Abstract
We aimed to know the risk-stratification-based prevalence of bacterial contamination of ambulance vehicle surfaces, equipment, and materials. This study was performed in a metropolitan area with fire-based single-tiered Basic Life Support ambulances. Total 13 out of 117 ambulances (11.1%) were sampled and 33 sites per each ambulance were sampled using a soft rayon swab and aseptic containers. These samples were then plated onto a screening media of blood agar and MacConkey agar. Specific identification with antibiotic susceptibility was performed. We categorized sampling sites into risk stratification-based groups (Critical, Semi-critical, and Non-critical equipment) related to the likelihood of direct contact with patients' mucosa. Total 214 of 429 samples showed positive results (49.9%) for any bacteria. Four of these were pathogenic (0.9%) (MRSA, MRCoNS, and K. pneumoniae), and 210 of these were environmental flora (49.0%). However, the prevalence (positive/number of sample) of bacterial contamination in critical, semi-critical airway, semi-critical breathing apparatus group was as high as 15.4% (4/26), 30.7% (16/52), and 46.2% (48/104), respectively. Despite current formal guidelines, critical and semi-critical equipments were contaminated with pathogens and normal flora. This study suggests the need for strict infection control and prevention for ambulance services.
Keywords: Bacterial Infections, Contamination, Ambulances
INTRODUCTION
Ambulances can possibly be a source for various pathogens to be transmitted because they transport many patients with various diseases or infections. To prevent the ambulance from being a source for transmission of infection to patients or ambulance crews, strict infection control protocols should be implemented and monitored. Ambulance services are increasingly being recognized around the world as being an important part of public health system. However, although hospital-based infection control programs are being currently emphasized (1-3), prehospital infection control has not been recognized as an essential part of public health. Existing research related to prehospital infection have usually been regarding the prevalence of pathogens in samples from the surface of ambulances or devices, contamination rates of specific pathogens, and the possibility of sterilization for the cultured microorganisms (4-6). To prevent the ambulance from being a source of contamination, we should have an evidence-based and cost-effective infection control protocol for ambulances and its equipment. Medical devices and material are usually classified into three categories (critical, semi-critical, and non-critical) according to the likelihood of being contaminated (7). For example, devices like the blade of laryngoscope, being directly in contact with the airway mucous membrane of patients, are considered as a critical device. Because ambulance devices and materials are too many and very various, this schematic approach according to this risk stratification-based surveillance (RSS) for contamination will be very helpful for the implementation and quality assurance of infection control.
Few studies on the prevalence of microorganism according to RSS (Critical, Semi-critical, Non-critical) have been done. This RSS-based prevalence will help ambulance authorities make a cost-effective infection control guideline and monitoring system.
This study aimed to know the prevalence of microorganism contaminated in ambulance devices and material according to RSS.
MATERIALS AND METHODS
Study design and setting
This study was a surveillance and descriptive study. This study was done in a metropolitan emergency medical service (EMS), which is a single tiered, fire-based, basic life support (BLS) EMS. The metropolitan city has about 10 million population, 250,000 annual ambulance transports in 2008, and 117 ambulances for prehospital transport.
The metropolitan ambulance authority (city fire department) follows the national standard operating procedures (SOP) for infection control which was first made by the national headquarters of the fire department in 2005. The national SOP included the goal of infection control, role of EMS authority, infection control committee and education program, environmental control of the ambulance station, personal protective equipment, field precautions and post-return precautions. This was revised to be stricter in January 2008. According to these SOP, ambulance crews should wash the decontaminated surface of ambulances using an appropriate cleaner, sterilize the devices, and change the material if disposable.
Selection of sampling sites
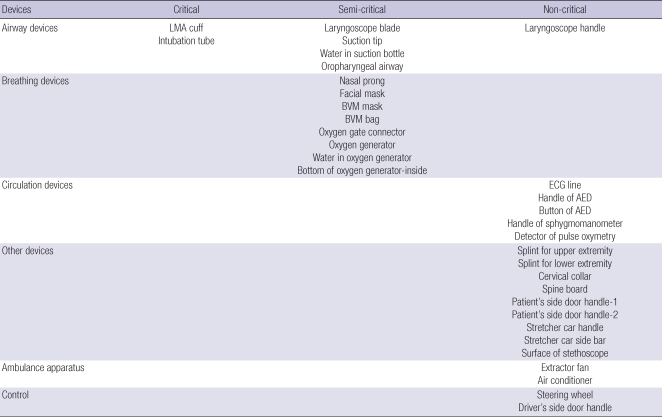
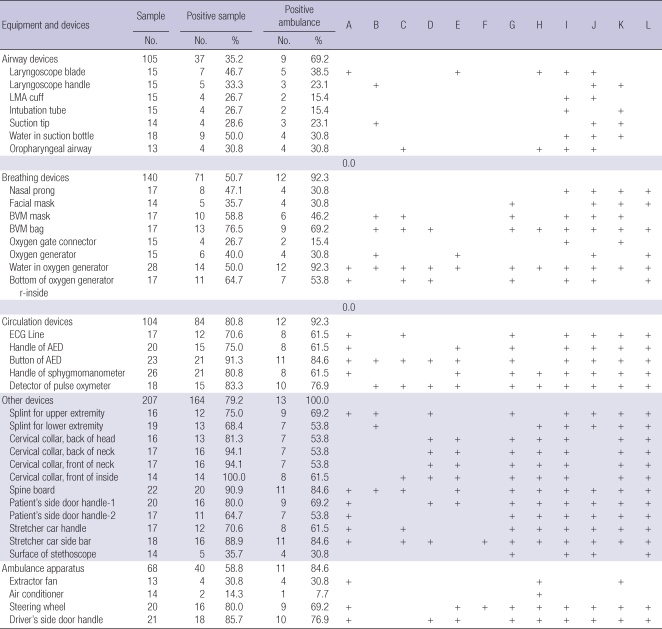
We used a convenience sampling method. Sampling time also decided with a convenience method. Among 117 ambulances, 13 ambulances (11.3%) were selected. For each ambulance, the same thirty three sampling sites were decided (total 429 sites), according to risk stratification. Each sampling was also categorized according to type of device: airway devices, breathing devices, circulation devices, other devices, and ambulance apparatus. Driver site was used as a control (Table 1). Sampling was done with blinding to ambulance crews in April, 2009.
Table 1.
Sampling sites according to risk stratification for contamination
LMA, laryngeal mask airway; BVM, bag valve mask; ECG, electrocardiography; AED, automatic external defibrillator.
Data collection and processing
Sampling was done by surface swabbing using soft rayon swabs (COPAN Italia S.p.A., Brescia, Italy). The samples were put on the blood agar plate and MacConkey agar plate and then screening was done for Methicillin-resistant Staphylococcus aureus (MRSA) and Vancomycin-resistant Enterococcus (VRE) contamination. Fluid samples were put into an aseptic container and transported to the microbiology laboratory center of the study institution for cultivation. Fluid from the oxygen filter tank or suction bottle was filtered using an analytical test filter funnel with 0.2 µm size and samples from surface of the filter were swabbed and tested for Legionella antigen. After one night, the fluids were also put on blood agar and MacConkey agar plates and then screening was done for MRSA and VRE contamination. Interpretation was done by the certified board of the division of microbiology (laboratory medicine) as a routine clinical interpretation.
Outcome measures and primary data analysis
We investigated the positive rate for whole bacteria including MRSA and VRE for the 429 samples according to risk stratification groups (Critical, Semi-critical, and Non-critical). We calculated the positive culture rate and its 95% confidence intervals (95% CIs) for descriptive analysis.
Ethics statement
This study was exempted for review by the institutional board review of the Seoul National University Hospital because this study did not enroll human subjects or animals.
RESULTS
Demographics of participating ambulance
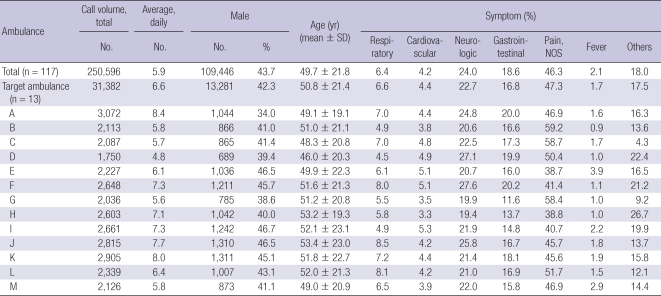
All ambulances participating in this study had very similar configuration to type II of Federal Specification for Ambulances KKK-A-1822 of the USA (8) and were made in Korea. Demographics on patient transport of participating ambulances of 2008 were described in Table 2. Daily average transport volume per a ambulance was 6.6 per a day (range 4.8-8.4). Male was 42.3% (range 34.0%-46.7%) and mean age was 50.8 ± 21.4. Proportion of respiratory symptom or fever was 6.6% and 1.7%, respectively.
Table 2.
Demographic findings of transported patients by ambulance in 2008
NOS, not otherwise specified.
Prevalence rate of microorganisms according to sampling site
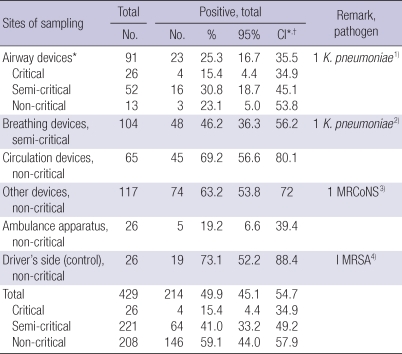
The total positive culture rate among 429 samples was 214 (49.9%, 95% CI; 45.1%-54.7%), which was the highest for circulation devices (69.2%, 95% CI; 56.6%-80.1%) and the lowest in ambulance apparatus (19.2%, 95% CI; 6.6%-39.4%) (Table 3). Critical, semi-critical, and non-critical devices showed 15.4% (95% CI, 4.4%-34.9%), 41.0 (95% CI, 33.2%-49.2%), and 59.1% (95% CI, 44.0%-57.9%), respectively. Pathogens were found the following four sites; 1) Extended spectrum beta lactamase (ESBL) positive-Klebsiella pneumoniae in the water of suction bottles (airway devices). 2) ESBL positive-K. pneumoniae in the Bag-Valve Mask (BVM) bag (breathing devices). 3) Methicillin resistant coagulase negative Staphylococcus (MRCoNS) in stretcher side bars (other devices). 4) MRSA in the driver's side door handle (Control site).
Table 3.
Prevalence rate of microorganisms according to risk stratification-based sampling sites
*Critical airway equipments were intubation tube and laryngeal mask airway cuff. Semi-critical airway equipments were laryngoscope blade, suction tip, water in suction bottle and oropharyngeal airway. Laryngoscope handle was classified into noncritical equipment. All breathing devices were semi-critical group. Circulation, and other devices, ambulance apparatus, and driver's side was non-critical group. 1) One Extended spectrum beta lactamase (ESBL) positive-K. pneumoniae was cultured in water of suction bottle among airway equipment; 2) One ESBL positive-K. pneumoniae was cultured in BVM bag among breathing equipment; 3) One Methicillin resistant Coagulase Negative Staphylococcus was cultured in stretcher car side bar; 4) One Methicillin resistant Staphylococcus aureus was cultured in driver's side door handle; †95% confidence interval.
Cultured microorganism and features
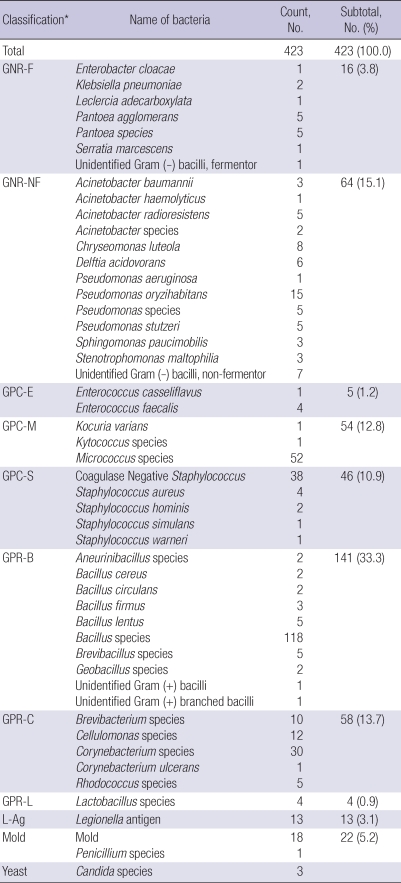
From 429 sampling sites, 624 sample cultures were investigated. Positive rate for any microorganism was 63.5% (396/624). Of these, four pathogens were identified. The others were environmental or normal flora, which are all susceptible to antibiotics (Table 4).
Table 4.
Identification of microorganism: environmental and normal flora
*Microorganisms were classified as follows. GNR-F, Gram-negative rods-fermentor; GNR-NF, Gram-negative rods-nonfermentor; GPC-E, Gram positive coccus-enterococcus; GPC-M, Gram-positive coccus-micrococcus; GPC-S, Gram-positive coccus-staphylococcus; GPR-B, Gram-positive rods-bacillus; GPR-C, Gram-positive rods-corynebacterium; GPR-L, Gram-positive rods-Lactobacillus; L-ag, Legionella antigen.
When we described the positive rate according to type of devices and participating ambulance, positive rate was 69.2% (9/13 ambulances) for airway devices which are a kind of critical devices, 92.3% (12/13 ambulances) for breathing devices which are a kind of semi-critical devices (Table 5).
Table 5.
Prevalence of microorganism culture by ambulance, equipment and device
LMA, laryngeal mask airway; BVM, bag valve mask; ECG, electrocardiography; AED, automatic external defibrillator.
DISCUSSION
The prevalence rate for microorganisms in a metropolitan ambulance surveillance was 49%, of which a few were pathogenic and most environmental or normal flora. This prevalence rate is not likely to be important, unless in critical or semi-critical devices. Medical devices in ambulance are classified into critical, semi-critical, and non-critical. Critical devices like intubation equipment, which should be sterilized until use, showed a 15.4% positive rate. 45.2% of semi-critical devices sampled were also positive. This finding is a surrogate marker for poor infection control for ambulance equipment (1, 2). Critical and semi-critical devices should be sterilized to clear up all microorganisms. A disposable device will be an alternative option for this goal (5). Non-critical devices include any external monitor apparatus for ECG, defibrillator, and so on. These devices are not important even though there are any microorganisms contaminated. Risk stratification-based surveillance (RSS) will guide us to make a feasible approach for maintenance of disinfection and quality.
Ambulance apparatus or driver's sides are classified into non-critical devices, generally not needing any sterilizing. For example, ambulance driver's sides showed very high contamination rate (73.1%). These findings are not serious.
Environmental microorganisms also will be problematic for immune compromised patients (9). However, environmental flora like Acinetobacter or Pseudomonas, which usual grow in soil or water, were found in this study. Those flora mean that minimum cleaning and washing for the ambulance was insufficient. This finding suggests disinfection for ambulances was poor.
Four pathologic microorganisms were indentified (0.9%). MRSA was from the driver's site and MRCoNS was from the stretcher bar. These pathogens should not be present in ambulances and devices. Ambulance crews as well as drivers take part in transfer of patients, which can deliver pathogen to new patients from these side devices. MRSA has been known to be a common pathogen in hospital-based surveys, particularly in intensive care units (10). In recent reports, nosocomial MRSA infections are spreading to community, which are very different from hospital-acquired MRSA in terms of molecular analysis or clinical risk factors and features (11, 12). In this study, only one sample was MRSA positive, which was not identified on the basis of molecular biologic analysis.
The positive rate for MRSA in an ambulance sample conducted in the USA was 12.4%, which was very high compared to that of our study. However, our study was conducted using samples of devices and ambulance apparatus, not from the human body including hands, which are very relevant for MRSA infection. For future studies, to investigate the positive rate of MRSA, we should test samples from hands of ambulances crews.
Another pathologic microorganism was K. pneumoniae, which was extended spectrum beta lactamase (ESBL) positive. These bacteria were cultured from water in the suction bottle and surface of a bag-valve-mask bag, which means these can cause pneumonia in patients directly. K. pneumoniae also may cause septicemia and septic shock in the immune compromised host (13, 14). Although the identified pathogens were few, strict infection control should be emphasized.
This study has limitations. The number of selected ambulances was 13 (11.1%), which was conveniently sampled. Therefore, study results could be biased from selection method. In particular four ambulances were washed using alcohol and tap water before sampling, which could have affected the results as routine practice. However, the positive rate of normal flora was similar in medical devices between pre-washed ambulances and non-washed ambulances because the most of medical devices were not washed or sterilized.
Different EMS systems and infection control guidelines maybe there, which also make us this result to be generalized to external world. This study was not related with infection rate or contamination rate for patients transported by these ambulances. Therefore these results are not related with clinical outcomes.
The risk stratification-based surveillance for contamination in metropolitan ambulances showed very high prevalence of environmental and normal flora infection in critical and semi-critical devices. And a few pathogens were also found. All kinds of pathogens are important to infection control for non-critical devices in ambulance as well as semi-critical, or critical. For critical devises, all normal flora are serious in terms of contaminated devices and should be targeted for being sterilized.
To prevent the ambulance from being a source of contamination, more strict infection control and monitoring protocol should be implemented.
ACKNOWLEDGMENTS
All authors had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. We would like to thank and acknowledge the contributions of Emergency Medical Technicians (Doo Ho Kim and Yeon Jeong Cho) of Seoul National University Hospital on sampling and the Division of Rescue and Emergency Medical Service, National Emergency Management Agency for cooperation.
This study was financially supported by the office of Mrs. Ae Joo Lee, a member of National Assembly. All authors (Noh H, Shin SD, Kim NJ, Oh HS, Joo SI, Kim JI, Ro YS, and Ong ME) are not related with any other conflicts of interest in this study.
AUTHOR SUMMARY
Risk Stratification-based Surveillance of Bacterial Contamination in Metropolitan Ambulances
Hyun Noh, Sang Do Shin, Nam Joong Kim, Young Sun Ro, Hyang Soon Oh, Se Ik Joo, Jung In Kim, and Marcus Eng Hock Ong
This study was performed to know the risk-stratification-based prevalence of bacterial contamination of Seoul Metropolitan City-Fire department's ambulance vehicle surfaces, equipment, and materials. Total 13 out of 117 ambulances (11.1%) were sampled and 33 sites per each ambulance were sampled and specific identification was performed. We categorized sampling sites into risk stratification-based groups (Critical, Semi-critical, and Non-critical equipment) related to the likelihood of direct contact with patients' mucosa. The prevalence (positive/number of sample) of bacterial contamination in critical, semi-critical airway, semi-critical breathing apparatus group was as high as 15.4% (4/26), 30.7% (16/52), and 46.2% (48/104), respectively. Despite current formal guidelines, critical and semi-critical equipments were contaminated with pathogens and normal flora. This study suggests the need for strict infection control and prevention for ambulance services.
References
- 1.Greenwood D, Slack RB, Peutherer JF. Hospital infection. In: Greenwood D, editor. Medical microbiology: a guide to microbial infections: pathogenesis, immunity, laboratory diagnosis, and control. 16th ed. Edinburgh, NY: Churchill Livingstone; 2002. pp. 662–669. [Google Scholar]
- 2.WHO. Department of communicable disease, Surveillance and response; [accessed on 1 Jun 2010]. Prevention of hospital-acquired infections. A practice guide, 2nd ed. Available at http://www.who.int/csr/resources/publications/drugresist/whocdscsreph200212.pdf. [Google Scholar]
- 3.Pittet D, Tarara D, Wenzel RP. Nosocomial bloodstream infection in critically ill patients. Excess length of stay, extra costs, and attributable mortality. JAMA. 1994;271:1598–1601. doi: 10.1001/jama.271.20.1598. [DOI] [PubMed] [Google Scholar]
- 4.Nigam Y, Cutter J. A preliminary investigation into bacterial contamination of Welsh emergency ambulances. Emerg Med J. 2003;20:479–482. doi: 10.1136/emj.20.5.479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Roline CE, Crumpecker C, Dunn TM. Can methicillin-resistant Staphylococcus aureus be found in an ambulance fleet? Prehosp Emerg Care. 2007;11:241–244. doi: 10.1080/10903120701205125. [DOI] [PubMed] [Google Scholar]
- 6.Alves DW, Bissell RA. Bacterial pathogens in ambulances: results of unannounced sample collection. Prehosp Emerg Care. 2008;12:218–224. doi: 10.1080/10903120801906721. [DOI] [PubMed] [Google Scholar]
- 7.Block SS. Chemical disinfection of medical and surgical materials. In: Block SS, editor. Disinfection, sterilization, and preservation. 4th ed. Philadelphia: Lea & Febiger; 1991. pp. 617–641. [Google Scholar]
- 8.Ambulances-current AMD Standards. The Information Resource for the Emergency Management Industry. [accessed on 22 Nov 2009]. Available at http://www.idsemergencymanagement.com/emergency_management/corporate/ambulance_manufacturers_division_/ambulance_manufacturers_division_/20_0/g_supplier_5.html.
- 9.Jarvis WR. The epidemiology of colonization. Infect Control Hosp Epidemiol. 1996;17:47–52. doi: 10.1086/647189. [DOI] [PubMed] [Google Scholar]
- 10.National Nosocomial Infections Surveillance System. National Nosocomial Infections Surveillance (NNIS) System Report, data summary from January 1992 to June 2002, issued August 2002. Am J Infect Control. 2002;30:458–475. doi: 10.1067/mic.2002.130032. [DOI] [PubMed] [Google Scholar]
- 11.Herold BC, Immergluck LC, Maranan MC, Lauderdale DS, Gaskin RE, Boyle-Vavra S, Leitch CD, Daum RS. Community-acquired methicillin-resistant Staphylococcus aureus in children with no identified predisposing risk. JAMA. 1998;279:593–598. doi: 10.1001/jama.279.8.593. [DOI] [PubMed] [Google Scholar]
- 12.Kowalski TJ, Berbari EF, Osmon DR. Epidemiology, treatment, and prevention of community-acquired methicillin-resistant Staphylococcus aureus infections. Mayo Clin Proc. 2005;80:1201–1207. doi: 10.4065/80.9.1201. [DOI] [PubMed] [Google Scholar]
- 13.Chisti MJ, Tebruegge M, La Vincente S, Graham SM, Duke T. Pneumonia in severely malnourished children in developing countries-mortality risk, aetiology and validity of WHO clinical signs: a systematic review. Trop Med Int Health. 2009;14:1173–1189. doi: 10.1111/j.1365-3156.2009.02364.x. [DOI] [PubMed] [Google Scholar]
- 14.Meatherall BL, Gregson D, Ross T, Pitout JD, Laupland KB. Incidence, risk factors, and outcomes of Klebsiella pneumoniae bacteremia. Am J Med. 2009;122:866–873. doi: 10.1016/j.amjmed.2009.03.034. [DOI] [PubMed] [Google Scholar]