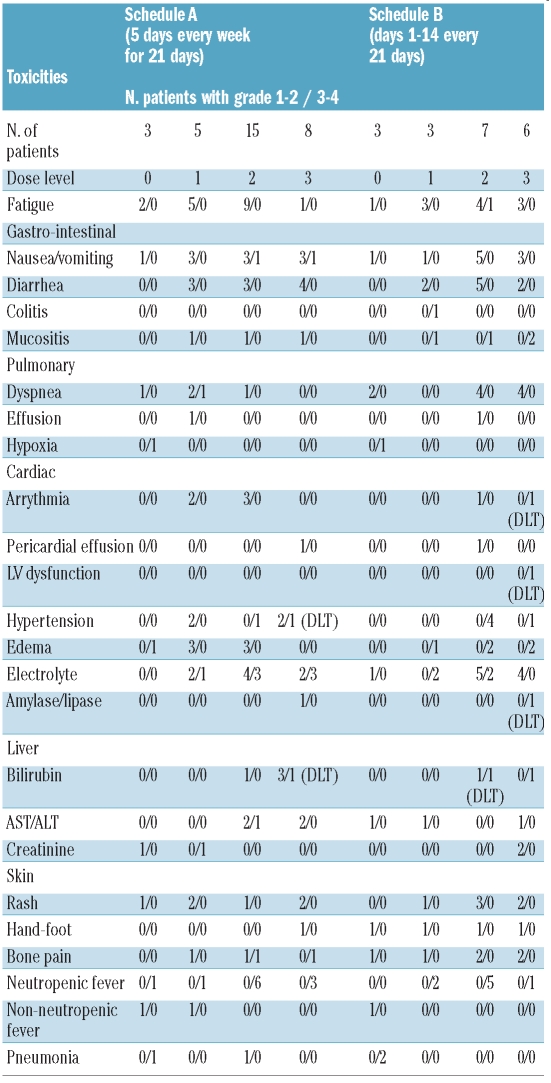
Treatment overall was well tolerated. The most common adverse events (regardless of causality) were fatigue in 29 (58%), nausea/vomiting in 22 (44%), diarrhea in 18 (36%) and dyspnea in 15 (30%) patients (Table 3). Grade 3-4 non-DLT defining toxicities included fatigue in one patient, nausea/vomiting in 2 patients, dyspnea in one, creatinine elevation in one patient, transient electrolyte abnormalities in 14 patients and bone pain in one patient. Grade 1-2 skin rash was encountered in 12 (24%) patients and 5 (10%) patients developed grade 1-2 hand-foot syndrome. There was no clear difference in the incidence or type of adverse events between the two treatment schedules. On Schedule A, two DLTs (grade 3 hyperbilirubinemia and hypertension, respectively) were encountered at dose level 3 (i.e. 600 mg twice daily for five days per week). A total of 15 patients (one received less than 21 days of therapy and was replaced) were treated at dose level 2 (400 mg twice daily) without any DLT. On Schedule B one DLT (grade 3 hyperbilirubinemia) was encountered at dose level 2 (400 mg twice daily) with no additional DLT after expansion of the cohort to 6 patients. Two DLTs (congestive heart failure/atrial fibrillation and asymptomatic elevation of amylase/lipase, respectively) were encountered in the next dose cohort. Thus, for both schedules, dose level 2 (400mg twice daily) was deemed to be the MTD. Toxicities regardless of attribution and according to schedule and dose level are summarized in Table 3.
Table 3.
Toxicities irrespective of attribution.
Responses
A total of 5 (10%) patients responded, 3 with CR and 2 with CRp (Schedule A=3, Schedule B=2) (Online Supplementary Figure S1). The age range of the responders was 21–75 years and median number of prior therapies was 3 (range 1–4). All but one of the responders had reduction of bone marrow blasts to below 5% by C1D21. Two of the responders proceeded with stem cell transplant and in the other 3 patients responses lasted for four weeks, four weeks, and more than six months, respectively. The patient with the longest response (Patient 41), with AML evolving from CMML, had received 2 prior therapies (with a brief response to decitabine) and had FLT3-ITD. On Schedule B at dose of 600 mg twice daily, he developed elevation of amylase and lipase after five days of administration of sorafenib requiring interruption of therapy. Still, by day 15 his bone marrow blast had decreased from 40% to 2%. He had also cleared his circulating blasts by day 5 and his platelets improved from 25×109/L to 103×109/L by day 8. Upon resolution of toxicity, he resumed therapy at 400 mg twice daily and is currently on cycle 12 of treatment.
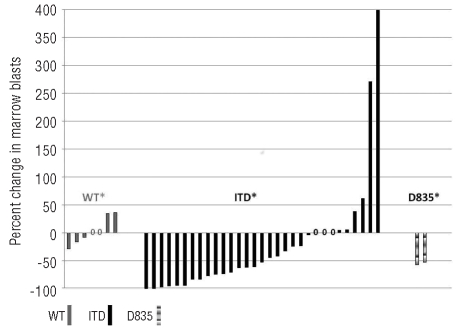
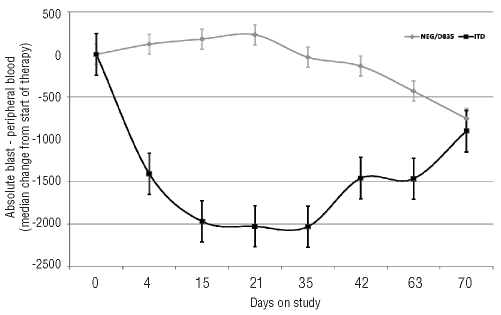
In addition, 3 patients (all FLT3-ITD) had clearance of marrow blasts (morphological leukemia free status) from pre-treatment blast count of 85%, 83%, and 55%, respectively without recovery of blood counts; one of these patients proceeded to stem cell transplant (SCT). Twelve (24%) additional patients had reduction in bone marrow blasts (≥50% in 9 and 25–49% in 3 patients) (Figure 1). The improvement in blast count lasted four weeks or longer in 11 of these patients. Two additional patients had a reduction of over 50% in circulating blasts that lasted for four weeks. In total, 7 patients (one achieving CR, one CRp and 5 with significant reduction in marrow blasts) were able to proceed to SCT after responding to sorafenib. The changes in peripheral blood blasts are shown in Figure 2 stratified by FLT3 ITD status and the marrow blast changes are summarized in the Online Supplementary Table S1 and S2.
Figure 1.
Percentage changes in bone marrow blasts from baseline according to FLT3 mutation status. Patients with both internal tandem duplication (ITD) and D835 mutation are counted as ITD mutation. Only information from patients with at least one follow-up bone marrow examination in addition to the baseline is included. *= no follow-up marrow done due to disease progression (ITD=4, WT=3, D835=3). 0=no change in marrow blast percentage.
Figure 2.
Changes in median number of peripheral blood absolute blast count (total white blood cell count X % blasts) with time according to FLT3 mutation status. Patients with both internal tandem duplication (ITD) and D835 mutation are counted as ITD mutation. (N, ITD=34, Wild-type/D835=16) in a compassionate use
Responses were seen at all dose levels but confined to patients with FLT3 mutation. No response was seen in patients without FLT3 mutation or among the 3 patients with FLT3-ITD mutation who had received prior therapy with other FLT3 inhibitors. These last 3 patients had transient reductions in marrow blasts without achieving remission on their prior therapy with other FLT3 inhibitors.
Translational studies
Data for translational studies is available for 24 patients. Induction of apoptosis and changes in mitochondrial membrane potential increased in peripheral blood mononuclear cells on days +1 and +4 compared to baseline in patients with FLT3 ITD and ITD+D835 dual mutation. In contrast, there was no statistically significant change in patients with D835 mutation or wild-type FLT3 (Figure 3). Protein lysate for immunoblots was available from 20 patient samples. Inhibition of FLT3 phosphorylation was seen in 6 out of 14 patient samples with FLT3 ITD mutation (Online Suplementary Figure S2). Five out of these 6 patients with demonstrable inhibition of FLT3 phosphorylation had clinical response (CR=one, reduction in marrow blast=4). Inhibition of ERK phosphorylation was seen in 6 patient samples (both ERK and FLT3 inhibition in 3). Three out of 6 patients without FLT3 ITD mutation who were tested showed reduction in FLT3 and/or ERK phosphorylation.
Figure 3.
Apoptosis and changes in mitochondrial membrane potential in peripheral blood mononuclear cells (PBMC). Apoptosis induction in PBMCs (collected prior to sorafenib and on days 1 and 4) was measured by annexin V binding and percentage of cells with changes in mitochondrial membrane potential was measured by staining with chloromethyl-X-rosamine (CMXRos, Invitrogen Co) using flow cytometry.
Discussion
In this phase I study we identify sorafenib as a valuable agent for the treatment of patients with AML and FLT3 ITD. Activity of sorafenib in this setting has been reported report involving 6 patients.19 Based on our study, the recommended phase II dose for sorafenib in acute leukemia is 400 mg twice daily given either for five days every week or for two consecutive weeks every three weeks. The MTD is similar to that found in the first phase I study in solid tumors (400 mg twice daily), although in that study sorafenib was administered continuously.20 In another phase I trial in solid tumors using a one week on and one week off schedule, the MTD was established at a higher dose of 600 mg twice daily.21 In phase I studies with hematologic malignancies, one study established MTD at 400 mg twice daily for 21 days in a 28 day cycle22 while a second study established MTD at 300 mg twice daily administered continuously.23 In our study, continuous dosing was not explored. The DLTs in our study included hyperbilirubinemia (in 2 patients), hypertension, hyperamylasemia and congestive heart failure/atrial fibrillation (one patient each). Other studies in solid tumors reported additional DLTs including fatigue, skin rash, hand-foot syndrome, and hypertension.20, 21
Sorafenib showed single-agent clinical anti-leukemic activity in patients with AML at multiple dose levels including CR in 3 patients and CRp in 2 patient. While CR/CRps have been reported with AC220, other FLT3 inhibitors as single agents have shown mostly reduction in blast counts, but rarely CR/CRp.24–27 Besides clinical activity, these studies have shown that effective FLT3 inhibition is necessary for clinical response.25,26 In our study, 5 of the 6 patients who had demonstrable FLT3 inhibition by Western blot had clinical responses (one CR, 4 reduction in marrow blasts). Our data show statistically higher apoptosis induction in FLT3 ITD±D835 samples, but results should be taken with caution because of the low number of samples from patients with FLT3 WT, partly due to the lower WBC count in these samples.
Unfortunately, responses with sorafenib in the present study and in studies with other FLT3 inhibitors have usually only been of short duration. This could either mean that alternative survival pathways are able to rescue AML cells despite blockade of FLT3 activation, or that other events (e.g. development of mutations in FLT3) make inhibition with the inhibitor no longer effective.28,29 Interestingly, we did not observe clinical activity in patients with FLT3 mutations who had been previously exposed to other FLT3 inhibitors. Investigation of the mechanism of secondary resistance to sorafenib and other FLT3 inhibitors is ongoing.
The ideal schedule for administration of FLT3 inhibitors in general and sorafenib in particular remains to be determined. We investigated two schedules with interrupted administration hoping that it would allow higher dose administration to achieve deeper FLT3 inhibition. Despite the discontinuous schedule, the MTD was no different than that achieved with a continuous administration in solid tumors.
The transient nature of the responses reported here with sorafenib and elsewhere with other FLT3 inhibitors25–27 suggests that combination therapy with other agents should be explored. Flt3-ITD up-regulates the chemokine receptor CXCR430 and indeed, inhibition of CXCR4 in combination with sorafenib resulted in enhanced anti-leukemia activity in vitro31 and this concept is presently being investigated in a clinical trial. Combination of sorafenib with the Bcl-2 inhibitor ABT-737 has shown synergistic activity32 and the new FLT3-ITD inhibitor Fl-700 was shown to also neutralize Mcl-1, a major anti-apoptotic protein.33 Combinations with chemotherapy may be particularly dependent on the schedule of administration, at least for some of the FLT3 inhibitors.34,35 Studies of sorafenib in combination with chemotherapy for patients with AML have been initiated and early reports suggest high response rate in patients with FLT3-ITD.36
We conclude that administration of sorafenib to patients with acute leukemias can be accomplished with a favorable toxicity profile, with a recommended phase II dose of 400 mg twice daily, similar to that used in solid tumor studies. Sorafenib has significant anti-leukemia activity in patients with FLT3 ITD. Further studies of sorafenib in these patients, including studies exploring combination with other agents are warranted.
Footnotes
Funding: NIH Grant U01CA062461.
Authorship and Disclosures
The information provided by the authors about contributions from persons listed as authors and in acknowledgments is available with the full text of this paper at www.haematologica.org.
Financial and other disclosures provided by the authors using the ICMJE (www.icmje.org) Uniform Format for Disclosure of Competing Interests are also available at www.haematologica.org.
References
- 1.Milella M, Kornblau SM, Andreeff M. The mitogen-activated protein kinase signaling module as a therapeutic target in hematologic malignancies. Rev Clin Exp Hematol. 2003;7(2):160–90. [PubMed] [Google Scholar]
- 2.Ricciardi MR, McQueen T, Chism D, Milella M, Estey E, Kaldjian E, et al. Quantitative single cell determination of ERK phosphorylation and regulation in relapsed and refractory primary acute myeloid leukemia. Leukemia. 2005;19(9):1543–9. doi: 10.1038/sj.leu.2403859. [DOI] [PubMed] [Google Scholar]
- 3.Kornblau SM, Womble M, Qiu YH, Jackson CE, Chen W, Konopleva M, et al. Simultaneous activation of multiple signal transduction pathways confers poor prognosis in acute myelogenous leukemia. Blood. 2006;108(7):2358–65. doi: 10.1182/blood-2006-02-003475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhang W, Konopleva M, Ruvolo VR, McQueen T, Evans RL, Bornmann WG, et al. Sorafenib induces apoptosis of AML cells via Bim-mediated activation of the intrinsic apoptotic pathway. Leukemia. 2008;22(4):808–18. doi: 10.1038/sj.leu.2405098. [DOI] [PubMed] [Google Scholar]
- 5.Deng X, Kornblau SM, Ruvolo PP, May WS., Jr Regulation of Bcl2 phosphorylation and potential significance for leukemic cell chemoresistance. J Natl Cancer Inst Monogr. 2001(28):30–7. doi: 10.1093/oxfordjournals.jncimonographs.a024254. [DOI] [PubMed] [Google Scholar]
- 6.Yu C, Bruzek LM, Meng XW, Gores GJ, Carter CA, Kaufmann SH, et al. The role of Mcl-1 downregulation in the proapoptotic activity of the multikinase inhibitor BAY 43-9006. Oncogene. 2005;24(46):6861–9. doi: 10.1038/sj.onc.1208841. [DOI] [PubMed] [Google Scholar]
- 7.Milella M, Kornblau SM, Estrov Z, Carter BZ, Lapillonne H, Harris D, et al. Therapeutic targeting of the MEK/MAPK signal transduction module in acute myeloid leukemia. J Clin Invest. 2001;108 (6):851–9. doi: 10.1172/JCI12807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Adnane L, Trail PA, Taylor I, Wilhelm SM. Sorafenib (BAY 43-9006, Nexavar), a dual-action inhibitor that targets RAF/MEK/ERK pathway in tumor cells and tyrosine kinases VEGFR/PDGFR in tumor vasculature. Methods Enzymol. 2006;407:597–612. doi: 10.1016/S0076-6879(05)07047-3. [DOI] [PubMed] [Google Scholar]
- 9.Sridhar SS, Hedley D, Siu LL. Raf kinase as a target for anticancer therapeutics. Mol Cancer Ther. 2005;4(4):677–85. doi: 10.1158/1535-7163.MCT-04-0297. [DOI] [PubMed] [Google Scholar]
- 10.Gilliland DG, Griffin JD. The roles of FLT3 in hematopoiesis and leukemia. Blood. 2002;100(5):1532–42. doi: 10.1182/blood-2002-02-0492. [DOI] [PubMed] [Google Scholar]
- 11.Nakao M, Yokota S, Iwai T, Kaneko H, Horiike S, Kashima K, et al. Internal tandem duplication of the flt3 gene found in acute myeloid leukemia. Leukemia. 1996;10(12):1911–8. [PubMed] [Google Scholar]
- 12.Whitman SP, Ruppert AS, Radmacher MD, Mrozek K, Paschka P, Langer C, et al. FLT3 D835/I836 mutations are associated with poor disease-free survival and a distinct gene-expression signature among younger adults with de novo cytogenetically normal acute myeloid leukemia lacking FLT3 internal tandem duplications. Blood. 2008;111 (3):1552–9. doi: 10.1182/blood-2007-08-107946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hayakawa F, Towatari M, Kiyoi H, Tanimoto M, Kitamura T, Saito H, et al. Tandem-duplicated Flt3 constitutively activates STAT5 and MAP kinase and introduces autonomous cell growth in IL-3-dependent cell lines. Oncogene. 2000;19 (5):624–31. doi: 10.1038/sj.onc.1203354. [DOI] [PubMed] [Google Scholar]
- 14.Auclair D, Miller D, Yatsula V, Pickett W, Carter C, Chang Y, et al. Antitumor activity of sorafenib in FLT3-driven leukemic cells. Leukemia. 2007;21(3):439–45. doi: 10.1038/sj.leu.2404508. [DOI] [PubMed] [Google Scholar]
- 15.Zhang W, Konopleva M, Shi YX, McQueen T, Harris D, Ling X, et al. Mutant FLT3: a direct target of sorafenib in acute myelogenous leukemia. J Natl Cancer Inst. 2008;100(3):184–98. doi: 10.1093/jnci/djm328. [DOI] [PubMed] [Google Scholar]
- 16.Cheson BD, Bennett JM, Kopecky KJ, Buchner T, Willman CL, Estey EH, et al. Revised recommendations of the International Working Group for Diagnosis, Standardization of Response Criteria, Treatment Outcomes, and Reporting Standards for Therapeutic Trials in Acute Myeloid Leukemia. J Clin Oncol. 2003;21(24):4642–9. doi: 10.1200/JCO.2003.04.036. [DOI] [PubMed] [Google Scholar]
- 17.Konopleva M, Tsao T, Estrov Z, Lee RM, Wang RY, Jackson CE, et al. The synthetic triterpenoid 2-cyano-3,12-dioxooleana-1,9-dien-28-oic acid induces caspase-dependent and -independent apoptosis in acute myelogenous leukemia. Cancer Res. 2004;64(21):7927–35. doi: 10.1158/0008-5472.CAN-03-2402. [DOI] [PubMed] [Google Scholar]
- 18.Clodi K, Kliche KO, Zhao S, Weidner D, Schenk T, Consoli U, et al. Cell-surface exposure of phosphatidylserine correlates with the stage of fludarabine-induced apoptosis in chronic lymphocytic leukemia and expression of apoptosis-regulating genes. Cytometry. 2000;40(1):19–25. doi: 10.1002/(sici)1097-0320(20000501)40:1<19::aid-cyto3>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- 19.Metzelder S, Wang Y, Wollmer E, Wanzel M, Teichler S, Chaturvedi A, et al. Compassionate use of sorafenib in FLT3-ITD-positive acute myeloid leukemia: sustained regression before and after allogeneic stem cell transplantation. Blood. 2009;113(26):6567–71. doi: 10.1182/blood-2009-03-208298. [DOI] [PubMed] [Google Scholar]
- 20.Strumberg D, Richly H, Hilger RA, Schleucher N, Korfee S, Tewes M, et al. Phase I clinical and pharmacokinetic study of the Novel Raf kinase and vascular endothelial growth factor receptor inhibitor BAY 43-9006 in patients with advanced refractory solid tumors. J Clin Oncol. 2005;23(5):965–72. doi: 10.1200/JCO.2005.06.124. [DOI] [PubMed] [Google Scholar]
- 21.Clark JW, Eder JP, Ryan D, Lathia C, Lenz HJ. Safety and pharmacokinetics of the dual action Raf kinase and vascular endothelial growth factor receptor inhibitor, BAY 43-9006, in patients with advanced, refractory solid tumors. Clin Cancer Res. 2005;11(15):5472–80. doi: 10.1158/1078-0432.CCR-04-2658. [DOI] [PubMed] [Google Scholar]
- 22.Pratz KW, Cho E, Karp J, Levis M, Zhao M, Rudek M, et al. Phase I dose escalation trial of sorafenib as a single agent for adults with relapsed and refractory acute leukemias. J Clin Oncol. [Abstract] 2009;27(15S (abstr 7065)):371s. [Google Scholar]
- 23.Crump M, Hedley D, Kamel-Reid S, Leber B, Wells R, Brandwein J, et al. A randomized phase I clinical and biologic study of two schedules of sorafenib in patients with myelodysplastic syndrome or acute myeloid leukemia: a NCIC (National Cancer Institute of Canada) Clinical Trials Group Study. Leuk Lymphoma. 2010;51(2):252–60. doi: 10.3109/10428190903585286. [DOI] [PubMed] [Google Scholar]
- 24.Stone RM, DeAngelo DJ, Klimek V, Galinsky I, Estey E, Nimer SD, et al. Patients with acute myeloid leukemia and an activating mutation in FLT3 respond to a small-molecule FLT3 tyrosine kinase inhibitor, PKC412. Blood. 2005;105(1):54–60. doi: 10.1182/blood-2004-03-0891. [DOI] [PubMed] [Google Scholar]
- 25.Smith BD, Levis M, Beran M, Giles F, Kantarjian H, Berg K, et al. Single-agent CEP-701, a novel FLT3 inhibitor, shows biologic and clinical activity in patients with relapsed or refractory acute myeloid leukemia. Blood. 2004;103(10):3669–76. doi: 10.1182/blood-2003-11-3775. [DOI] [PubMed] [Google Scholar]
- 26.Pratz KW, Cortes J, Roboz GJ, Rao N, Arowojolu O, Stine A, et al. A pharmaco-dynamic study of the FLT3 inhibitor KW-2449 yields insight into the basis for clinical response. Blood. 2009;113(17):3938–46. doi: 10.1182/blood-2008-09-177030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cortes J, Foran J, Ghirdaladze D, DeVetten M, Zodelava M, Holman P, et al. AC220, a potent, selective, second generation FLT3 receptor tyrosine kinase (RTK) inhibitor, in a first-in-human (FIH) phase 1 AML study Blood 200911422 (abstr 636)26419414860 [Google Scholar]
- 28.von Bubnoff N, Engh RA, Aberg E, Sanger J, Peschel C, Duyster J. FMS-like tyrosine kinase 3-internal tandem duplication tyrosine kinase inhibitors display a nonoverlap-ping profile of resistance mutations in vitro. Cancer Res. 2009;69(7):3032–41. doi: 10.1158/0008-5472.CAN-08-2923. [DOI] [PubMed] [Google Scholar]
- 29.Heidel F, Solem FK, Breitenbuecher F, Lipka DB, Kasper S, Thiede MH, et al. Clinical resistance to the kinase inhibitor PKC412 in acute myeloid leukemia by mutation of Asn-676 in the FLT3 tyrosine kinase domain. Blood. 2006;107(1):293–300. doi: 10.1182/blood-2005-06-2469. [DOI] [PubMed] [Google Scholar]
- 30.Rombouts EJ, Pavic B, Lowenberg B, Ploemacher RE. Relation between CXCR-4 expression, Flt3 mutations, and unfavorable prognosis of adult acute myeloid leukemia. Blood. 2004;104(2):550–7. doi: 10.1182/blood-2004-02-0566. [DOI] [PubMed] [Google Scholar]
- 31.Zeng Z, Shi YX, Samudio IJ, Wang RY, Ling X, Frolova O, et al. Targeting the leukemia microenvironment by CXCR4 inhibition overcomes resistance to kinase inhibitors and chemotherapy in AML. Blood. 2009;113(24):6215–24. doi: 10.1182/blood-2008-05-158311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kohl TM, Hellinger C, Ahmed F, Buske C, Hiddemann W, Bohlander SK, et al. BH3 mimetic ABT-737 neutralizes resistance to FLT3 inhibitor treatment mediated by FLT3-independent expression of BCL2 in primary AML blasts. Leukemia. 2007;21 (8):1763–72. doi: 10.1038/sj.leu.2404776. [DOI] [PubMed] [Google Scholar]
- 33.Kojima K, Konopleva M, Tsao T, Andreeff M, Ishida H, Shiotsu Y, et al. Selective FLT3 inhibitor FI-700 neutralizes Mcl-1 and enhances p53-mediated apoptosis in AML cells with activating mutations of FLT3 through Mcl-1/Noxa axis. Leukemia. 2010;24(1):33–43. doi: 10.1038/leu.2009.212. [DOI] [PubMed] [Google Scholar]
- 34.Stone RM, Fischer T, Paquette R, Schiller G, Schiffer CA, Ehninger G, et al. Phase IB study of PKC412, an oral FLT3 kinase inhibitor, in sequential and simultaneous combinations with daunorubicin and cytarabine (DA) induction and high-dose cytarabine consolidation in newly diagnosed adult patients (pts) with acute myeloid leukemia (AML) under age 61. Blood. 2006;108(11 (abstr 157)):50a. [Google Scholar]
- 35.Levis M, Pham R, Smith BD, Small D. In vitro studies of a FLT3 inhibitor combined with chemotherapy: sequence of administration is important to achieve synergistic cyto-toxic effects. Blood. 2004;104(4):1145–50. doi: 10.1182/blood-2004-01-0388. [DOI] [PubMed] [Google Scholar]
- 36.Ravandi Kashani F, Cortes J, Faderl S, Jones D, Byrd A, Brandt M, et al. Phase I/II study of idarubicin (Ida), high-dose ara-C, and sorafenib (S) in patients (pts) younger than 65 years with acute myeloid leukemia (AML) J Clin Oncol. 2009;27(15s (abstr 7018)):360s. [Google Scholar]