Abstract
Dasatinib is considered an effective drug in imatinib-resistant chronic myeloid leukemia. Although reported to be well-tolerated, severe events such as pleural or pericardial effusion have been reported at 140 mg daily. We examined our chronic myeloid leukemia patients treated with dasatinib at 100 mg or 50 mg daily and identified 4 of 13 patients who developed marked effusion formation. In 2 patients, grade III/IV pleural and/or pericardial effusions were recorded. All 4 patients had received previous anti-leukemia therapy but none had pre-existing cardiac or pulmonary diseases. In 3 patients, dasatinib had to be discontinued despite treatment with diuretics and glucocorticosteroids. In conclusion, dasatinib-treated chronic myeloid leukemia patients are at risk for the development of pleural and pericardial effusions even when the drug is administered at 100 mg or 50 mg daily. Therefore, all patients should be examined for pre-existing comorbidity and risk factors before starting dasatinib and all should have repeated chest X-rays during long-term dasatinib therapy.
Keywords: chronic myeloid leukemia, BCR/ABL, dasatinib, side-effects
Introduction
Therapy for imatinib-resistant chronic myeloid leukemia (CML) is an emerging challenge in clinical hematology.1–3 In these patients, novel BCR/ABL tyrosine kinase inhibitors (TKI) are prescribed.1–5 One highly effective second-generation BCR/ABL TKI is dasatinib.5–8 This drug reportedly produces complete cytogenetic responses (CCyR) in a substantial number of imatinib-resistant patients.6–10 Initially, dasatinib was announced as a dual inhibitor of Src kinases and BCR/ABL.5 However, recent data suggest that dasatinib interacts with multiple kinase targets11 which may explain superior growth-inhibitory effects on CML cells and also side-effects. The initially approved standard dose of dasatinib was 70 mg per os twice daily. However, several studies have shown that dasatinib at 140 mg daily produces various side-effects, including cytopenia and pleural effusions.6–10,12,13 Therefore, the recommended standard dose of dasatinib has been reduced to 100 mg once daily in chronic phase CML. At this dose, dasatinib reportedly retains its anti-leukemic efficacy but seems to be less toxic.14 In patients in whom side-effects occur with 100 mg dasatinib daily, dose reduction to 50 mg daily, treatment interruption, or switching to alternative drugs may be considered. However, long-term data with low-dose dasatinib are not yet available. Furthermore, some stimulating effects of dasatinib on immune cells, such as enhancement of IgE-dependent histamine-liberation from basophils in vitro, are particularily seen at low concentrations of dasatinib.15
We report on 4 patients with imatinib-resistant CML who were treated with dasatinib at 100 mg or 50 mg per os daily and developed extensive pericardial and/or pleural effusions.
Design and Methods
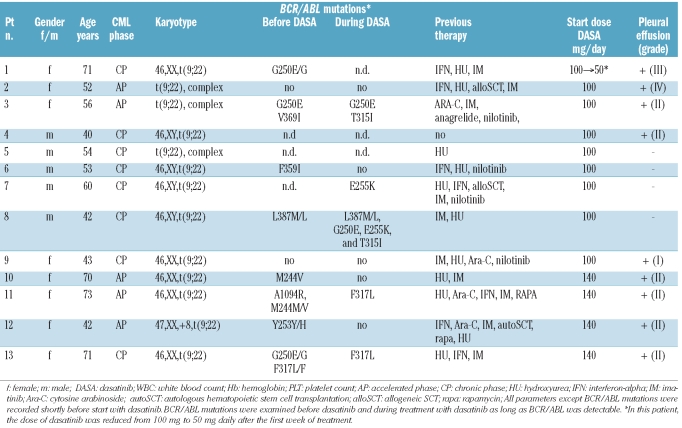
The objective of the study was the evaluation of consecutive CML patients treated with low-dose dasatinib. Thirteen patients with CML received dasatinib at 100 mg or 50 mg daily for at least three months (range 3–38 months) in our center (observation period March 2005 to June 2010). Patients’ characteristics are shown in Table 1. Two patients (CP) received dasatinib front line in a phase II study (clinicaltrials.gov identifier: NCT00481247). All patients gave written informed consent.
Table 1.
Patients’ characteristics
Case Reports
Patient 1, a 70-year old female patient presented with imatinib-resistant CML-AP in December 2007. Chronic myeloid leukemia had been diagnosed in 2001. Previous therapies included interferon-alpha (IFNα), hydroxyurea (HU), and imatinib. No relevant comorbidities had been reported except pneumonia in 2001 and herpes zoster in 2003. In December 2007, the white blood count (WBC) was 20×109/L, hemoglobin 12.5 g/dL, and platelets were 832×109/L. Sequencing analysis revealed BCR/ABL G250E. Therapy with dasatinib (100 mg daily) was initiated in January 2008. One week later, the patient developed grade I pulmonary edema. Dose-reduction to 50 mg dasatinib daily was followed by regression of symptoms. Over the following months the patient entered major molecular response (BCR/ABL<0.01%). In February 2009, the patient developed symptomatic pleural effusions, and received diuretics and glucocorticosteroids. In March 2009, the patient presented with grade III pleural effusion and pneumonia requiring hospitalization. In May 2009, the patient also relapsed with herpes zoster. Dasatinib was discontinued. Treatment with nilotinib was initiated in June 2009.
Patient 2, a 52-year old female patient presented with imatinib-resistant CML-AP in February 2009. Chronic myeloid leukemia had been diagnosed in 1993. In December 1995, she underwent HLA-identical unrelated donor allogeneic stem cell transplantation (SCT). After SCT, she developed graft-versus-host disease (skin/liver). In November 1998, she experienced a cytogenetic relapse. Discontinuation of immunosuppressive therapy and donor-lymphocytes produced a transient response. In 2001, imatinib (400 mg daily) was started and resulted in complete cytogenetic response and major molecular response. Previous comorbidities unrelated to stem cell transplantion were a malignant melanoma (right leg) resected in 2000 and a carcinoma of the tongue resected in 2007. No pre-existing pulmonary or cardiac disorders had been reported. In February 2009, the white blood cell count was 4.7×109/L, hemoglobin 8.0 g/dL, and platelets were 962 ×109/L. Cytogenetic analysis revealed a complex Ph+ karyotype. No BCR/ABL mutation was detected. Dasatinib (100 mg/day) was started in March 2009. A few days later, pneumonia and massive pleural effusions with consecutive right heart failure developed. The patient was hospitalized and received antibiotics and diuretics. Dasatinib was discontinued for two weeks. We then tried once again to start dasatinib. However, in May 2009, she developed gastrointestinal bleeding, pleural effusions, and pneumonia. A few weeks later, the patient presented with massive pericardial effusion requiring intubation and transfer to the intensive care unit (pleural drainage and pericardial fenestration). Dasatinib was stopped and low-dose prednisolone was initiated. The patient’s clinical condition improved over the following weeks. Nilotinib was started in June 2009.
Patient 3, a 57-year old female patient without known comorbidities was diagnosed with CML-AP in June 2003. Imatinib (400 mg/day) was started. Imatinib was discontinued in February 2008 because of intolerance and resistance (BCR/ABL V379I). Between April 2008 and September 2008 the patient received nilotinib. Despite prolonged pancytopenia, no major response was recorded and in September 2008 BCR/ABL G250E and V379I were detected. In August 2009 dasatinib (100 mg/daily) was started. However, after four months dasatinib had to be discontinued because of grade IV cytopenia and pericardial effusion. Dasatinib was again started in January 2010 at 50 mg daily. However, after one month the patient developed a gastrointestinal infection as well as pericardial and pleural effusions. At that time BCR/ABL T315I was detected and progression to blast phase recorded. She then received one cycle of polychemotherapy with cytarabine and mitoxantrone. After chemotherapy, the mutation status revealed a persistent BCR/ABL V379I but no T315I or G250E. Since May 2010 the patient has been receiving nilotinib (800 mg/day).
Patient 4, a 40-year old patient was diagnosed with CML-CP in April 2008. The blood count at diagnosis was: white blood count 13.010×109/L, hemoglobin 13.9 g/dL, and platelets 601×109/L. The patient had been suffering from psoriasis vulgaris since 1987, and received continuous methotrexate therapy p.o. No other relevant comorbidity had been reported. In April 2008, dasatinib at 100 mg daily was started. During the following months, the patient entered a complete cytogenetic response and a major molecular response (BCR/ABL 0.01% in May 2010). No relevant side-effects were recorded until May 2010 when the patient developed bronchitis followed by severe dyspnea. A chest X-ray revealed marked pleural effusion without pneumonia. The patient received diuretics and prednisolone (25 mg/day) which resulted in a rapid improvement in clinical condition. The patient is currently still on dasatinib.
Results and Discussion
The most frequent relevant non-hematologic adverse event in dasatinib-treated patients is the occurrence of pleural effusions.6–10,12,13 This side-effect has also been referred to as serosal inflammation and apparently develops more commonly in patients with advanced disease.16,17 In initial reports, the incidence of grade III or IV pleural effusions was reported to be rather low. On the other hand, effusion formation accumulates over time. More recently it has been reported that the risk of effusion formation is lower in patients treated with 100 mg dasatinib once daily compared to patients treated with 140 mg dasatinib.14,17 However, pleural effusions may develop even in freshly diagnosed patients treated with dasatinib at 100 mg daily.17 The frequency of symptomatic pleural effusions in these patients was reported to be 13% but only one out of 62 patients (2%) developed grade III/IV pleural effusion.17
We examined 13 CML patients treated with dasatinib at 100 mg or 50 mg daily in our center. Four of the 13 patients developed clinically relevant pleural or pericardial effusions: 2 of these 4 patients had grade III or IV effusions and one developed a life-threatening pericardial effusion. We also observed effusion formation in one of the 2 patients with CML-CP who started front-line therapy with dasatinib at 100 mg daily. However, in this patient only grade II pleural effusions developed which is in line with the data of Cortes et al.17 The more aggressive clinical course in the other patients may be explained by the advanced disease-phase, previous therapy, and/or comorbidity.
Of the 13 patients examined, 4 had previously received 140 mg dasatinib daily. In these 4 patients, the dose of dasatinib was reduced to 100 mg or 50 mg daily because of pleural effusions. In the other 9 patients, the initial dose of dasatinib was 100 mg/day. Of these 9 patients, 2 CP patients received front-line dasatinib. One of these 2 patients developed pleural effusions. Three other patients also developed pleural effusions during treatment with dasatinib at 100 mg or 50 mg daily and pericardial effusions were also recorded in 2 of them. Of all 13 patients analyzed, 9 (69%) developed pleural effusions and of the 9 patients who started with dasatinib at 100 mg/day, 5 (56%) developed pleural effusions.
In 3 of the 4 patients with marked effusion formation, dasatinib had to be discontinued. At that time, Patient 1 was in complete cytogenetic respone and major molecular response whereas Patients 2 and 3 were in hematologic and molecular relapse (BCR/ABL 39.1% in Patient 2 and 54.3% in Patient 3). No BCR/ABL mutation was detected in Patient 2. The BCR/ABL mutants detectable in Patient 1 (G250E) and Patient 3 (V379I) are known to be responsive to nilotinib. Therefore we switched to nilotinib in these patients. During nilotinib therapy, Patient 1 and Patient 2 entered major molecular response with a decrease of BCR/ABL to 0.0% in Patient 1 and to 0.008% in Patient 2. In Patient 3, no molecular follow up is available. Patient 4 is still in major molecular response and complete cytogenetic response. So far, no further pleural effusions have been recorded in the 4 patients.
The mechanisms underlying the development of effusion formation during dasatinib treatment are currently unknown. Most likely, several different factors contribute to effusion formation.16,18 An interesting aspect is that dasatinib binds to and blocks major kinases of the immune system including Lyn, Btk, and Src, with consecutive deactivation of immune cells.11,13,15 At low concentration, however, dasatinib may even trigger the activation of certain immune cells. Likewise, low-dose dasatinib even promotes IgE-dependent histamine release in basophils.15 Although it has not formally been proven that basophil/mast cell activation and histamine release is involved in serosal inflammation/effusion formation caused by low-dose dasatinib, it seems clear that dose reduction of dasatinib may lower the risk of various side effects but does not eliminate the risk of effusion formation. This may also be true for patients with freshly diagnosed CML.
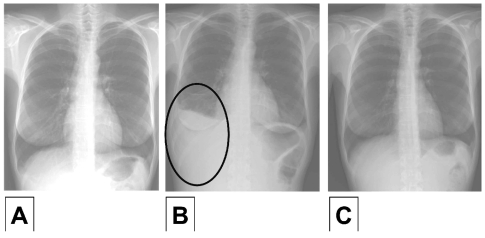
Figure 1.
Radiographic findings during dasatinib therapy. (A) Chest X-ray in Patient 2 one day before starting dasatinib. No pleural effusions were noted. (B) Six weeks after initiation of dasatinib, massive pleural effusions were recorded when the patient suffered from dyspnea. (C) After discontinuation of dasatinib, pleural effusions disappeared almost completely. The patient is currently treated with nilotinib.
One important factor concerning the risk of effusion formation may be pre-existing comorbidities, such as cardiac or pulmonary disorders.13,16 In addition, viral reactivation or infection, and an increase in activated lymphocytes (LGL cells) have been discussed as potential factors contributing to effusion formation.19,20 In the 4 patients with pleural effusions (on 100 mg dasatinib) described in this study, no relevant pre-existing pulmonary or cardiac diseases had been reported. However, one patient was heavily pre-treated and suffered from chronic graft-versus-host disease of the skin. Therefore, we believe that several factors and comorbidities may act together to predispose for effusion formation in patients treated with dasatinib.
In general, pleural effusions occurring during dasatinib are managed by treatment interruption or dose reduction as well as supportive therapy.14,21,22 Whereas diuretics alone are usually without a long-lasting effect, glucocorticosteroids are often effective in these patients.14,21,22 Therefore, we also applied low-dose glucocortico isteroids. However, because of the severity and recurrence of adverse events, dasatinib had to be discontinued in 3 of 4 patients.
An important aspect is early recognition and prevention of effusion formation in CML patients treated with dasatinib. As mentioned above, one critical point is to screen for possible comorbidities before starting dasatinib. A second important point is to recommend that dasatinib-treated CML patients have a repeated chest X-ray (e.g. once or twice a year) in their follow up even if no risk factors or symptoms are recorded.
Currently, two BCR/ABL inhibitors, dasatinib and nilotinib are available for treatment of imatinib-resistant CML and both have shown similar efficacy in these patients.5–9,23 It has also been described that both novel TKIs can be administered in sequence when drug resistance or intolerance occurs.24 Similarly, in our patients we switched to nilotinib and so far no relapse and no further effusion events have been recorded.
In summary, we describe 4 patients who received low-dose dasatinib and developed marked or even grade III/IV pericardial and/or pleural effusions. We recommend that all patients should be screened for relevant co-morbidities and potential risk factors before starting dasatinib (at any dose) and all patients who receive long-term dastatinib should have repeated X-rays and careful monitoring of symptoms in their follow up.
Footnotes
Supported by: Fonds zur Förderung der Wissenschaftlichen Forschung in Österreich.
Authorship and Disclosures
The information provided by the authors about contributions from persons listed as authors and in acknowledgments is available with the full text of this paper at www.haematologica.org.
Financial and other disclosures provided by the authors using the ICMJE (www.icmje.org) Uniform Format for Disclosure of Competing Interests are also available at www.haematologica.org.
References
- 1.Martinelli G, Soverini S, Rosti G, Cilloni D, Baccarani M. New tyrosine kinase inhibitors in chronic myeloid leukemia. Haematologica. 2005;90(4):534–41. [PubMed] [Google Scholar]
- 2.Valent P. Emerging stem cell concepts for imatinib-resistant CML: implications for the biology, management, and therapy of the disease. Br J Haematol. 2008;142(3):361–78. doi: 10.1111/j.1365-2141.2008.07197.x. [DOI] [PubMed] [Google Scholar]
- 3.Saglio G, Ulisciani S, Bosa M, Cilloni D, Rege-Cambrin G. New therapeutic approaches and prognostic factors in chronic myeloid leukemia. Leuk Lymphoma. 2008;49(4):625–8. doi: 10.1080/10428190801896210. [DOI] [PubMed] [Google Scholar]
- 4.Cortes J, Kantarjian H. Beyond dose escalation: clinical options for relapse or resistance in chronic myelogenous leukemia. J Natl Compr Canc Netw. 2008;6(S2):S22–S30. [PubMed] [Google Scholar]
- 5.Shah NP, Tran C, Lee FY, Chen P, Norris D, Sawyers CL. Overriding imatinib resistance with a novel ABL kinase inhibitor. Science. 2004;305(5682):399–401. doi: 10.1126/science.1099480. [DOI] [PubMed] [Google Scholar]
- 6.Hochhaus A. Dasatinib for the treatment of Philadelphia chromosome-positive chronic myelogenous leukaemia after imatinib failure. Expert Opin Pharmacother. 2007;8 (18):3257–64. doi: 10.1517/14656566.8.18.3257. [DOI] [PubMed] [Google Scholar]
- 7.Talpaz M, Shah NP, Kantarjian H, Donato N, Nicoll J, Paquette R, et al. Dasatinib in imatinib-resistant Philadelphia chromosome-positive leukemias. N Engl J Med. 2006;354(24):2531–41. doi: 10.1056/NEJMoa055229. [DOI] [PubMed] [Google Scholar]
- 8.Quintas-Cardama A, Kantarjian H, Jones D, Nicaise C, O’Brien S, Giles F, et al. Dasatinib (BMS-354825) is active in Philadelphia chromosome-positive chronic myelogenous leukemia after imatinib and nilotinib (AMN107) therapy failure. Blood. 2007;109 (2):497–9. doi: 10.1182/blood-2006-07-035493. [DOI] [PubMed] [Google Scholar]
- 9.Guilhot F, Apperley J, Kim DW, Bullorsky EO, Baccarani M, Roboz GJ, et al. Dasatinib induces significant hematologic and cytogenetic responses in patients with imatinib-resistant or -intolerant chronic myeloid leukemia in accelerated phase. Blood. 2007;109(10):4143–50. doi: 10.1182/blood-2006-09-046839. [DOI] [PubMed] [Google Scholar]
- 10.Cortes J, Rousselot P, Kim DW, Ritchie E, Hamerschlak N, Coutre S, et al. Dasatinib induces complete hematologic and cytogenetic responses in patients with imatinib-resistant or -intolerant chronic myeloid leukemia in blast crisis. Blood. 2007;109(8):3207–13. doi: 10.1182/blood-2006-09-046888. [DOI] [PubMed] [Google Scholar]
- 11.Rix U, Hantschel O, Dürnberger G, Remsing Rix LL, Planyavsky M, Fernbach NV, et al. Chemical proteomic profiling of the BCR-ABL inhibitors imatinib, nilotinib, and dasatinib reveal different interaction networks and novel kinase and non-kinase targets. Blood. 2007;110(1):4055–63. doi: 10.1182/blood-2007-07-102061. [DOI] [PubMed] [Google Scholar]
- 12.Quintás-Cardama A, Kantarjian H, O’Brien S, Borthakur G, Bruzzi J, Munden R, et al. Pleural effusion in patients with chronic myelogenous leukemia treated with dasatinib after imatinib failure. J Clin Oncol. 2007;25(25):3908–14. doi: 10.1200/JCO.2007.12.0329. [DOI] [PubMed] [Google Scholar]
- 13.Sillaber C, Herrmann H, Bennett K, Rix U, Baumgartner C, Böhm A, Herndlhofer S, Tschachler E, Superti-Furga G, Jäger U, Valent P. Immunosuppression and atypical infections in CML patients treated with dasatinib at 140 mg daily. Eur J Clin Invest. 2009;39(12):1098–109. doi: 10.1111/j.1365-2362.2009.02206.x. [DOI] [PubMed] [Google Scholar]
- 14.Shah NP, Kantarjian HM, Kim DW, Réa D, Dorlhiac-Llacer PE, Milone JH, et al. Intermittent target inhibition with dasatinib 100 mg once daily preserves efficacy and improves tolerability in imatinib-resistant and –intolerant chronic-phase chronic myeloid leukemia. J Clin Oncol. 2008;26(19):3204–12. doi: 10.1200/JCO.2007.14.9260. [DOI] [PubMed] [Google Scholar]
- 15.Kneidinger M, Schmidt U, Rix U, Gleixner KV, Vales A, Baumgartner C, et al. The effects of dasatinib on IgE receptor-dependent activation and histamine release in human basophils. Blood. 2008;111(6):3097–107. doi: 10.1182/blood-2007-08-104372. [DOI] [PubMed] [Google Scholar]
- 16.Kelly K, Swords R, Mahalingam D, Padmanabhan S, Giles FJ. Serosal inflammation (pleural and pericardial effusions) related to tyrosine kinase inhibitors. Target Oncol. 2009;4(2):99–105. doi: 10.1007/s11523-009-0110-4. [DOI] [PubMed] [Google Scholar]
- 17.Cortes JE, Jones D, O’Brien S, Jabbour E, Ravandi F, Koller C, et al. Results of dasatinib therapy in patients with early chronic-phase chronic myeloid leukemia. J Clin Oncol. 2010;28(3):398–404. doi: 10.1200/JCO.2009.25.4920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.de Lavallade H, Punnialingam S, Milojkovic D, Bua M, Khorashad JS, Gabriel IH, et al. Pleural effusions in patients with chronic myeloid leukaemia treated with dasatinib may have an immune-mediated pathogenesis. Br J Haematol. 2008;141(5):745–7. doi: 10.1111/j.1365-2141.2008.07108.x. [DOI] [PubMed] [Google Scholar]
- 19.Mustjoki S, Ekblom M, Arstila TP, Dybedal I, Epling-Burnette PK, Guilhot F, et al. Clonal expansion of T/NK-cells during tyrosine kinase inhibitor dasatinib therapy. Leukemia. 2009;23(8):1398–405. doi: 10.1038/leu.2009.46. [DOI] [PubMed] [Google Scholar]
- 20.Kreutzman A, Juvonen V, Kairisto V, Ekblom M, Stenke L, Seggewiss R, et al. Mono/oligo-clonal T and NK cells are common in chronic myeloid leukemia patients at diagnosis and expand during dasatinib therapy. Blood. 2010;116(5):772–82. doi: 10.1182/blood-2009-12-256800. [DOI] [PubMed] [Google Scholar]
- 21.Masiello D, Gorospe G, 3rd, Yang AS. The occurrence and management of fluid retention associated with TKI therapy in CML, with a focus on dasatinib. J Hematol Oncol. 2009;2:46. doi: 10.1186/1756-8722-2-46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mauro MJ, Deininger MW. Management of drug toxicities in chronic myeloid leukaemia. Best Pract Res Clin Haematol. 2009;22(3):409–29. doi: 10.1016/j.beha.2009.06.001. [DOI] [PubMed] [Google Scholar]
- 23.Rosti G, Castagnetti F, Gugliotta G, Palandri F, Martinelli G, Baccarani M. Dasatinib and nilotinib in imatinib-resistant Philadelphia-positive chronic myelogenous leukemia: a ‘head-to-head comparison’. Leuk Lymphoma. 2010;51(4):583–91. doi: 10.3109/10428191003637282. [DOI] [PubMed] [Google Scholar]
- 24.Mauro MJ. Appropriate sequencing of tyro-sine kinase inhibitors in chronic myelogenous leukemia: when to change? A perspective in 2009. Curr Opin Hematol. 2009;16 (2):135–9. doi: 10.1097/MOH.0b013e3283257b2b. [DOI] [PubMed] [Google Scholar]