Abstract
The only way to cure leukemia is by cooperative research. To optimize research, the European LeukemiaNet integrates 105 national leukemia trial groups and networks, 105 interdisciplinary partner groups and about 1,000 leukemia specialists from 175 institutions. They care for tens of thousands of leukemia patients in 33 countries across Europe. Their ultimate goal is to cure leukemia. Since its inception in 2002, the European LeukemiaNet has steadily expanded and has unified leukemia research across Europe. The European LeukemiaNet grew from two major roots: 1) the German Competence Network on Acute and Chronic Leukemias; and 2) the collaboration of European Investigators on Chronic Myeloid Leukemia. The European LeukemiaNet has improved leukemia research and management across Europe. Its concept has led to funding by the European Commission as a network of excellence. Other sources (European Science Foundation; European LeukemiaNet-Foundation) will take over when the support of the European Commission ends.
Keywords: Cooperative leukemia research, European LeukemiaNet, transnational and interdisciplinary cooperation on leukemia, cure of leukemia, leukemia management guidelines
Introduction
Before the creation of the European LeukemiaNet (ELN), there were a number of pre-existing networks in Europe that were each individually developing diagnostic methodology, running clinical trials and producing management guidelines.1 The main goal of the ELN was to create an environment in which these organizations could work more closely together, to harmonize their efforts and bring their advances to a wider community in a more timely fashion.
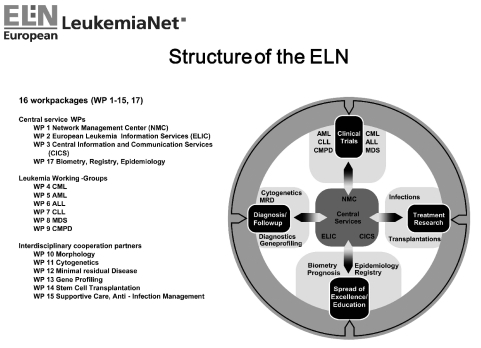
The first step was to bring together national leukemia trial groups in an attempt to provide common definitions and standards, to share information on ongoing and planned trials, to work together to avoid duplicating activities, and to share the benefits of infrastructure. In a second step, the interdisciplinary cooperation partners common to all leukemia trial groups were included (Figure 1). The structure of the ELN is shown in Figure 2.
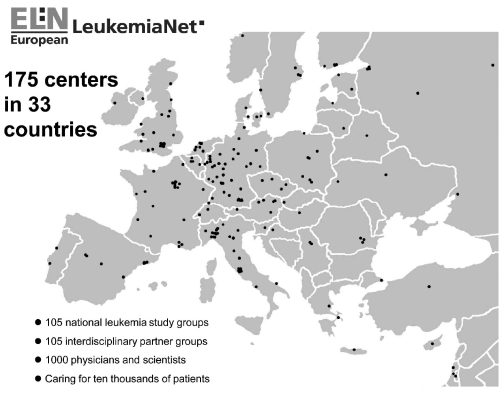
Figure 1.
Participants of the European LeukemiaNet according to their location. According to the rules of the European Commission, only institutions can be participants. One participating institution may comprise more than one leukemia trial or interdisciplinary partner group. Participating institutions are listed in the Online Supplementary Appendix.
Figure 2.
Working-groups and organigram of the European LeukemiaNet.
Achievements
The ELN is a model of transnational cooperation. Working together successfully has created a spirit of cooperation and mutual trust.
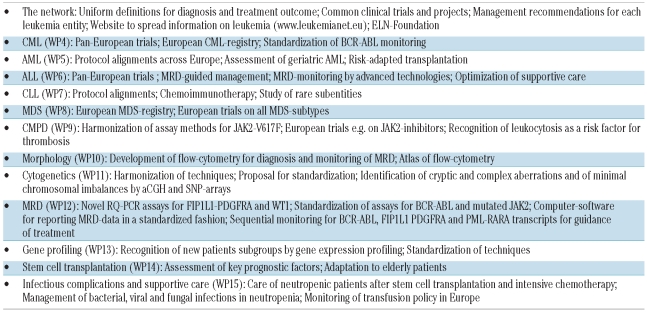
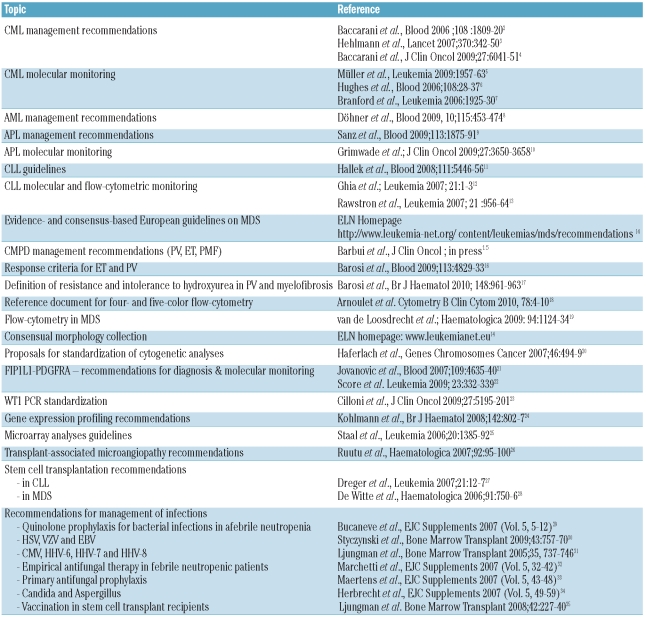
The most visible results are: 1) those due to the cooperative research projects and trials (Table 1) as reflected by a large number of high impact publications; 2) the guidelines and management recommendations for virtually every leukemia and interdisciplinary speciality (Table 2) which have lain the groundwork for uniform definitions and standards required for common clinical trials and projects; and 3) the website of ELN’s leukemia information center for physicians, patients, their carers and the general public (www.leukemia-net.org, www.leukemianet.eu).
Table 1.
Key results.
Table 2.
Recommendations and Guidelines
The CML working-group (Work-Package WP 4) originated from the European Investigators on Chronic Myeloid Leukemia (EI-CML) which itself met for the first time in 1992. EI-CML has an impressive heritage of accomplishments including meta-analyses, long-term observation of cytogenetic responders, and the development of a new prognostic CML-score (Euro-score). WP4 coordinates clinical trials between participating countries and facilitates pan-European trials whenever feasible, e.g. discontinuation of imatinib in stable complete molecular responders. International management recommendations were first published in 20062–3 and updated in 20094 (Table 2). WP4 was the first group to initiate a public-private partnership with industry (Novartis) known as the European Treatment and Outcome Study [EUTOS] for CML: to build a European CML registry together with the Registry working-group (WP17) (currently close to 5,000 patients registered and followed for outcome annually), to standardize molecular and pharmacological monitoring across Europe (58 laboratories in 29 countries standardized for BCR-ABL monitoring) and to spread the information to non-participating colleagues and countries by annual educational symposia (five events since 2006), training events for young hematologists, and lectures.
The AML working-group (WP5) evolved from 16 European AML study-groups: MRC, GOELAMS, ALFA, Polish AML-group, Russian AML-group, GIMEMA, EORTC, HOVON, SAKK, Swedish AML-group, CET-LAM, PETHEMA and four German AML study groups cooperating in the German AML Intergroup to study cross trial comparability with upfront randomization into a common standard arm. The AML working-group currently uses three approaches to improve the prognosis of AML: 1) harmonization of criteria for the alignment of protocols with stratification according to molecular and cytogenetic risk markers, thus creating a platform for meta-analyses; 2) establishment of a European network on AML-management including geriatric assessment of elderly AML which represents a poor risk population and the largest proportion of AML patients;36 3) consensus approach for risk adapted integration of transplantation in AML balancing risk of disease versus risk of transplantation, including a newly defined frailty index. A European network on management of de novo and relapsed acute promyelocytic leukemia (APL) has been established. Management recommendations have been completed for AML8 and APL9 (Table 2).
The ALL working-group (WP6) brings together pediatric and adult hematologists and has successfully used the advent of advanced technologies for monitoring residual disease to optimize the outcome of ALL. The rarity of ALL has accelerated the formation of a European Working Party for ALL (EWALL) and the performance of common trials in several European countries. New drugs are under study (nelarabine, clofarabine, herceptin, anti-CD22, dasatinib, depocyte and forodesine), and a chemotherapy backbone for elderly ALL was activated by three groups (GMALL, GRAALL and PETHEMA). The latter have activated the first joint European trial with dasatinib for older patients with Ph+ ALL. Supportive care and infection prophylaxis were optimized on a European basis. EWALL has recently been recognized as a scientific working-group within the European Hematology Association (EHA), thereby achieving synergy between the ELN and the EHA.
The CLL working-group (WP7) has formally cooperated since the foundation of the European Research Initiative for CLL (ERIC) in 2001. ERIC is an incorporated legal entity (ERIC e.V.) in Germany, and in 2009 ERIC was recognized as a scientific working-group within EHA. The development of new potentially curative treatment modalities for CLL is one of the long-term goals of WP7/ERIC. Several protocol exchanges addressing immunochemotherapy have been made between European CLL-groups (German and French groups). Rare subentities are addressed by combined immunochemotherapy with fludarabine, mitoxantrone, cyclophosphamide and alemtuzumab for T-prolymphocytic leukemia (T-PLL) (lead group Austria), with fludarabine, cyclophosphamide and rituximab for B-PLL (lead group in Erfurt, Germany) and recommendations for stem cell transplantation in T-PLL (lead group in Heidelberg and Cologne, Germany). The harmonization of clinical protocols between national CLL study-groups is ongoing. Several guidelines on diagnostic procedures and therapy in CLL have been published11–13 (Table 2).
The MDS working-group (WP8) is the second WP to start a European registry (EUMDS) with a private partner (Novartis). About 650 low and intermediate risk-1 patients have so far been registered. Extensive data on transfusions and associated iron load are being collected. An extension to high-risk patients is in progress. WP8 conducts European trials on all MDS-subtypes with various agents (lenalidomide, bortezomib, demethylating agents [azacy-tidine, decitabine], cytarabine and growth factors: erythropoietin, GCSF, AMG531) and explores the impact of iron chelation (deferasirox) on the prognosis of MDS. Recommendations for diagnosis and treatment of MDS are published on the ELN-website (Table 2). Also the MDS working-group was recently recognized as a scientific working-group within the EHA.
The CMPD working-group (WP9) in cooperation with groups in North America has explored the impact of JAK2 mutations on the diagnosis and therapy of myeloproliferative neoplasms (MPN). Harmonization of assay methods for JAK2-V617F has been undertaken in close collaboration with WP12. Several consensus protocols were published on response criteria in essential thrombocythemia (ET) and polycythemia vera (PV),16 and on the use of hydroxyurea in PV and myelofibrosis17 (Table 2). A new risk factor (leukocytosis) relevant for the management of thrombosis in MPN has been identified. Management recommendations for PV, ET and myelofibrosis have been completed.15 European trials are being developed to test proteasome- and JAK2-inhibitors and other drugs (e.g. pomalidomide) in myelofibrosis.
A good example of the potential of networking is provided by the diagnostic working-groups (WP10-13) with the Microarray Innovations in Leukemia (MILE) study. The MILE-study, which was coordinated by WP13, involved 11 laboratories (7 from ELN, 3 from the US, one from Singapore) and integrated data from morphology, cytogenetics, molecular genetics, immunophenotyping and gene expression profiling from 3,334 patients to reveal new patient subgroups with specific prognosis and survival.17,37 The MILE-study analyzes patients with all types of leukemia in cooperation with WP4-9. Recommendations for gene expression profiling and microarray analyses have been published (Table 2).
The morphology working-group (WP10) has developed recommendations for immunophenotyping, an atlas of flow-cytometry of normal bone marrow and a consensual morphology collection of hematopoietic cells posted on the ELN-website (Table 2). WP10 has closely collaborated with the clinical groups regarding the development of flow-cytometry for the diagnosis and monitoring of minimal residual disease,38 particularly in MDS.19
A major challenge for the cytogenetics working-group (WP 11) has been the harmonization of techniques and the identification of cryptic and complex chromosome aberrations. Consensus protocols for the diagnostic workup of all types of leukemia and related syndromes, and a proposal for standardization of cytogenetic analyses have been developed20 (Table 2). In order to identify minimal chromosomal imbalances not detectable by classical chromosome banding or FISH analysis, comparative genomic hybridization using arrays (aCGH) has been performed, and loss of heterozygosity, a phenomenon often found in leukemias, is studied by single nucleotide polymorphism (SNP) arrays.
The monitoring of minimal residual disease (MRD) by WP12 has gained great importance by the advent of well defined molecular markers with prognostic relevance in virtually all leukemias. WP12 has developed novel assays to increase the proportion of patients with myeloid/myeloproliferative disorders who might benefit from MRD monitoring such as RQ-PCR assays for FIP1L1-PDGFRA21,22 in chronic eosinophilic leukemia and assays to detect overexpression of the Wilms’ Tumor gene (WT1) in AML23 (Table 2). This has been complemented by standardization of established assays (e.g. RQ-PCR for BCR-ABL5,6 and JAK2-V617F, in collaboration with WP4 and WP9, respectively) and the development of a tailor-made computer software-package to standardize reporting of MRD-data. Using the optimized ELN-WT1 assay, WP12 has shown that the kinetics of disease response provide an independent prognostic factor in AML, and WP12 has highlighted, through studies involving RQ-PCR detection of BCR-ABL, FIP1L1-PDGFRA and PML-RARA transcripts,5,6,9,10,21,22 how sequential MRD monitoring can be used to track response to molecularly targeted therapies in a more individualized approach.
The stem cell transplantation working-group (WP14) makes use of their productive collaboration with the European Group for Blood and Marrow Transplantation (EBMT). The main activities include regular surveys on transplantation activity in Europe, recommendations for the use of stem cell transplantation (Table 2), assessment of key factors responsible for outcome39 and, as a current focus, the adaptation of transplantation conditions to the needs of elderly patients, mainly with AML and ALL. In CML, an improvement in transplantation outcome has been achieved with low transplantation mortality (<10%) and 3-year survival rates of approximately 90% in chronic phase and more than 50% in advanced phase patients.40 These favorable developments are mediated by improvements in patient and donor selection, transplantation procedures and supportive care.
The Working-group on management of infectious complications, infection prophylaxis and supportive care (WP15) has addressed the management of neutropenic patients after stem cell transplantation or intensive chemotherapy. Recommendations on the diagnosis and management of bacterial, viral and fungal infections have been published (Table 2). Guidelines for the management of hepatic, respiratory and adenovirus infections are in preparation as well as protocols to assess the genetic risks for fungal infections and to monitor transfusion policy in Europe.
Perspectives
It is not easy to measure the individual contribution of the ELN towards the general advancement of research and improvement of prognosis in the field of leukemia. However, we can point to the number of common clinical trials, projects and publications, and the steadily increasing numbers of ELN participants to demonstrate its success. Various ELN-studies have been completed41,42 and ELN-criteria are widely used.43–45 A number of activities point to sustainability and further development of the ELN.
Common observational and interventional studies on a European level continue in realization of the need for cooperation on rare diseases such as the leukemias.
Leukemia-registries will expand, answer questions, and promote progress of leukemia research. Multiple public-private partnerships are envisaged.
New projects and trials will be defined by working-groups and delivered with support by the ELN-Foundation, the European Science Foundation and other sources.
In view of current legislation which threatens treatment optimization studies, the ELN-Foundation might serve as ‘Sponsor’. The ELN supports every effort to achieve a modification of the European drug legislation for treatment optimization studies.
Due to its structured and long-term cooperation, the ELN is likely to have a durable impact on leukemia research in Europe. Infrastructure and the productive collaboration provided by the ELN have provided a valuable contribution to progress in the field of leukemia.
By promoting cooperation over the competition that is necessary for good research, the ELN provides a competitive advantage for all participants to the benefit of every patient with leukemia worldwide.
Acknowledgments
the authors would like to thank Clara Bloomfield (The Ohio State University, Columbus, OH), Ching-Hon Pui (University of Tennessee Health Science Center, Memphis, TN) and Jan Willem van de Loo (European Commission, Brussels) for continuous advice and support. The contribution of Ute Kossak, Gabriele Bartsch, Matthias Dumke, Barbara Müller, Nicole Schomber and Catherine Sodan-Boyer (Medizinische Fakultät Mannheim, Universität Heidelberg) is acknowledged.
Footnotes
Funding: this work was supported by the European Commission (LSHC-CT-2004-503216).
Authorship and Disclosures
The information provided by the authors about contributions from persons listed as authors and in acknowledgments is available with the full text of this paper at www.haematologica.org.
Financial and other disclosures provided by the authors using the ICMJE (www.icmje.org) Uniform Format for Disclosure of Competing Interests are also available at www.haematologica.org.
References
- 1.Hehlmann R, Berger U, Aul C, Büchner T, Döhner H, Ehninger G, et al. The German competence network ‘Acute and chronic leukemias’. Leukemia. 2004;18(4):665–9. doi: 10.1038/sj.leu.2403317. [DOI] [PubMed] [Google Scholar]
- 2.Baccarani M, Saglio G, Goldman J, Hochhaus A, Simonsson B, Appelbaum F, et al. Evolving concepts in the management of chronic myeloid leukemia. Recommendations from an expert panel on behalf of the European LeukemiaNet. Blood. 2006;108(6):1809–20. doi: 10.1182/blood-2006-02-005686. [DOI] [PubMed] [Google Scholar]
- 3.Hehlmann R, Hochhaus A, Baccarani M. Chronic myeloid leukaemia. Lancet. 2007;370:342–50. doi: 10.1016/S0140-6736(07)61165-9. [DOI] [PubMed] [Google Scholar]
- 4.Baccarani M, Cortes J, Pane F, Niederwieser D, Saglio G, Apperley J, et al. Chronic myeloid leukemia: an update of concepts and management recommendations of European LeukemiaNet. J Clin Oncol. 2009;27(35):6041–51. doi: 10.1200/JCO.2009.25.0779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Müller MC, Cross NC, Erben P, Schenk T, Hanfstein B, Ernst T, et al. Harmonization of molecular monitoring of CML therapy in Europe. Leukemia. 2009;23(11):1957–63. doi: 10.1038/leu.2009.168. [DOI] [PubMed] [Google Scholar]
- 6.Hughes T, Deininger M, Hochhaus A, Branford S, Radich J, Kaeda J, et al. Monitoring CML patients responding to treatment with tyrosine kinase inhibitors: review and recommendations for harmonizing current methodology for detecting BCR-ABL transcripts and kinase domain mutations and for expressing results. Blood. 2006;108(1):28–37. doi: 10.1182/blood-2006-01-0092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Branford S, Cross NC, Hochhaus A, Radich J, Saglio G, Kaeda J, et al. Rationale for the recommendations for harmonizing current methodology for detecting BCR-ABL transcripts in patients with chronic myeloid leukaemia. Leukemia. 2006;20:1925–30. doi: 10.1038/sj.leu.2404388. [DOI] [PubMed] [Google Scholar]
- 8.Döhner H, Estey EH, Amadori S, Appelbaum FR, Büchner T, Burnett AK, et al. Diagnosis and management of acute myeloid leukemia in adults: recommendations from an international expert panel, on behalf of the European LeukemiaNet. Blood. 2010;115(3):453–74. doi: 10.1182/blood-2009-07-235358. [DOI] [PubMed] [Google Scholar]
- 9.Sanz MA, Grimwade D, Tallman MS, Lowenberg B, Fenaux P, Estey EH, et al. Management of acute promyelocytic leukemia: recommendations from an expert panel on behalf of the European LeukemiaNet. Blood. 2009;113(9):1875–91. doi: 10.1182/blood-2008-04-150250. [DOI] [PubMed] [Google Scholar]
- 10.Grimwade D, Jovanovic JV, Hills RK, Nugent EA, Patel Y, Flora R, et al. Prospective minimal residual disease monitoring to predict relapse of acute promyelocytic leukemia and to direct pre-emptive arsenic trioxide therapy. J Clin Oncol. 2009;27(22):3650–8. doi: 10.1200/JCO.2008.20.1533. [DOI] [PubMed] [Google Scholar]
- 11.Hallek M, Cheson BD, Catovsky D, Caligaris-Cappio F, Dighiero G, Döhner H, et al. Guidelines for the diagnosis and treatment of chronic lymphocytic leukemia: a report from the International Workshop on Chronic Lymphocytic Leukemia updating the National Cancer Institute-Working Group 1996 guidelines. Blood. 2008;111 (12):5446–56. doi: 10.1182/blood-2007-06-093906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ghia P, Stamatopoulos K, Belessi C, Moreno C, Stilgenbauer S, Stevenson F, et al. ERIC recommendations on IGHV gene mutational status analysis in chronic lymphocytic leukemia. Leukemia. 2007;21(1):1–3. doi: 10.1038/sj.leu.2404457. [DOI] [PubMed] [Google Scholar]
- 13.Rawstron AC, Villamor N, Ritgen M, Bottcher S, Ghia P, Zehnder JL, et al. International standardized approach for flow cytometric residual disease monitoring in chronic lymphocytic leukaemia. Leukemia. 2007;21(5):956–64. doi: 10.1038/sj.leu.2404584. [DOI] [PubMed] [Google Scholar]
- 14.ELN Homepage. http://www.leukemi-anet.eu.
- 15.Barbui T, Barosi G, Birgegard G, Cervantes F, Finazzi G, Griesshammer M, et al. Philadelphia-Negative Classical Myeloproliferative Neoplasms: Critical Concepts and Management Recommendations from European Leukemia. Net J Clin Oncol. 2010 doi: 10.1200/JCO.2010.31.8436. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Barosi G, Birgegard G, Finazzi G, Griesshammer M, Harrison C, Hasselbalch HC, et al. Response criteria for essential thrombocythemia and polycythemia vera: result of a European LeukemiaNet consensus conference. Blood. 2009;113(20):4829–33. doi: 10.1182/blood-2008-09-176818. [DOI] [PubMed] [Google Scholar]
- 17.Barosi G, Birgegard G, Finazzi G, Griesshammer M, Harrison C, Hasselbalch H, et al. A unified definition of clinical resistance and intolerance to hydroxycarbamide in polycythaemia vera and primary myelofibrosis: results of a European LeukemiaNet (ELN) consensus process. BrJ Haematol. 2010;148(6):961–3. doi: 10.1111/j.1365-2141.2009.08019.x. [DOI] [PubMed] [Google Scholar]
- 18.Arnoulet C, Béné MC, Durrieu F, Feuillard J, Fossat C, Husson B, et al. Four- and five-color flow cytometry analysis of leukocyte differentiation pathways in normal bone marrow: a reference document based on a systematic approach by the GTLLF and GEIL. Cytometry B Clin Cytom. 2010;78:4–10. doi: 10.1002/cyto.b.20484. [DOI] [PubMed] [Google Scholar]
- 19.van de Loosdrecht AA, Alhan C, Bene MC, Della Porta MG, Drager AM, Feuillard J, et al. Standardization of flow cytometry in myelodysplastic syndromes: report from the first European LeukemiaNet working conference on flow cytometry in myelodysplastic syndromes. Haematologica. 2009;94(8):1124–34. doi: 10.3324/haematol.2009.005801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Haferlach C, Rieder H, Lillington DM, Dastugue N, Hagemeijer A, Harbott J, et al. Proposals for standardized protocols for cytogenetic analyses of acute leukemias, chronic lymphocytic leukemia, chronic myeloid leukemia, chronic myeloproliferative disorders, and myelodysplastic syndromes. Genes Chromosomes Cancer. 2007;46(5):494–9. doi: 10.1002/gcc.20433. [DOI] [PubMed] [Google Scholar]
- 21.Jovanovic JV, Score J, Waghorn K, Cilloni D, Gottardi E, Metzgeroth G, et al. Low-dose imatinib mesylate leads to rapid induction of major molecular responses and achievement of complete molecular remission in FIP1L1-PDGFRA-positive chronic eosinophilic leukemia. Blood. 2007;109(11):4635–40. doi: 10.1182/blood-2006-10-050054. [DOI] [PubMed] [Google Scholar]
- 22.Score J, Walz C, Jovanovic JV, Jones AV, Waghorn K, Hidalgo-Curtis C, et al. Detection and molecular monitoring of FIP1L1-PDGFRA-positive disease by analysis of patient-specific genomic DNA fusion junctions. Leukemia. 2009;23(2):332–9. doi: 10.1038/leu.2008.309. [DOI] [PubMed] [Google Scholar]
- 23.Cilloni D, Renneville A, Hermitte F, Hills RK, Daly S, Jovanovic JV, et al. Real-time quantitative polymerase chain reaction detection of minimal residual disease by standardized WT1 assay to enhance risk stratification in acute myeloid leukemia: a European LeukemiaNet study. J Clin Oncol. 2009;27(31):5195–201. doi: 10.1200/JCO.2009.22.4865. [DOI] [PubMed] [Google Scholar]
- 24.Kohlmann A, Kipps TJ, Rassenti LZ, Downing JR, Shurtleff SA, Mills KI, et al. An international standardization programme towards the application of gene expression profiling in routine leukaemia diagnostics: the Microarray Innovations in Leukemia study prephase. Br J Haematol. 2008;142(5):802–7. doi: 10.1111/j.1365-2141.2008.07261.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Staal FJ, Cario G, Cazzaniga G, Haferlach T, Heuser M, Hofmann WK, et al. Consensus guidelines for microarray gene expression analyses in leukemia from three European leukemia networks. Leukemia. 2006;20(8):1385–92. doi: 10.1038/sj.leu.2404274. [DOI] [PubMed] [Google Scholar]
- 26.Ruutu T, Barosi G, Benjamin RJ, Clark RE, George JN, Gratwohl A, et al. Diagnostic criteria for hematopoietic stem cell transplant-associated microangiopathy: results of a consensus process by an International Working Group. Haematologica. 2007;92 (1):95–100. doi: 10.3324/haematol.10699. [DOI] [PubMed] [Google Scholar]
- 27.Dreger P, Corradini P, Kimby E, Michallet M, Milligan D, Schetelig J, et al. Indications for allogeneic stem cell transplantation in chronic lymphocytic leukemia: the EBMT transplant consensus. Leukemia. 2007;21(1):12–7. doi: 10.1038/sj.leu.2404441. [DOI] [PubMed] [Google Scholar]
- 28.De Witte T, Brand R, van Biezen A, Delforge M, Biersack H, Or R, et al. The role of stem cell source in autologous hematopoietic stem cell transplantation for patients with myelodysplastic syndromes. Haematologica. 2006;91(6):750–6. [PubMed] [Google Scholar]
- 29.Bucaneve G, Castagnola E, Viscoli C, Leibovici L, Menichetti F. Quinolone prophylaxis for bacterial infections in afebrile high risk neutropenic patients. EJC Supplements. 2007;5:5–12. [Google Scholar]
- 30.Styczynski J, Reusser P, Einsele H, de la Camara R, Cordonnier C, Ward KN, et al. Management of HSV, VZV and EBV infections in patients with hematological malignancies and after SCT: guidelines from the Second European Conference on Infections in Leukemia. Bone Marrow Transplant. 2009;43(10):757–70. doi: 10.1038/bmt.2008.386. [DOI] [PubMed] [Google Scholar]
- 31.Ljungman P, Engelhard D, de la Camara R, Einsele H, Locasciulli A, Martino R, et al. Vaccination of stem cell transplant recipients: recommendations of the Infectious Diseases Working Party of the EBMT. Bone Marrow Transplant. 2005;35(8):737–46. doi: 10.1038/sj.bmt.1704870. [DOI] [PubMed] [Google Scholar]
- 32.Marchetti O, Cordonnier C, Calandra T. Empirical antifungal therapy in neutropienic cancer patients with persistent fever. EJC Supplements. 2007;5:32–42. [Google Scholar]
- 33.Maertens JA, Frére P, Lass-Flörl C, Heinz W, Cornely O. Primary antifungal prophylaxis in leukaemia patients. EJC Supplements. 2007;5:43–8. [Google Scholar]
- 34.Herbrechet R, Flückiger U, Gachot B, Ribaud P, Thiebaut A, Cordonnier C. Treatment of invasive Candida and invasive Aspergillus infections in adult haematological patients. EJC Supplements. 2007;5:49–59. [Google Scholar]
- 35.Ljungman P, de la Camara R, Cordonnier C, Einsele H, Engelhard D, Reusser P, et al. Management of CMV, HHV-6, HHV-7 and Kaposi-sarcoma herpesvirus (HHV-8) infections in patients with hematological malignancies and after SCT. Bone Marrow Transplant. 2008;42(4):227–40. doi: 10.1038/bmt.2008.162. [DOI] [PubMed] [Google Scholar]
- 36.Büchner T, Berdel WE, Haferlach C, Haferlach T, Schnittger S, Müller-Tidow C, et al. Age-related risk profile and chemotherapy dose response in acute myeloid leukemia: a study by the German Acute Myeloid Leukemia Cooperative Group. J Clin Oncol. 2009;27(1):61–9. doi: 10.1200/JCO.2007.15.4245. [DOI] [PubMed] [Google Scholar]
- 37.Haferlach T, Kohlmann A, Wieczorek L, Basso G, Kronnie GT, Bene MC, et al. Clinical utility of microarray-based gene expression profiling in the diagnosis and subclassification of leukemia: report from the International Microarray Innovations in Leukemia Study Group. J Clin Oncol. 2010;28(15):2529–37. doi: 10.1200/JCO.2009.23.4732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bene MC, Kaeda JS. How and why minimal residual disease studies are necessary in leukemia: a review from WP10 and WP12 of the European LeukaemiaNet. Haematologica. 2009;94(8):1135–50. doi: 10.3324/haematol.2008.004267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gratwohl A, Stern M, Brand R, Apperley J, Baldomero H, de Witte T, et al. Risk score for outcome after allogeneic hematopoietic stem cell transplantation: a retrospective analysis. Cancer. 2009;115(20):4715–26. doi: 10.1002/cncr.24531. [DOI] [PubMed] [Google Scholar]
- 40.Saussele S, Lauseker M, Gratwohl A, Beelen DW, Bunjes D, Schwerdtfeger R, et al. Allogeneic hematopoietic stem cell transplantation (allo SCT) for chronic myeloid leukemia in the imatinib era: evaluation of its impact within a subgroup of the randomized German CML Study IV. Blood. 2010;115(10):1880–5. doi: 10.1182/blood-2009-08-237115. [DOI] [PubMed] [Google Scholar]
- 41.Baccarani M, Rosti G, Castagnetti F, Haznedaroglu I, Porkka K, Abruzzese E, et al. Comparison of imatinib 400 mg and 800 mg daily in the front-line treatment of high-risk, Philadelphia-positive chronic myeloid leukemia: a European LeukemiaNet Study. Blood. 2009;113(19):4497–504. doi: 10.1182/blood-2008-12-191254. [DOI] [PubMed] [Google Scholar]
- 42.Rinaldi CR, Rinaldi P, Gemei M, Grimaldi F, Battipaglia G, Del Vecchio L, et al. JAK2V617F mutation persists in blasts and mature cells of transformed JAK2V617F-positive-myeloproliferative neoplasia: a European Leukemia Net ENL study. Am J Hematol. 2010;85(5):383–6. doi: 10.1002/ajh.21687. [DOI] [PubMed] [Google Scholar]
- 43.Marin D, Milojkovic D, Olavarria E, Khorashad JS, de Lavallade H, Reid AG, et al. European LeukemiaNet criteria for failure or suboptimal response reliably identify patients with CML in early chronic phase treated with imatinib whose eventual outcome is poor. Blood. 2008;112(12):4437–44. doi: 10.1182/blood-2008-06-162388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Alvarado Y, Kantarjian H, O’Brien S, Faderl S, Borthakur G, Burger J, et al. Significance of suboptimal response to imatinib, as defined by the European LeukemiaNet, in the long-term outcome of patients with early chronic myeloid leukemia in chronic phase. Cancer. 2009;115(16):3709–18. doi: 10.1002/cncr.24418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Carobbio A, Finazzi G, Antonioli E, Vannucchi AM, Barosi G, Ruggeri M, et al. Hydroxyurea in essential thrombocythemia: rate and clinical relevance of responses by European LeukemiaNet criteria. Blood. 2010;116(7):1051–5. doi: 10.1182/blood-2010-03-272179. [DOI] [PubMed] [Google Scholar]