Abstract
Objective
To assess the efficacy and safety of everolimus in Japanese patients with metastatic renal cell carcinoma.
Methods
A subgroup analysis of the pivotal Phase III, randomized, double-blind, placebo-controlled trial of everolimus 10 mg/day in patients with disease progression after treatment with sorafenib, sunitinib or both assessed outcomes in Japanese participants. Results were compared with those for the overall study population.
Results
The final trial analysis included 24 Japanese patients (everolimus, n= 15; placebo, n = 9). Median progression-free survival in the Japanese subpopulation was 5.75 months (95% confidence interval, 4.90 months to not reached) with everolimus and 3.61 months (95% confidence interval, 1.91–9.03 months) with placebo (hazard ratio, 0.19; 95% confidence interval, 0.05–0.83). Median overall survival was not reached with everolimus and was 14.9 months (95% confidence interval, 11.0–16.8 months) with placebo (hazard ratio, 0.30; 95% confidence interval, 0.07–1.27). Overall, efficacy and safety were similar when comparing the Japanese and overall populations. In the Japanese subpopulation, the most common adverse events with everolimus were stomatitis, infections and rash. Four Japanese subjects (27%) developed Grade 1 (n = 2) or 2 (n = 2) pneumonitis (all reversible and allowing for continuation of therapy, after interruption, steroids and dose reduction for both Grade 2 cases), with a lower pneumonitis incidence of 14% in the overall population (albeit associated with a Grade 3 incidence of 4%).
Conclusions
These findings suggest that the demonstrated benefits of everolimus in the overall trial population are similar in Japanese patients with metastatic renal cell carcinoma.
Keywords: everolimus, renal cell carcinoma, mTOR
INTRODUCTION
The incidence of renal cell carcinoma (RCC) in Japan is increasing. Results of a survey conducted from January 2002 through December 2002 in all 47 prefectures revealed the crude incidence rates of RCC to be ∼8.2 males and 3.6 females per 100 000 persons, an increase of ∼1000 individuals compared with results obtained in a similarly conducted 1997 survey (1). In general, approximately one-third of patients with RCC present with metastatic disease (mRCC), which has a 5 year survival rate of 10% (2).
The vascular endothelial growth factor receptor-tyrosine kinase inhibitors (VEGFr-TKI) sorafenib and sunitinib are now approved in Japan, Europe and the USA for the treatment of unresectable or metastatic RCC based on their demonstrated benefit in clinical trials (3,4). These targeted agents have become the standard therapy for mRCC. Until recently, however, no standard therapy existed for patients with mRCC that progressed after treatment with VEGFr-TKI-targeted agents.
Everolimus is an orally administered inhibitor of the mammalian target of rapamycin (mTOR) and was approved by the United States Food & Drug Administration (US FDA) in March 2009, the European Medicines Agency (EMEA) in August 2009 and in Japan in January 2010 for the treatment of unresectable or metastatic RCC. mTOR is a cytoplasmic serine/threonine kinase that acts as an integration point for three key inputs: (i) extracellular stimulation by growth factors including VEGF; (ii) nutrient availability; and (iii) intracellular energy status. The convergence of these upstream signals, combined with positive and negative feedback mechanisms, determines whether mTOR is activated. Once activated, mTOR initiates a downstream cascade that triggers the cell's translational machinery to produce proteins required for a variety of cellular functions, including metabolism, growth, proliferation and angiogenesis. Dysregulation of signaling elements both upstream and downstream of mTOR has been implicated in many cancers (5–8).
In a Phase II trial, everolimus demonstrated antitumor activity in patients with mRCC who experienced disease progression after treatment with cytokines, chemotherapy or erlotinib and bevacizumab (9). Everolimus was subsequently evaluated in RECORD-1 (Renal Cell cancer treatment with Oral RAD001 given Daily), the pivotal, Phase III, randomized, placebo-controlled trial of patients with mRCC who had progressed on VEGFr-TKI. Results of this trial, which enrolled patients from 10 countries, including Japan, led to the US FDA and EMEA approval of everolimus. RECORD-1 demonstrated that treatment with everolimus plus best supportive care (BSC) prolonged progression-free survival (PFS) compared with placebo plus BSC (4.9 vs. 1.9 months, respectively; P < 0.001; hazard ratio [HR], 0.33; 95% confidence interval [CI], 0.25–0.43) (10). Consequently, everolimus represents a viable treatment option for patients with VEGFr-TKI-refractory disease.
Results of a Phase I clinical and pharmacokinetic study of everolimus in previously treated Japanese patients with advanced solid tumors demonstrated that its pharmacokinetics and tolerability were similar to those observed in previous studies with large populations of Caucasian patients, for whom the most common drug-related toxicities included rash, stomatitis and fatigue. No dose-limiting toxicities were noted (11). The current analysis was initiated to assess the efficacy and safety of everolimus in the Japanese subgroup of patients who participated in RECORD-1.
PATIENTS AND METHODS
Patients
The RECORD-1 inclusion and exclusion criteria have been described in detail previously (12). Briefly, patients ≥18 years of age were eligible for enrollment if they had been diagnosed with metastatic carcinoma and had histologic or cytologic confirmation of clear cell RCC, had measurable disease, showed disease progression on or within 6 months of treatment with sunitinib, sorafenib or both, and exhibited adequate Karnofsky performance status (≥70%), blood counts and serum chemistry. Prior therapy with bevacizumab and cytokines was permitted. Written informed consent was obtained from each patient before screening procedures were initiated.
Study Design
RECORD-1 was a prospective, randomized, double-blind, placebo-controlled, international, multicenter, parallel-group Phase III trial (NCT00410124). It contained five phases: (i) screening/baseline; (ii) blinded treatment; (iii) open-label treatment; (iv) follow-up; and (v) extension treatment. The study design incorporated two planned interim analyses and a final analysis. The protocol specified that the interim analyses were to be carried out when ∼30 and 60% of the 290 PFS events (per central radiology) required for the final analysis had been reached. The protocol and all amendments issued prior to or during the study were reviewed by the local independent ethics committee or institutional review board for each center, and the study was conducted according to the ethical principles of the Declaration of Helsinki.
Patients were randomized in a 2:1 ratio to receive everolimus 10 mg/day (n = 277) or matching placebo (n = 139) in conjunction with BSC. Randomization was stratified by the number of prior VEGFr-TKI therapies (1 or 2) and Memorial Sloan-Kettering Cancer Center (MSKCC) prognostic criteria for patients with previously treated mRCC (favorable, intermediate or poor) (10,13). Treatment cycles were 28 days in length.
Patients continued on blinded treatment until tumor progression or unacceptable toxicity, death or discontinuation for any other reason. Dose modifications were permitted for clinically significant hematologic or other adverse events, as described previously (12). Patients randomized to receive placebo who demonstrated evidence of progression by investigator assessment were unblinded and permitted to cross over to receive open-label everolimus (12).
Efficacy Analyses
The primary efficacy endpoint was PFS by central review. Tumor response was assessed at scheduled intervals with the Response Evaluation Criteria in Solid Tumors (RECIST) (14), based on imaging studies by the investigators and independent central radiology review (12). Secondary efficacy endpoints included overall survival (OS) and tumor response.
Safety Analyses
Safety analyses were carried out as described previously (12). Briefly, all adverse events were monitored and recorded, and laboratory parameters, vital signs, physical examinations and concomitant therapies were assessed regularly and recorded. The National Cancer Institute's Common Terminology Criteria (NCI-CTC) version 3.0 were used to grade adverse events and laboratory abnormalities (12). To detect radiologic lung changes suggestive of pneumonitis, a central radiology review of chest computed tomography scans and chest X-rays was performed.
Statistical Analyses
The full analysis set included all randomized patients; the safety population consisted of all patients who received at least one dose of study drug and who had at least one post-baseline safety assessment.
PFS and OS curves in each treatment group were estimated with Kaplan–Meier analysis. HR for the Japanese subpopulation was obtained from an unstratified Cox proportional hazard model. As previously reported for the overall population (12), PFS and OS were statistically compared among the groups with a stratified, one-sided log-rank test, adjusting for strata defined by the MSKCC risk criteria. HRs were obtained from a stratified Cox proportional hazards model, using the strata defined by the MSKCC risk criteria.
The blinded phase of RECORD-1 was stopped on 28 February 2008, based on the efficacy of everolimus shown in the second interim analysis (10). Compared with data for the second interim analysis (collected up to 15 October 2007; 191 PFS events), data for the final analysis were based on 75 additional PFS events, 6 additional patients accrued and 4.5 months of additional blinded follow-up. The cutoff date of follow-up for OS was 15 November 2008.
RESULTS
Patient Demographics and Disposition
Of the 416 patients randomized to treatment in the overall trial, the subpopulation of Japanese patients in the final analysis included 15 patients who received everolimus and 9 patients who received placebo. The baseline characteristics and prior therapies of these 24 Japanese patients and the 416-patient overall population are shown in Table 1 (10). The Japanese subpopulation was 79% male and the median age was 63.5 years. The Japanese subpopulation was similar to the overall trial population, with the exceptions that the predominant VEGFr-TKI therapy of the Japanese patients was sorafenib instead of sunitinib and that almost all Japanese patients had received prior treatment with interferon.
Table 1.
Baseline characteristics and prior therapies in RECORD-1 (10)
| Characteristic | Overall population |
Japanese patients |
||
|---|---|---|---|---|
| Everolimus (n = 277) | Placebo (n = 139) | Everolimus (n = 15) | Placebo (n = 9) | |
| Sex, n (%) | ||||
| Female | 61 (22) | 33 (24) | 1 (7) | 4 (44) |
| Male | 216 (78) | 106 (76) | 14 (93) | 5 (56) |
| Median age (range), year | 61 (27–85) | 60 (29–79) | 65 (48–77) | 62 (46–73) |
| KPS, ≥90/≤80, % | 64/36 | 68/32 | 80/20 | 100/0 |
| % MSKCC risk | ||||
| Favorable/intermediate/poor | 29/56/14 | 28/57/15 | 33/60/7 | 44/44/11 |
| Sites of metastases, % | ||||
| Lung | 73 | 81 | 87 | 89 |
| Bone | 37 | 30 | 33 | 11 |
| Liver | 33 | 38 | 20 | 33 |
| Prior therapies, % | ||||
| Nephrectomy | 97 | 96 | 100 | 100 |
| Radiotherapy | 31 | 27 | 20 | 11 |
| VEGFr-TKI therapy | ||||
| Sunitinib | 45 | 43 | 13 | 22 |
| Sorafenib | 29 | 31 | 80 | 78 |
| Both | 26 | 26 | 7 | 0 |
| Other systemic therapy | ||||
| Immunotherapy | 65 | 67 | 100 | 89 |
| Chemotherapy | 13 | 16 | 13 | 11 |
| Hormone therapy | 2 | 4 | 0 | 0 |
| Other | 5 | 3 | 0 | 0 |
KPS, Karnofsky performance status; MSKCC, Memorial Sloan-Kettering Cancer Center; VEGFr-TKI, vascular endothelial growth factor receptor-tyrosine kinase inhibitor.
By the end of the double-blind phase, five Japanese patients in the everolimus group had discontinued (disease progression, n = 4; withdrew consent, n = 1) and eight patients in the placebo group had discontinued (all due to disease progression). These eight patients from the placebo group then crossed over to receive open-label everolimus. All 24 Japanese patients were included in the full analysis set and in the safety population. In the overall population, 202 patients in the everolimus group and 133 patients in the placebo group had discontinued therapy, with the primary reasons being disease progression and adverse events in the everolimus group (n = 137 and n = 36, respectively) and disease progression and death for the placebo group (n = 124 and n = 4, respectively).
Treatment Administration
The median duration of treatment in the Japanese subpopulation was 135 days in the everolimus group and 96 days in the placebo group (overall population: 141 and 60 days, respectively). The median cumulative dose of everolimus was 1160 mg, the median dose intensity was 9.9 mg/day and the median relative dose intensity was 0.99 (overall population: 1252.5 mg, 10.0 mg/day and 1.0, respectively). In the Japanese subpopulation, dose reduction and/or interruption was necessary in eight patients in the everolimus group (53.3%; for five of eight patients, this was due to an adverse event) and in no patients in the placebo group; corresponding rates for the overall population were 46 and 15%, respectively, most commonly for an adverse event in both groups (39 and 9%, respectively).
Efficacy Assessment
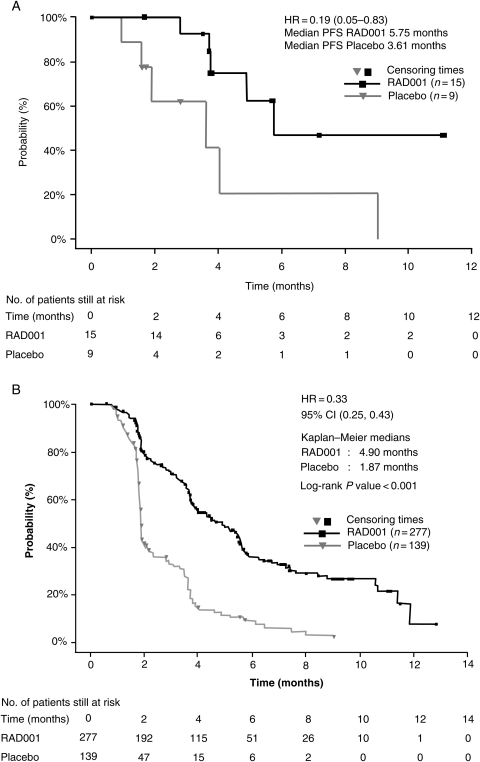
Everolimus appeared to prolong PFS compared with placebo in the Japanese subpopulation. By independent central radiology review, median PFS was 5.75 months (95% CI, 4.90 months to not reached) with everolimus and 3.61 months (95% CI, 1.91–9.03 months) with placebo (Fig. 1A). The HR was 0.19, with a 95% CI of 0.05–0.83. In the overall population, median PFS was 4.90 months (95% CI, 3.98–5.52 months) with everolimus and 1.87 months (95% CI, 1.84–1.94 months) with placebo, translating into a highly significant HR of 0.33 (95% CI, 0.25–0.43; P < 0.001; Fig. 1B).
Figure 1.
Median progression-free survival in the (A) Japanese subpopulation and (B) overall population (10) of RECORD-1 by central radiology review. Figure 1B reprinted with permission from Wiley & Sons. Copyright 2010. All rights reserved (10).
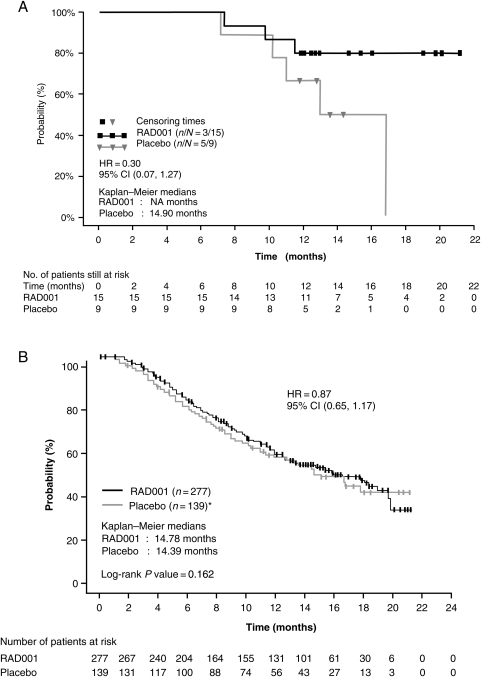
Median OS in the Japanese subpopulation was not reached in the everolimus group and was 14.9 months (95% CI, 11.0–16.8 months) in the placebo group at the cutoff date of 15 November 2008 (Fig. 2A). The HR was 0.30, with a 95% CI of 0.07–1.27. There were no on-treatment deaths among Japanese patients in either treatment group. One Japanese patient who had been treated with everolimus died during the follow-up period, over 6 months after the last dose. In the overall population, median OS was 14.8 months with everolimus and 14.4 months with placebo, translating into a non-significant HR of 0.87 (95% CI, 0.65–1.17; P = 0.16; Fig. 2B) that was influenced by the 80% rate of crossover from placebo to open-label everolimus (10).
Figure 2.
Median overall survival in the (A) Japanese subpopulation and (B) overall population (10) of RECORD-1. Figure 2B reprinted with permission from Wiley & Sons. Copyright 2010. All rights reserved (10).
The best overall response based on central radiologic assessment was stable disease (SD) in 14 of 15 patients who received everolimus (the response of 1 patient was unknown) and SD in 6 of 9 patients who received placebo (the other 3 patients had progressive disease). No patient achieved a partial response (PR) at the final analysis, but in a subsequent efficacy assessment made in an open-label extension phase only for Japanese subjects, one patient achieved a PR as an investigator-assessed best overall response. In the overall population, the SD rates were 185 of 277 (67%) with everolimus and 45 of 139 (32%) with placebo, with an additional 5 patients in the everolimus group (1.8%) achieving a PR.
Safety
As summarized in Table 2, stomatitis, infections and rash were the predominant adverse events among Japanese patients in the everolimus group during the blinded-study phase, with incidences that were notably higher than the incidences in the overall population. Most adverse events were mild (Grade 1) to moderate (Grade 2) in severity and were resolved with dose interruption and/or reduction. In the Japanese subpopulation, there were two reports of Grade 2 interstitial lung disease and two reports of Grade 1 pneumonitis during everolimus therapy, for a total on-treatment occurrence of pneumonitis (based on grouped terms) of 27%. The corresponding pneumonitis incidence among the everolimus group in the overall population was 14%, including Grade 1/2 (n = 27) as well as Grade 3 (n = 10) events. None of the Japanese patients stopped therapy permanently because of pneumonitis. With respect to the two Japanese cases of Grade 2 pneumonitis (reported as interstitial lung disease), study treatment was interrupted, corticosteroid therapy was instituted and everolimus was resumed at a reduced dose, with eventual resolution of the interstitial lung disease. Despite increased reporting of non-infectious pneumonitis for Japanese patients compared with the overall study population, central review of chest computed tomography scans for radiologic lung changes suggestive of pneumonitis found a similar incidence of radiologic changes in the Japanese and the overall population.
Table 2.
Incidence of adverse events, irrespective of relationship to treatment and laboratory abnormalities in the Japanese subpopulation of RECORD-1
| Overall population |
Japanese patients |
|||||||
|---|---|---|---|---|---|---|---|---|
| Everolimus (n = 274), n (%) |
Placebo (n = 137), n (%) |
Everolimus (n = 15), n (%) |
Placebo (n = 9), n (%) |
|||||
| All Grades | Grade 3/4 | All Grades | Grade 3/4 | All Grades | Grade 3/4 | All Grades | Grade 3/4 | |
| Adverse eventa (total) | 265 (97) | 142 (52)/36 (13) | 128 (93) | 32 (23)/7 (5) | 15 (100) | 6 (40)/0 | 5 (56) | 0/0 |
| Stomatitisb | 120 (44) | 11 (4)/1 (<1) | 11 (8) | 0/0 | 11 (73) | 0/0 | 1 (11) | 0/0 |
| Infectionsc | 101 (37) | 19 (7)/7 (3) | 25 (18) | 2 (1)/0 | 10 (67) | 1 (7)/0 | 2 (22) | 0/0 |
| Rash | 80 (29) | 3 (1)/0 | 9 (7) | 0/0 | 10 (67) | 0/0 | 0 | 0/0 |
| Dysgeusia | 28 (10) | 0/0 | 3 (2) | 0/0 | 7 (47) | 0/0 | 0 | 0/0 |
| Epistaxis | 49 (18) | 0/0 | 0 | 0/0 | 6 (40) | 0/0 | 0 | 0/0 |
| Diarrhea | 81 (30) | 4 (1)/0 | 9 (7) | 0/0 | 6 (40) | 0/0 | 0 | 0/0 |
| Cough | 82 (30) | 2 (<1)/0 | 22 (16) | 0/0 | 5 (33) | 0/0 | 2 (22) | 0/0 |
| Edema peripheral | 68 (25) | 2 (<1)/0 | 11 (8) | 1 (<1)/0 | 5 (33) | 0/0 | 0 | 0/0 |
| Pneumonitisd | 37 (14) | 10 (4)/0 | 0 | 0/0 | 4 (27) | 0/0 | 0 | 0/0 |
| Nail disorder | 14 (5) | 0/0 | 0 | 0/0 | 4 (27) | 0/0 | 0 | 0/0 |
| Constipation | 53 (19) | 1 (<1)/0 | 24 (18) | 1 (<1)/0 | 4 (27) | 0/0 | 1 (11) | 0/0 |
| Anorexia | 69 (25) | 4 (1)/0 | 19 (14) | 1 (<1)/0 | 3 (20) | 0/0 | 0 | 0/0 |
| Cheilitis | 4 (1) | 0/0 | 0 | 0/0 | 3 (20) | 0/0 | 0 | 0/0 |
| Eyelid edema | 11 (4) | 0/0 | 0 | 0/0 | 3 (20) | 0/0 | 0 | 0/0 |
| Arthralgia | 28 (10) | 3 (1)/0 | 14 (10) | 2 (1)/0 | 3 (20) | 0/0 | 1 (11) | 0/0 |
| Hemorrhoid | 15 (6) | 0/0 | 1 (<1) | 0/0 | 3 (20) | 0/0 | 0 | 0/0 |
| Nausea | 72 (26) | 4 (1)/0 | 26 (19) | 0/0 | 3 (20) | 0/0 | 0 | 0/0 |
| Vomiting | 56 (20) | 6 (2)/0 | 16 (12) | 0/0 | 3 (20) | 0/0 | 1 (11) | 0/0 |
| Fatigue | 84 (31) | 15 (6)/0 | 37 (27) | 4 (3)/1 (<1) | 3 (20) | 0/0 | 1 (11) | 0/0 |
| Pyrexia | 54 (20) | 2 (<1)/0 | 12 (9) | 0/0 | 3 (20) | 0/0 | 0 | 0/0 |
| Laboratory abnormality | ||||||||
| Hematology | ||||||||
| Hemoglobin decreased | 253 (92) | 33 (12)/3 (1) | 108 (79) | 7 (5)/1 (<1) | 14 (93) | 0/0 | 6 (67) | 0/0 |
| Lymphocytes decreased | 139 (51) | 43 (16)/6 (2) | 39 (28) | 7 (5)/0 | 6 (40) | 2 (13)/0 | 3 (33) | 0/0 |
| Platelets decreased | 64 (23) | 3 (1)/0 | 3 (2) | 0/1 (<1) | 4 (27) | 1 (7)/0 | 0 | 0/0 |
| Neutrophils decreased | 37 (14) | 0/1 (<1) | 5 (4) | 0/0 | 4 (27) | 0/0 | 1 (11) | 0/0 |
| Biochemistry | ||||||||
| Cholesterol increased | 212 (77) | 12 (4)/0 | 48 (35) | 0/0 | 13 (87) | 0/0 | 7 (78) | 0/0 |
| Triglycerides increased | 200 (73) | 2 (<1)/0 | 46 (34) | 0/0 | 9 (60) | 1 (7)/0 | 4 (44) | 0/0 |
| Glucose increased | 157 (57) | 42 (15)/1 (<1) | 34 (25) | 2 (1)/0 | 8 (53) | 1 (7)/0 | 0 | 0/0 |
| Creatinine increased | 137 (50) | 4 (1)/0 | 46 (34) | 0/0 | 6 (40) | 0/0 | 3 (33) | 0/0 |
| Phosphate decreased | 102 (37) | 17 (6)/0 | 11 (8) | 0/0 | 6 (40) | 0/0 | 2 (22) | 0/0 |
| Aspartate transaminase increased | 68 (25) | 1 (<1)/1 (<1) | 9 (7) | 0/0 | 4 (27) | 0/0 | 0 | 0/0 |
| Alanine transaminase increased | 58 (21) | 3 (1)/0 | 5 (4) | 0/0 | 5 (33) | 0/0 | 0 | 0/0 |
| Bilirubin increased | 8 (3) | 2 (<1)/1 (<1) | 3 (2) | 0/0 | 0 | 0/0 | 0 | 0/0 |
aIncludes events that occurred in ≥3 patients in the Japanese subpopulation and corresponding data for the overall population. Data for the overall population are not all inclusive, only capturing the most common events in the Japanese subpopulation.
bIncludes aphthous stomatitis, mouth ulceration and tongue ulceration.
cIncludes all infections.
dIncludes interstitial lung disease, lung infiltration, pneumonitis, pulmonary alveolar hemorrhage, alveolitis, pneumopathy and pulmonary toxicity.
Treatment-related Grade 3/4 adverse events were infrequently reported, with no specific type reported in >1 patient in the Japanese subpopulation (all of which were Grade 3) and incidences of individual events typically <5% in the overall population (with a few exceptions with respect to laboratory abnormalities). No Grade 4 adverse events were reported. In the Japanese subpopulation, no adverse event led to study discontinuation in the double-blind phase, whereas the adverse event-related discontinuation rates in the overall population were 13.9 and 2.9% for the everolimus and placebo groups, respectively. The most common adverse events resulting in everolimus discontinuation were dyspnea and pneumonitis (n = 7 [3.6%] for each).
DISCUSSION
The results of RECORD-1 established that daily treatment with oral everolimus prolongs PFS in patients with mRCC that has progressed on VEGFr-TKI therapies and generally is well tolerated, fulfilling an unmet medical need in this patient population. The results of this subgroup analysis of patients in RECORD-1 suggest that a similar benefit is expected for Japanese patients.
The Japanese subpopulation analysis was limited by the small number of patients in the trial (n = 24); however, the PFS results in the Japanese subgroup aligned with the PFS results obtained in the overall trial population. It was speculated that the longer PFS of both everolimus and placebo groups in the Japanese subpopulation compared with the overall population may have been caused by the better overall condition of patients enrolled in the study. Although OS was likely confounded by the crossover design of the trial, results trended toward a benefit for everolimus compared with placebo, similar to findings in the overall trial population.
In addition, the types of adverse events occurring in the Japanese patients were similar to those occurring in the overall trial population. The most common event in the everolimus group was rash, followed in decreasing order by stomatitis, dysgeusia, diarrhea, epistaxis, cough and peripheral edema. These events also occurred with a relatively high frequency (≥10% incidence) in the overall population of RECORD-1. The pharmacokinetic profile of everolimus in a Phase I study in previously treated Japanese patients with advanced solid tumors was similar to that observed in previous studies with large populations of Caucasian patients (11), suggesting no difference in treatment exposure between these two ethnic populations. The increased incidence of adverse events may be due to differences in ethnicity, stricter investigation of the Japanese physicians in identifying adverse events, or the small number of Japanese patients in this study. Most adverse events were mild/moderate in severity and there was no evidence of worsening adverse events. The majority of adverse events requiring treatment resolved after dose interruption and/or reduction, and patients were able to continue receiving everolimus.
Non-infectious pneumonitis is a known class effect of rapamycin and its derivatives, possibly representing a hypersensitivity reaction; however, its etiology has not been fully characterized (15,16). A diagnosis of non-infectious pneumonitis should be considered in patients presenting with non-specific respiratory signs and symptoms (to include pyrexia, cough or dyspnea) and in whom infectious, neoplastic and other non-medicinal causes have been excluded by appropriate investigation. According to the data reported here for everolimus in mRCC, the incidence of non-infectious pneumonitis was increased among Japanese patients, at 27 vs. 11% for the overall population; the impact of the small sample size on this disparate finding is unknown. However, it was noted during the central review of results that the radiological changes observed did not support the higher incidence of non-infectious pneumonitis reported in Japanese when compared with the overall population. Nonetheless, it is encouraging that the four Japanese cases were limited to Grade 1/2 severity and all were successfully managed, resulting in the resolution of the toxicity and the ability to continue treatment (with treatment interruption and dose reduction for the 2 patients with Grade 2 events).
In conclusion, the results of this subgroup analysis suggest that the benefits of everolimus in Japanese patients with mRCC are similar to those observed in the overall pivotal Phase III trial population. These findings, along with those of previous studies of everolimus in Japanese patients, suggest that everolimus is a valuable treatment option for Japanese patients with mRCC that has progressed on VEGFr-TKI therapy.
Funding
Financial support for medical editorial assistance was provided by Novartis Pharmaceuticals.
Conflict of interest statement
Drs Takeshi Tajima, Akio Kasuga, Yoshie Fujita are employed by Novartis Pharma K.K. Dr Andrea Kay is employed by and owns stock in Novartis Pharma K.K. Drs Hideyuki Akaza, Hiro-omi Kanayama, Hirotsugu Uemura and Nobuo Shinohara declare the following potential conflict of interest: Medical advisor of everolimus on Novartis Pharma K. K. (compensated); Seminar presentation in the seminar hosted by Novartis Pharma K.K. (compensated).
Acknowledgements
We thank Amy Zannikos for editorial assistance with this manuscript.
Appendix
In addition to the authors listed in the author field, following are the authors who contributed equally to this study.
Takeshi Tajima, Akio Kasuga, Yoshie Fujita: Novartis Pharma K.K, Tokyo, Japan.
Andrea Kay: Novartis Pharma Corporation, Florham Park, NJ, USA.
References
- 1.Marumo K, Kanayama H, Miyao N, Nakazawa H, Ozono S, Horie S, et al. Prevalence of renal cell carcinoma: A nation-wide survey in Japan, 2002. Int J Urol. 2007;14:479–82. doi: 10.1111/j.1442-2042.2007.01739.x. doi:10.1111/j.1442-2042.2007.01739.x. [DOI] [PubMed] [Google Scholar]
- 2.Molina AM, Motzer RJ. Current algorithms and prognostic factors in the treatment of metastatic renal cell carcinoma. Clin Genitourin Cancer. 2008;6:s7–13. doi: 10.3816/CGC.2008.s.002. doi:10.3816/CGC.2008.s.002. [DOI] [PubMed] [Google Scholar]
- 3.Motzer RJ, Hutson TE, Tomczak P, Michaelson MD, Bukowski RM, Rixe O, et al. Sunitinib versus interferon alfa in metastatic renal cell carcinoma. N Engl J Med. 2007;356:115–24. doi: 10.1056/NEJMoa065044. doi:10.1056/NEJMoa065044. [DOI] [PubMed] [Google Scholar]
- 4.Escudier B, Eisen T, Stadler WM, Szczylik C, Oudard S, Siebels M, et al. for the TARGET Study Group. Sorafenib in advanced clear-cell renal cell carcinoma. N Engl J Med. 2007;356:125–34. doi: 10.1056/NEJMoa060655. doi:10.1056/NEJMoa060655. [DOI] [PubMed] [Google Scholar]
- 5.Abraham RT, Gibbons JJ. The mammalian target of rapamycin signaling pathway: twists and turns in the road to cancer therapy. Clin Cancer Res. 2007;13:3109–14. doi: 10.1158/1078-0432.CCR-06-2798. doi:10.1158/1078-0432.CCR-06-2798. [DOI] [PubMed] [Google Scholar]
- 6.Albanell J, Dalmases A, Rovira A, Rojo F. mTOR signalling in human cancer. Clin Transl Oncol. 2007;9:484–93. doi: 10.1007/s12094-007-0092-6. doi:10.1007/s12094-007-0092-6. [DOI] [PubMed] [Google Scholar]
- 7.Dancey JE. Therapeutic targets: MTOR and related pathways. Cancer Biol Ther. 2006;5:1065–73. doi: 10.4161/cbt.5.9.3175. [DOI] [PubMed] [Google Scholar]
- 8.Jiang B-H, Liu L-Z. Role of mTOR in anticancer drug resistance: perspectives for improved drug treatment. Drug Resist Updat. 2008;11:63–76. doi: 10.1016/j.drup.2008.03.001. doi:10.1016/j.drup.2008.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jac J, Giessinger S, Khan M, Willis J, Chiang S, Amato R. A phase II trial of RAD001 in patients (pts) with metastatic renal cell carcinoma (MRCC) J Clin Oncol. 2007;25(Suppl 18):261S. Abstract 5107. [Google Scholar]
- 10.Motzer RJ, Escudier B, Oudard S, Hutson TE, Porta C, Bracarda S, et al. for the RECORD-1 Study Group. Phase 3 trial of everolimus for metastatic renal cell carcinoma: final results and analysis of prognostic factors. Cancer. 2010;116:4256–65. doi: 10.1002/cncr.25219. [DOI] [PubMed] [Google Scholar]
- 11.Okamoto I, Doi T, Ohtsu A, Miyazaki M, Tsuya A, Kurei K, et al. Phase I clinical and pharmacokinetic study of RAD001 (everolimus) administered daily to Japanese patients with advanced solid tumors. Jpn J Clin Oncol. 2010;41:17–23. doi: 10.1093/jjco/hyp120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Motzer RJ, Escudier B, Oudard S, Hutson TE, Porta C, Bracarda S, et al. for the RECORD-1 Study Group. Efficacy of everolimus in advanced renal cell carcinoma: a double-blind, randomised, placebo-controlled phase III trial. Lancet. 2008;372:449–56. doi: 10.1016/S0140-6736(08)61039-9. doi:10.1016/S0140-6736(08)61039-9. [DOI] [PubMed] [Google Scholar]
- 13.Motzer RJ, Bacik J, Schwartz LH, Reuter V, Russo P, Marion S, et al. Prognostic factors for survival in previously treated patients with metastatic renal cell carcinoma. J Clin Oncol. 2004;22:454–63. doi: 10.1200/JCO.2004.06.132. doi:10.1200/JCO.2004.06.132. [DOI] [PubMed] [Google Scholar]
- 14.Therasse P, Arbuck SG, Eisenhauer EA, Wanders J, Kaplan RS, Rubinstein L, et al. New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst. 2000;92:205–16. doi: 10.1093/jnci/92.3.205. doi:10.1093/jnci/92.3.205. [DOI] [PubMed] [Google Scholar]
- 15.Champion L, Stern M, Israël-Biet D, Mamzer-Bruneel MF, Peraldi MN, Kreis H, et al. Brief communication: sirolimus-associated pneumonitis: 24 cases in renal transplant recipients. Ann Intern Med. 2006;144:505–9. doi: 10.7326/0003-4819-144-7-200604040-00009. [DOI] [PubMed] [Google Scholar]
- 16.Morelon E, Stern M, Israël-Biet D, Correas JM, Danel C, Mamzer-Bruneel MF, et al. Characteristics of sirolimus-associated interstitial pneumonitis in renal transplant patients. Transplantation. 2001;72:787–90. doi: 10.1097/00007890-200109150-00008. doi:10.1097/00007890-200109150-00008. [DOI] [PubMed] [Google Scholar]