The t(15;17) chromosome translocation in acute promyelocytic leukemia is classified as a favorable cytogenetic feature among acute myeloid leukemia patients.1–4 However, the prognostic impact of additional chromosomal abnormalities (ACAs) in acute promyelocytic leukemia has been debated.5–9 We analyzed the clinical features, biological markers and clinical outcome of Japanese acute promyelocytic leukemia patients with or without ACAs who were treated by all-trans retinoic acid (ATRA) and chemotherapy, and tried to determine the role of ACAs on a 10-year follow up.
Adult patients with previously untreated de novo acute promyelocytic leukemia were registered consecutively into the JALSG APL97 study.4 This study was approved by the institutional review boards of each participating institution and registered at http://www.umin.ac.jp/ctrj/underC000000206. Informed consent was obtained from patients before registration in the study in accordance with the Declaration of Helsinki.
Chromosomes analyzed by G-banding on bone marrow samples from patients before treatment were classified according to the 1995 International System for Human Cytogenetic Nomenclature (ISCN). Patients were categorized into two groups: those with t(15;17) and ACAs, and those with t(15;17) but without ACAs. Patients with der(17)t(15;17), der(15)t(15;17) or three-way translocation were placed in the group with ACAs.
Details of treatment protocol have been described previously.4 In brief, remission induction consisted of ATRA and chemotherapy including idarubicin and cytarabine. Dose and duration of chemotherapy were based on initial leukocyte count. After completion of consolidation chemotherapy, patients negative for the PML-RARA transcript were randomly allocated either to receive 6 courses of intensified maintenance chemotherapy or to observation. Patients who were positive for the PML-RARA fusion transcript received late ATRA therapy followed by maintenance therapy, and received allogeneic hematopoietic stem cell transplantation if they had a human leukocyte antigen-identical donor.
Hematologic response was evaluated by standard criteria according to a previous report.2 Hematologic and molecular relapse detected by RT-PCR analysis of PML-RARA was considered a relapse event.
The primary end point of the JALSG APL97 study was overall survival and disease free survival of patients who achieved complete remission. Overall survival for all patients was calculated from the first day of therapy to death or last visit. Disease free survival was measured from the date of complete remission to relapse, death from any cause or last visit. We also evaluated overall and disease free survival from the time of randomization to maintenance chemotherapy or observation.
Clinical and biological characteristics were compared between patients with or without ACAs by the χ2 test or Fisher’s exact test for categorical data, and Wilcoxon’s rank-sum test for continuous data. Overall and disease free survival were estimated by the Kaplan-Meier method and then compared by the log rank test. Clinical outcomes were updated on January 2009 and the median follow-up period is 7.3 years. Statistical analyses were performed using SPSS 11.0 software (SPSS Inc, Chicago, IL, USA).
Among 302 patients enrolled between May 1997 and June 2002, 283 patients were evaluable.4 Of these, 58 patients were excluded because of insufficient data for ACAs status. Thus, the present analysis was carried out on 225 patients.
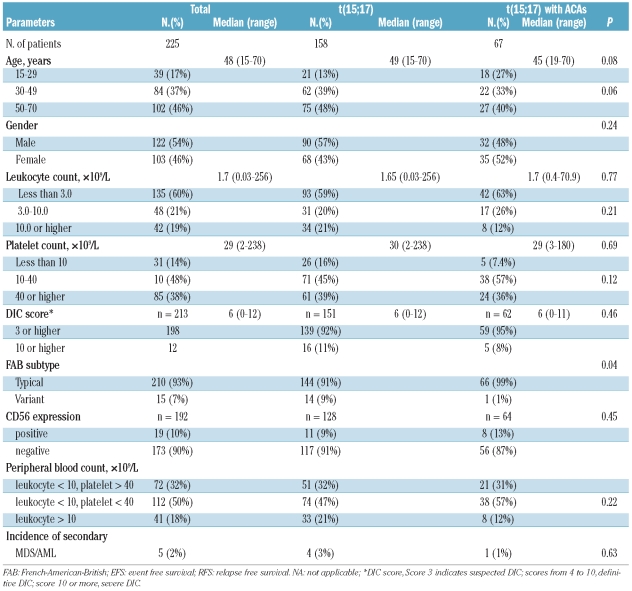
Sixty-seven (30%) of 225 patients had ACAs. Trisomy 8 was the most frequently observed ACA and detected in 21 cases (31%). Seven cases (11%) had ACAs in chromosome 15 in addition to t(15;17), 6 (9%) in chromosome 9, 6 (9%) in chromosome 7, 4 (6%) in chromosome 15, and 4 (6%) in chromosome 6. There was no significant difference in clinical or biological characteristics between the two groups, except the frequency of M3v (1% vs. 9%, P=0.04) (Table 1).
Table 1.
Clinical features of patients.
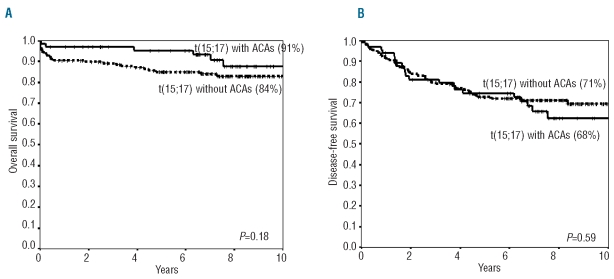
Complete remission rates in patients with or without ACAs were 97% and 95%, respectively (P=0.72). There was no difference in cumulative incidence of early death at 50 days, severe hemorrhagic complication or retinoic acid syndrome between the two groups (P=0.16, P=0.46 and P=0.16, respectively). There was also no difference in overall survival, disease free survival or cumulative incidence of relapse between the two groups (91% vs. 84%, P=0.18; 68% vs. 71%, P=0.59; 26% vs. 22%, P=0.51, respectively). Overall and disease free survival are shown in Figure 1A and B. In addition, clinical outcome was analyzed among subgroups of patients with ACAs. However, ACAs including chromosome 8, 7, 9, 15 and 17 did not influence outcomes.
Figure 1.
Overall survival and disease free survival of APL patients between with or without additional chromosomal abnormalities in addition to t(15;17). (A) Overall survival (91% vs. 84% at 10 years, P=0.18), (B) Disease-free survival (68% versus 71% at 10 years, P=0.59) were similar between two groups.
Clinical and biological characteristics have been compared between patients with or without ACAs. ACAs have been detected in 26% to 33% of newly diagnosed acute promyelocytic leukemia patients in whom trisomy 8 was consistently the most frequent ACA.5–9 In this study, 67 patients (30%) had ACAs, and trisomy 8 was the most frequent (31%). There was no significant difference in overall survival, disease free survival or relapse rate between patients with or without trisomy 8.
The frequency of M3v was significantly lower among our patients with ACAs. This agrees with the report by Schoch et al.,10 although several previous studies showed that the morphology of M3v was not related to the presence of ACAs.5,6,8 The inconsistency of these results may be caused by a considerably smaller number of M3v cases (16% to 27% of APL). Some authors have reported that the morphology of M3v is related to fms-like tyrosine kinase 3 mutations.8,11,12 Future analysis of this with ACAs is needed.
Several authors have discussed the clinical importance of ACAs in acute promyelocytic leukemia patients treated with ATRA and chemotherapy. Cervera et al.9 found in the LPA99 trial that ACAs were associated with lower relapse free survival in univariate analysis but not in multivariate analysis. Schlenk et al.8 analyzed 82 patients and reported that ACAs were an unfavorable prognostic marker for overall survival due to early death during the induction therapy. On the contrary, Botton et al.6 and Hernandez et al.7 reported that ACAs had no impact on clinical outcome. In our study, ACAs also did not show any prognostic significance. One of the reasons for this discrepancy would be that the clinical outcome of acute promyelocytic leukemia has recently improved dramatically. The outcome of each subgroup has also been greatly improved, although with some limitations, because patients have been stratified according to risk factors and consequently recent studies have used risk-adapted therapies. Thus, it may become more difficult to identify prognostic factors in acute promyelocytic leukemia.
Acknowledgments
the authors express their sincere gratitude to all of the clinicians participating in the Japan Adult Leukemia Study Group (JALSG) APL97 study.
Footnotes
Funding: this work was supported in part by the Grants for Cancer from the Ministry of Health, Welfare and Labour Government of Japan.
The information provided by the authors about contributions from persons listed as authors and in acknowledgments is available with the full text of this paper at www.haematologica.org.
Financial and other disclosures provided by the authors using the ICMJE (www.icmje.org) Uniform Format for Disclosure of Competing Interests are also available at www.haematologica.org.
References
- 1.Sanz MA. Treatment of acute promyelocytic leukemia. Hematology Am Soc Hematol Educ Program. 2006:147–55. doi: 10.1182/asheducation-2006.1.147. [DOI] [PubMed] [Google Scholar]
- 2.Sanz MA, Grimwade D, Tallman MS, Lowenberg B, Fenaux P, Estey EH, et al. Management of acute promyelocytic leukemia: recommendations from an expert panel on behalf of the European LeukemiaNet. Blood. 2009;113(9):1875–91. doi: 10.1182/blood-2008-04-150250. [DOI] [PubMed] [Google Scholar]
- 3.Sanz MA, Lo Coco F. Standard practice and controversial issues in front-line therapy of acute promyelocytic leukemia. Haematologica. 2005;90(6):840–5. [PubMed] [Google Scholar]
- 4.Asou N, Kishimoto Y, Kiyoi H, Okada M, Kawai Y, Tsuzuki M, et al. A randomized study with or without intensified maintenance chemotherapy in patients with acute promyelocytic leukemia who have become negative for PML-RARalpha transcript after consolidation therapy: the Japan Adult Leukemia Study Group (JALSG) APL97 study. Blood. 2007;110(1):59–66. doi: 10.1182/blood-2006-08-043992. [DOI] [PubMed] [Google Scholar]
- 5.Grimwade D, Walker H, Oliver F, Wheatley K, Harrison C, Harrison G, et al. The importance of diagnostic cytogenetics on outcome in AML: analysis of 1,612 patients entered into the MRC AML 10 trial. The Medical Research Council Adult and Children’s Leukaemia Working Parties. Blood. 1998;92(7):2322–33. [PubMed] [Google Scholar]
- 6.De Botton S, Chevret S, Sanz M, Dombret H, Thomas X, Guerci A, et al. Additional chromosomal abnormalities in patients with acute promyelocytic leukaemia (APL) do not confer poor prognosis: results of APL 93 trial. Br J Haematol. 2000;111(3):801–6. doi: 10.1046/j.1365-2141.2000.02442.x. [DOI] [PubMed] [Google Scholar]
- 7.Hernandez JM, Martin G, Gutierrez NC, Cervera J, Ferro MT, Calasanz MJ, et al. Additional cytogenetic changes do not influence the outcome of patients with newly diagnosed acute promyelocytic leukemia treated with an ATRA plus anthracyclin based protocol. A report of the Spanish group PETHEMA. Haematologica. 2001;86(8):807–13. [PubMed] [Google Scholar]
- 8.Schlenk RF, Germing U, Hartmann F, Glasmacher A, Fischer JT, del Valle y Fuentes F, et al. High-dose cytarabine and mitoxantrone in consolidation therapy for acute promyelocytic leukemia. Leukemia. 2005;19(6):978–83. doi: 10.1038/sj.leu.2403766. [DOI] [PubMed] [Google Scholar]
- 9.Cervera J, Montesinos P, Hernandez-Rivas JM, Calasanz MJ, Aventin A, Ferro MT, et al. Additional chromosome abnormalities in patients with acute promyelocytic leukemia treated with all-trans retinoic acid and chemotherapy. Haematologica. 2010;95(3):424–31. doi: 10.3324/haematol.2009.013243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Schoch C, Haase D, Haferlach T, Freund M, Link H, Lengfelder E, et al. Incidence and implication of additional chromosome aberrations in acute promyelocytic leukaemia with translocation t(15;17)(q22;q21): a report on 50 patients. Br J Haematol. 1996;94(3):493–500. doi: 10.1046/j.1365-2141.1996.d01-1829.x. [DOI] [PubMed] [Google Scholar]
- 11.Callens C, Chevret S, Cayuela JM, Cassinat B, Raffoux E, de Botton S, et al. Prognostic implication of FLT3 and Ras gene mutations in patients with acute promyelocytic leukemia (APL): a retrospective study from the European APL Group. Leukemia. 2005;19(7):1153–60. doi: 10.1038/sj.leu.2403790. [DOI] [PubMed] [Google Scholar]
- 12.Gale RE, Hills R, Pizzey AR, Kottaridis PD, Swirsky D, Gilkes AF, et al. Relationship between FLT3 mutation status, biologic characteristics, and response to targeted therapy in acute promyelocytic leukemia. Blood. 2005;106(12):3768–76. doi: 10.1182/blood-2005-04-1746. [DOI] [PubMed] [Google Scholar]