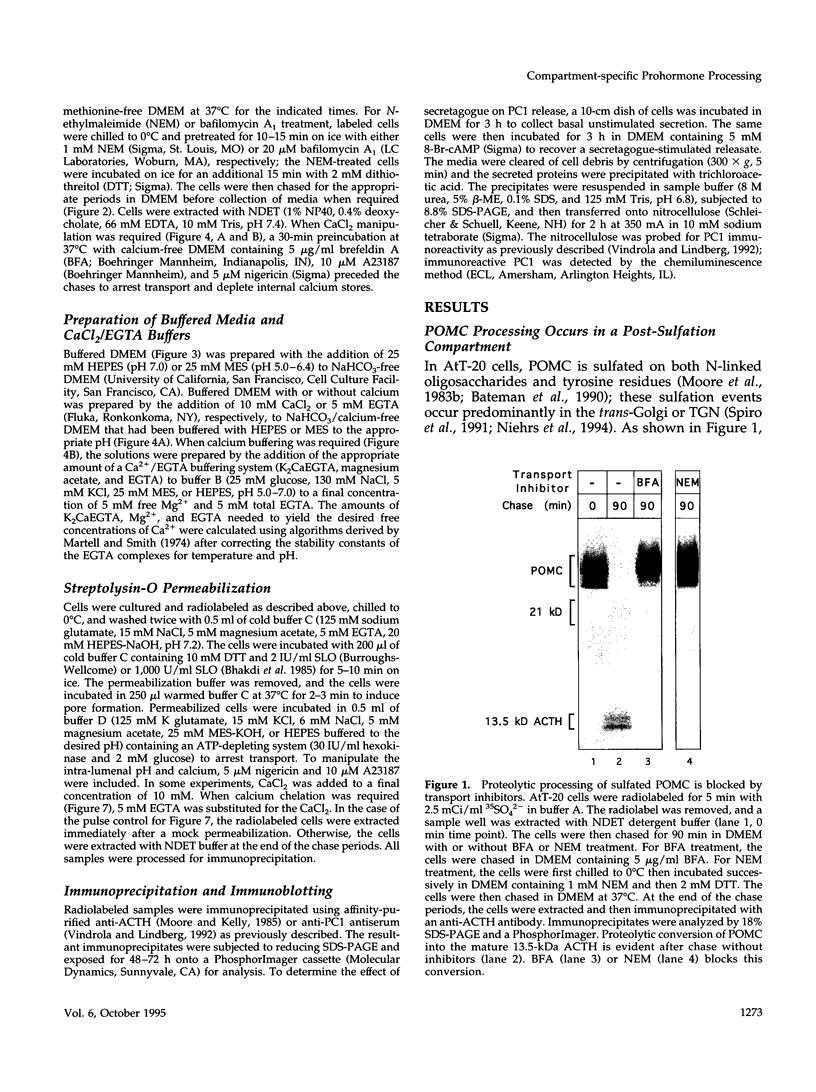
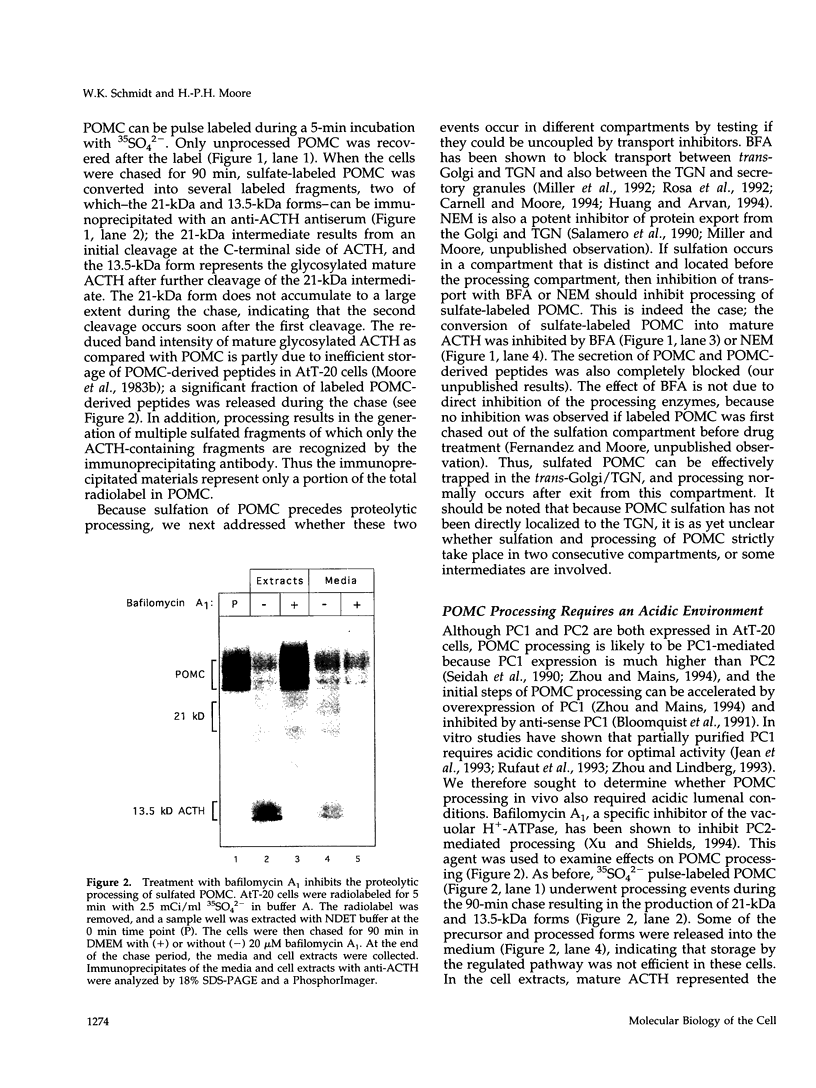
Abstract
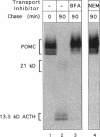
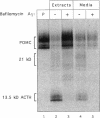
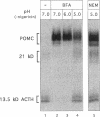
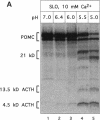
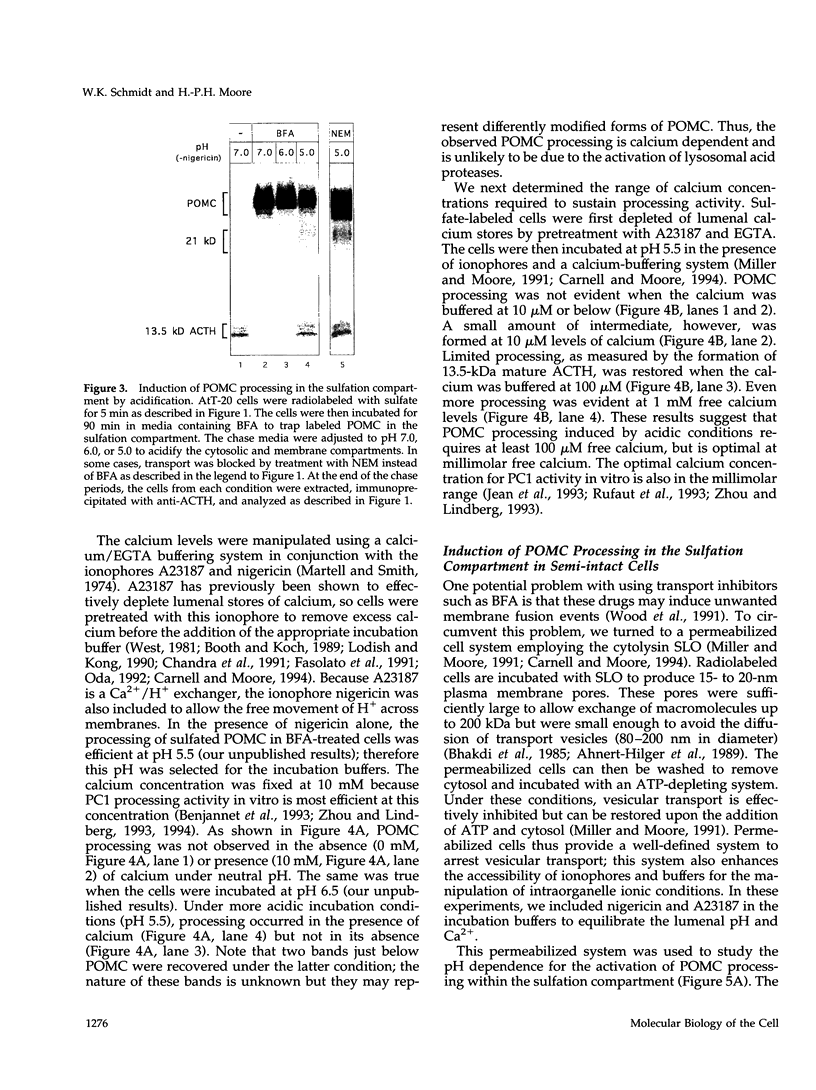
Newly synthesized prohormones and their processing enzymes transit through the same compartments before being packaged into regulated secretory granules. Despite this coordinated intracellular transport, prohormone processing does not occur until late in the secretory pathway. In the mouse pituitary AtT-20 cell line, conversion of pro-opiomelanocortin (POMC) to mature adrenocorticotropic hormone involves the prohormone convertase PC1. The mechanism by which this proteolytic processing is restricted to late secretory compartments is unknown; PC1 activity could be regulated by compartment-specific activators/inhibitors, or through changes in the ionic milieu that influence its activity. By arresting transport in a semi-intact cell system, we have addressed whether metabolically labeled POMC trapped in early secretory compartments can be induced to undergo conversion if the ionic milieu in these compartments is experimentally manipulated. Prolonged incubation of labeled POMC trapped in the endoplasmic reticulum or Golgi/trans-Golgi network did not result in processing, thereby supporting the theory that processing is normally a post-Golgi/trans-Golgi network event. However, acidification of these compartments allowed effective processing of POMC to the intermediate and mature forms. The observed processing increased sharply at a pH below 6.0 and required millimolar calcium, regardless of the compartment in which labeled POMC resided. These conditions also resulted in the coordinate conversion of PC1 from the 84/87 kDa into the 74-kDa and 66-kDa forms. We propose that POMC processing is predominantly restricted to acidifying secretory granules, and that a change in pH within these granules is both necessary and sufficient to activate POMC processing.
Full text
PDF


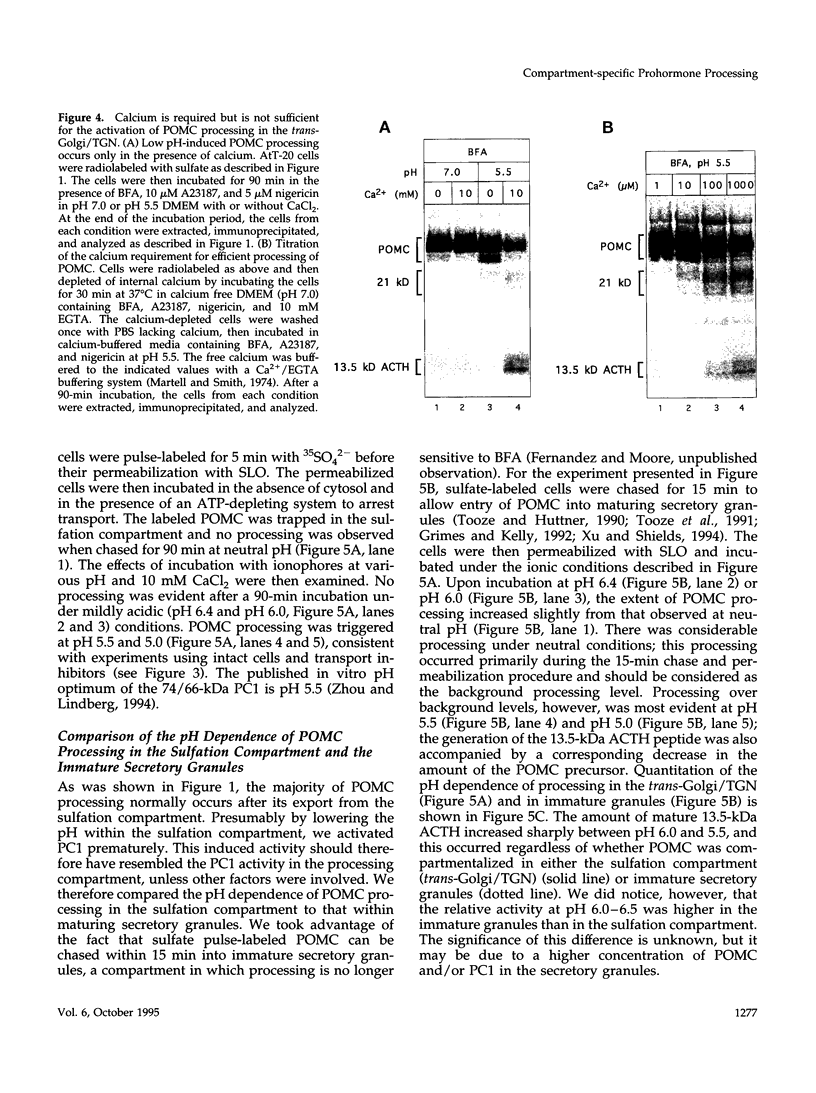

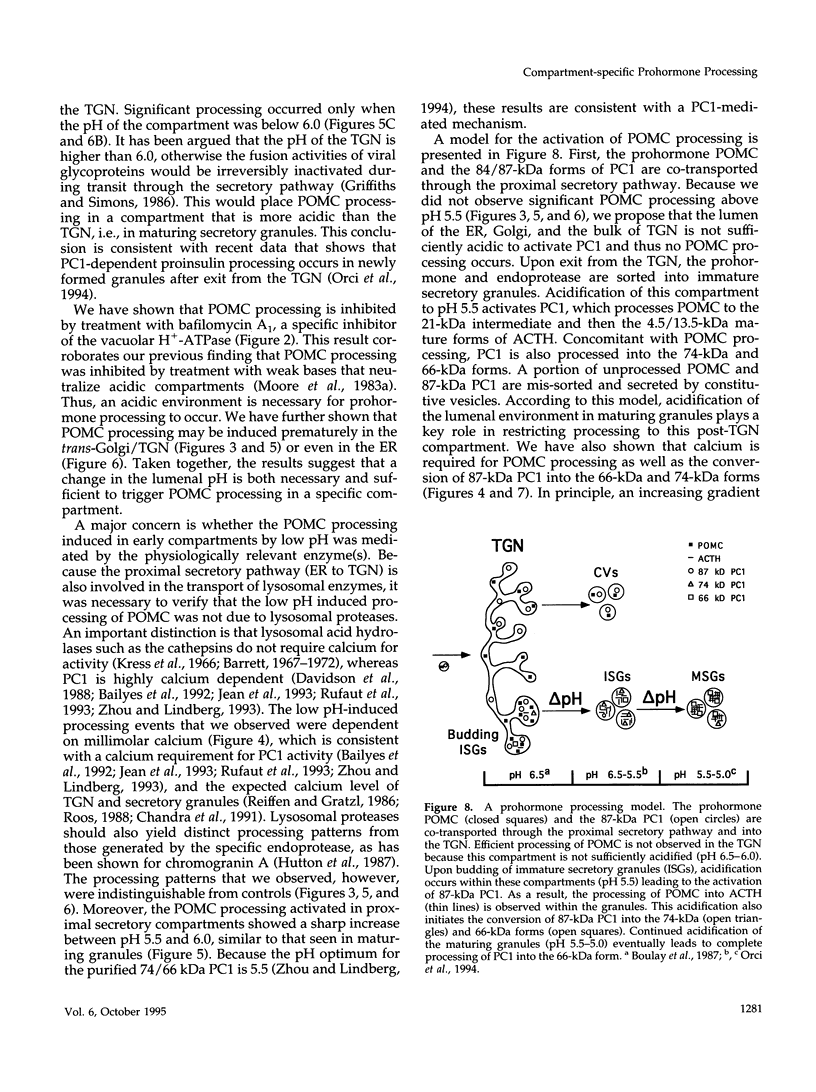



Images in this article
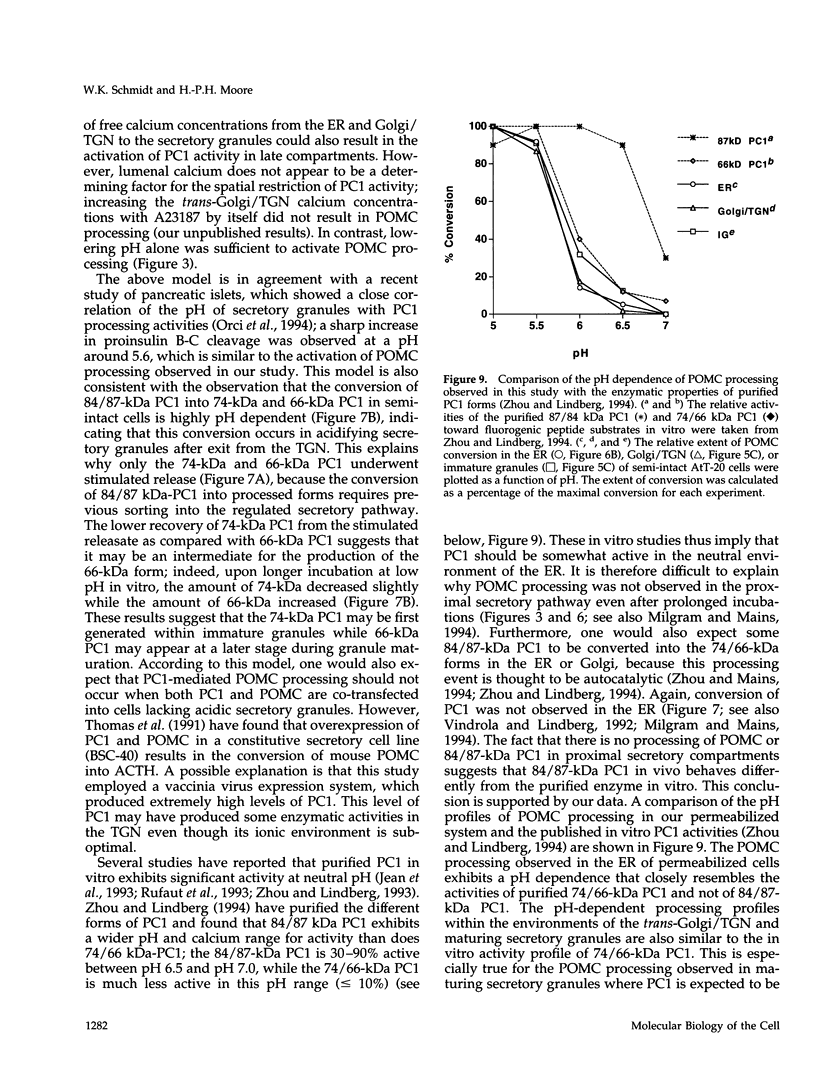
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
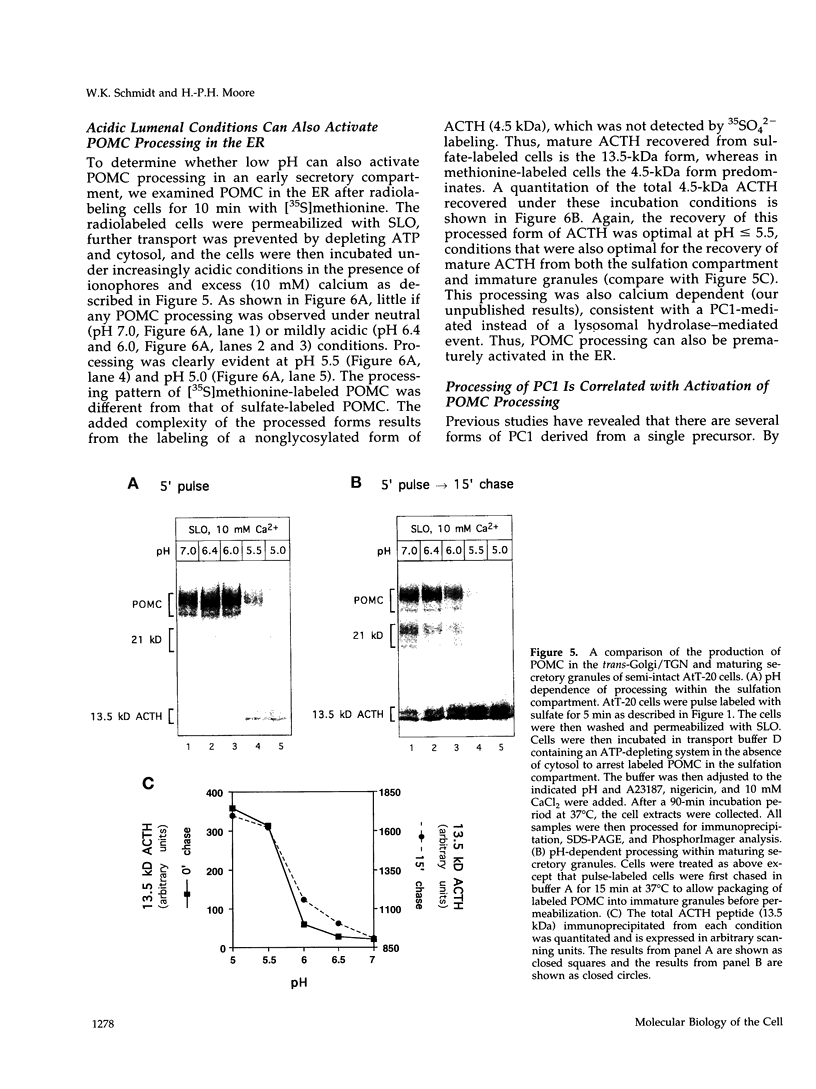
- Ahnert-Hilger G., Bader M. F., Bhakdi S., Gratzl M. Introduction of macromolecules into bovine adrenal medullary chromaffin cells and rat pheochromocytoma cells (PC12) by permeabilization with streptolysin O: inhibitory effect of tetanus toxin on catecholamine secretion. J Neurochem. 1989 Jun;52(6):1751–1758. doi: 10.1111/j.1471-4159.1989.tb07253.x. [DOI] [PubMed] [Google Scholar]
- Anderson R. G., Pathak R. K. Vesicles and cisternae in the trans Golgi apparatus of human fibroblasts are acidic compartments. Cell. 1985 Mar;40(3):635–643. doi: 10.1016/0092-8674(85)90212-0. [DOI] [PubMed] [Google Scholar]
- Aubert L., Motais R. Molecular features of organic anion permeablity in ox red blood cell. J Physiol. 1975 Mar;246(1):159–179. doi: 10.1113/jphysiol.1975.sp010884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bailyes E. M., Shennan K. I., Seal A. J., Smeekens S. P., Steiner D. F., Hutton J. C., Docherty K. A member of the eukaryotic subtilisin family (PC3) has the enzymic properties of the type 1 proinsulin-converting endopeptidase. Biochem J. 1992 Jul 15;285(Pt 2):391–394. doi: 10.1042/bj2850391. [DOI] [PMC free article] [PubMed] [Google Scholar]
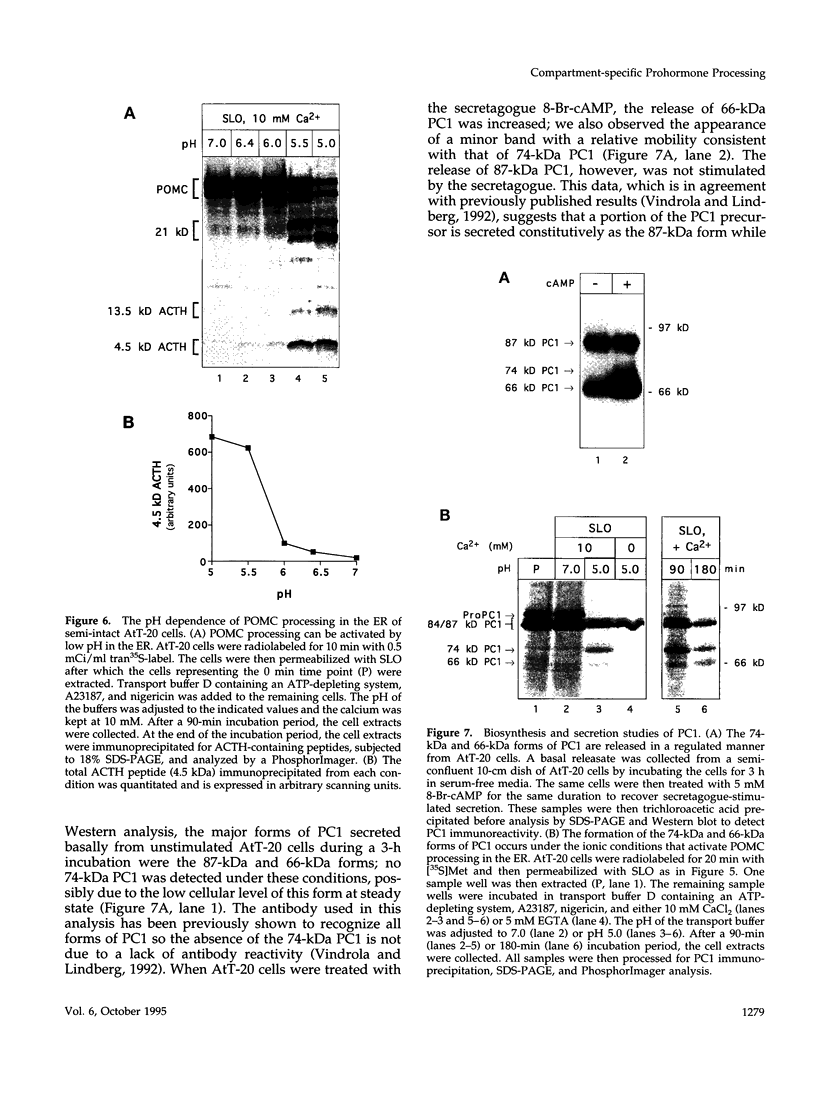
- Barrett A. J. Lysosomal acid proteinase of rabbit liver. Biochem J. 1967 Aug;104(2):601–608. doi: 10.1042/bj1040601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bateman A., Solomon S., Bennett H. P. Post-translational modification of bovine pro-opiomelanocortin. Tyrosine sulfation and pyroglutamate formation, a mass spectrometric study. J Biol Chem. 1990 Dec 25;265(36):22130–22136. [PubMed] [Google Scholar]
- Benjannet S., Rondeau N., Day R., Chrétien M., Seidah N. G. PC1 and PC2 are proprotein convertases capable of cleaving proopiomelanocortin at distinct pairs of basic residues. Proc Natl Acad Sci U S A. 1991 May 1;88(9):3564–3568. doi: 10.1073/pnas.88.9.3564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benjannet S., Rondeau N., Paquet L., Boudreault A., Lazure C., Chrétien M., Seidah N. G. Comparative biosynthesis, covalent post-translational modifications and efficiency of prosegment cleavage of the prohormone convertases PC1 and PC2: glycosylation, sulphation and identification of the intracellular site of prosegment cleavage of PC1 and PC2. Biochem J. 1993 Sep 15;294(Pt 3):735–743. doi: 10.1042/bj2940735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett D. L., Bailyes E. M., Nielsen E., Guest P. C., Rutherford N. G., Arden S. D., Hutton J. C. Identification of the type 2 proinsulin processing endopeptidase as PC2, a member of the eukaryote subtilisin family. J Biol Chem. 1992 Jul 25;267(21):15229–15236. [PubMed] [Google Scholar]
- Bhakdi S., Tranum-Jensen J., Sziegoleit A. Mechanism of membrane damage by streptolysin-O. Infect Immun. 1985 Jan;47(1):52–60. doi: 10.1128/iai.47.1.52-60.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhakdi S., Weller U., Walev I., Martin E., Jonas D., Palmer M. A guide to the use of pore-forming toxins for controlled permeabilization of cell membranes. Med Microbiol Immunol. 1993 Sep;182(4):167–175. doi: 10.1007/BF00219946. [DOI] [PubMed] [Google Scholar]
- Bloomquist B. T., Eipper B. A., Mains R. E. Prohormone-converting enzymes: regulation and evaluation of function using antisense RNA. Mol Endocrinol. 1991 Dec;5(12):2014–2024. doi: 10.1210/mend-5-12-2014. [DOI] [PubMed] [Google Scholar]
- Booth C., Koch G. L. Perturbation of cellular calcium induces secretion of luminal ER proteins. Cell. 1989 Nov 17;59(4):729–737. doi: 10.1016/0092-8674(89)90019-6. [DOI] [PubMed] [Google Scholar]
- Boulay F., Doms R. W., Wilson I., Helenius A. The influenza hemagglutinin precursor as an acid-sensitive probe of the biosynthetic pathway. EMBO J. 1987 Sep;6(9):2643–2650. doi: 10.1002/j.1460-2075.1987.tb02555.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braks J. A., Martens G. J. 7B2 is a neuroendocrine chaperone that transiently interacts with prohormone convertase PC2 in the secretory pathway. Cell. 1994 Jul 29;78(2):263–273. doi: 10.1016/0092-8674(94)90296-8. [DOI] [PubMed] [Google Scholar]
- Carnell L., Moore H. P. Transport via the regulated secretory pathway in semi-intact PC12 cells: role of intra-cisternal calcium and pH in the transport and sorting of secretogranin II. J Cell Biol. 1994 Nov;127(3):693–705. doi: 10.1083/jcb.127.3.693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chandra S., Kable E. P., Morrison G. H., Webb W. W. Calcium sequestration in the Golgi apparatus of cultured mammalian cells revealed by laser scanning confocal microscopy and ion microscopy. J Cell Sci. 1991 Dec;100(Pt 4):747–752. doi: 10.1242/jcs.100.4.747. [DOI] [PubMed] [Google Scholar]
- Davidson H. W., Rhodes C. J., Hutton J. C. Intraorganellar calcium and pH control proinsulin cleavage in the pancreatic beta cell via two distinct site-specific endopeptidases. Nature. 1988 May 5;333(6168):93–96. doi: 10.1038/333093a0. [DOI] [PubMed] [Google Scholar]
- Davoust J., Gruenberg J., Howell K. E. Two threshold values of low pH block endocytosis at different stages. EMBO J. 1987 Dec 1;6(12):3601–3609. doi: 10.1002/j.1460-2075.1987.tb02691.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Day N. C., Lin H., Ueda Y., Meador-Woodruff J. H., Akil H. Characterization of pro-opiomelanocortin processing in heterologous neuronal cells that express PC2 mRNA. Neuropeptides. 1993 May;24(5):253–262. doi: 10.1016/0143-4179(93)90013-z. [DOI] [PubMed] [Google Scholar]
- Day R., Schafer M. K., Watson S. J., Chrétien M., Seidah N. G. Distribution and regulation of the prohormone convertases PC1 and PC2 in the rat pituitary. Mol Endocrinol. 1992 Mar;6(3):485–497. doi: 10.1210/mend.6.3.1316544. [DOI] [PubMed] [Google Scholar]
- Fasolato C., Zottini M., Clementi E., Zacchetti D., Meldolesi J., Pozzan T. Intracellular Ca2+ pools in PC12 cells. Three intracellular pools are distinguished by their turnover and mechanisms of Ca2+ accumulation, storage, and release. J Biol Chem. 1991 Oct 25;266(30):20159–20167. [PubMed] [Google Scholar]
- Friedman T. C., Loh Y. P., Birch N. P. In vitro processing of proopiomelanocortin by recombinant PC1 (SPC3). Endocrinology. 1994 Sep;135(3):854–862. doi: 10.1210/endo.135.3.8070378. [DOI] [PubMed] [Google Scholar]
- Gianoulakis C., Seidah N. G., Routhier R., Chrétien M. Biosynthesis and characterization of adrenocorticotropic hormone, alpha-melanocyte-stimulating hormone, and an NH2-terminal fragment of the adrenocorticotropic hormone/beta-lipotropin precursor from rat pars intermedia. J Biol Chem. 1979 Dec 10;254(23):11903–11906. [PubMed] [Google Scholar]
- Griffiths G., Simons K. The trans Golgi network: sorting at the exit site of the Golgi complex. Science. 1986 Oct 24;234(4775):438–443. doi: 10.1126/science.2945253. [DOI] [PubMed] [Google Scholar]
- Grimes M., Kelly R. B. Intermediates in the constitutive and regulated secretory pathways released in vitro from semi-intact cells. J Cell Biol. 1992 May;117(3):539–549. doi: 10.1083/jcb.117.3.539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gumbiner B., Kelly R. B. Secretory granules of an anterior pituitary cell line, AtT-20, contain only mature forms of corticotropin and beta-lipotropin. Proc Natl Acad Sci U S A. 1981 Jan;78(1):318–322. doi: 10.1073/pnas.78.1.318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gumbiner B., Kelly R. B. Two distinct intracellular pathways transport secretory and membrane glycoproteins to the surface of pituitary tumor cells. Cell. 1982 Jan;28(1):51–59. doi: 10.1016/0092-8674(82)90374-9. [DOI] [PubMed] [Google Scholar]
- Heuser J. Effects of cytoplasmic acidification on clathrin lattice morphology. J Cell Biol. 1989 Feb;108(2):401–411. doi: 10.1083/jcb.108.2.401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang X. F., Arvan P. Formation of the insulin-containing secretory granule core occurs within immature beta-granules. J Biol Chem. 1994 Aug 19;269(33):20838–20844. [PubMed] [Google Scholar]
- Hutton J. C., Davidson H. W., Peshavaria M. Proteolytic processing of chromogranin A in purified insulin granules. Formation of a 20 kDa N-terminal fragment (betagranin) by the concerted action of a Ca2+-dependent endopeptidase and carboxypeptidase H (EC 3.4.17.10). Biochem J. 1987 Jun 1;244(2):457–464. doi: 10.1042/bj2440457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutton J. C. Subtilisin-like proteinases involved in the activation of proproteins of the eukaryotic secretory pathway. Curr Opin Cell Biol. 1990 Dec;2(6):1131–1142. doi: 10.1016/0955-0674(90)90167-d. [DOI] [PubMed] [Google Scholar]
- Jean F., Basak A., Rondeau N., Benjannet S., Hendy G. N., Seidah N. G., Chrétien M., Lazure C. Enzymic characterization of murine and human prohormone convertase-1 (mPC1 and hPC1) expressed in mammalian GH4C1 cells. Biochem J. 1993 Jun 15;292(Pt 3):891–900. doi: 10.1042/bj2920891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kornfeld S., Mellman I. The biogenesis of lysosomes. Annu Rev Cell Biol. 1989;5:483–525. doi: 10.1146/annurev.cb.05.110189.002411. [DOI] [PubMed] [Google Scholar]
- Kornfeld S. Structure and function of the mannose 6-phosphate/insulinlike growth factor II receptors. Annu Rev Biochem. 1992;61:307–330. doi: 10.1146/annurev.bi.61.070192.001515. [DOI] [PubMed] [Google Scholar]
- Kress L. F., Peanasky R. J., Klitgaard H. M. Purification, properties, and specificity of hog thyroid proteinase. Biochim Biophys Acta. 1966 Feb 14;113(2):375–389. doi: 10.1016/s0926-6593(66)80076-0. [DOI] [PubMed] [Google Scholar]
- L'Allemain G., Paris S., Pouysségur J. Growth factor action and intracellular pH regulation in fibroblasts. Evidence for a major role of the Na+/H+ antiport. J Biol Chem. 1984 May 10;259(9):5809–5815. [PubMed] [Google Scholar]
- Lindberg I. Evidence for cleavage of the PC1/PC3 pro-segment in the endoplasmic reticulum. Mol Cell Neurosci. 1994 Jun;5(3):263–268. doi: 10.1006/mcne.1994.1030. [DOI] [PubMed] [Google Scholar]
- Lindberg I. The new eukaryotic precursor processing proteinases. Mol Endocrinol. 1991 Oct;5(10):1361–1365. doi: 10.1210/mend-5-10-1361. [DOI] [PubMed] [Google Scholar]
- Lodish H. F., Kong N. Perturbation of cellular calcium blocks exit of secretory proteins from the rough endoplasmic reticulum. J Biol Chem. 1990 Jul 5;265(19):10893–10899. [PubMed] [Google Scholar]
- Mains R. E., Cullen E. I., May V., Eipper B. A. The role of secretory granules in peptide biosynthesis. Ann N Y Acad Sci. 1987;493:278–291. doi: 10.1111/j.1749-6632.1987.tb27213.x. [DOI] [PubMed] [Google Scholar]
- Mains R. E., Eipper B. A. Coordinate synthesis of corticotropins and endorphins by mouse pituitary tumor cells. J Biol Chem. 1978 Feb 10;253(3):651–655. [PubMed] [Google Scholar]
- Mains R. E., Eipper B. A., Ling N. Common precursor to corticotropins and endorphins. Proc Natl Acad Sci U S A. 1977 Jul;74(7):3014–3018. doi: 10.1073/pnas.74.7.3014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mains R. E., Eipper B. A. Synthesis and secretion of corticotropins, melanotropins, and endorphins by rat intermediate pituitary cells. J Biol Chem. 1979 Aug 25;254(16):7885–7894. [PubMed] [Google Scholar]
- Martens G. J., Braks J. A., Eib D. W., Zhou Y., Lindberg I. The neuroendocrine polypeptide 7B2 is an endogenous inhibitor of prohormone convertase PC2. Proc Natl Acad Sci U S A. 1994 Jun 21;91(13):5784–5787. doi: 10.1073/pnas.91.13.5784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mellman I., Fuchs R., Helenius A. Acidification of the endocytic and exocytic pathways. Annu Rev Biochem. 1986;55:663–700. doi: 10.1146/annurev.bi.55.070186.003311. [DOI] [PubMed] [Google Scholar]
- Milgram S. L., Mains R. E. Differential effects of temperature blockade on the proteolytic processing of three secretory granule-associated proteins. J Cell Sci. 1994 Mar;107(Pt 3):737–745. doi: 10.1242/jcs.107.3.737. [DOI] [PubMed] [Google Scholar]
- Miller S. G., Carnell L., Moore H. H. Post-Golgi membrane traffic: brefeldin A inhibits export from distal Golgi compartments to the cell surface but not recycling. J Cell Biol. 1992 Jul;118(2):267–283. doi: 10.1083/jcb.118.2.267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller S. G., Moore H. P. Reconstitution of constitutive secretion using semi-intact cells: regulation by GTP but not calcium. J Cell Biol. 1991 Jan;112(1):39–54. doi: 10.1083/jcb.112.1.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore H. P., Gumbiner B., Kelly R. B. A subclass of proteins and sulfated macromolecules secreted by AtT-20 (mouse pituitary tumor) cells is sorted with adrenocorticotropin into dense secretory granules. J Cell Biol. 1983 Sep;97(3):810–817. doi: 10.1083/jcb.97.3.810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore H. P., Gumbiner B., Kelly R. B. Chloroquine diverts ACTH from a regulated to a constitutive secretory pathway in AtT-20 cells. 1983 Mar 31-Apr 6Nature. 302(5907):434–436. doi: 10.1038/302434a0. [DOI] [PubMed] [Google Scholar]
- Moore H. P., Kelly R. B. Secretory protein targeting in a pituitary cell line: differential transport of foreign secretory proteins to distinct secretory pathways. J Cell Biol. 1985 Nov;101(5 Pt 1):1773–1781. doi: 10.1083/jcb.101.5.1773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niehrs C., Beisswanger R., Huttner W. B. Protein tyrosine sulfation, 1993--an update. Chem Biol Interact. 1994 Jun;92(1-3):257–271. doi: 10.1016/0009-2797(94)90068-x. [DOI] [PubMed] [Google Scholar]
- Oda K. Calcium depletion blocks proteolytic cleavages of plasma protein precursors which occur at the Golgi and/or trans-Golgi network. Possible involvement of Ca(2+)-dependent Golgi endoproteases. J Biol Chem. 1992 Aug 25;267(24):17465–17471. [PubMed] [Google Scholar]
- Orci L., Halban P., Perrelet A., Amherdt M., Ravazzola M., Anderson R. G. pH-independent and -dependent cleavage of proinsulin in the same secretory vesicle. J Cell Biol. 1994 Sep;126(5):1149–1156. doi: 10.1083/jcb.126.5.1149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orci L., Ravazzola M., Amherdt M., Madsen O., Perrelet A., Vassalli J. D., Anderson R. G. Conversion of proinsulin to insulin occurs coordinately with acidification of maturing secretory vesicles. J Cell Biol. 1986 Dec;103(6 Pt 1):2273–2281. doi: 10.1083/jcb.103.6.2273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reiffen F. U., Gratzl M. Ca2+ binding to chromaffin vesicle matrix proteins: effect of pH, Mg2+, and ionic strength. Biochemistry. 1986 Jul 29;25(15):4402–4406. doi: 10.1021/bi00363a034. [DOI] [PubMed] [Google Scholar]
- Rhodes C. J., Thorne B. A., Lincoln B., Nielsen E., Hutton J. C., Thomas G. Processing of proopiomelanocortin by insulin secretory granule proinsulin processing endopeptidases. J Biol Chem. 1993 Feb 25;268(6):4267–4275. [PMC free article] [PubMed] [Google Scholar]
- Roberts J. L., Herbert E. Characterization of a common precursor to corticotropin and beta-lipotropin: identification of beta-lipotropin peptides and their arrangement relative to corticotropin in the precursor synthesized in a cell-free system. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5300–5304. doi: 10.1073/pnas.74.12.5300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts J. L., Phillips M., Rosa P. A., Herbert E. Steps involved in the processing of common precursor forms of adrenocorticotropin and endorphin in cultures of mouse pituitary cells. Biochemistry. 1978 Aug 22;17(17):3609–3618. doi: 10.1021/bi00610a030. [DOI] [PubMed] [Google Scholar]
- Roos N. A possible site of calcium regulation in rat exocrine pancreas cells: an X-ray microanalytical study. Scanning Microsc. 1988 Mar;2(1):323–329. [PubMed] [Google Scholar]
- Rosa P., Barr F. A., Stinchcombe J. C., Binacchi C., Huttner W. B. Brefeldin A inhibits the formation of constitutive secretory vesicles and immature secretory granules from the trans-Golgi network. Eur J Cell Biol. 1992 Dec;59(2):265–274. [PubMed] [Google Scholar]
- Rufaut N. W., Brennan S. O., Hakes D. J., Dixon J. E., Birch N. P. Purification and characterization of the candidate prohormone-processing enzyme SPC3 produced in a mouse L cell line. J Biol Chem. 1993 Sep 25;268(27):20291–20298. [PubMed] [Google Scholar]
- Salamero J., Sztul E. S., Howell K. E. Exocytic transport vesicles generated in vitro from the trans-Golgi network carry secretory and plasma membrane proteins. Proc Natl Acad Sci U S A. 1990 Oct;87(19):7717–7721. doi: 10.1073/pnas.87.19.7717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnabel E., Mains R. E., Farquhar M. G. Proteolytic processing of pro-ACTH/endorphin begins in the Golgi complex of pituitary corticotropes and AtT-20 cells. Mol Endocrinol. 1989 Aug;3(8):1223–1235. doi: 10.1210/mend-3-8-1223. [DOI] [PubMed] [Google Scholar]
- Seidah N. G., Day R., Chrétien M. The family of pro-hormone and pro-protein convertases. Biochem Soc Trans. 1993 Aug;21(3):685–691. doi: 10.1042/bst0210685. [DOI] [PubMed] [Google Scholar]
- Seidah N. G., Gaspar L., Mion P., Marcinkiewicz M., Mbikay M., Chrétien M. cDNA sequence of two distinct pituitary proteins homologous to Kex2 and furin gene products: tissue-specific mRNAs encoding candidates for pro-hormone processing proteinases. DNA Cell Biol. 1990 Jul-Aug;9(6):415–424. doi: 10.1089/dna.1990.9.415. [DOI] [PubMed] [Google Scholar]
- Seidah N. G., Marcinkiewicz M., Benjannet S., Gaspar L., Beaubien G., Mattei M. G., Lazure C., Mbikay M., Chrétien M. Cloning and primary sequence of a mouse candidate prohormone convertase PC1 homologous to PC2, Furin, and Kex2: distinct chromosomal localization and messenger RNA distribution in brain and pituitary compared to PC2. Mol Endocrinol. 1991 Jan;5(1):111–122. doi: 10.1210/mend-5-1-111. [DOI] [PubMed] [Google Scholar]
- Shennan K. I., Taylor N. A., Jermany J. L., Matthews G., Docherty K. Differences in pH optima and calcium requirements for maturation of the prohormone convertases PC2 and PC3 indicates different intracellular locations for these events. J Biol Chem. 1995 Jan 20;270(3):1402–1407. doi: 10.1074/jbc.270.3.1402. [DOI] [PubMed] [Google Scholar]
- Smeekens S. P., Avruch A. S., LaMendola J., Chan S. J., Steiner D. F. Identification of a cDNA encoding a second putative prohormone convertase related to PC2 in AtT20 cells and islets of Langerhans. Proc Natl Acad Sci U S A. 1991 Jan 15;88(2):340–344. doi: 10.1073/pnas.88.2.340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smeekens S. P., Steiner D. F. Identification of a human insulinoma cDNA encoding a novel mammalian protein structurally related to the yeast dibasic processing protease Kex2. J Biol Chem. 1990 Feb 25;265(6):2997–3000. [PubMed] [Google Scholar]
- Spiro R. C., Freeze H. H., Sampath D., Garcia J. A. Uncoupling of chondroitin sulfate glycosaminoglycan synthesis by brefeldin A. J Cell Biol. 1991 Dec;115(5):1463–1473. doi: 10.1083/jcb.115.5.1463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steiner D. F., James D. E. Cellular and molecular biology of the beta cell. Diabetologia. 1992 Dec;35 (Suppl 2):S41–S48. doi: 10.1007/BF00586278. [DOI] [PubMed] [Google Scholar]
- Thomas L., Leduc R., Thorne B. A., Smeekens S. P., Steiner D. F., Thomas G. Kex2-like endoproteases PC2 and PC3 accurately cleave a model prohormone in mammalian cells: evidence for a common core of neuroendocrine processing enzymes. Proc Natl Acad Sci U S A. 1991 Jun 15;88(12):5297–5301. doi: 10.1073/pnas.88.12.5297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tooze J., Hollinshead M., Frank R., Burke B. An antibody specific for an endoproteolytic cleavage site provides evidence that pro-opiomelanocortin is packaged into secretory granules in AtT20 cells before its cleavage. J Cell Biol. 1987 Jul;105(1):155–162. doi: 10.1083/jcb.105.1.155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tooze S. A., Flatmark T., Tooze J., Huttner W. B. Characterization of the immature secretory granule, an intermediate in granule biogenesis. J Cell Biol. 1991 Dec;115(6):1491–1503. doi: 10.1083/jcb.115.6.1491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tooze S. A., Huttner W. B. Cell-free protein sorting to the regulated and constitutive secretory pathways. Cell. 1990 Mar 9;60(5):837–847. doi: 10.1016/0092-8674(90)90097-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vindrola O., Lindberg I. Biosynthesis of the prohormone convertase mPC1 in AtT-20 cells. Mol Endocrinol. 1992 Jul;6(7):1088–1094. doi: 10.1210/mend.6.7.1508222. [DOI] [PubMed] [Google Scholar]
- Vindrola O. Rapid cleavage of the endogenous PC3 prosegment and slow conversion to 74 kDa and 66 kDa proteins in AtT-20 cells. Neuropeptides. 1994 Aug;27(2):109–120. doi: 10.1016/0143-4179(94)90051-5. [DOI] [PubMed] [Google Scholar]
- West D. W. Energy-dependent calcium sequestration activity in a Golgi apparatus fraction derived from lactating rat mammary glands. Biochim Biophys Acta. 1981 Apr 3;673(4):374–386. doi: 10.1016/0304-4165(81)90469-4. [DOI] [PubMed] [Google Scholar]
- Wood S. A., Park J. E., Brown W. J. Brefeldin A causes a microtubule-mediated fusion of the trans-Golgi network and early endosomes. Cell. 1991 Nov 1;67(3):591–600. doi: 10.1016/0092-8674(91)90533-5. [DOI] [PubMed] [Google Scholar]
- Xu H., Shields D. Prohormone processing in the trans-Golgi network: endoproteolytic cleavage of prosomatostatin and formation of nascent secretory vesicles in permeabilized cells. J Cell Biol. 1993 Sep;122(6):1169–1184. doi: 10.1083/jcb.122.6.1169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu H., Shields D. Prosomatostatin processing in permeabilized cells. Endoproteolytic cleavage is mediated by a vacuolar ATPase that generates an acidic pH in the trans-Golgi network. J Biol Chem. 1994 Sep 9;269(36):22875–22881. [PubMed] [Google Scholar]
- Zhou A., Bloomquist B. T., Mains R. E. The prohormone convertases PC1 and PC2 mediate distinct endoproteolytic cleavages in a strict temporal order during proopiomelanocortin biosynthetic processing. J Biol Chem. 1993 Jan 25;268(3):1763–1769. [PubMed] [Google Scholar]
- Zhou A., Mains R. E. Endoproteolytic processing of proopiomelanocortin and prohormone convertases 1 and 2 in neuroendocrine cells overexpressing prohormone convertases 1 or 2. J Biol Chem. 1994 Jul 1;269(26):17440–17447. [PubMed] [Google Scholar]
- Zhou Y., Lindberg I. Enzymatic properties of carboxyl-terminally truncated prohormone convertase 1 (PC1/SPC3) and evidence for autocatalytic conversion. J Biol Chem. 1994 Jul 15;269(28):18408–18413. [PubMed] [Google Scholar]
- Zhou Y., Lindberg I. Purification and characterization of the prohormone convertase PC1(PC3). J Biol Chem. 1993 Mar 15;268(8):5615–5623. [PubMed] [Google Scholar]