Abstract
Background
Hematopoietic stem cell transplantation has become an established procedure worldwide. Severe early and late complications are well described. Little is known about more subtle changes in general health status of very long-term survivors. The study objective was to assess health status of very long-term survivors in comparison with their respective human leukocyte antigen-identical sibling donors.
Design and Methods
Case matched comparison in a cross-sectional cohort was performed in a tertiary university hospital and referral center for hematopoietic stem cell transplantation. Forty-four pairs of recipients and their respective donors with a very long-term (17.5 years median; 11–26 years range) follow up after allogeneic hematopoietic stem cell transplantation were included. A comparative clinical evaluation and examination of routine clinical chemistry tests was carried out.
Results
Recipients more frequently had a lower Karnofsky score (P=0.05), hypertension (P=0.015) and dyslipidemia (P=0.002) but were less likely to be smokers (P=0.016). Recipients showed systematically lower glomerular filtration rates (P<0.0001), higher liver function tests (P=0.0004 for Aspartat-Amino-Transferase) and reduced thyroid function (P=0.002) despite normal or near normal values, and independent of presence or absence of chronic graft-versus-host disease. Indicators of inflammation were more frequent in recipients (9 of 44) with ongoing chronic graft-versus-host disease as measured by higher C-reactive protein (P=0.001) and higher von Willebrand factor (P=0.002).
Conclusions
Clinically very long-term survivors after an allogeneic hematopoietic stem cell transplantation present more frequently with cardiovascular risk factors and with subtle signs of altered organ function compared to their sibling donors. Even minimal ongoing chronic graft-versus-host disease remains associated with elevated laboratory indicators of inflammation. The clinical significance of these findings needs to be defined.
Keywords: stem cell transplantation, late effects
Introduction
Allogeneic hematopoietic stem cell transplantation (HSCT) has become a curative treatment option for a variety of malignant and non-malignant disorders worldwide.1 Overall survival has improved substantially over the last decade, and the number of procedures, and consequently the number of long-term survivors, is continuously increasing.2 Nevertheless, HSCT remains associated with considerable early and late treatment-related morbidity and mortality.3 Pre-transplant patient and donor characteristics can give a reasonable risk estimate of allogeneic HSCT.4 Over recent years, the focus has shifted to long-term survivors. Patients do expect to recover their initial health status after HSCT and to lead a normal life with complete social integration. However, when compared to a matched general population, mortality remains increased.5 Many late complications, such as secondary cancer,6–8 cataract development,9,10 infertility,11,12 endocrine dysfunctions,13 bone and joint degeneration,14,15 and cardiovascular and cardiac complications16–18 have been well described. Other organ dysfunctions have been discussed but an unequivocal relationship with the transplant procedure remains an open question. In theory, however, any organ or tissue could be a target.
Late effects of HSCT can be the consequence of the initial disease, of pre-transplant comorbidity, of the treatments used before transplantation, of the conditioning regimen, of acute and chronic graft-versus-host disease (GVHD) and its treatment or of infectious complications. Moderate and severe side-effects are easily recognized and during the first year after allogeneic HSCT anomalies of laboratory function tests are frequent. When discontinuation of immunosuppressive drugs becomes feasible, most of these parameters tend to normalize. Little attention is usually given to discrete changes in clinical chemistry parameters during follow up. However, changes persisting over years and decades could eventually lead to significant late organ dysfunction. We were, therefore, interested in systematically searching for subtle signs of organ dysfunction in very long-term survivors of allogeneic HSCT and used their respective sibling donor as comparator.
Design and Methods
Study design
This was a case matched comparison in a cross-sectional cohort single center study of the division of Hematology of the University Hospitals Basel, Switzerland to assess health status, hematopoietic function and organ alterations in very long-term survivors after HSCT compared with their respective HLA-identical sibling stem cell donors. In brief, all recipients transplanted more than ten years ago from an HLA-identical sibling donor, living in the region and still in complete remission at the last follow up were invited to present together with his or her sibling donor on the same day to our outpatient clinic. All donors and recipients gave written informed consent. The study was approved by the Beider Basel Ethics Committee.
Patient population
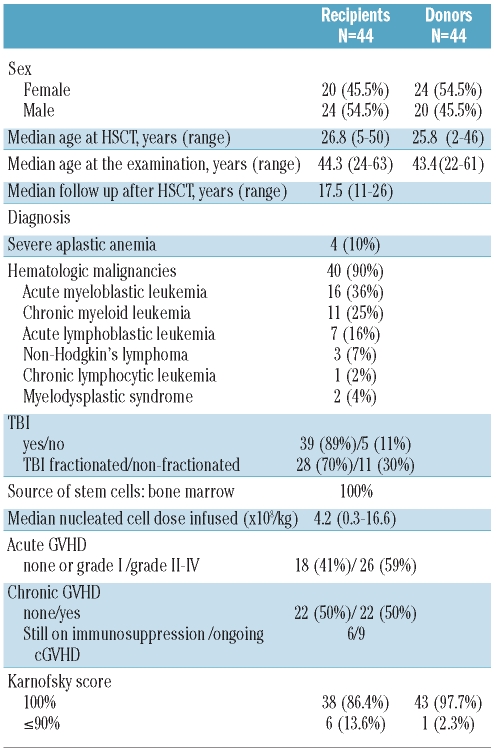
From 82 pairs contacted, 44 (54%) agreed to participate in the study. There were no differences in characteristics between those pairs included in the study compared to those not included. The characteristics of the recipients and their respective donors evaluated in this study are summarized in Table 1. The transplant had been performed 11 to 26 years (median 17.5 years) earlier. There were slightly more male recipients (55%) and slightly more female donors (55%) (P=0.55) with no difference in age between donors and recipients. Slightly more recipients presented with a Karnofsky score below 100 (P=0.05; χ2 test).
Table 1.
Characteristics of recipients and donors.
Total body irradiation (TBI) was part of the conditioning for 39 (89%) of the recipients. Of these, acute GVHD was observed in 26 (59%) and chronic GVHD in 22 (50%). Through the follow up of this cohort, chronic GVHD affected the following organs: skin n=13 recipients, oral mucosa n=11, eye n=6, vagina n=4, liver n=3, gastrointestinal n=2, lung n=1. At time of study, 9 recipients had ongoing chronic GVHD (oral mucosa n=6, vagina n=4, skin n=3, liver n=2 and lung n=1) and 6 recipients were still under immuno-suppressive therapy (cyclosporine n=4, prednisone n=1, cyclosporine and prednisone n=1, mycophenolate mofetil n=1).
Examinations
A complete clinical and biological examination was performed for both the recipient and the donor. In order to assess the general health status, all participants were asked to fill in a standardized questionnaire: the Short Form 36 (SF-36) Health Survey for quality of life assessment.19–21 Moreover, all participants filled in a detailed questionnaire focused on organ symptoms, past and current diseases, and current medication. For most participants, we also received full medical information from the primary care physician. The evaluated biological parameters included a complete blood count with lymphocyte subpopulations and telomere length determination as published elsewhere.22 For the present study, we carried out paired analysis to compare the results of the routine clinical chemistry tests including inflammatory parameters (C-reactive protein (CRP), albumin, von Willebrand factor antigen (vWF), liver tests (bilirubine, ASAT, ALAT, Gamma-GT), renal function (creatinine, glomerular filtration rate), serum lipids (triglycerides, cholesterol, HDL-cholesterol, LDL-cholesterol, cholesterol/HDL-cholesterol ratio), and thyroid function (TSH). Moreover, pre-transplant values for renal function evaluation were collected from the donors’ and recipients’ clinical files; results before starting conditioning were considered as pre-transplant values.
Statistical analysis
To compare clinical chemistry values between recipients and their respective donors, as well as pre-treatment and current data, we used the non-parametric Wilcoxon’s Signed Rank Test. Diagnosis and staging of chronic GVHD were assessed according to NIH consensus criteria.23 To evaluate the impact of chronic GVHD, a comparison between recipients with and recipients without chronic GVHD, and between donors and recipients with and without chronic GVHD was performed. We considered the constellation of a significant difference between donors and recipients with chronic GVHD without a significant difference between donors and recipients without chronic GVHD as an indicator for an impact of chronic GVHD on the respective laboratory value. In any other constellation we considered that chronic GVHD could not be the unique factor involved.
The interactions between risk factors and clinical chemistry parameters were evaluated using linear regression analysis. Differences between the results of comparative tests were considered significant if the two-sided P value was less than 0.05. Statistical analysis was performed using SPSS statistical software (SPSS for Windows, Release 17, SPSS, Inc., Chicago, Illinois, USA).
Results
There were significant differences between donors and recipients regarding the absolute values of the individual laboratory function tests, regarding the percentage of abnormal values, and regarding the evolution from pre-transplant values to time of follow up.
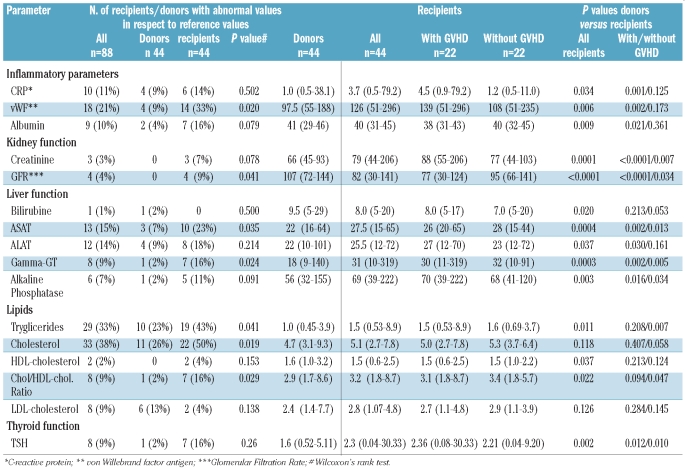
Recipients showed a decreased kidney function with systematically higher creatinine levels and systematically lower GFR than their donors (P<0.001), had systematically higher liver function tests values (Figure 1A) except for bilirubin (for respective P values see Table 2) and systematically higher TSH values (P=0.002) independent of presence or absence of chronic GVHD (Table 2). This was also reflected by a significantly higher number of abnormal GFR (9% vs. 0%; P=0.041) and abnormal liver function tests (23% vs. 7% for ASAT; P=0.035). There was no difference in laboratory signs of inflammation and for total cholesterol, LDL- and HDL-cholesterol between donors and recipients without chronic GVHD. Interestingly, recipients without chronic GVHD had higher triglycerides (P=0.007) and a higher cholesterol/HDL ratio (P=0.047) than their respective donors.
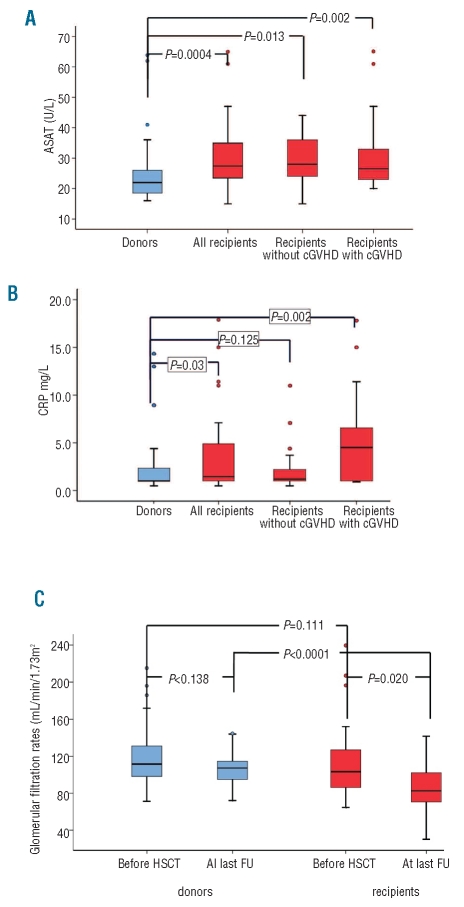
Figure 1.
Laboratory values of 44 recipients and their respective HLA-identical sibling donors 17.5 years (median) after an allogeneic HSCT. Figure presents median and 75% confidence interval for donors (blue) and recipients (red). (A) The figure illustrates the liver function test for ASAT and presents results globally (red left) and subdivided in recipients with and without chronic GVHD. Data reflect an example with a systematic elevation in recipients, independent of presence or absence of chronic GVHD. (B) The figure illustrates the CRP values and presents results globally (red left) and subdivided in recipients with and without chronic GVHD. Data reflect an example with a systematic elevation in recipients with ongoing chronic GVHD. (C) The figure illustrates the glomerular filtration rates (GFR) in donors and recipients at time of HSCT and at time of follow up. Data reflect the systematic decrease of GFR in recipients since time of transplant and the stable values in donors.
Table 2.
(Left) Number of recipients/donors with abnormal values in respect to reference values. (Right) Clinical chemistry parameters: comparison between long-term survivors and their respective donor (with and without GVHD).
In contrast, recipients with ongoing chronic GVHD more frequently had laboratory signs of inflammation and significantly higher CRP (P=0.001) (Figure 1B) and vWF (P=0.002), and significantly lower albumin values (P=0.021) (Table 2).
Organ dysfunction, defined as two or more laboratory values out of normal range (Table 2) for CRP, GFR, albumin, vWF, liver tests or dyslipidemia, was significantly more frequent in recipients (28 of 44; 64%) than in donors (7 of 44; 16%) (P<0.0001); this was due to the higher rate of organ dysfunction in recipients with (13 of 22; 59%) than without (5 of 22; 23%) chronic GVHD (P=0.014).
At time of transplantation, there was no difference in GFRs of donors and recipients (P=0.1) and GFRs of donors did not change over time (P=0.138). In contrast, as mentioned above, GFRs of recipients declined significantly over time (P=0.02). Consequently, at study time, there were significant differences in GFR between recipients and their respective donors (P<0.0001) (Figure 1C).
Significantly more recipients presented with arterial hypertension (22 of 44 vs. 11 of 44; P=0.015) and dyslipidemia (27 of 44 vs. 13 of 44; P=0.002) but more donors reported a history of cigarette smoking (P=0.016). There was no difference in diabetes, body mass index (BMI), continuous cigarette smoking since HSCT, and physical activity. Recipients were significantly more often treated for their hypertension (16 of 22; 73%) when compared to donors (4 of 11; 36%: P=0.044). In contrast, only 5 of 27 (18%) of the recipients and 3 of 13 (23%) of the donors were treated for dyslipidemia (P=0.736) independent of immunosuppression status of the recipients. At time of study, 25 of 44 (75%) recipients reported regular physical activity and 9 of 44 (20%) still continued smoking.
No familiar predisposition for cardiovascular risk factors could be identified in this small series. Arterial hypertension was observed 17 times in recipients only, 6 times in donors only, and 5 times in both recipient and donor. Dyslipidemia was found in 19 recipients, in 4 donors, and in 8 pairs. Four recipients and one donor had developed an arterial event since time of HSCT.
Discussion
Data from this unique cohort of very long-term survivors after allogeneic HSCT show a consistent difference in the number of biologically relevant laboratory values between recipients and their respective stem cell donor (Table 2). The recipients, at a similar median age, showed systematically lower glomerular filtration rates, higher liver function tests, reduced thyroid function, higher levels of indicators for inflammation and higher serum lipid levels. These differences were subtle but significant. Most of the recipients had normal or near normal values with only a small proportion out of the reference range. The abnormalities of inflammatory parameters, CRP, vWF antigen and albumin were related to a history of chronic GVHD; the differences in renal, liver and thyroid function and serum lipid tests were not explained by chronic GVHD.
Severe late organ complications after HSCT such as chronic kidney disease,24,25 liver complications or endocrine dysfunction13 have been well described. Moreover, long-term survivors of HSCT remain at an increased risk of developing metabolic syndrome, diabetes, dyslipidemia, and hypertension compared to sibling donors. In a cross sectional study, a high prevalence of metabolic syndrome and elevated triglycerides was reported among adult survivors of allogeneic HSCT compared with a general population.26,27 Another study revealed a high proportion of undiagnosed or untreated cardiovascular risk factors.28 According to the interpretation of the authors, physicians seemed to be reluctant to initiate a treatment for cardiovascular risk factors, possibly in the expectation that diabetes, dyslipidemia or hypertension would improve or disappear upon discontinuation of immunosuppressive treatment. We confirm and extend these findings. Cardiovascular risk factors and the threats for cardiovascular complications do persist after immuno-suppression is discontinued. Even though most recipients were correctly treated for their arterial hypertension, this was not the case for dyslipidemia: among all recipients who fulfilled the criteria for dyslipidemia less than 20% were treated accordingly.29 Furthermore, smoking was still an issue despite repeated medical advice to stop.
These concerns are substantiated by our findings of discrete but systematic biological changes in otherwise apparently healthy long-term survivors. We followed with this new approach of evaluation after HSCT, the concept derived from general population studies. Indeed, increased CRP values, even within the normal range were shown to enhance the global coronary risk as assessed by the Framingham Score30 or to be associated with an increased risk of death due to chronic diseases.31 Likewise, serum cholesterol concentrations even within the so called normal range have been directly related to morbidity for coronary heart disease.32 Therefore, increased, but near normal clinical chemistry values might indicate an ongoing risk for the premature cardiovascular disease after allogeneic HSCT. This might relate to patients with and without chronic GVHD. So far, no clear relationship has been demonstrated between cardiovascular complications and chronic GVHD. Still, an alloreactive effect seems to play a role; recipients treated with allogeneic compared to autologous HSCT suffer significantly more frequently from such complications.33 It is, therefore, of interest that an inflammatory state was observed more frequently in patients with chronic GVHD. Abnormal values of clinical chemistry parameters, such as CRP or serum lipids, could help to detect patients at risk and to initiate preventive strategies.
The systematic decrease in GFR in all recipients, with or without chronic GVHD fits with this concept. Multiple mechanisms can explain chronic renal dysfunction in recipients of allogeneic HSCT. They have been treated with intensive conditioning with or without TBI, have been exposed to multiple drug toxicities, and have suffered from acute and chronic GVHD and infectious complications.23,24 The metabolic syndrome with its associated microalbuminuria could enhance the premature aging in the endothelial compartment and renal vascular network, as has been demonstrated for hematopoietic cells.22 The numbers of patients examined in our series were too small to document such an association.
What are the consequences of these findings of subtle altered organ function in otherwise apparently clinically healthy very long-term survivors after HSCT? Clearly, the clinical consequences of these findings, of their early detection and of the introduction of therapeutic measures have to be assessed in a larger group of patients. These results demonstrate that individuals undergoing allogeneic HSCT, even when cured, will never become “non-patients”. Allogeneic HSCT is a lifelong commitment for all involved: the patient, his or her family, the primary care physician, the transplantation team, and the healthcare providers.
Acknowledgments
we thank Katie Perret for her efficient support in the organization of this study.
Footnotes
Funding: this work was supported by the Swiss National Research Foundation (Nr 3200B0-118176), the Horten Foundation and the Werner Geissberger Foundation
Authorship and Disclosures
The information provided by the authors about contributions from persons listed as authors and in acknowledgments is available with the full text of this paper at www.haematologica.org.
Financial and other disclosures provided by the authors using the ICMJE (www.icmje.org) Uniform Format for Disclosure of Competing Interests are also available at www.haematologica.org.
References
- 1.Copelan EA. Hematopoietic stem-cell transplantation. N Engl J Med. 2006;354 (17):1813–26. doi: 10.1056/NEJMra052638. [DOI] [PubMed] [Google Scholar]
- 2.Gratwohl A, Baldomero H, Aljurf M, Pasquini MC, Bouzas LF, Yoshimi A, et al. Hematopoietic stem cell transplantation: a global perspective. JAMA. 2010;303(16):1617–24. doi: 10.1001/jama.2010.491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tichelli A, Rovó A, Passweg J, Schwarze CP, Van Lint MT, Arat M, et al. Late complications after hematopoietic stem cell transplantation. Expert Review of Hematology. 2009;2(5):583–601. doi: 10.1586/ehm.09.48. [DOI] [PubMed] [Google Scholar]
- 4.Gratwohl A, Stern M, Brand R, Apperley J, Baldomero H, de WT, et al. Risk score for outcome after allogeneic hematopoietic stem cell transplantation: a retrospective analysis. Cancer. 2009;115(20):4715–26. doi: 10.1002/cncr.24531. [DOI] [PubMed] [Google Scholar]
- 5.Bhatia S, Francisco L, Carter A, Sun CL, Baker KS, Gurney JG, et al. Late mortality after allogeneic hematopoietic cell transplantation and functional status of long-term survivors: report from the Bone Marrow Transplant Survivor Study. Blood. 2007;110(10):3784–92. doi: 10.1182/blood-2007-03-082933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Friedman DL, Rovo A, Leisenring W, Locasciulli A, Flowers ME, Tichelli A, et al. Increased risk of breast cancer among survivors of allogeneic hematopoietic cell transplantation: a report from the FHCRC and the EBMT-Late Effect Working Party. Blood. 2008;111(2):939–44. doi: 10.1182/blood-2007-07-099283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Socie G, Curtis RE, Deeg HJ, Sobocinski KA, Filipovich AH, Travis LB, et al. New malignant diseases after allogeneic marrow transplantation for childhood acute leukemia. J Clin Oncol. 2000;18(2):348–57. doi: 10.1200/JCO.2000.18.2.348. [DOI] [PubMed] [Google Scholar]
- 8.Rizzo JD, Curtis RE, Socie G, Sobocinski KA, Gilbert E, Landgren O, et al. Solid cancers after allogeneic hematopoietic cell transplantation. Blood. 2009;113(5):1175–83. doi: 10.1182/blood-2008-05-158782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Belkacemi Y, Labopin M, Vernant JP, Prentice HG, Tichelli A, Schattenberg A, et al. Cataracts after total body irradiation and bone marrow transplantation in patients with acute leukemia in complete remission: a study of the European Group for Blood and Marrow Transplantation. Int J Radiat Oncol Biol Phys. 1998;41(3):659–68. doi: 10.1016/s0360-3016(98)00077-7. [DOI] [PubMed] [Google Scholar]
- 10.Tichelli A, Gratwohl A, Egger T, Roth J, Prunte A, Nissen C, et al. Cataract Formation after Bone Marrow Transplantation. Annals of Internal Medicine. 1993;119(12):1175–80. doi: 10.7326/0003-4819-119-12-199312150-00004. [DOI] [PubMed] [Google Scholar]
- 11.Rovo A, Tichelli A, Passweg JR, Heim D, Meyer-Monard S, Holzgreve W, et al. Spermatogenesis in long-term survivors after allogeneic hematopoietic stem cell transplantation is associated with age, time interval since transplantation, and apparently absence of chronic GvHD. Blood. 2006;108(3):1100–5. doi: 10.1182/blood-2006-01-0176. [DOI] [PubMed] [Google Scholar]
- 12.Salooja N, Szydlo RM, Socie G, Rio B, Chatterjee R, Ljungman P, et al. Pregnancy outcomes after peripheral blood or bone marrow transplantation: a retrospective survey. Lancet. 2001;358(9278):271–6. doi: 10.1016/s0140-6736(01)05482-4. [DOI] [PubMed] [Google Scholar]
- 13.Cohen A, Bekassy AN, Gaiero A, Faraci M, Zecca S, Tichelli A, et al. Endocrinological late complications after hematopoietic SCT in children. Bone Marrow Transplant. 2008;41(Suppl 2):S43–48. doi: 10.1038/bmt.2008.54. [DOI] [PubMed] [Google Scholar]
- 14.Campbell S, Sun CL, Kurian S, Francisco L, Carter A, Kulkarni S, et al. Predictors of avascular necrosis of bone in long-term survivors of hematopoietic cell transplantation. Cancer. 2009;115(18):4127–35. doi: 10.1002/cncr.24474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Socie G, Cahn JY, Carmelo J, Vernant JP, Jouet JP, Ifrah N, et al. Avascular necrosis of bone after allogeneic bone marrow transplantation: analysis of risk factors for 4388 patients by the Societe Francaise de Greffe de Moelle (SFGM) Br J Haematol. 1997;97(4):865–70. doi: 10.1046/j.1365-2141.1997.1262940.x. [DOI] [PubMed] [Google Scholar]
- 16.Armenian SH, Bhatia S. Cardiovascular disease after hematopoietic cell transplantation – lessons learned. Haematologica. 2008;93(8):1132–6. doi: 10.3324/haematol.13514. [DOI] [PubMed] [Google Scholar]
- 17.Tichelli A, Passweg J, Wojcik D, Rovo A, Harousseau JL, Masszi T, et al. Late cardiovascular events after allogeneic hematopoietic stem cell transplantation: a retrospective multicenter study of the Late Effects Working Party of the European Group for Blood and Marrow Transplantation. Haematologica. 2008;93(8):1203–10. doi: 10.3324/haematol.12949. [DOI] [PubMed] [Google Scholar]
- 18.Tichelli A, Rovo A, Gratwohl A. Late pulmonary, cardiovascular, and renal complications after hematopoietic stem cell transplantation and recommended screening practices. Hematology Am Soc Hematol Educ Program. 2008:125–33. doi: 10.1182/asheducation-2008.1.125. [DOI] [PubMed] [Google Scholar]
- 19.Rovo A, Daikeler T, Stern M, Halter J, Studt JD, Buser A, et al. Physical and not mental health is impaired in very long-term survivors after HSCT compared with their respective donors: a paired analysis. Blood. 2008;111(3):1740–1. doi: 10.1182/blood-2007-10-115964. [DOI] [PubMed] [Google Scholar]
- 20.McHorney CA, Ware JE, Jr, Raczek AE. The MOS 36-Item Short-Form Health Survey (SF-36): II. Psychometric and clinical tests of validity in measuring physical and mental health constructs. Med Care. 1993;31(3):247–63. doi: 10.1097/00005650-199303000-00006. [DOI] [PubMed] [Google Scholar]
- 21.Ware JE, Jr, Sherbourne CD. The MOS 36-item short-form health survey (SF-36). I. Conceptual framework and item selection. Med Care. 1992;30(6):473–83. [PubMed] [Google Scholar]
- 22.Baerlocher GM, Rovo A, Muller A, Matthey S, Stern M, Halter J, et al. Cellular senescence of white blood cells in very long-term survivors after allogeneic hematopoietic stem cell transplantation: the role of chronic graft-versus-host disease and female donor sex. Blood. 2009;114 (1):219–22. doi: 10.1182/blood-2009-03-209833. [DOI] [PubMed] [Google Scholar]
- 23.Filipovich AH, Weisdorf D, Pavletic S, Socie G, Wingard JR, Lee SJ, et al. National Institutes of Health consensus development project on criteria for clinical trials in chronic graft-versus-host disease: I. Diagnosis and staging working group report. Biol Blood Marrow Transplant. 2005;11(12):945–56. doi: 10.1016/j.bbmt.2005.09.004. [DOI] [PubMed] [Google Scholar]
- 24.Choi M, Sun CL, Kurian S, Carter A, Francisco L, Forman SJ, et al. Incidence and predictors of delayed chronic kidney disease in long-term survivors of hematopoietic cell transplantation. Cancer. 2008;113(7):1580–7. doi: 10.1002/cncr.23773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hingorani S, Guthrie KA, Schoch G, Weiss NS, McDonald GB. Chronic kidney disease in long-term survivors of hematopoietic cell transplant. Bone Marrow Transplant. 2007;39(4):223–9. doi: 10.1038/sj.bmt.1705573. [DOI] [PubMed] [Google Scholar]
- 26.Annaloro C, Usardi P, Airaghi L, Giunta V, Forti S, Orsatti A, et al. Prevalence of metabolic syndrome in long-term survivors of hematopoietic stem cell transplantation. Bone Marrow Transplant. 2008;41(9):797–804. doi: 10.1038/sj.bmt.1705972. [DOI] [PubMed] [Google Scholar]
- 27.Taskinen M, Saarinen-Pihkala UM, Hovi L, Lipsanen-Nyman M. Impaired glucose tolerance and dyslipidaemia as late effects after bone-marrow transplantation in childhood. Lancet. 2000;356(9234):993–7. doi: 10.1016/S0140-6736(00)02717-3. [DOI] [PubMed] [Google Scholar]
- 28.Majhail NS, Flowers ME, Ness KK, Jagasia M, Carpenter PA, Arora M, et al. High prevalence of metabolic syndrome after allogeneic hematopoietic cell transplantation. Bone Marrow Transplant. 2009;43 (1):49–54. doi: 10.1038/bmt.2008.263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Grundy SM, Cleeman JI, Merz CN, Brewer HB, Jr, Clark LT, Hunninghake DB, et al. Implications of recent clinical trials for the National Cholesterol Education Program Adult Treatment Panel III Guidelines. J Am Coll Cardiol. 2004;44(3):720–32. doi: 10.1016/j.jacc.2004.07.001. [DOI] [PubMed] [Google Scholar]
- 30.Koenig W, Lowel H, Baumert J, Meisinger C. C-reactive protein modulates risk prediction based on the Framingham Score: implications for future risk assessment: results from a large cohort study in southern Germany. Circulation. 2004;109(11):1349–53. doi: 10.1161/01.CIR.0000120707.98922.E3. [DOI] [PubMed] [Google Scholar]
- 31.Koenig W, Khuseyinova N, Baumert J, Meisinger C. Prospective study of high-sensitivity C-reactive protein as a determinant of mortality: results from the MONICA/KORA Augsburg Cohort Study, 1984–1998. Clin Chem. 2008;54(2):335–42. doi: 10.1373/clinchem.2007.100271. [DOI] [PubMed] [Google Scholar]
- 32.Chen Z, Peto R, Collins R, MacMahon S, Lu J, Li W. Serum cholesterol concentration and coronary heart disease in population with low cholesterol concentrations. BMJ. 1991;303(6797):276–82. doi: 10.1136/bmj.303.6797.276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tichelli A, Bucher C, Rovo A, Stussi G, Stern M, Paulussen M, et al. Premature cardiovascular disease after allogeneic hematopoietic stem-cell transplantation. Blood. 2007;110(9):3463–71. doi: 10.1182/blood-2006-10-054080. [DOI] [PubMed] [Google Scholar]