Abstract
Stent fracture (SF) has been implicated as a risk factor for in-stent restenosis, but its incidence and clinical characteristics are not well established. Therefore we investigated the conditions associated with stent fracture and its clinical presentation and outcome. Between 2004 and 2007, consecutive cases of SF were collected from the Seoul National University Hospital. Clinical characteristics and outcome of patients with fractured stents were compared with a ten-fold cohort of age and gender matched controls (n = 236). A total of 4,845 patients received percutaneous coronary intervention and 3,315 patients (68.4%) underwent angiographic follow-up. Twenty-eight fractured stents were observed in 24 patients. The incidence of SF was 0.89% for sirolimus-eluting stents (SES) and 0.09% for paclitaxel-eluting stents. Chronic kidney disease, stent implantation in the right coronary artery (RCA), and SES use were independent predictors of drug-eluting stent fracture by multivariate analysis. SF was significantly associated with binary restenosis (11.4% vs 41.7%, P < 0.001) and increased risk of target lesion revascularization (8.1% vs 33.3%, P = 0.001). Patients with SF but without significant restenosis showed excellent outcome despite only medical treatment. In conclusion, SF is associated with increased rates of restenosis and repeat revascularization. Significant risk factors include chronic kidney disease, RCA intervention, and SES use.
Keywords: DES Fracture, Risk Factors, In-stent Restenosis, Target Vessel Revascularization, Clinical Outcome
INTRODUCTION
The development of the drug-eluting stent (DES) has revolutionized the field of interventional cardiology by significantly reducing the restenosis rates and need for repeat revascularization (1-4). However, fracture of stent struts in a DES with consequent interruption may lead to insufficient drug delivery resulting in attenuated inhibition of neointimal formation and restenosis. Stent fracture was not a big concern in the bare metal stent (BMS) era because neointima formation and restenosis were a much more frequent phenomenon and investigators were not focused on evaluating the angiography for possible disruptions in stent struts. Furthermore, the greater neointimal coverage in the early stages of post-stent implantation could have protected struts from occurrence of fracture in the BMS era (5, 6).
In the present study, we investigated the conditions associated with stent fracture and the clinical consequences of stent fracture by consecutive analysis of percutaneous coronary intervention (PCI) cases performed at two major cardiovascular centers in Korea. We also analyzed possible factors associated with binary restenosis in lesions with fractured stent struts.
MATERIALS AND METHODS
Patients
We reviewed consecutive PCI cases performed between June 2004 and December 2007 at Seoul National University Hospital and Bundang Hospital. A total of 4845 patients received PCI during this period, where 4,132 sirolimus-eluting stents (SES) (CYPHER® Stent, Cordis Corporation, a Johnson & Johnson Company, Warren, NJ, USA) and 2,966 paclitaxel-eluting stents (PES) (TAXUS®, Boston Scientific Cooperation, Boston, MA, USA) were implanted. Of these patients, 3,315 patients (68.42%) received routine angiographic follow-up between 6 to 12 months post-PCI.
The patients with stent fracture were matched 10-fold with age- and gender-matched controls. Clinical, angiographic, and procedural information were recorded and follow-up report on angiographies and procedures were available for all patients since we closely monitor all our PCI patients. In addition, we collected the follow-up data after detection of stent fracture in these patients for at least an additional 9 months. Median follow-up duration of patients with fractured stents was 30 (24.5-37.5) months since index PCI, and 23 (16.25-27.75) months since diagnosis of stent strut fracture, respectively. All medical records were reviewed by independent clinical data managers that were unaware of the purpose of the study.
Definitions
Stent fracture was defined as the presence of an angiographically visible interrupted connection of stent struts or fewer visible stent struts at the suspected site than normal looking stented area on intravascular ultrasound. Binary restenosis was defined as in-segment diameter stenosis greater than 50%, and target lesion revascularization (TLR) as repeated revascularization of a previously implanted stent with binary restenosis.
Procedure
We performed PCI according to the standard guidelines. The choice between sirolimus-eluting stents and paclitaxel-eluting stents was up to the operators' discretion as well as performing pre- and post-dilatation for optimal stent expansion, and the use of glycoprotein IIb/IIIa antagonists. A loading dose of 300 mg aspirin and 300-600 mg clopidogrel was administered prior to PCI. All patients were recommended to take aspirin indefinitely and clopidogrel for at least 6 months post-PCI.
Statistics
Data was presented as numbers and frequencies for categorical variables, and mean ± standard deviation for continuous variables. For comparison between fractured stents with age gender matched controls, chi-square and Fisher's exact test were used for categorical variables and Student's t-test for continuous variables. For comparison within the fractured stent group, we applied non-parametric Mann-Whitney U test for continuous variables. A multivariate logistic regression analysis was used to identify independent predictors of stent fracture. Statistical tests were performed using SPSS version 17 (SPSS Inc., Chicago, IL, USA).
Ethics statement
The study was approved by the institutional review board of Seoul National University Hospital (IRB Number: H-1006-104-322). Informed consent was exempted by the board.
RESULTS
Incidence of stent fracture
Twenty eight fractured struts in 26 stents were observed in 24 of 3315 patients (one patient had two fractured struts in one SES, another patient had 3 fractured struts in 2 overlapped SES, a third had one fractured strut in each of the 2 implanted stents). The location of fracture was 34.6% in left anterior descending artery (LAD), 11.5% in left circumflex artery (LCX), and 53.8% in right coronary artery (RCA) respectively. As for the severity of stent fracture, there were 13 type I fractures (50%), 2 type II fractures (7.7%), 10 type III fractures (38.4%), and 1 type IV fracture (3.9%), and 0 type V fracture according to stent fracture grading (7). Therefore the incidence of stent fracture was 0.52% (26 of 4993 stents) in total but there was a preponderance for stent fracture in SES (0.89%, 24 of 2709 SES) compared with the PES (0.09%, 2 of 2284 PES). Most cases (21 of 24) were detected during asymptomatic routine surveillance angiography between 6 to 12 months after index PCI, while 3 patients received coronary angiography for evaluation of chest pain.
Baseline characteristics and risk factors of stent fracture
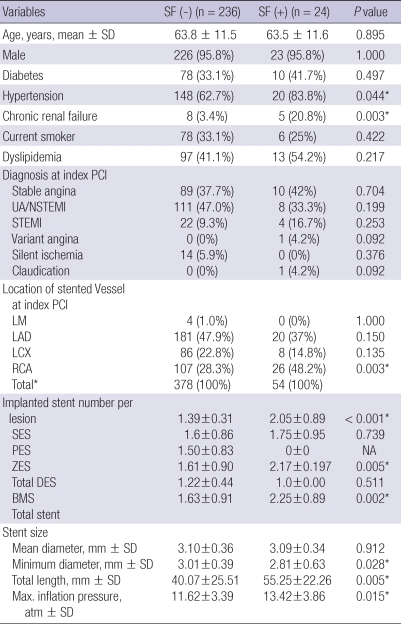
To find characteristics associated with stent fracture, the 24 patients with stent fracture were matched 10-fold with age- and gender-matched controls. Ten age- and gender-matched control patients per stent fracture patient was possible in all but one patient (n = 230). Only six matching controls could be identified for that one individual and thus characteristics of 24 patients with stent fracture were compared with 236 age- and gender-matched controls. The baseline clinical and procedural characteristics of the patients with stent fracture compared with those without stent fracture are shown in Table 1. Baseline clinical characteristics were mostly comparable between the two groups. Hypertension (62.7% vs 83.8%, P = 0.044) and chronic kidney disease (3.4% vs 20.8%, P = 0.003) were more common in the stent fracture group. No difference was found regarding the initial diagnosis necessitating stent implantation.
Table 1.
Baseline clinical, procedural characteristics
*Numbers are rounded and may not total 100%. BMS, bare metal stent; DES, drug-eluting stent; LAD, left anterior descending artery; LCX, left circumflex artery; LM, left main artery; Max. inflation pressure, maximal inflation pressure (atm); NSTEMI, non-ST elevation myocardial infarction; PES, paclitaxel-eluting stent; RCA, right coronary artery; SES, sirolimus-eluting stent; UA, unstable angina; ZES, zotarolimus-eluting stent.
As for angiographic and procedural characteristics, there were several differences. The average number of implanted stents was greater (2.17 ± 0.19 vs 1.61 ± 0.91, P = 0.005), the total stent length longer, (55.25 ± 22.26 mm vs 40.07 ± 25.51 mm, P = 0.005), and the maximal stent inflation pressure higher (13.42 ± 3.86 atm vs 11.62 ± 3.39 atm, P = 0.015) in the stent fracture group compared with the control group.
Although the distribution of implanted stents in the control group was 47.9% in LAD, 22.8% in LCX and 28.3% in RCA, the distribution of fractured stents in the stent fracture group was 34.6% in LAD, 11.5% in LCX, and 53.8% in RCA respectively, suggesting that stent fracture was more prevalent in RCA implanted stents.
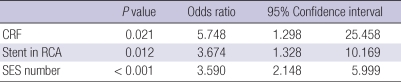
To find independent predictors of DES fracture, we performed a logistic multivariate analysis entering previously reported risk factors of stent fracture such as total stent length, SES, minimum stent diameter, maximal inflation pressure (5, 8, 9) along with variables that were found to be significant in univariate analysis in the present cohort. On multivariate analysis, we found that chronic kidney disease, stent implantation in the RCA, and SES were independent predictors of DES fracture (Table 2).
Table 2.
Independent predictors of stent fracture after multivariate analysis (entering hypertension, chronic kidney disease, stent in RCA, SES number, minimum stent diameter, maximum inflation pressure)
CRF, chromic renal failure; RCA, right coronary artery; SES, sirolimus-eluting stent.
Clinical presentation at stent fracture diagnosis
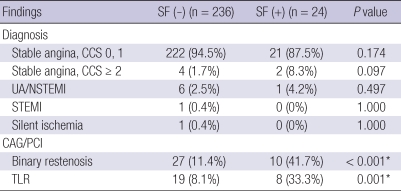
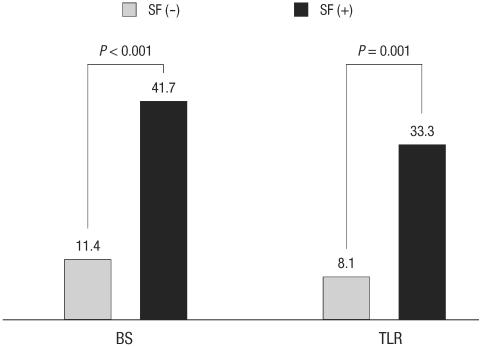
We compared the clinical and angiographic presentation of those that had stent fracture versus those that did not. At the time of angiographic follow-up (where the diagnosis of stent fracture was possible), clinical presentation of patients with fractured stents did not differ from those without stent fracture. The severity of angina according to Canadian Cardiovascular Society (CCS) Functional Classification and the incidence of acute coronary syndrome (ASC) were not different in both groups (CCS ≤ 1: 94.5% vs 87.5%, P = 0.174, CCS ≥ 2: 1.7% vs 8.3%, P = 0.097, including unstable angina/non-ST elevation myocardial infarction (NSTEMI) 2.5% vs 4.2%, P = 0.497, STEMI 0.4% vs 0%, P = 1.000, silent ischemia 0.4% vs 0%, P = 1.000) (Table 3). However, the binary restenosis rate was significantly higher in stent fracture group compared with the control group (41.7% vs 11.4%, P < 0.001) as well as the TLR (33.3% vs 8.1%, P = 0.001) (Fig. 1).
Table 3.
Clinical presentation, angiographic finding of patients at follow up coronary angiography
CCS, Canadian Cardiovascular Society Classification; FU CAG, Follow up coronary angiography; NSTEMI, non-ST elevation myocardial infarction; STEMI, ST elevation myocardial infarction; TLR, Target lesion revascularization; UA, unstable angina.
Fig. 1.
Clinical outcome of patients regarding binary restenosis (BS) and target lesion revascularization (TLR) rate. Patients with fractured stents (SF) have higher binary restenosis and target lesion revascularization rate compared with those without fracture.
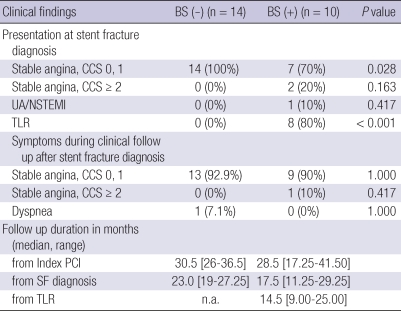
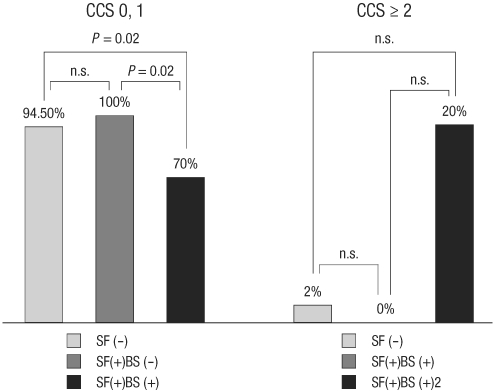
Subgroup analysis of patients with fractured stents showed that all patients without binary restenosis in their fractured stents had CCS ≤ 1, while 2 (20%) of patients with binary restenosis complained of chest pain CCS ≥ 2 and one patients presented with NSTEMI (Table 4, Fig. 2).
Table 4.
The rate of stent fracture
BS, binary restenosis; CCS, Canadian Cardiology Society Functional Classification; NSTEMI, non ST-elevation myocardial infarction; SA, stable angina; SF, stent fracture; TLR, target lesion revascularization; PCI, percutaneous coronary intervention; UA, unstable angina.
Fig. 2.
Clinical presentation at 6 month follow up CAG or stent fracture diagnosis. Patients with angina score CCS 0 or 1 were less common among those with stent fracture (SF) and binary restenosis (BS) compared with those without stent fracture or stent fracture without binary restenosis. CCS, Canadian Cardiology Society functional classification; n.s., not significant.
Clinical outcome of stent fracture
Among the 24 patients with fractured stents, binary restenosis was present in 10 patients (41.7%). Of the 10 patients, TLR was performed in 8 patients, where 3 patients were symptomatic while five were asymptomatic. The reason for performing TLR in those that were asymptomatic was in-stent restenosis greater than 70%. Of the eight patients that received TLR, three were treated with balloon angioplasty only, three with PES, and two with zotarolimus eluting stent (ZES). Of the 10 patients with binary restenosis, repeat intervention was not performed in two patients who showed adequate coronary flow with acceptable fractional flow reserve (FFR). After TLR, only 1 patient developed chest pain CCS ≥ 2. Those that received TLR showed an excellent clinical course with no occurrence of adverse events (Table 4).
Of the 14 patients without binary restenosis despite stent fracture at the time of stent fracture diagnosis, none experienced an adverse cardiac event. Although all 14 patients were managed medically without repeat PCI, no patient required TLR during follow up (median follow up since diagnosis of stent fracture: 30.5 months [26.0-36.5]).
DISCUSSION
Stent fracture is a rare complication of DES implantation. Although the clinical consequence of stent fracture may be much milder than stent thrombosis, growing interest exists regarding stent fracture due to its possible association with restenosis. In addition, due to the increase in PCI and the large numbers of implanted DES, stent fracture prevalence is increasing.
In the present study, we found that the incidence of stent fracture was relatively rare, with greater risk of stent fracture in SES. Amongst various univariate risk factors, chronic kidney disease, SES, and implantation in RCA were independent predictors of stent fracture. Also, stent fracture was associated with a significantly higher risk for binary restenosis and TLR. When stent fracture was not associated with significant restenosis, the prognosis was good with only medical follow-up. However, when associated with significant restenosis, symptoms were more likely to occur and thus patients received repeated procedures.
The first case report on DES fracture was reported by Sianos et al. in 2004 (9, 10). Since then, various studies have reported the rate of DES fracture to be between 0.84% and 3.2% (11-13). The overall incidence of stent fracture in this study was 0.58%, is therefore lower than those reported in the literature. We cannot rule out possible underestimation since stent fractures are not always easily identified during a routine coronary angiography.
Despite this low incidence of stent fracture, more cases of stent fractures have been reported due to high volume of DES placements, which makes now up to 90% of all implanted stents (1, 14). Earlier studies have shown that the risk of focal restenosis is greater in DES with fracture. The proposed mechanism for the greater risk of neointimal growth in fractured stents is that when the stent strut becomes disrupted, sufficient sustained local drug delivery is not assured, and this can lead to possible focal neointimal overgrowth and in-stent restenosis. Binary restenosis rates have been reported to be between 37.5% and 65% in fractured stents, and some have suggested that DES stent fracture could account for 1%-2% of all DES target vessel revascularizations.
The incidence of stent fracture for SES and PES was 0.89% and 0.09%, respectively in the present study. Previous data in the literature have all shown a greater incidence of stent fracture in SES (5, 13). This may be due to the difference in stent design between the two types of stents. SES with its closed cell design, on one hand contributes to even distribution of drug in the stented vessel, but on the other hand it is more rigid due to its closed cell design compared with PES, which had an open cell design. Hence SES is less deformable during dynamic cardiac movement, resulting in transmission of shear force possibly resulting in breakage of stent strut. Regarding second generation DES, there is limited data with only one case report on ZES fracture (15). The Endeavor® stent is a ZES based on the Driver® platform with its open cell design and cobalt alloy struts. Xience V® is an everolimus eluting stents based on the Multi-link Vision® platform, which is a cobalt chromium alloy with open cell and nonlinear link design making the stent flexible and more conformable to the vessel wall. Clinical experience must prove the stability of DES with open cell design regarding stent fracture.
In the present study, we identified several risk factors on univariate and multivariate analysis, such as multiple stenting, long stent length, chronic renal failure, implantation in RCA, SES, and higher maximal inflation pressure. This finding is comparable to previous published data (16, 17), and a recent metaanalysis from UCLA Medical Center (18). Mechanical stress may be an important factor in causing stent fracture. Liao et al. (19) illustrated how deployed stent resulted in vessel straightening with a mean curvature decrease by 22%. Stents deployed in vessels with greater tortuosity such as RCA will experience greater straightening after stent implantation than LAD or LCX. This makes stents in the RCA more vulnerable to stent fracture, and is also consistent with our data, as 48.2% of the stent fracture cases occurred in the RCA.
Although stent fracture was associated with an increased risk for TLR, stent fracture itself was not associated with significant symptom aggravation. Only fractured stents with binary stenosis lead to chest pain aggravation. Also, all of the lesions with stent fracture but less than 50% diameter stenosis were treated medically without any further repeat intervention, and these patients did very well with no adverse events up to median follow-up of 30 months, suggesting that the natural course of stent fracture without significant stenosis is relatively benign. However, since the number of cases with stent fracture were very small, we cannot exclude the possibility that stent fracture could predispose to stent thrombosis as suggested previously in anecdotal case reports (20). Currently stent thrombosis and in-stent restenosis requiring TLR are considered DES failure. Therefore the stent fracture might be considered as a significant risk factor for DES failure, even it has a benign prognosis.
The major limitation of current study is the selection of control group for identification of predictors of stent fractures. Although the predictors of stent fracture in the current study are concordant with results from previous studies, our study population may not represent the real population.
In conclusion, stent fracture is a rare complication of DES implantation, which is associated with chronic renal failure, stent implantation in the RCA, and SES. Although its clinical course seems rather benign, due to the high implantation volume, it is associated with higher restenosis rates and repeated revascularization. Precautions to avoid stent fracture need to be considered during PCI.
Footnotes
This study was supported by a grant from the Clinical Research Center for Ischemic Heart Disease, Seoul, Republic of Korea (0412-CR02-0704-0001) and a grant from the Innovative Research Institute for Cell Therapy, Seoul National University Hospital (A062260), sponsored by the Ministry of Health & Welfare, Republic of Korea.
AUTHOR SUMMARY
Clinical Characteristics of Coronary Drug-Eluting Stent Fracture: Insights from a Two-Center DES Registry
Kyung Woo Park, Jin Joo Park, In-Ho Chae, Jae-Bin Seo, Han-Mo Yang, Hae-Young Lee, Hyun-Jae Kang, Young-Seok Cho, Tae-Jin Yeon, Woo-Young Chung, Bon-Kwon Koo, Dong-Ju Choi, Byung-Hee Oh, Young-Bae Park, and Hyo-Soo Kim
Stent fracture (SF) has been implicated as a risk factor for in-stent restenosis (ISR), but its incidence, clinical characteristics as well as clinical outcome are not well established. Between 2004 and 2007, consecutive cases of SF were collected from the Seoul National University Hospital. Clinical characteristics and outcome of 24 patients with fractured stents were compared with a ten-fold cohort of age and gender matched controls (n = 236). A total of 4845 patients received PCI and 3315 patients (68.4%) underwent angiographic follow-up. The incidence of SF was 0.89% for sirolimus-eluting stents (SES) and 0.09% for paclitaxel-eluting stents (PES). Chronic kidney disease, stent implantation in the right coronary artery (RCA), and SES use were independent predictors of DES fracture by multivariate analysis. Although SF was significantly associated with binary restenosis and increased risk of target lesion revascularization, patients with SF but without significant restenosis showed excellent outcome despite only medical treatment.
References
- 1.Shaikh F, Maddikunta R, Djelmami-Hani M, Solis J, Allaqaband S, Bajwa T. Stent fracture, an incidental finding or a significant marker of clinical in-stent restenosis? Catheter Cardiovasc Interv. 2008;71:614–618. doi: 10.1002/ccd.21371. [DOI] [PubMed] [Google Scholar]
- 2.Betriu A, Masotti M, Serra A, Alonso J, Fernández-Avilés F, Gimeno F, Colman T, Zueco J, Delcan JL, Garcia E, Calabuig J. Randomized comparison of coronary stent implantation and balloon angioplasty in the treatment of de novo coronary artery lesions (START): a four-year follow-up. J Am Coll Cardiol. 1999;34:1498–1506. doi: 10.1016/s0735-1097(99)00366-6. [DOI] [PubMed] [Google Scholar]
- 3.Al Suwaidi J, Holmes DR, Jr, Salam AM, Lennon R, Berger PB. Impact of coronary artery stents on mortality and nonfatal myocardial infarction: meta-analysis of randomized trials comparing a strategy of routine stenting with that of balloon angioplasty. Am Heart J. 2004;147:815–822. doi: 10.1016/j.ahj.2003.11.025. [DOI] [PubMed] [Google Scholar]
- 4.Bae JH, Hyun DW, Kim KY, Yoon HJ, Nakamura S. Drug-eluting stent strut fracture as a cause of restenosis. Korean Circ J. 2005;35:787–789. [Google Scholar]
- 5.Lee SH, Park JS, Shin DG, Kim YJ, Hong GR, Kim W, Shim BS. Frequency of stent fracture as a cause of coronary restenosis after sirolimus-eluting stent implantation. Am J Cardiol. 2007;100:627–630. doi: 10.1016/j.amjcard.2007.03.073. [DOI] [PubMed] [Google Scholar]
- 6.Doi H, Maehara A, Mintz GS, Tsujita K, Kubo T, Castellanos C, Liu J, Yang J, Oviedo C, Aoki J, Franklin-Bond T, Dasgupta N, Lansky AJ, Dangas GD, Stone GW, Moses JW, Mehran R, Leon MB. Classification and potential mechanisms of intravascular ultrasound patterns of stent fracture. Am J Cardiol. 2009;103:818–823. doi: 10.1016/j.amjcard.2008.11.051. [DOI] [PubMed] [Google Scholar]
- 7.Jaff M, Dake M, Pompa J, Ansel G, Yoder T. Standardized evaluation and reporting of stent fractures in clinical trials of noncoronary devices. Catheter Cardiovasc Interv. 2007;70:460–462. doi: 10.1002/ccd.21240. [DOI] [PubMed] [Google Scholar]
- 8.Yang TH, Kim DI, Park SG, Seo JS, Cho HJ, Seol SH, Kim SM, Kim DK, Kim DS. Clinical characteristics of stent fracture after sirolimus-eluting stent implantation. Int J Cardiol. 2009;131:212–216. doi: 10.1016/j.ijcard.2007.10.059. [DOI] [PubMed] [Google Scholar]
- 9.Umeda H, Gochi T, Iwase M, Izawa H, Shimizu T, Ishiki R, Inagaki H, Toyama J, Yokota M, Murohara T. Frequency, predictors and outcome of stent fracture after sirolimus-eluting stent implantation. Int J Cardiol. 2009;133:321–326. doi: 10.1016/j.ijcard.2007.12.067. [DOI] [PubMed] [Google Scholar]
- 10.Sianos G, Hofma S, Ligthart JM, Saia F, Hoye A, Lemos PA, Serruys PW. Stent fracture and restenosis in the drug-eluting stent era. Catheter Cardiovasc Interv. 2004;61:111–116. doi: 10.1002/ccd.10709. [DOI] [PubMed] [Google Scholar]
- 11.Kim EJ, Rha SW, Wani SP, Suh SY, Choi CU, Kim JW, Park CG, Seo HS, Oh DJ. Coronary stent fracture and restenosis in the drug-eluting stent era: do we have clues of management? Int J Cardiol. 2007;120:417–419. doi: 10.1016/j.ijcard.2006.08.019. [DOI] [PubMed] [Google Scholar]
- 12.Lee HS, Hur SH, Nam CW, Cho YK, Kim H, Han SW, Kim KB, Kim YN. A case of stent strut fracture of a paclitaxel-eluting stent at the time of sent implantation in a complex coronary lesion. Korean Circ J. 2008;38:387–389. [Google Scholar]
- 13.Lee MS, Jurewitz D, Aragon J, Forrester J, Makkar RR, Kar S. Stent fracture associated with drug-eluting stents: clinical characteristics and implications. Catheter Cardiovasc Interv. 2007;69:387–394. doi: 10.1002/ccd.20942. [DOI] [PubMed] [Google Scholar]
- 14.Makaryus AN, Lefkowitz L, Lee AD. Coronary artery stent fracture. Int J Cardiovasc Imaging. 2007;23:305–309. doi: 10.1007/s10554-006-9151-2. [DOI] [PubMed] [Google Scholar]
- 15.Park JS, Cho IH, Kim YJ. Stent fracture and restenosis after zotarolimus-eluting stent implantation. Int J Cardiol. 2009 doi: 10.1016/j.ijcard.2009.01.030. doi: 10.1016/j.ijcard.2009.01.030. [DOI] [PubMed] [Google Scholar]
- 16.Chung WS, Park CS, Seung KB, Kim PJ, Lee JM, Koo BK, Jang YS, Yang JY, Yoon JH, Kim DI, Yoon YW, Park JS, Cho YH, Park SJ. The incidence and clinical impact of stent strut fractures developed after drug-eluting stent implantation. Int J Cardiol. 2008;125:325–331. doi: 10.1016/j.ijcard.2007.02.033. [DOI] [PubMed] [Google Scholar]
- 17.Kim HS, Kim YH, Lee SW, Park DW, Lee CW, Hong MK, Park SW, Ko JK, Park JH, Lee JH, Choi SW, Seong IW, Cho YH, Lee NH, Kim JH, Chun KJ, Park SJ Long-DES-II study investigators. Incidence and predictors of drug-eluting stent fractures in long coronary disease. Int J Cardiol. 2009;133:354–358. doi: 10.1016/j.ijcard.2008.01.005. [DOI] [PubMed] [Google Scholar]
- 18.Canan T, Lee MS. Drug-eluting stent fracture: incidence, contributing factors, and clinical implications. Catheter Cardiovasc Interv. 2010;75:237–245. doi: 10.1002/ccd.22212. [DOI] [PubMed] [Google Scholar]
- 19.Liao R, Green NE, Chen SY, Messenger JC, Hansgen AR, Groves BM, Carroll JD. Three-dimensional analysis of in vivo coronary stent--coronary artery interactions. Int J Cardiovasc Imaging. 2004;20:305–313. doi: 10.1023/b:caim.0000041950.84736.e6. [DOI] [PubMed] [Google Scholar]
- 20.Nakazawa G, Finn AV, Vorpahl M, Ladich E, Kutys R, Balazs I, Kolodgie FD, Virmani R. Incidence and predictors of drug-eluting stent fracture in human coronary artery a pathologic analysis. J Am Coll Cardiol. 2009;54:1924–1931. doi: 10.1016/j.jacc.2009.05.075. [DOI] [PubMed] [Google Scholar]