Abstract
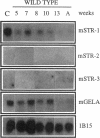
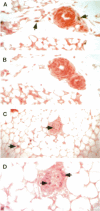
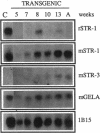
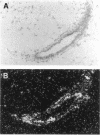
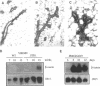
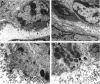
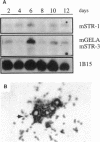
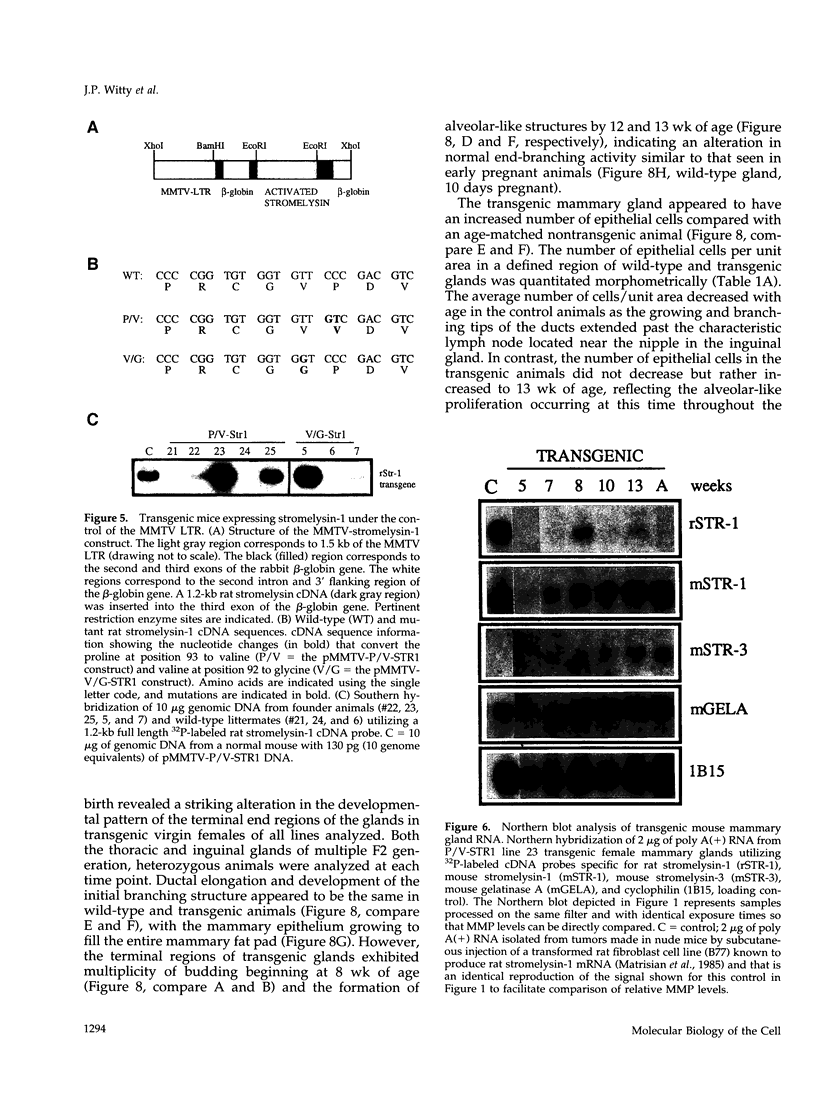
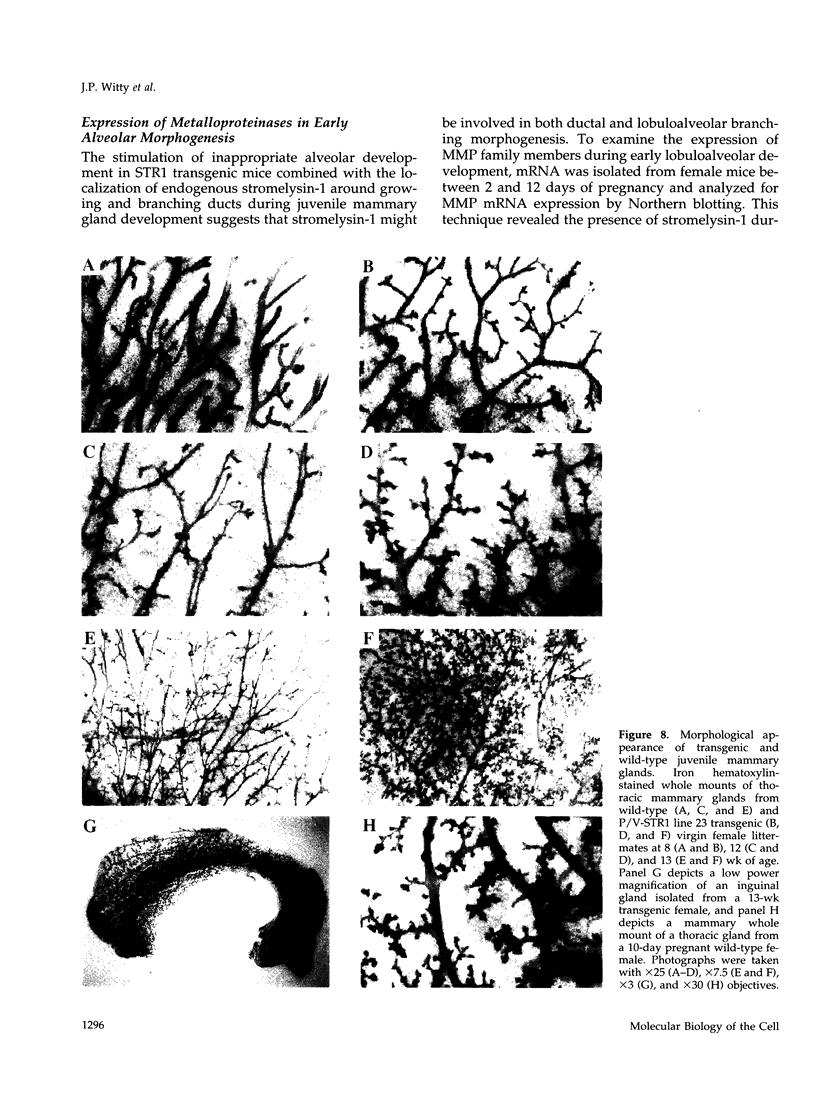
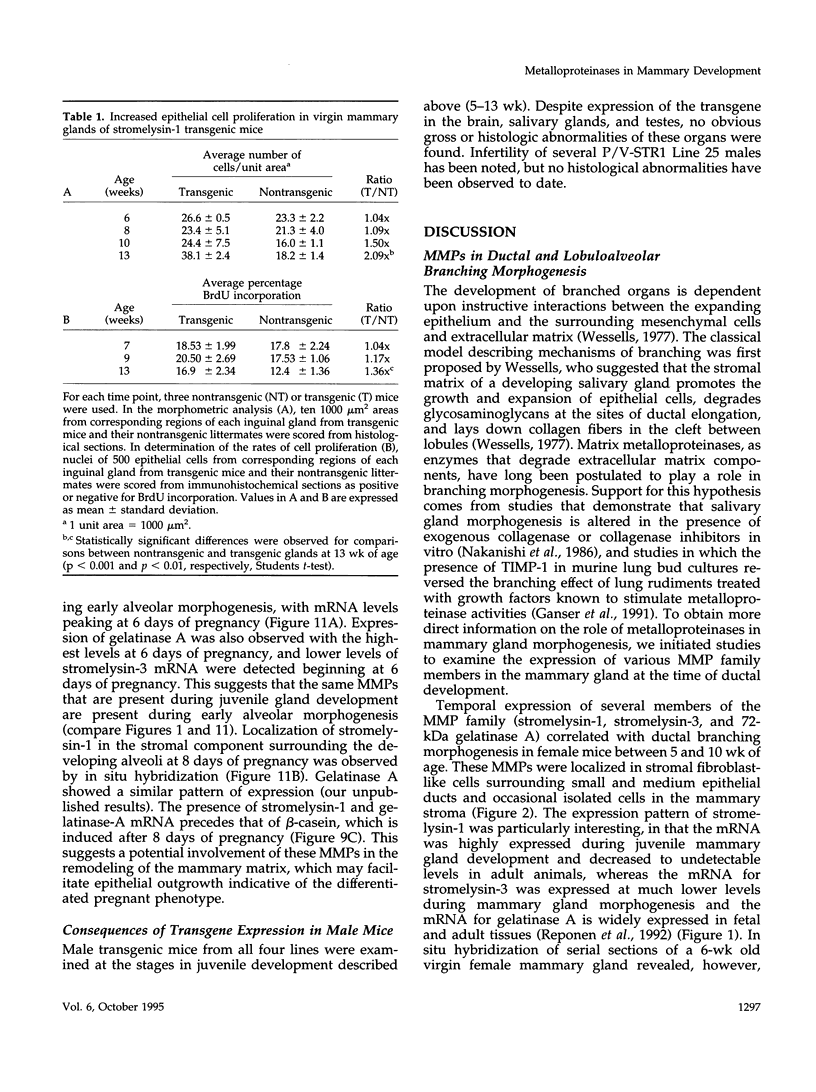
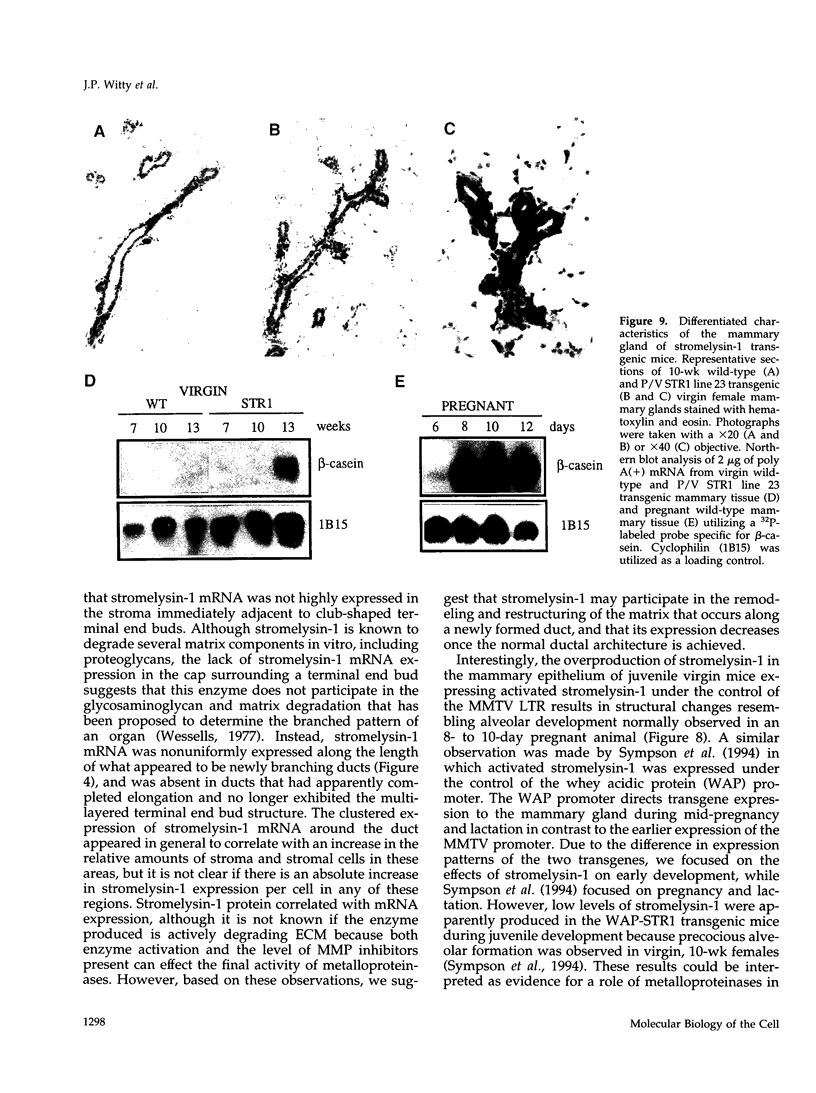
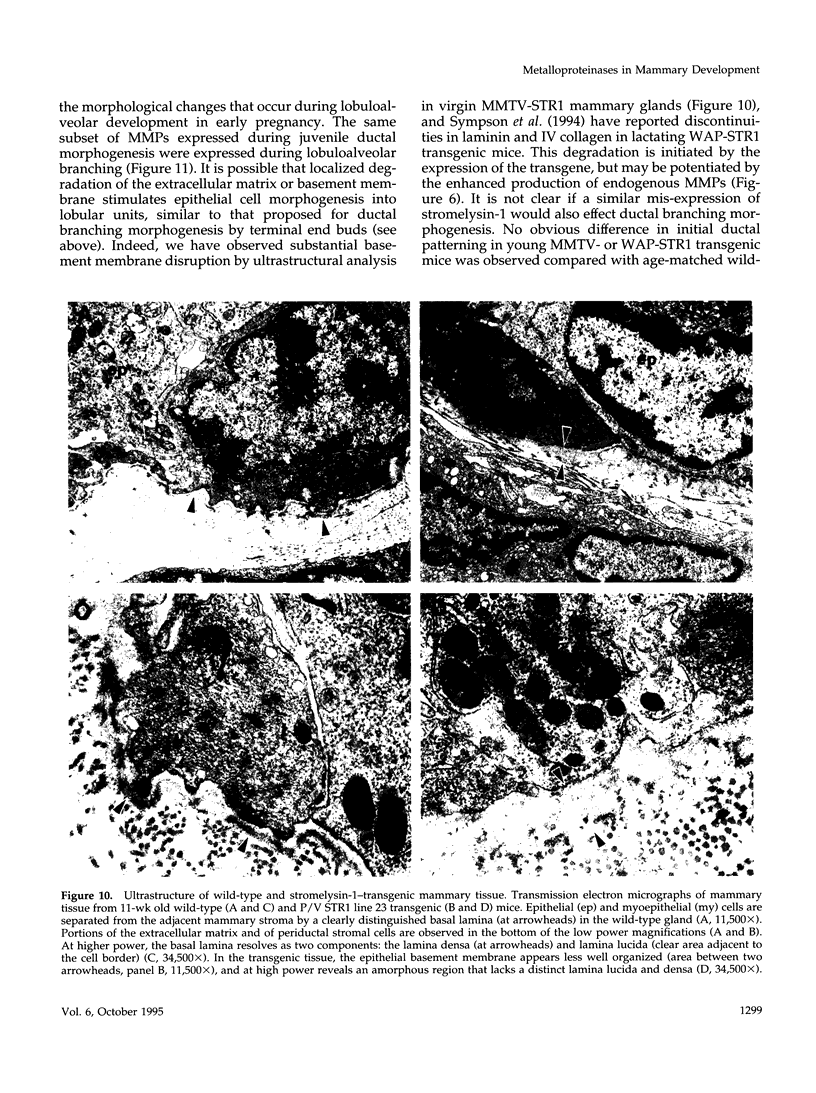
The matrix-degrading metalloproteinases stromelysin-1, stromelysin-3, and gelatinase A are expressed during ductal branching morphogenesis of the murine mammary gland. Stromelysin-1 expression in particular correlates with ductal elongation, and in situ hybridization and three-dimensional reconstruction studies revealed that stromelysin-1 mRNA was concentrated in stromal fibroblasts along the length of advancing ducts. Transgenic mice expressing an activated form of stromelysin-1 under the control of the MMTV promoter/enhancer exhibited inappropriate alveolar development in virgin females. Ultrastructural analysis demonstrated that the basement membrane underlying epithelial and myoepithelial cells was amorphous and discontinuous compared with the highly ordered basal lamina in control mammary glands. Transgenic mammary glands had at least a twofold increase in the number of cells/unit area and a 1.4-fold increase in the percent of cycling cells by 13 wk of age compared with nontransgenic littermates. In addition, transgenic glands expressed beta-casein mRNA, but not protein, and resembled the proliferative and differentiated state of an animal between 8 and 10 days pregnant. An analysis of metalloproteinase expression in the glands of normal pregnant females demonstrated that the same matrix metalloproteinase family members, including stromelysin-1, were expressed in connective tissue cells surrounding epithelial clusters during the time of lobuloalveolar development. These results suggest that metalloproteinases may assist in remodeling ECM during normal ductal and alveolar branching morphogenesis, and that disruption of the basement membrane by an activated metalloproteinase can affect basic cellular processes of proliferation and differentiation.
Full text
PDF











Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barcellos-Hoff M. H., Aggeler J., Ram T. G., Bissell M. J. Functional differentiation and alveolar morphogenesis of primary mammary cultures on reconstituted basement membrane. Development. 1989 Feb;105(2):223–235. doi: 10.1242/dev.105.2.223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birkedal-Hansen H., Moore W. G., Bodden M. K., Windsor L. J., Birkedal-Hansen B., DeCarlo A., Engler J. A. Matrix metalloproteinases: a review. Crit Rev Oral Biol Med. 1993;4(2):197–250. doi: 10.1177/10454411930040020401. [DOI] [PubMed] [Google Scholar]
- Blair H. C., Teitelbaum S. L., Ehlich L. S., Jeffrey J. J. Collagenase production by smooth muscle: correlation of immunoreactive with functional enzyme in the myometrium. J Cell Physiol. 1986 Oct;129(1):111–123. doi: 10.1002/jcp.1041290116. [DOI] [PubMed] [Google Scholar]
- Boudreau N., Sympson C. J., Werb Z., Bissell M. J. Suppression of ICE and apoptosis in mammary epithelial cells by extracellular matrix. Science. 1995 Feb 10;267(5199):891–893. doi: 10.1126/science.7531366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breathnach R., Matrisian L. M., Gesnel M. C., Staub A., Leroy P. Sequences coding for part of oncogene-induced transin are highly conserved in a related rat gene. Nucleic Acids Res. 1987 Feb 11;15(3):1139–1151. doi: 10.1093/nar/15.3.1139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brinckerhoff C. E. Joint destruction in arthritis: metalloproteinases in the spotlight. Arthritis Rheum. 1991 Sep;34(9):1073–1075. doi: 10.1002/art.1780340902. [DOI] [PubMed] [Google Scholar]
- Danielson P. E., Forss-Petter S., Brow M. A., Calavetta L., Douglass J., Milner R. J., Sutcliffe J. G. p1B15: a cDNA clone of the rat mRNA encoding cyclophilin. DNA. 1988 May;7(4):261–267. doi: 10.1089/dna.1988.7.261. [DOI] [PubMed] [Google Scholar]
- Davies B., Brown P. D., East N., Crimmin M. J., Balkwill F. R. A synthetic matrix metalloproteinase inhibitor decreases tumor burden and prolongs survival of mice bearing human ovarian carcinoma xenografts. Cancer Res. 1993 May 1;53(9):2087–2091. [PubMed] [Google Scholar]
- Dickson S. R., Warburton M. J. Enhanced synthesis of gelatinase and stromelysin by myoepithelial cells during involution of the rat mammary gland. J Histochem Cytochem. 1992 May;40(5):697–703. doi: 10.1177/40.5.1315355. [DOI] [PubMed] [Google Scholar]
- Edwards D. R., Waterhouse P., Holman M. L., Denhardt D. T. A growth-responsive gene (16C8) in normal mouse fibroblasts homologous to a human collagenase inhibitor with erythroid-potentiating activity: evidence for inducible and constitutive transcripts. Nucleic Acids Res. 1986 Nov 25;14(22):8863–8878. doi: 10.1093/nar/14.22.8863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gack S., Vallon R., Schaper J., Rüther U., Angel P. Phenotypic alterations in fos-transgenic mice correlate with changes in Fos/Jun-dependent collagenase type I expression. Regulation of mouse metalloproteinases by carcinogens, tumor promoters, cAMP, and Fos oncoprotein. J Biol Chem. 1994 Apr 8;269(14):10363–10369. [PubMed] [Google Scholar]
- Galardy R. E., Grobelny D., Foellmer H. G., Fernandez L. A. Inhibition of angiogenesis by the matrix metalloprotease inhibitor N-[2R-2-(hydroxamidocarbonymethyl)-4-methylpentanoyl)]-L-tryptophan methylamide. Cancer Res. 1994 Sep 1;54(17):4715–4718. [PubMed] [Google Scholar]
- Ganser G. L., Stricklin G. P., Matrisian L. M. EGF and TGF alpha influence in vitro lung development by the induction of matrix-degrading metalloproteinases. Int J Dev Biol. 1991 Dec;35(4):453–461. [PubMed] [Google Scholar]
- Gearing A. J., Beckett P., Christodoulou M., Churchill M., Clements J., Davidson A. H., Drummond A. H., Galloway W. A., Gilbert R., Gordon J. L. Processing of tumour necrosis factor-alpha precursor by metalloproteinases. Nature. 1994 Aug 18;370(6490):555–557. doi: 10.1038/370555a0. [DOI] [PubMed] [Google Scholar]
- Henriet P., Rousseau G. G., Eeckhout Y. Cloning and sequencing of mouse collagenase cDNA. Divergence of mouse and rat collagenases from the other mammalian collagenases. FEBS Lett. 1992 Sep 28;310(2):175–178. doi: 10.1016/0014-5793(92)81323-e. [DOI] [PubMed] [Google Scholar]
- Ingber D. E. Fibronectin controls capillary endothelial cell growth by modulating cell shape. Proc Natl Acad Sci U S A. 1990 May;87(9):3579–3583. doi: 10.1073/pnas.87.9.3579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lefebvre O., Wolf C., Limacher J. M., Hutin P., Wendling C., LeMeur M., Basset P., Rio M. C. The breast cancer-associated stromelysin-3 gene is expressed during mouse mammary gland apoptosis. J Cell Biol. 1992 Nov;119(4):997–1002. doi: 10.1083/jcb.119.4.997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li F., Strange R., Friis R. R., Djonov V., Altermatt H. J., Saurer S., Niemann H., Andres A. C. Expression of stromelysin-1 and TIMP-1 in the involuting mammary gland and in early invasive tumors of the mouse. Int J Cancer. 1994 Nov 15;59(4):560–568. doi: 10.1002/ijc.2910590421. [DOI] [PubMed] [Google Scholar]
- Li M. L., Aggeler J., Farson D. A., Hatier C., Hassell J., Bissell M. J. Influence of a reconstituted basement membrane and its components on casein gene expression and secretion in mouse mammary epithelial cells. Proc Natl Acad Sci U S A. 1987 Jan;84(1):136–140. doi: 10.1073/pnas.84.1.136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- López-De León A., Rojkind M. A simple micromethod for collagen and total protein determination in formalin-fixed paraffin-embedded sections. J Histochem Cytochem. 1985 Aug;33(8):737–743. doi: 10.1177/33.8.2410480. [DOI] [PubMed] [Google Scholar]
- Marbaix E., Donnez J., Courtoy P. J., Eeckhout Y. Progesterone regulates the activity of collagenase and related gelatinases A and B in human endometrial explants. Proc Natl Acad Sci U S A. 1992 Dec 15;89(24):11789–11793. doi: 10.1073/pnas.89.24.11789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matrisian L. M., Bowden G. T., Krieg P., Fürstenberger G., Briand J. P., Leroy P., Breathnach R. The mRNA coding for the secreted protease transin is expressed more abundantly in malignant than in benign tumors. Proc Natl Acad Sci U S A. 1986 Dec;83(24):9413–9417. doi: 10.1073/pnas.83.24.9413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matrisian L. M., Glaichenhaus N., Gesnel M. C., Breathnach R. Epidermal growth factor and oncogenes induce transcription of the same cellular mRNA in rat fibroblasts. EMBO J. 1985 Jun;4(6):1435–1440. doi: 10.1002/j.1460-2075.1985.tb03799.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matrisian L. M. Metalloproteinases and their inhibitors in matrix remodeling. Trends Genet. 1990 Apr;6(4):121–125. doi: 10.1016/0168-9525(90)90126-q. [DOI] [PubMed] [Google Scholar]
- Matrisian L. M. The matrix-degrading metalloproteinases. Bioessays. 1992 Jul;14(7):455–463. doi: 10.1002/bies.950140705. [DOI] [PubMed] [Google Scholar]
- McDonnell S., Navre M., Coffey R. J., Jr, Matrisian L. M. Expression and localization of the matrix metalloproteinase pump-1 (MMP-7) in human gastric and colon carcinomas. Mol Carcinog. 1991;4(6):527–533. doi: 10.1002/mc.2940040617. [DOI] [PubMed] [Google Scholar]
- McGeehan G. M., Becherer J. D., Bast R. C., Jr, Boyer C. M., Champion B., Connolly K. M., Conway J. G., Furdon P., Karp S., Kidao S. Regulation of tumour necrosis factor-alpha processing by a metalloproteinase inhibitor. Nature. 1994 Aug 18;370(6490):558–561. doi: 10.1038/370558a0. [DOI] [PubMed] [Google Scholar]
- Murphy G., Segain J. P., O'Shea M., Cockett M., Ioannou C., Lefebvre O., Chambon P., Basset P. The 28-kDa N-terminal domain of mouse stromelysin-3 has the general properties of a weak metalloproteinase. J Biol Chem. 1993 Jul 25;268(21):15435–15441. [PubMed] [Google Scholar]
- Nakanishi Y., Sugiura F., Kishi J., Hayakawa T. Collagenase inhibitor stimulates cleft formation during early morphogenesis of mouse salivary gland. Dev Biol. 1986 Jan;113(1):201–206. doi: 10.1016/0012-1606(86)90122-3. [DOI] [PubMed] [Google Scholar]
- Ostrowski L. E., Finch J., Krieg P., Matrisian L., Patskan G., O'Connell J. F., Phillips J., Slaga T. J., Breathnach R., Bowden G. T. Expression pattern of a gene for a secreted metalloproteinase during late stages of tumor progression. Mol Carcinog. 1988;1(1):13–19. doi: 10.1002/mc.2940010106. [DOI] [PubMed] [Google Scholar]
- Park A. J., Matrisian L. M., Kells A. F., Pearson R., Yuan Z. Y., Navre M. Mutational analysis of the transin (rat stromelysin) autoinhibitor region demonstrates a role for residues surrounding the "cysteine switch". J Biol Chem. 1991 Jan 25;266(3):1584–1590. [PubMed] [Google Scholar]
- Reponen P., Sahlberg C., Huhtala P., Hurskainen T., Thesleff I., Tryggvason K. Molecular cloning of murine 72-kDa type IV collagenase and its expression during mouse development. J Biol Chem. 1992 Apr 15;267(11):7856–7862. [PubMed] [Google Scholar]
- Richards D. A., Rodgers J. R., Supowit S. C., Rosen J. M. Construction and preliminary characterization of the rat casein and alpha-lactalbumin cDNA clones. J Biol Chem. 1981 Jan 10;256(1):526–532. [PubMed] [Google Scholar]
- Robinson S. D., Roberts A. B., Daniel C. W. TGF beta suppresses casein synthesis in mouse mammary explants and may play a role in controlling milk levels during pregnancy. J Cell Biol. 1993 Jan;120(1):245–251. doi: 10.1083/jcb.120.1.245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodgers W. H., Matrisian L. M., Giudice L. C., Dsupin B., Cannon P., Svitek C., Gorstein F., Osteen K. G. Patterns of matrix metalloproteinase expression in cycling endometrium imply differential functions and regulation by steroid hormones. J Clin Invest. 1994 Sep;94(3):946–953. doi: 10.1172/JCI117461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saarialho-Kere U. K., Pentland A. P., Birkedal-Hansen H., Parks W. C., Welgus H. G. Distinct populations of basal keratinocytes express stromelysin-1 and stromelysin-2 in chronic wounds. J Clin Invest. 1994 Jul;94(1):79–88. doi: 10.1172/JCI117351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchez-Lopez R., Nicholson R., Gesnel M. C., Matrisian L. M., Breathnach R. Structure-function relationships in the collagenase family member transin. J Biol Chem. 1988 Aug 25;263(24):11892–11899. [PubMed] [Google Scholar]
- Sapino A., Macrì L., Gugliotta P., Bussolati G. Immunocytochemical identification of proliferating cell types in mouse mammary gland. J Histochem Cytochem. 1990 Nov;38(11):1541–1547. doi: 10.1177/38.11.2212615. [DOI] [PubMed] [Google Scholar]
- Smith K. M., Lawn R. M., Wilcox J. N. Cellular localization of apolipoprotein D and lecithin:cholesterol acyltransferase mRNA in rhesus monkey tissues by in situ hybridization. J Lipid Res. 1990 Jun;31(6):995–1004. [PubMed] [Google Scholar]
- Stetler-Stevenson W. G., Aznavoorian S., Liotta L. A. Tumor cell interactions with the extracellular matrix during invasion and metastasis. Annu Rev Cell Biol. 1993;9:541–573. doi: 10.1146/annurev.cb.09.110193.002545. [DOI] [PubMed] [Google Scholar]
- Strange R., Li F., Saurer S., Burkhardt A., Friis R. R. Apoptotic cell death and tissue remodelling during mouse mammary gland involution. Development. 1992 May;115(1):49–58. doi: 10.1242/dev.115.1.49. [DOI] [PubMed] [Google Scholar]
- Streuli C. H., Bailey N., Bissell M. J. Control of mammary epithelial differentiation: basement membrane induces tissue-specific gene expression in the absence of cell-cell interaction and morphological polarity. J Cell Biol. 1991 Dec;115(5):1383–1395. doi: 10.1083/jcb.115.5.1383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Streuli C. H., Schmidhauser C., Bailey N., Yurchenco P., Skubitz A. P., Roskelley C., Bissell M. J. Laminin mediates tissue-specific gene expression in mammary epithelia. J Cell Biol. 1995 May;129(3):591–603. doi: 10.1083/jcb.129.3.591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stricklin G. P., Li L., Jancic V., Wenczak B. A., Nanney L. B. Localization of mRNAs representing collagenase and TIMP in sections of healing human burn wounds. Am J Pathol. 1993 Dec;143(6):1657–1666. [PMC free article] [PubMed] [Google Scholar]
- Sympson C. J., Talhouk R. S., Alexander C. M., Chin J. R., Clift S. M., Bissell M. J., Werb Z. Targeted expression of stromelysin-1 in mammary gland provides evidence for a role of proteinases in branching morphogenesis and the requirement for an intact basement membrane for tissue-specific gene expression. J Cell Biol. 1994 May;125(3):681–693. doi: 10.1083/jcb.125.3.681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talhouk R. S., Bissell M. J., Werb Z. Coordinated expression of extracellular matrix-degrading proteinases and their inhibitors regulates mammary epithelial function during involution. J Cell Biol. 1992 Sep;118(5):1271–1282. doi: 10.1083/jcb.118.5.1271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talhouk R. S., Chin J. R., Unemori E. N., Werb Z., Bissell M. J. Proteinases of the mammary gland: developmental regulation in vivo and vectorial secretion in culture. Development. 1991 Jun;112(2):439–449. doi: 10.1242/dev.112.2.439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka H., Hojo K., Yoshida H., Yoshioka T., Sugita K. Molecular cloning and expression of the mouse 105-kDa gelatinase cDNA. Biochem Biophys Res Commun. 1993 Feb 15;190(3):732–740. doi: 10.1006/bbrc.1993.1110. [DOI] [PubMed] [Google Scholar]
- Topper Y. J., Freeman C. S. Multiple hormone interactions in the developmental biology of the mammary gland. Physiol Rev. 1980 Oct;60(4):1049–1106. doi: 10.1152/physrev.1980.60.4.1049. [DOI] [PubMed] [Google Scholar]
- Williams J. M., Daniel C. W. Mammary ductal elongation: differentiation of myoepithelium and basal lamina during branching morphogenesis. Dev Biol. 1983 Jun;97(2):274–290. doi: 10.1016/0012-1606(83)90086-6. [DOI] [PubMed] [Google Scholar]
- Wilson C. L., Heppner K. J., Rudolph L. A., Matrisian L. M. The metalloproteinase matrilysin is preferentially expressed by epithelial cells in a tissue-restricted pattern in the mouse. Mol Biol Cell. 1995 Jul;6(7):851–869. doi: 10.1091/mbc.6.7.851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witty J. P., Lempka T., Coffey R. J., Jr, Matrisian L. M. Decreased tumor formation in 7,12-dimethylbenzanthracene-treated stromelysin-1 transgenic mice is associated with alterations in mammary epithelial cell apoptosis. Cancer Res. 1995 Apr 1;55(7):1401–1406. [PubMed] [Google Scholar]
- Woessner J. F., Jr, Taplin C. J. Purification and properties of a small latent matrix metalloproteinase of the rat uterus. J Biol Chem. 1988 Nov 15;263(32):16918–16925. [PubMed] [Google Scholar]
- Wright J. H., McDonnell S., Portella G., Bowden G. T., Balmain A., Matrisian L. M. A switch from stromal to tumor cell expression of stromelysin-1 mRNA associated with the conversion of squamous to spindle carcinomas during mouse skin tumor progression. Mol Carcinog. 1994 Aug;10(4):207–215. doi: 10.1002/mc.2940100405. [DOI] [PubMed] [Google Scholar]