Abstract
Background
The number of CD34+ cells mobilized from bone marrow to peripheral blood after administration of granulocyte colony-stimulating factor varies greatly among healthy donors. This fact might be explained, at least in part, by constitutional differences in genes involved in the interactions tethering CD34+ cells to the bone marrow.
Design and Methods
We analyzed genetic characteristics associated with CD34+ cell mobilization in 112 healthy individuals receiving granulocyte colony-stimulating factor (filgrastim; 10 μg/kg; 5 days).
Results
Genetic variants in VCAM1 and in CD44 were associated with the number of CD34+ cells in peripheral blood after granulocyte colony-stimulating factor administration (P=0.02 and P=0.04, respectively), with the quantity of CD34+ cells ×106/kg of donor (4.6 versus 6.3; P<0.001 and 7 versus 5.6; P=0.025, respectively), and with total CD34+ cells ×106 (355 versus 495; P=0.002 and 522 versus 422; P=0.012, respectively) in the first apheresis. Of note, granulocyte colony-stimulating factor administration was associated with complete disappearance of VCAM1 mRNA expression in peripheral blood. Moreover, genetic variants in granulocyte colony-stimulating factor receptor (CSF3R) and in CXCL12 were associated with a lower and higher number of granulocyte colony-stimulating factor-mobilized CD34+ cells/μL in peripheral blood (81 versus 106; P=0.002 and 165 versus 98; P=0.02, respectively) and a genetic variant in CXCR4 was associated with a lower quantity of CD34+ cells ×106/kg of donor and total CD34+ cells ×106 (5.3 versus 6.7; P=0.02 and 399 versus 533; P=0.01, respectively).
Conclusions
In conclusion, genetic variability in molecules involved in migration and homing of CD34+ cells influences the degree of mobilization of these cells.
Keywords: VCAM1, CD44, polymorphisms, CD34+ cell mobilization, CD34+ cell yield
Introduction
Allogeneic hematopoietic stem cell transplantation (HSCT) is used as a curative treatment for life-threatening hematologic diseases. Different sources of CD34+ cells can be used for allogeneic HSCT, including bone marrow (BM), peripheral blood (PB), and umbilical cord blood.1,2 Currently, more than 70% of allogeneic HSCT are performed using PB as the source of CD34+ cells.2 Advantages of using PB rather than BM for allogeneic HSCT are easier CD34+ cell collection, quicker post-transplant neutrophil and platelet recovery, a reduction of infectious complications, and lower transplant-related mortality for patients with advanced hematologic malignancies.3,4
The precise number of donor CD34+ cells that must be given to the recipient in order to achieve robust myeloid and lymphoid engraftment has not yet been defined, although transplantation of a large quantity of CD34+ cells seems to be associated with better survival.5 The number of CD34+ cells mobilized into PB following administration of granulocyte colony-stimulating factor (G-CSF) varies significantly among donors.6 Although different factors may influence CD34+ cell mobilization, such as age, gender, or weight,7,8 there are currently no reliable predictive factors of poor or good CD34+ cell mobilization in healthy donors. We hypothesized that individual genetic factors might explain, at least in part, the variability and that these factors might be used to predict CD34+ cell mobilization.
G-CSF is the predominant cytokine used for mobilizing CD34+ cells in healthy donors.1 During the G-CSF-induced mobilization process, the interactions tethering CD34+ cells to the BM stroma are disrupted. Some of these interactions include CXCR4 with CXCL12, very late antigen-4 (VLA-4) with vascular cell adhesion molecule-1 (VCAM-1), and CD44 with hyaluronic acid. G-CSF, through its receptor CSF3R, plays a pivotal role in mobilization of CD34+ cells by releasing proteolytic enzymes, such as neutrophil elastase (NE), catepsin-G (CG), matrix metalloproteinase-9 (MMP-9), and by down-regulating the expression of some of these genes.1 Other molecules more recently described to be involved in the trafficking and homing of CD34+ cells include β3-adrenergic receptor (ADRB3) and guanine-nucleotide-binding protein stimulatory alpha subunit (GNAS).9,10
An increasing number of studies have found evidence that genetic factors known as single nucleotide polymorphisms (SNP) explain, in part, the significant inter-individual variability in responses to drug administration.11 Identifying SNP predictive of a poor or good response to G-CSF, in terms of number of CD34+ cells mobilized, might be useful when discussing the possibility of using a different mobilizing agent or a different source of CD34+ cells for allogeneic HSCT.
The aim of this study was to evaluate a possible association between SNP in 16 genes involved in adhesive and chemotactic interactions to retain CD34+ cells within the BM with the number of G-CSF-mobilized CD34+ cells/kg of donor in the first apheresis in healthy donors.
Design and Methods
Study population
Two groups of healthy people were studied: group 1 consisted of 112 individuals who were given G-CSF (filgrastim 10 μg/kg subcutaneous for 5 days) to mobilize CD34+ cells for HLA-identical sibling allogeneic HSCT at the Hematology Department of Universitario Virgen del Rocío Hospital, Seville, between January 1999 and March 2008. Group 2 consisted of 107 voluntary blood donors from the Regional Centre for Blood Transfusion, Seville. Group 2 was included since the number of CD34+ cells in PB in steady state (i.e. without having received G-CSF) was not available for group 1. All donors were Caucasians from Spain. The local ethics committee of the Universitario Virgen del Rocío Hospital provided institutional review board-approval for this study, and informed consent was obtained from all donors in accordance with the Declaration of Helsinki. The main characteristics of the donors in both groups are presented in Online Supplementary Table S1.
Peripheral blood cell counts
White blood cell (WBC) and platelet counts were analyzed by a Sysmex XE-2100 auto-analyzer. The number of CD34+ cells in PB was quantified by conventional flow cytometry in a Becton Dickinson FACS-Calibur flow cytometer acquiring a total of 10,000 events and using monoclonal antibody CD34-PE. CD45-FITC monoclonal antibody was used to gate pure leukocyte populations. Both monoclonal antibodies were purchased from BD Biosciences, San Jose, CA (USA).
Selection of genes, single nucleotide polymorphisms and genotyping
Sixteen genes (CXCL12, CXCR4, VCAM-1, VLA-4, G-CSF, CSF3R, CD34, ADRB3, CXCL2, CXCR2, CD44, Kit ligand, c-Kit, MMP-9, CTSG, GNAS) were screened at the NCBI database searching for SNP that fulfilled the following criteria: (i) located at either 3′UTR or 5′UTR regions or at coding regions associated with an amino acid change, (ii) frequency of the less frequent homozygous allele more than 10% in Caucasians, and (iii) associated with a functional change of the gene or involved in the pathogenesis of diseases. When no SNP located at either 3′UTR or 5′UTR regions or at coding regions had a frequency higher than 10%, intronic SNP were selected. A total of 28 SNP were selected for genotyping (Online Supplementary Table S2). Genomic DNA isolation and allelic discrimination polymerase chain reaction (PCR) methods are described in the Online Supplementary Design and Methods.
RNA isolation and gene expression analysis
Details of total RNA isolation and the gene expression analysis are provided in the Online Supplementary Design and Methods.
Statistical analysis
SNP were analyzed for deviation from Hardy-Weinberg equilibrium, using a χ2 test. Allele frequencies and genotype frequencies were formulated by direct counting. In group 1, a univariate analysis was performed for clinical characteristics and each genotype to evaluate possible associations with the CD34+ cell count in PB on the fifth day of G-CSF, with the number of CD34+ cells/kg of donor obtained after the first apheresis and with the total number of CD34+ cells obtained after the first apheresis. In group 2, a univariate analysis was performed to evaluate a possible association of clinical characteristics and genotype with the CD34+ cell steady state count in PB. Pearson’s correlation test was used to analyze the effects of age on the CD34+ cell count in PB and with the number of CD34+ cells/kg of donor collected with the first apheresis. A t-test was used to analyze the difference in the CD34+ cell count in PB and the number of CD34+ cells/kg of donor between male and female donors. Three subgroups were established regarding the genotype for each polymorphism (homozygous more frequent, heterozygous, and homozygous less frequent), which were clustered afterwards into two sub-groups, regarding the presence or absence (in either homozygosis or heterozygosis) of the SNP allele. Homogeneity and normality in the variables of the study were checked using Levene’s test and the Shapiro-Wilk test, respectively. Comparisons between three subgroups were made by one-way analysis of variance, followed by Bonferroni’s test for the identification of statistically distinct groups. Comparisons between two subgroups were performed with a t-test, using Levene’s test to check for homogeneity of the variances. Age, gender and genetic polymorphisms were considered as independent variables, and CD34+ cell count in PB, number of CD34+ cells/kg of donor and the total number of CD34+ cells after the first apheresis were considered as dependent variables. Multivariate linear and multivariate logistic regression analyses were performed, including those variables showing an association or trend in the univariate analysis (P<0.1). Real time PCR results of mRNA of the different genes were analyzed statistically by either the Kruskal-Wallis test or Mann-Whitney U test, making comparisons among the different subgroups. The effect of G-CSF administration on gene expression was also analyzed by the Mann-Whitney U test. Differences were considered statistically significant when P values were less than 0.05. All statistical analyses were performed using the SPSS software v 15.0 (Chicago, IL, USA).
Results
Clinical and hematologic correlations with the number of CD34+ cells in peripheral blood in steady state and after granulocyte colony-stimulating factor
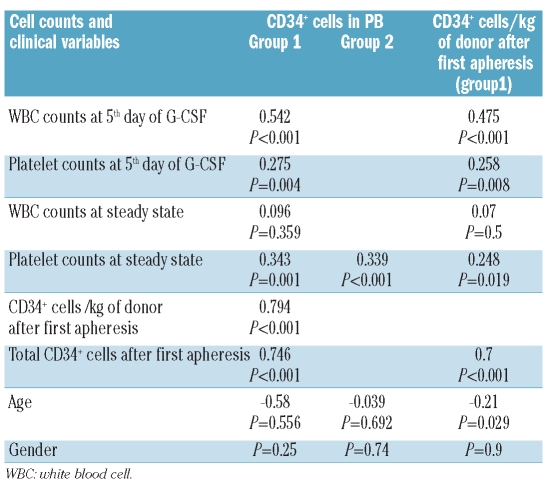
The number of CD34+ cells/μL in PB after 5 days of G-CSF, CD34+ cells ×106/kg of donor and total CD34+ cell count x 106 obtained after the first apheresis were: median [range] 99 [21–267], 6.3 [1–24] and 477 [84–2006], respectively. The median number [range] of CD34+ cells/μL in PB in steady state was 5.7 [1–51] (Online Supplementary Table S3). There was no correlation between CD34+ cell count in PB before or after G-CSF with age or gender. However, the number of CD34+ cells/kg of donor after the first apheresis was negatively correlated with age (r=−0.21, P=0.03) (Table 1).
Table 1.
Correlation analyses of CD34+ cell count/μL of PB and CD34+ cells/kg of donor after G-CSF (Group1) and in steady state (Group 2) with different cell counts and with clinical variables. Pearson’s correlation coefficients and P values are shown. P gender values were calculated by Students’ T test.
Single nucleotide polymorphisms and CD34+ cell numbers
Single nucleotide polymorphisms associated with CD34+ cell count in peripheral blood after granulocyte colony-stimulating factor, with CD34+ cells/kg of donor and with total CD34+ cells after the first apheresis
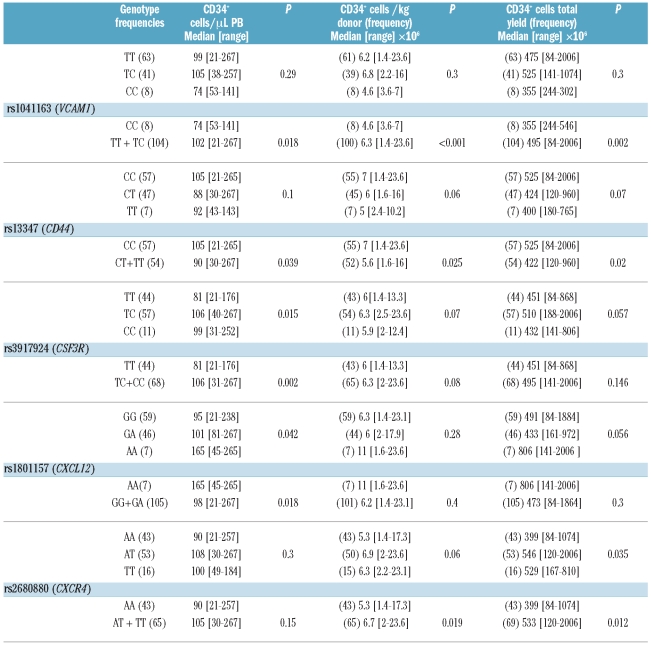
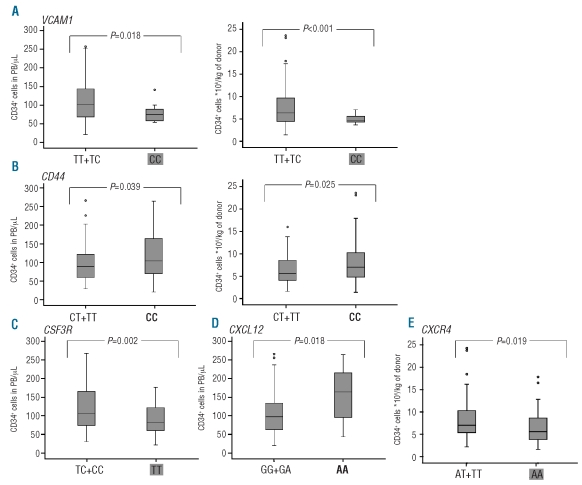
Two out of 28 SNP tested, one in VCAM1 and one in CD44, were significantly associated with CD34+ cell count in PB after G-CSF (Table 2). Both were in Hardy-Weinberg equilibrium. The genetic variant CC rs1041163 in VCAM1, corresponding to homozygous less frequent, was associated with a lower CD34+ cell count in PB after G-CSF (P=0.02), with a lower number of CD34+ cells/kg of donor after the first apheresis (P<0.001), and with the total number of CD34+ cells collected after the first apheresis (P=0.002) (Figure 1A and Table 2). The genetic variant CC rs13347 in CD44, corresponding to the homozygous most frequent, was associated with a higher CD34+ cell count in PB (P=0.04), with a higher number of CD34+ cells/kg of donor (P=0.025), and with the total number of CD34+ cells after the first apheresis (P=0.02) (Figure 1B and Table 2). Finally, multivariate logistic regression analysis was performed including the SNP with greatest clinical importance and the age of donor as independent variables, and the median value for CD34+ cells/kg as a dependent variable. rs1041163 in VCAM1 was the variable with the greatest impact on the number of CD34+ cells/kg of donor (P=0.05).
Table 2.
SNP associated with levels of CD34+ cells after G-CSF: associations of SNP with the number of CD34+ cells in PB, with the CD34+cells/kg of donor and with the total yield of CD34+ cells after the first apheresis.
Figure 1.
Association of SNP with CD34+ cell count in PB and with CD34+ cells/kg of donor: (A) rs1041163 in VCAM1. CC corresponds to homozygous recessive. (B) rs13347 in CD44. CC corresponds to homozygous dominant. (C) rs3917924 in CSF3R. TT corresponds to homozygous dominant. (D) rs1801157 in CXCL12. AA corresponds to homozygous recessive. (E) rs2680880 in CXCR4. AA corresponds to homozygous dominant. CD34+ cells/μL of PB values at fifth day of G-CSF and CD34+ cells/kg of donor after the first apheresis are shown in the different genotype groups. * and • indicate outliers. Bold and shaded genotypes are associated with lower and higher apheresis yields, respectively.
Single nucleotide polymorphisms associated only with the CD34+ cell count in peripheral blood after granulocyte colony-stimulating factor
Two SNP were associated with CD34+ cell count in PB after G-CSF without influencing the number of CD34+ cells/kg of donor after the first apheresis. The genetic variant TT rs3917924 in CSF3R, corresponding to homozygous most frequent, was associated with lower CD34+ cell count after G-CSF (P=0.002), showing a trend to an association with the number of CD34+ cells/kg of donor after the first apheresis (P=0.08) (Figure 1C and Table 2). The genetic variant AA rs1801157 in CXCL12, corresponding to homozygous less frequent, was associated with a higher CD34+ cell count in PB after G-CSF (P=0.02) (Figure 1D). Finally, multivariate linear regression analysis including these two SNP, the previous ones (in VCAM1 and in CD44), and the age of donor showed that rs3917924 in CSF3R was the variable with the greatest impact on the CD34+ cells in PB (P=0.02, B=−27). None of these SNP was associated with CD34+ cell count in PB in steady state.
Single nucleotide polymorphisms associated with number of CD34+ cells/kg of donor and with total CD34+ cells after the first apheresis
The genetic variant AA rs2680880 in CXCR4, corresponding to homozygous most frequent, was significantly associated with a lower number of CD34+ cells/kg of donor after the first apheresis (P=0.02) and with the total number of CD34+ cells collected after the first apheresis (P=0.01). This variant showed a tendency to an association with lower CD34+ cell levels in PB after G-CSF (P=0.15) (Figure 1D and Table 2).
Finally, results obtained for the different SNP associated with the amount of CD34+ cells after G-CSF were validated in a different center (Hospital Clinic, Barcelona). The characteristics and apheresis results of this population are shown in Online Supplementary Table S6. The apheresis results in this population were very different, with much lower apheresis yields than in the first population (P<0.001), which may be due to differences in the apheresis process. SNP associated with the number of CD34+ cells in PB/μL after G-CSF in the first cohort (SNP in VCAM1, CSF3R, CD44 and CXCL12) presented the same pattern in both cohorts for mean values of CD34+ cells in PB/μL after G-CSF (Online Supplementary Figure S1).
Association of single nucleotide polymorphisms with gene expression
In order to determine whether CC rs1041163 in VCAM1, CC rs13347 in CD44, TT rs3917924 in CSF3R, AA rs1801157 in CXCL12, and AA rs2680880 in CXCR4 had any influence on gene expression, mRNA levels of these genes were quantified in PB in steady state and after G-CSF. No differences were observed in mRNA expression among the different VCAM1 genotypes at steady state levels. However, VCAM1 mRNA was undetected in PB, in any of the different genotypes, after G-CSF.
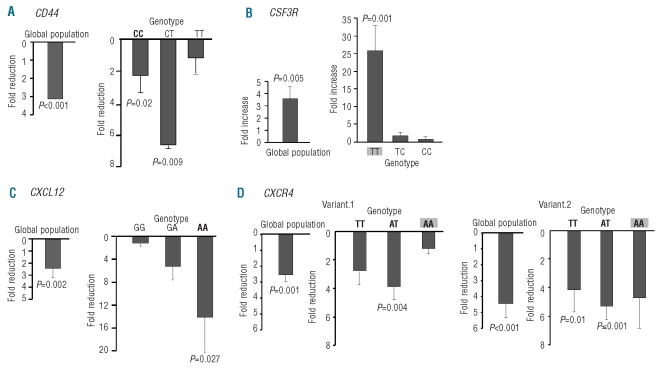
No differences were observed in mRNA expression among the different CD44 genotypes at steady state levels. G-CSF caused a global decrease in the expression of CD44 (P<0.001), this effect being significant in subjects with the CC (P=0.02) and CT variants (P=0.009), but not in those with the TT variant, the group with the lowest CD34+ cell yields (Figure 2A).
Figure 2.
Effect of G-CSF on mRNA levels of CD44, CSF3R, CXCL12 and CXCR4 in PB. (A) Reduction of CD44 expression in the global population and in the different genotype groups of rs13347 in CD44 after G-CSF. (C) Increase of CSF3R expression after G-CSF in the global population and in the different genotype groups of rs3917924 in CSF3R. (D) Decrease of CXCL12 expression after G-CSF in the global population and in the different genotype groups of rs1801157 in CXCL12. (E) Reduction of expression after G-CSF of CXCR4 variant.1 and variant.2 in the global population and in the different genotype groups of rs2680880 in CXCR4. Shaded and bold genotypes are associated with lower and higher amounts of CD34+ cells in PB after G-CSF administration, respectively. In all the cases, genotype groups at steady sate were used as calibrators. In the case of the global population, the mean of all steady state values was used as a calibrator. For the different genotypes, each genotype after G-CSF was calibrated with the value of the corresponding genotype at steady state. All data were normalized with β-actin. Data are given as mean ± S.E.M. All expression values are linear values relative to the calibrator group. Calibrator groups have a value equal to one; fold reduction or fold increase of the gene expression obtained is relative to that value.
As regards CSF3R, G-CSF increased mRNA expression by 26-fold in the group with the TT genotype (P=0.001), without causing significant changes in the groups with the other CSF3R variants. Overall quantification of mRNA levels for CSF3R before and after G-CSF, showed a global increased expression of CSF3R (P=0.005) (Figure 2B).
For CXCL12, with respect to donors with AG and GG variants, those with the AA genotype showed the lowest expression after G-CSF (P=0.03 for the comparison of the three genotypes and P=0.007 in the recessive model) (data not shown). Overall quantification of mRNA levels for CXCL12 showed a global decreased expression for CXCL12 after G-CSF (P=0.002). The AA genotype showed 14-fold lower mRNA expression after G-CSF (P=0.027), without significant changes in the other rs1801157 CXCL12 variants (Figure 2C).
In order to examine whether different genotypes in rs2680880 in CXCR4 influenced the two transcript variants of CXCR4 to different degrees and whether the effect of G-CSF differed in the two variants, mRNA levels of the two transcript variants were amplified. Variant 1 did not differ among the different genotypes either at steady state or after G-CSF. Variant 2, at steady state, showed a trend towards higher mRNA expression in the TT genotype with respect to AA genotype (P=0.06); this difference was statistically significant after G-CSF (P=0.02) (data not shown). Quantification of mRNA levels for the two variants, before and after G-CSF, showed overall decreased expression for both variants, with a more prominent effect in variant 2 (P=0.001 and P<0.001 for variant 1 and variant 2, respectively). A comparison of the effect of G-CSF in the different genotype groups showed a reduction of CXCR4 variant 2 in the TT and AT genotypes (P=0.01 and P<0.001, respectively) but not in the AA genotype which represents the group with the lowest CD34+ cell yield. Furthermore, the effect of G-CSF in variant 1 was only detected in the AT genotype (P=0.004), the group with the highest CD34+ cell yield (Figure 2D).
Association of multiple single nucleotide polymorphisms with the number of CD34+ cells in peripheral blood
A combinatory analysis of SNP was performed with the allelic variants associated with a higher number of CD34+ cells/μL of PB after G-CSF. Results showed a combination of T allele in VCAM1, C allele in CD44, C allele in CSF3R, A allele in CXCL12 and T allele in CXCR4 with a difference in the mean values of CD34+ cells/μL PB of 72 (95% CI, 42–104) (P<0.0001) with respect to the most frequent allele combination (Online Supplementary Figure S2). The estimated frequency of this allelic combination was 0.052.
Discussion
In the present study, genetic variants in VCAM1 (VCAM1-1591C), in CD44 (CD44-2392C), in CSF3R (TT rs3917924), and in CXCL12 (CXCL12-801A) were associated with the number of G-CSF-mobilized CD34+ cells in PB. VCAM1-1591C, CD44-2392C, and CXCR4-40A (rs2680880) were associated with the number of CD34+ cells/kg of donor and with the total number of CD34+ cells obtained with the first apheresis.
VCAM1 is essential for hematopoietic stem cell homing.12 It is expressed on stromal cells and in a subset of hematopoietic stem cells, the long-term hematopoietic stem cells.13 VCAM1 and other adhesion molecules and integrins in BM are responsible for dynamic cell-cell interactions which influence the fate of CD34+ cells.14 G-CSF first increases VCAM1 expression in BM promoting adhesion of CD34+ cells12 and then, 5 days after G-CSF administration, VCAM1 expression in BM decreases, which facilitates the egress of CD34+ cells into the PB.15 We observed a complete disappearance of VCAM1 expression in PB after G-CSF administration. This reduced expression in BM and in PB could lead to continuous circulation of CD34+ cells. In contrast to our results, Ulyanova et al. detected increased VCAM1 expression in PB after G-CSF. However, their results were obtained in mice, not in humans, and mechanisms mediating hematopoietic progenitor cell release may differ between species.9 Interestingly, the VCAM1-1591C allele has been associated with a decrease in both total white blood cells and CFU-GEMM progenitor cells among benzene-exposed workers,16 suggesting that it may be a factor in the regulation of hematopoiesis.
CD44 is a ubiquitously expressed transmembrane glycoprotein that regulates cell adhesion, movement and activation of cells. Depending on the molecules to which it binds, different responses are generated.17,18 Different variant isoforms are generated by alternative RNA splicing19 and a variety of microRNA affects its expression in cancer.20 After G-CSF mobilization, CD44 levels are diminished on CD34+ cells relative to BM-resident CD34+ cells.21 We observed an overall decrease of CD44 mRNA expression after G-CSF; however, this effect was not detected in individuals carrying the recessive TT genotype, who had the lowest mobilization yields. This scarce reduction of CD44 expression could be influential in retaining CD34+ cells in BM and lead to a lower mobilization yield. The mechanism of this effect in donors carrying the CD44-2392C SNP is unknown, but the position of the SNP in the 3′UTR region makes it susceptible to a different degree of mRNA degradation among the genetic variants and deserves further study. Thus, we show for first time that genetic variants in VCAM1 and CD44 affect the number of CD34+ cells collected by apheresis after G-CSF administration.
G-CSF induces CD34+ cell mobilization via stimulation of CSF3R, which is present in both CD34+ cells and neutrophil granulocytes, and has been found to have important biological functions. The currently accepted explanation for G-CSF-induced CD34+ cell mobilization is that this drug enriches the BM microenvironment with proteolytic enzymes released by neutrophils, which cleave the adhesion of CD34+ cells to the BM microenvironment.22 However, mice deficient in neutrophil proteases exhibit normal CD34+ cell mobilization in response to G-CSF,23 suggesting other possible mechanisms for CD34+ cell mobilization. G-CSF may act as a powerful chemotactic agent for human CD34+ cells, a function mediated through CSF3R.24 Moreover, the relatively minor importance of blood G-CSF level for effective CD34+ cell mobilization suggests that its ligand, CSF3R, may play a more significant role.7 In addition to CD34+ cell mobilization and myeloid development, G-CSF and CSF3R are involved in differentiation of adult neural stem cells, an effect which appears to be specifically mediated through CSF3R.25,26 These substantial functions of CSFR3 would explain the biological impact of a genetic polymorphism in CSF3R. We detected increased CSF3R mRNA expression after G-CSF administration in those individuals with the TT variant in CSF3R. However, whether this increase in mRNA CSF3R expression is the reason for the poor hematopoietic stem cell mobilization is presently unclear. The biological impact of the CSF3R SNP could also be due to its capacity to modify the functional properties or the sensitivity of CSF3R to G-CSF.
The association of CXCL12-801A with a higher number of G-CSF-mobilized CD34+ cells has been reported in healthy donors27 and in patients.28 The CXCL12/CXCR4 interaction plays an important role in the homing of hematopoietic progenitor cells and their egress to PB during situations of stress.29 The mechanisms leading to CD34+ cell mobilization involve decreased CXCL12 expression in both BM and PB.1,30 CXCL12-801A has been linked to both higher31 and lower32 CXCL12 protein plasma levels. We found that under steady state conditions, CXCL12-801A was not associated with different mRNA expression levels. However, after G-CSF, the CXCL12-801A allele was related to much lower mRNA CXCL12 expression than the other genetic variants. The CXCL12-801A allelic variant is a result of the rs1801157 SNP, which is located in a highly demethylated area of the 3′UTR region. This SNP confers a G to A transition in nucleotide position 801, resulting in a loss of a methylation site which could affect the methylating effect of G-CSF,33 and leading to a greater decrease in CXCL12 expression in the individuals carrying the polymorphism. Our results showing this reduced CXCL12 expression only in individuals carrying the CXCL12-801A allele and only after G-CSF would support this hypothesis. Of note, the CXCL12-801A allelic variant has been associated with a higher incidence of breast and lung cancer, acute myeloid leukemia, lymphoma and chronic myeloproliferative disease, and with a slower progression to AIDS.31,34–38
An interaction between CXCL12 and CXCR4 is critical for the trafficking of hematopoietic progenitor cells in the BM, and an interruption of this interaction can affect CD34+ cell mobilization by G-CSF.1 New mechanisms of mobilization using AMD3100 as a CXCR4 antagonist have shown efficacy in healthy volunteers and in patients.39,40 CXCR4 has two isoforms due to alternative splicing. Variant 2 contains a shorter N-terminus compared to variant 1. The rs2680880 SNP in CXCR4 is located in an intron at the 5′UTR region close to the region where the alternative splicing of the two variants takes place. High-affinity binding to CXCL12 requires the extracellular N-terminal domain of CXCR4,41 this binding is mediated through the first 38 amino acids at the N-terminus of CXCR4 (p38) present in both variants, where three Tyr residues play important roles.42 Previous studies of the structure responsible for the binding of CXCR4 to CXCL1241,42 have considered only variant 2. We reasoned that G-CSF may have a differential effect on expression levels of the two CXCR4 variants and found that while the G-CSF effect was found for both variants, it was much more pronounced for variant 2, indicating a more important role for this variant in G-CSF mobilization. Due to the longer N-terminus of variant 1 and the involvement of the N-terminus of CXCR4 in the binding of CXCL12 to CXCR4,42 we suggest that the longer N-terminus could cause a stronger interaction of CXCR4 variant 1 with CXCL12 leading to a lower mobilization. We also reasoned that the three CXCR4 genotypes could have a differential effect on the expression of the two isoforms. The lower levels of variants 1 and 2 in the AA genotype indicate a lesser effect of G-CSF, leading to lower mobilization. The TT genotype, with higher levels of variant 2, mobilizes less than AT genotype. This could be due to an additive effect of the reduction of the expression of the two variants in the AT genotype, which has the same levels as the two variants at steady state; this would, in turn, lead to greater mobilization in the AT genotype than in the TT genotype, where only the effect of variant 2 is observed. We, therefore, hypothesize that the rs2680880 SNP in CXCR4 could modulate the alternative splicing in CXCR4 to produce lower expression of CXCR4 variant 2 in the AA genotype.
In summary, the present study has shown that SNP in genes involved in homing and migration of CD34+ cells are strongly related to the degree of CD34+ cell mobilization after G-CSF. Moreover, some of these SNP are associated with a differential level of mRNA expression. We have shown for the first time that genetic variants in VCAM1 and CD44 are related to the number of CD34+ cells collected after G-CSF administration. We also report the effect of G-CSF on a genetic variant in CD44 influencing the number of CD34+ cells collected. We note the influence of a genetic variant in CSFR3 on the number of CD34+ cells mobilized by G-CSF, and that the previously reported association of the CXCL12-801A allele with the number of CD34+ cells after G-CSF administration27 might be due to a dramatic G-CSF-induced decrease in gene expression in donors with this genetic variant.
Footnotes
Funding: this work was supported by a grant from Project PI08/1137 from ISCIII (Instituto de Salud Carlos III), by Project RD06/0020/0012 from RTICC (Red Temática de Investigación Cooperativa en Cáncer) and by a grant from Consejería de Salud, Junta de Andalucía.
The online version of this article has a Supplementary Appendix.
Authorship and Disclosures
The information provided by the authors about contributions from persons listed as authors and in acknowledgments is available with the full text of this paper at www.haematologica.org.
Financial and other disclosures provided by the authors using the ICMJE (www.icmje.org) Uniform Format for Disclosure of Competing Interests are also available at www.haematologica.org.
References
- 1.Pusic I, DiPersio JF. The use of growth factors in hematopoietic stem cell transplantation. Curr Pharm Des. 2008;14(20):1950–61. doi: 10.2174/138161208785061427. [DOI] [PubMed] [Google Scholar]
- 2.Ljungman P, Urbano-Ispizua A, Cavazzana-Calvo M, Demirer T, Dini G, Einsele H, et al. Allogeneic and autologous transplantation for haematological diseases, solid tumours and immune disorders: definitions and current practice in Europe. Bone Marrow Transplant. 2006;37 (5):439–49. doi: 10.1038/sj.bmt.1705265. [DOI] [PubMed] [Google Scholar]
- 3.Bensinger WI, Martin PJ, Storer B, Clift R, Forman SJ, Negrin R, et al. Transplantation of bone marrow as compared with peripheral blood cells from HLA-identical relatives in patients with hematologic cancer. N Engl J Med. 2001;344(3):175–81. doi: 10.1056/NEJM200101183440303. [DOI] [PubMed] [Google Scholar]
- 4.Anderlini P, Rizzo JD, Nugent ML, Schmitz N, Champlin RE, Horowitz MM. Peripheral blood stem cell donation: an analysis from the International Bone Marrow Transplant Registry (IBMTR) and European Group for Blood and Marrow Transplant (EBMT) databases. Bone Marrow Transplant. 2001;27(7):689–92. doi: 10.1038/sj.bmt.1702875. [DOI] [PubMed] [Google Scholar]
- 5.Nakamura R, Bahceci E, Read EJ, Leitman SF, Carter CS, Childs R, et al. Transplant dose of CD34(+) and CD3(+) cells predicts outcome in patients with haematological malignancies undergoing T cell-depleted peripheral blood stem cell transplants with delayed donor lymphocyte add-back. Br J Haematol. 2001;115(1):95–104. doi: 10.1046/j.1365-2141.2001.02983.x. [DOI] [PubMed] [Google Scholar]
- 6.Takeyama K, Ohto H. PBSC mobilization. Transfus Apher Sci. 2004;31(3):233–43. doi: 10.1016/j.transci.2004.09.007. [DOI] [PubMed] [Google Scholar]
- 7.Suzuya H, Watanabe T, Nakagawa R, Watanabe H, Okamoto Y, Onishi T, et al. Factors associated with granulocyte colony-stimulating factor-induced peripheral blood stem cell yield in healthy donors. Vox Sang. 2005;89(4):229–35. doi: 10.1111/j.1423-0410.2005.00701.x. [DOI] [PubMed] [Google Scholar]
- 8.Okano A, Ashihara E, Shimazaki C, Uchiyama H, Inaba T, Taniguchi K, et al. Predictive parameters for granulocyte colony-stimulating factor-induced peripheral blood stem cell mobilization. J Clin Apher. 2008;23(6):171–7. doi: 10.1002/jca.20179. [DOI] [PubMed] [Google Scholar]
- 9.Méndez-Ferrer S, Lucas D, Battista M, Frenette PS. Haematopoietic stem cell release is regulated by circadian oscillations. Nature. 2008;452(7186):442–7. doi: 10.1038/nature06685. [DOI] [PubMed] [Google Scholar]
- 10.Adams GB, Alley IR, Chung UI, Chabner KT, Jeanson NT, Lo Celso C, et al. Haematopoietic stem cells depend on Galpha(s)-mediated signalling to engraft bone marrow. Nature. 2009;459(7243):103–7. doi: 10.1038/nature07859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hollegaard MV, Bidwell JL. Cytokine gene polymorphism in human disease: on-line databases, Supplement 3. Genes Immun. 2006;7(4):269–76. doi: 10.1038/sj.gene.6364301. [DOI] [PubMed] [Google Scholar]
- 12.Fuste B, Escolar G, Marin P, Mazzara R, Ordinas A, Diaz-Ricart M. G-CSF increases the expression of VCAM-1 on stromal cells promoting the adhesion of CD34+ hematopoietic cells: studies under flow conditions. Exp Hematol. 2004;32(8):765–72. doi: 10.1016/j.exphem.2004.05.023. [DOI] [PubMed] [Google Scholar]
- 13.Forsberg EC, Prohaska SS, Katzman S, Heffner GC, Stuart JM, Weissman IL. Differential expression of novel potential regulators in hematopoietic stem cells. PLoS Genet. 2005;1(3):e28. doi: 10.1371/journal.pgen.0010028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Forsberg EC, Smith-Berdan S. Parsing the niche code: the molecular mechanisms governing hematopoietic stem cell adhesion and differentiation. Haematologica. 2009;94 (11):1477–81. doi: 10.3324/haematol.2009.013730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ulyanova T, Scott LM, Priestley GV, Jiang Y, Nakamoto B, Koni PA, et al. VCAM-1 expression in adult hematopoietic and non-hematopoietic cells is controlled by tissue-inductive signals and reflects their developmental origin. Blood. 2005;106(1):86–94. doi: 10.1182/blood-2004-09-3417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lan Q, Zhang L, Shen M, Smith MT, Li G, Vermeulen R, et al. Polymorphisms in cytokine and cellular adhesion molecule genes and susceptibility to hematotoxicity among workers exposed to benzene. Cancer Res. 2005;65(20):9574–81. doi: 10.1158/0008-5472.CAN-05-1419. [DOI] [PubMed] [Google Scholar]
- 17.Weber GF, Ashkar S, Glimcher MJ, Cantor H. Receptor-ligand interaction between CD44 and osteopontin (Eta-1) Science. 1996;271(5248):509–12. doi: 10.1126/science.271.5248.509. [DOI] [PubMed] [Google Scholar]
- 18.Aruffo A, Stamenkovic I, Melnick M, Underhill CB, Seed B. CD44 is the principal cell surface receptor for hyaluronate. Cell. 1990;61(7):1303–13. doi: 10.1016/0092-8674(90)90694-a. [DOI] [PubMed] [Google Scholar]
- 19.Sherman L, Wainwright D, Ponta H, Herrlich P. A splice variant of CD44 expressed in the apical ectodermal ridge presents fibroblast growth factors to limb mesenchyme and is required for limb outgrowth. Genes Dev. 1998;12(7):1058–71. doi: 10.1101/gad.12.7.1058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Huang Q, Gumireddy K, Schrier M, le Sage C, Nagel R, Nair S, et al. The microRNAs miR-373 and miR-520c promote tumour invasion and metastasis. Nat Cell Biol. 2008;10(2):202–10. doi: 10.1038/ncb1681. [DOI] [PubMed] [Google Scholar]
- 21.Lee S, Im SA, Yoo ES, Nam EM, Lee MA, Ahn JY, et al. Mobilization kinetics of CD34(+) cells in association with modulation of CD44 and CD31 expression during continuous intravenous administration of G-CSF in normal donors. Stem Cells. 2000;18(4):281–6. doi: 10.1634/stemcells.18-4-281. [DOI] [PubMed] [Google Scholar]
- 22.Lévesque JP, Hendy J, Takamatsu Y, Williams B, Winkler IG, Simmons PJ. Mobilization by either cyclophosphamide or granulocyte colony-stimulating factor transforms the bone marrow into a highly proteolytic environment. Exp Hematol. 2002;30(5):440–9. doi: 10.1016/s0301-472x(02)00788-9. [DOI] [PubMed] [Google Scholar]
- 23.Levesque JP, Liu F, Simmons PJ, Betsuyaku T, Senior RM, Pham C, et al. Characterization of hematopoietic progenitor mobilization in protease-deficient mice. Blood. 2004;104(1):65–72. doi: 10.1182/blood-2003-05-1589. [DOI] [PubMed] [Google Scholar]
- 24.Zhang Y, Cheng G, Yang K, Fan R, Xu Z, Chen L, et al. A novel function of granulocyte colony-stimulating factor in mobilization of human hematopoietic progenitor cells. Immunol Cell Biol. 2009;87(5):428–32. doi: 10.1038/icb.2009.9. [DOI] [PubMed] [Google Scholar]
- 25.Frank T, Schlachetzki JC, Göricke B, Meuer K, Rohde G, Dietz GP, et al. Both systemic and local application of granulocyte-colony stimulating factor (G-CSF) is neuroprotective after retinal ganglion cell axotomy. BMC Neurosci. 2009;10:49. doi: 10.1186/1471-2202-10-49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Schneider A, Krüger C, Steigleder T, Weber D, Pitzer C, Laage R, et al. The hematopoietic factor G-CSF is a neuronal ligand that counteracts programmed cell death and drives neurogenesis. J Clin Invest. 2005;115(8):2083–98. doi: 10.1172/JCI23559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bogunia-Kubik K, Gieryng A, Dlubek D, Lange A. The CXCL12-30A allele is associated with a higher mobilization yield of CD34 progenitors to the peripheral blood of healthy donors for allogeneic transplantation. Bone Marrow Transplant. 2009;44 (5):273–8. doi: 10.1038/bmt.2009.30. [DOI] [PubMed] [Google Scholar]
- 28.Benboubker L, Watier H, Carion A, Georget MT, Desbois I, Colombat P, et al. Association between the SDF-1-30A allele and high levels of CD34+ progenitor cells mobilized into peripheral blood in humans. Br J Haematol. 2001;113(1):247–50. doi: 10.1046/j.1365-2141.2001.02717.x. [DOI] [PubMed] [Google Scholar]
- 29.Abkowitz JL, Robinson AE, Kale S, Long MW, Chen J. Mobilization of hematopoietic stem cells during homeostasis and after cytokine exposure. Blood. 2003;102(4):1249–53. doi: 10.1182/blood-2003-01-0318. [DOI] [PubMed] [Google Scholar]
- 30.Gazitt Y, Qun L. Plasma levels of SDF-1 and expression of SDF-1 receptor on CD34+ cells in mobilized peripheral blood of non-Hodgkin’s lymphoma patients. Stem cells. 2001;19(1):37–45. doi: 10.1634/stemcells.19-1-37. [DOI] [PubMed] [Google Scholar]
- 31.Gerli G, Vanelli C, Turri O, Erario M, Gardellini A, Pugliano M, et al. SDF1-3′A gene polymorphism is associated with chronic myeloproliferative disease and thrombotic events. Clin Chem. 2005;51 (12):2411–4. doi: 10.1373/clinchem.2005.057802. [DOI] [PubMed] [Google Scholar]
- 32.Soriano A, Martínez C, García F, Plana M, Palou E, Lejeune M, et al. Plasma stromal cell-derived factor (SDF)-1 levels, SDF1-3′A genotype, and expression of CXCR4 on T lymphocytes: their impact on resistance to human immunodeficiency virus type 1 infection and its progression. J Infect Dis. 2002;186(7):922–31. doi: 10.1086/343741. [DOI] [PubMed] [Google Scholar]
- 33.Nagler A, Korenstein-Ilan A, Amiel A, Avivi L. Granulocyte colony-stimulating factor generates epigenetic and genetic alterations in lymphocytes of normal volunteer donors of stem cells. Exp Hematol. 2004;32(1):122–30. doi: 10.1016/j.exphem.2003.09.007. [DOI] [PubMed] [Google Scholar]
- 34.Dommange F, Cartron G, Espanel C, Gallay N, Domenech J, Benboubker L, et al. CXCL12 polymorphism and malignant cell dissemination/tissue infiltration in acute myeloid leukemia. FASEB J. 2006;20(11):1913–5. doi: 10.1096/fj.05-5667fje. [DOI] [PubMed] [Google Scholar]
- 35.de Oliveira CE, Cavassin GG, Perim AL, Nasser TF, de Oliveira KB, Fungaro MH, et al. Stromal cell-derived factor-1 chemokine gene variant in blood donors and chronic myelogenous leukemia patients. J Clin Lab Anal. 2007;21(1):49–54. doi: 10.1002/jcla.20142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zafiropoulos A, Crikas N, Passam AM, Spandidos DA. Significant involvement of CCR2-64I and CXCL12-3a in the development of sporadic breast cancer. J Med Genet. 2004;41(5):e59. doi: 10.1136/jmg.2003.013649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Razmkhah M, Doroudchi M, Ghayumi SM, Erfani N, Ghaderi A. Stromal cell-derived factor-1 (SDF-1) gene and susceptibility of Iranian patients with lung cancer. Lung Cancer. 2005;49(3):311–5. doi: 10.1016/j.lungcan.2005.04.014. [DOI] [PubMed] [Google Scholar]
- 38.Winkler C, Modi W, Smith MW, Nelson GW, Wu X, Carrington M, et al. Genetic restriction of AIDS pathogenesis by an SDF-1 chemokine gene variant. ALIVE Study, Hemophilia Growth and Development Study (HGDS), Multicenter AIDS Cohort Study (MACS), Multicenter Hemophilia Cohort Study (MHCS), San Francisco City Cohort (SFCC) Science. 1998;279(5349):389–93. doi: 10.1126/science.279.5349.389. [DOI] [PubMed] [Google Scholar]
- 39.Devine SM, Flomenberg N, Vesole DH, Liesveld J, Weisdorf D, Badel K, et al. Rapid mobilization of CD34+ cells following administration of the CXCR4 antagonist AMD3100 to patients with multiple myeloma and non-Hodgkin’s lymphoma. J Clin Oncol. 2004;22(6):1095–102. doi: 10.1200/JCO.2004.07.131. [DOI] [PubMed] [Google Scholar]
- 40.Liles WC, Broxmeyer HE, Rodger E, Wood B, Hübel K, Cooper S, et al. Mobilization of hematopoietic progenitor cells in healthy volunteers by AMD3100, a CXCR4 antagonist. Blood. 2003;102(8):2728–30. doi: 10.1182/blood-2003-02-0663. [DOI] [PubMed] [Google Scholar]
- 41.Veldkamp CT, Seibert C, Peterson FC, De la Cruz NB, Haugner JC, Basnet H, et al. Structural basis of CXCR4 sulfotyrosine recognition by the chemokine SDF-1/CXCL12. Sci Signal. 2008;1(37):ra4. doi: 10.1126/scisignal.1160755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Doranz BJ, Orsini MJ, Turner JD, Hoffman TL, Berson JF, Hoxie JA, et al. Identification of CXCR4 domains that support coreceptor and chemokine receptor functions. J Virol. 1999;73(4):2752–61. doi: 10.1128/jvi.73.4.2752-2761.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]