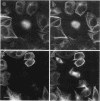
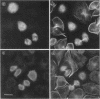
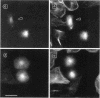
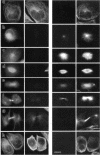
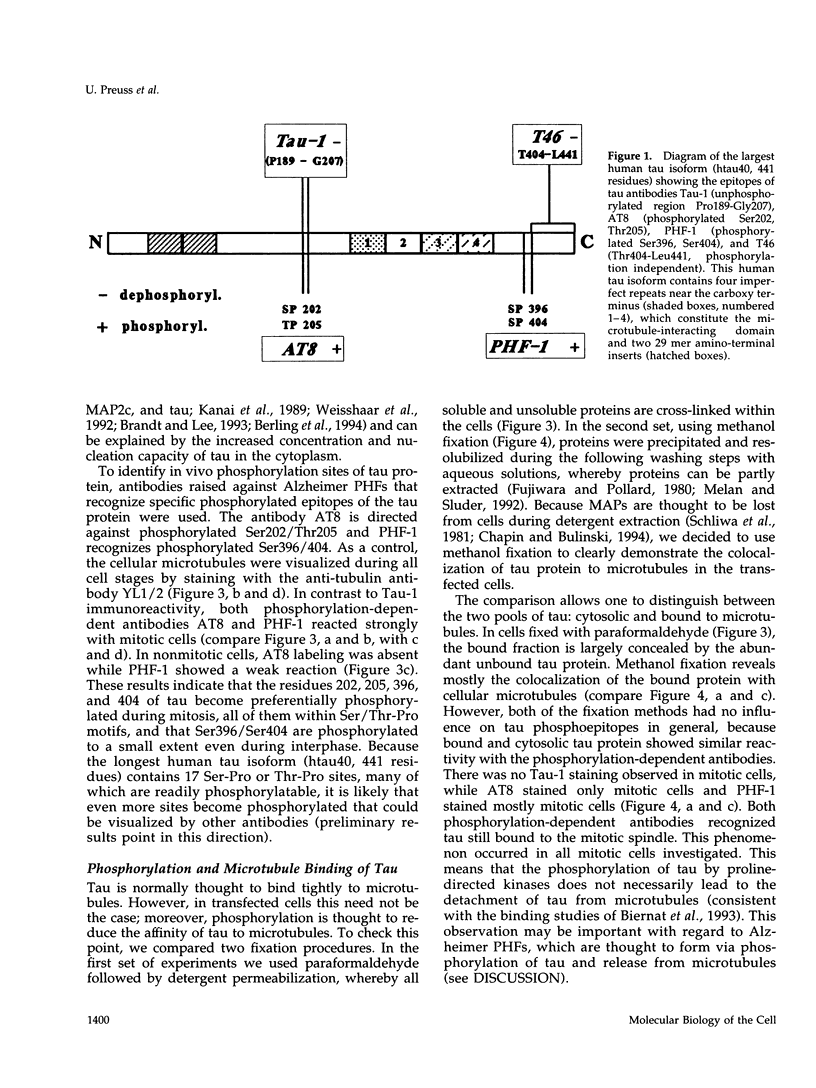
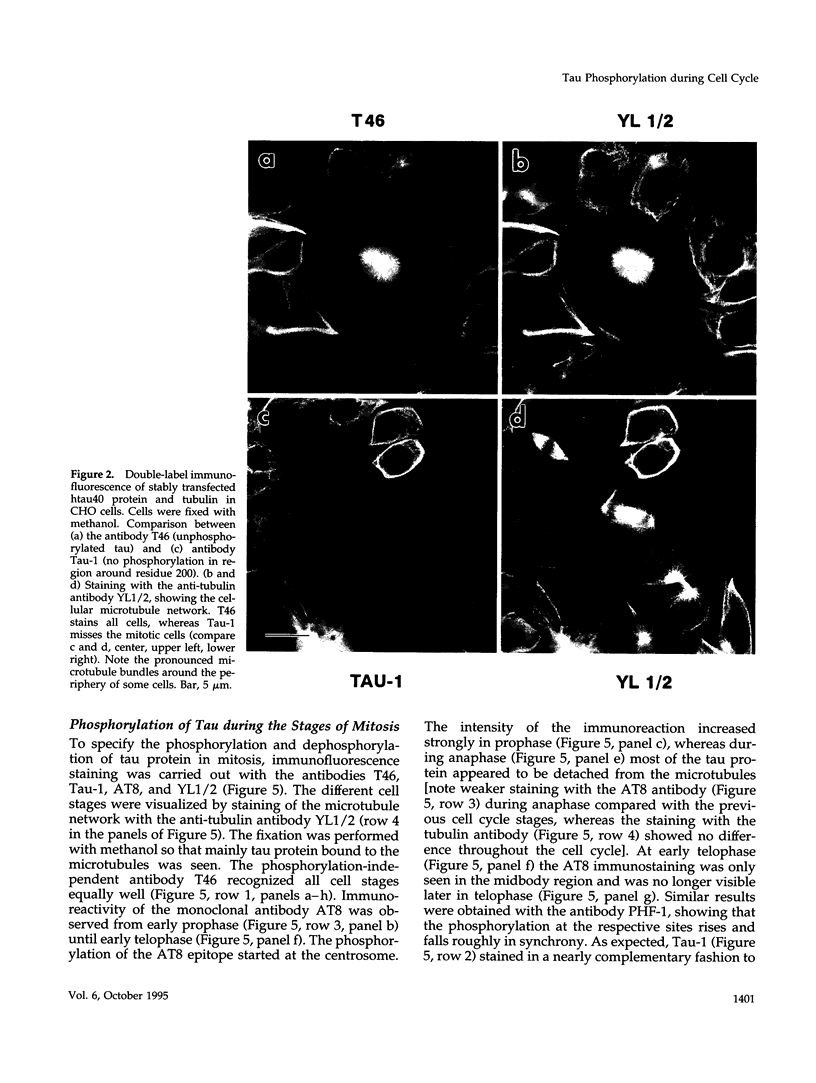
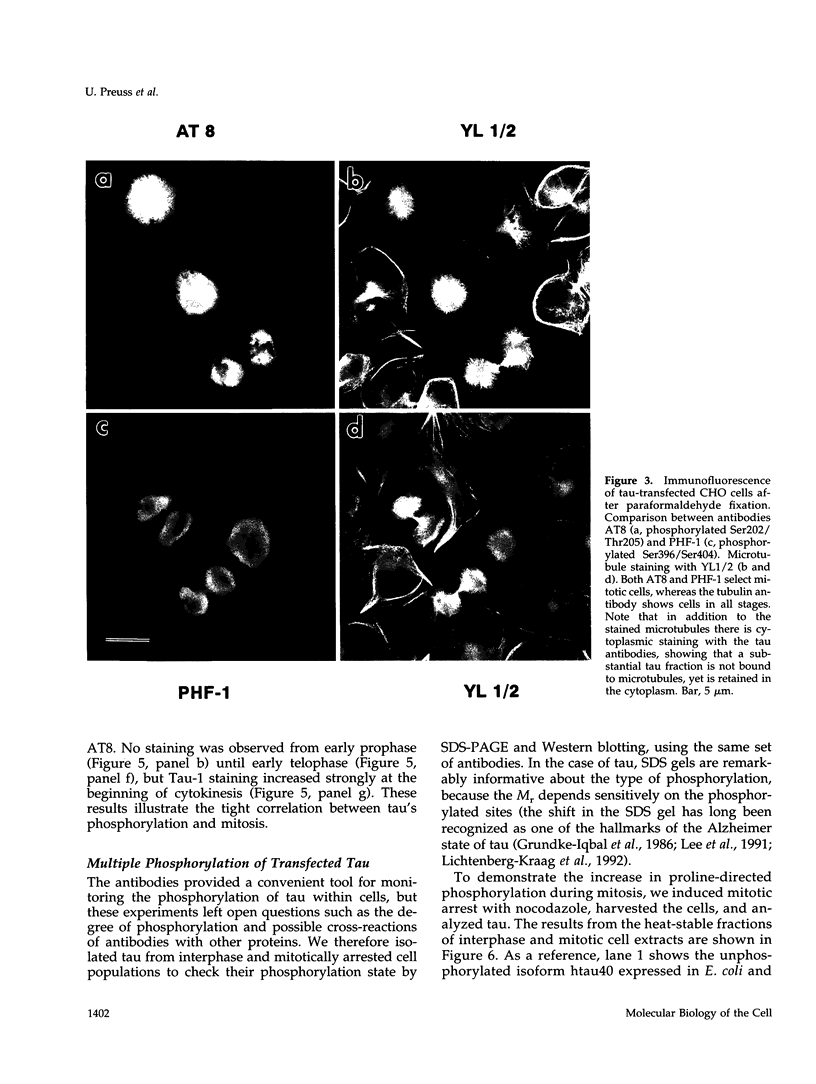
Abstract
Tau protein, a neuronal microtubule-associated protein, is phosphorylated in situ and hyperphosphorylated when aggregated into the paired helical filaments of Alzheimer's disease. To study the phosphorylation of tau protein in vivo, we have stably transfected htau40, the largest human tau isoform, into Chinese hamster ovary cells. The distribution and phosphorylation of tau was monitored by gel shift, autoradiography, immunofluorescence, and immunoblotting, using the antibodies Tau-1, AT8, AT180, and PHF-1, which are sensitive to the phosphorylation of Ser202, Thr205, Thr231, Ser235, Ser396, and Ser404 and are used in the diagnosis of Alzheimer tau. In interphase cells, tau becomes phosphorylated to some extent, partly at these sites; most of the tau is associated with microtubules. In mitosis, the above Ser/Thr-Pro sites become almost completely phosphorylated, causing a pronounced shift in M(r) and an antibody reactivity similar to that of Alzheimer tau. Moreover, a substantial fraction of tau is found in the cytoplasm detached from microtubules. Autoradiographs of metabolically labeled Chinese hamster ovary cells in interphase and mitosis confirmed that tau protein is more highly phosphorylated during mitosis. The understanding of tau phosphorylation under physiological conditions might help elucidate possible mechanisms for the hyperphosphorylation in Alzheimer's disease.
Full text
PDF


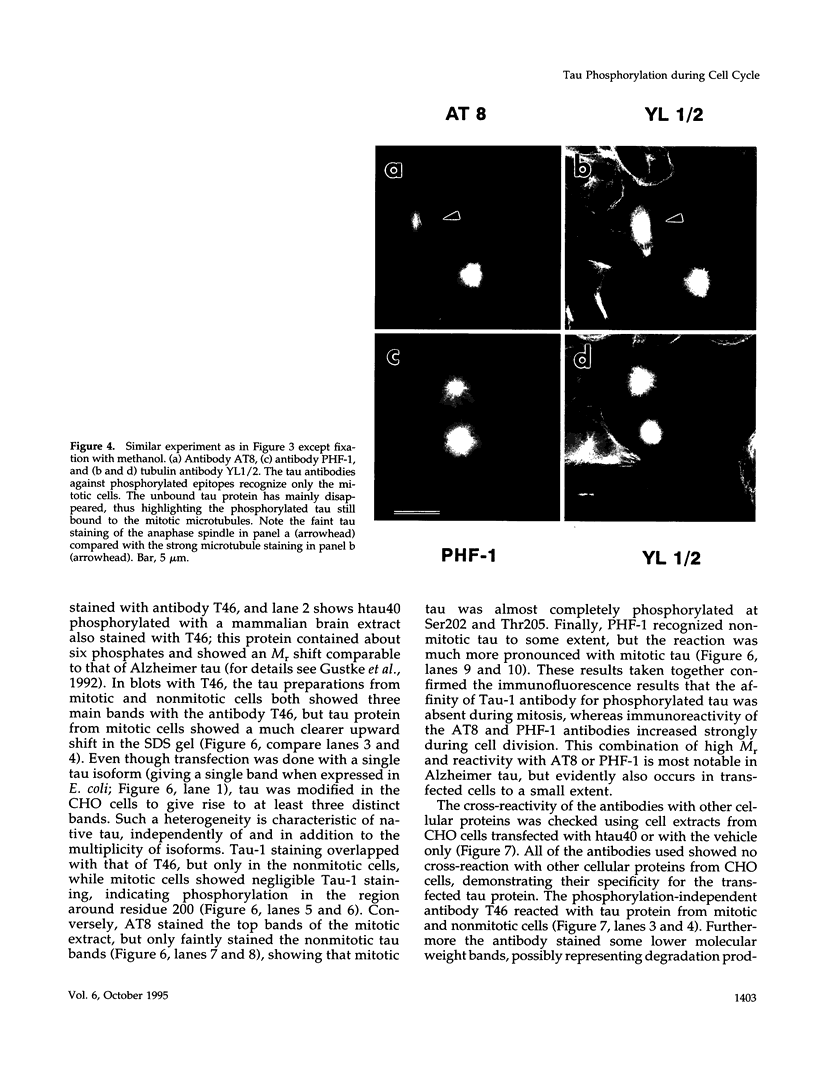
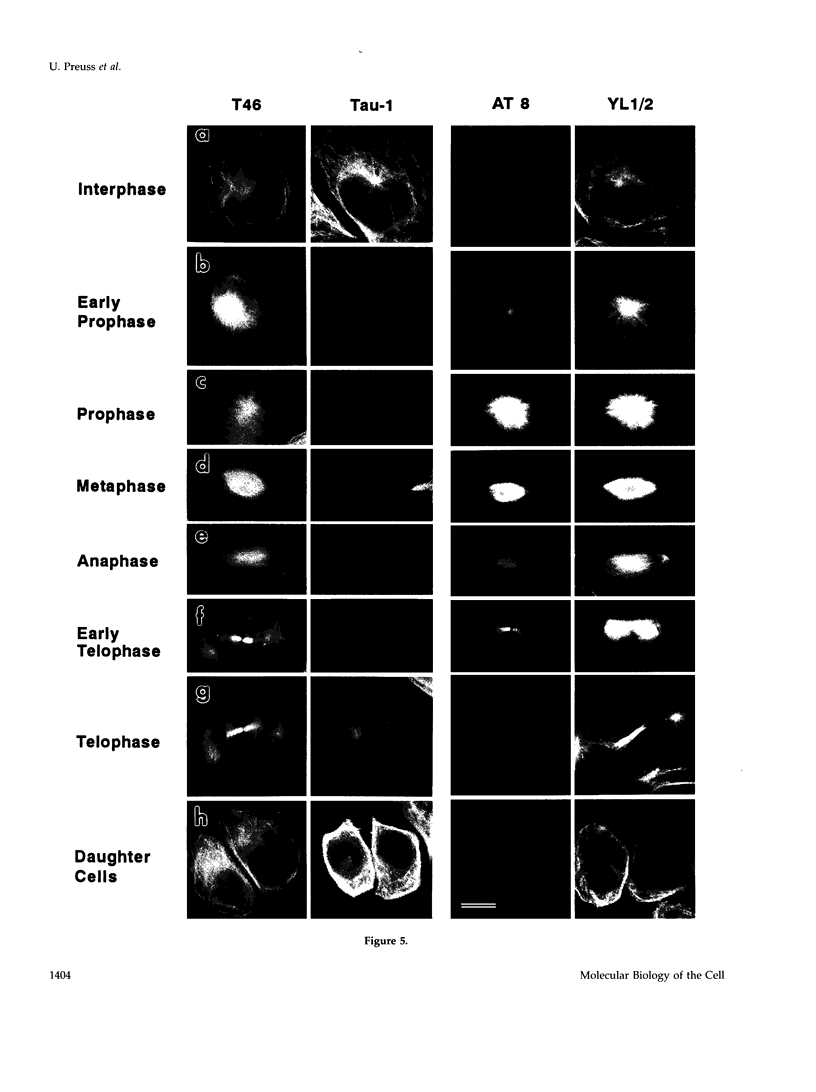



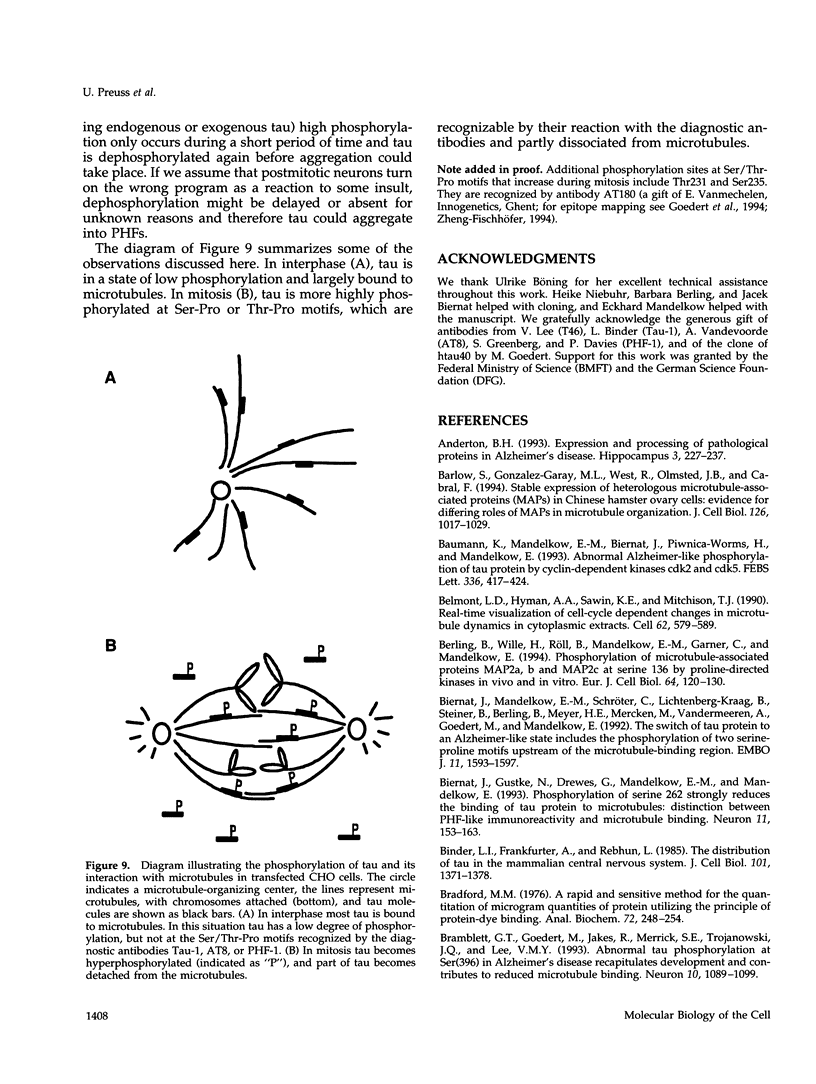


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderton B. H. Expression and processing of pathologic proteins in Alzheimer's disease. Hippocampus. 1993;3(Spec No):227–237. [PubMed] [Google Scholar]
- Barlow S., Gonzalez-Garay M. L., West R. R., Olmsted J. B., Cabral F. Stable expression of heterologous microtubule-associated proteins (MAPs) in Chinese hamster ovary cells: evidence for differing roles of MAPs in microtubule organization. J Cell Biol. 1994 Aug;126(4):1017–1029. doi: 10.1083/jcb.126.4.1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baumann K., Mandelkow E. M., Biernat J., Piwnica-Worms H., Mandelkow E. Abnormal Alzheimer-like phosphorylation of tau-protein by cyclin-dependent kinases cdk2 and cdk5. FEBS Lett. 1993 Dec 28;336(3):417–424. doi: 10.1016/0014-5793(93)80849-p. [DOI] [PubMed] [Google Scholar]
- Belmont L. D., Hyman A. A., Sawin K. E., Mitchison T. J. Real-time visualization of cell cycle-dependent changes in microtubule dynamics in cytoplasmic extracts. Cell. 1990 Aug 10;62(3):579–589. doi: 10.1016/0092-8674(90)90022-7. [DOI] [PubMed] [Google Scholar]
- Berling B., Wille H., Röll B., Mandelkow E. M., Garner C., Mandelkow E. Phosphorylation of microtubule-associated proteins MAP2a,b and MAP2c at Ser136 by proline-directed kinases in vivo and in vitro. Eur J Cell Biol. 1994 Jun;64(1):120–130. [PubMed] [Google Scholar]
- Biernat J., Gustke N., Drewes G., Mandelkow E. M., Mandelkow E. Phosphorylation of Ser262 strongly reduces binding of tau to microtubules: distinction between PHF-like immunoreactivity and microtubule binding. Neuron. 1993 Jul;11(1):153–163. doi: 10.1016/0896-6273(93)90279-z. [DOI] [PubMed] [Google Scholar]
- Biernat J., Mandelkow E. M., Schröter C., Lichtenberg-Kraag B., Steiner B., Berling B., Meyer H., Mercken M., Vandermeeren A., Goedert M. The switch of tau protein to an Alzheimer-like state includes the phosphorylation of two serine-proline motifs upstream of the microtubule binding region. EMBO J. 1992 Apr;11(4):1593–1597. doi: 10.1002/j.1460-2075.1992.tb05204.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Binder L. I., Frankfurter A., Rebhun L. I. The distribution of tau in the mammalian central nervous system. J Cell Biol. 1985 Oct;101(4):1371–1378. doi: 10.1083/jcb.101.4.1371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Bramblett G. T., Goedert M., Jakes R., Merrick S. E., Trojanowski J. Q., Lee V. M. Abnormal tau phosphorylation at Ser396 in Alzheimer's disease recapitulates development and contributes to reduced microtubule binding. Neuron. 1993 Jun;10(6):1089–1099. doi: 10.1016/0896-6273(93)90057-x. [DOI] [PubMed] [Google Scholar]
- Brandt R., Lee G. Functional organization of microtubule-associated protein tau. Identification of regions which affect microtubule growth, nucleation, and bundle formation in vitro. J Biol Chem. 1993 Feb 15;268(5):3414–3419. [PubMed] [Google Scholar]
- Brugg B., Matus A. Phosphorylation determines the binding of microtubule-associated protein 2 (MAP2) to microtubules in living cells. J Cell Biol. 1991 Aug;114(4):735–743. doi: 10.1083/jcb.114.4.735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buendia B., Draetta G., Karsenti E. Regulation of the microtubule nucleating activity of centrosomes in Xenopus egg extracts: role of cyclin A-associated protein kinase. J Cell Biol. 1992 Mar;116(6):1431–1442. doi: 10.1083/jcb.116.6.1431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butner K. A., Kirschner M. W. Tau protein binds to microtubules through a flexible array of distributed weak sites. J Cell Biol. 1991 Nov;115(3):717–730. doi: 10.1083/jcb.115.3.717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapin S. J., Bulinski J. C. Cellular microtubules heterogeneous in their content of microtubule-associated protein 4 (MAP4). Cell Motil Cytoskeleton. 1994;27(2):133–149. doi: 10.1002/cm.970270205. [DOI] [PubMed] [Google Scholar]
- Chapin S. J., Bulinski J. C. Microtubule stabilization by assembly-promoting microtubule-associated proteins: a repeat performance. Cell Motil Cytoskeleton. 1992;23(4):236–243. doi: 10.1002/cm.970230403. [DOI] [PubMed] [Google Scholar]
- Chapin S. J., Bulinski J. C. Non-neuronal 210 x 10(3) Mr microtubule-associated protein (MAP4) contains a domain homologous to the microtubule-binding domains of neuronal MAP2 and tau. J Cell Sci. 1991 Jan;98(Pt 1):27–36. doi: 10.1242/jcs.98.1.27. [DOI] [PubMed] [Google Scholar]
- Drewes G., Lichtenberg-Kraag B., Döring F., Mandelkow E. M., Biernat J., Goris J., Dorée M., Mandelkow E. Mitogen activated protein (MAP) kinase transforms tau protein into an Alzheimer-like state. EMBO J. 1992 Jun;11(6):2131–2138. doi: 10.1002/j.1460-2075.1992.tb05272.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drewes G., Trinczek B., Illenberger S., Biernat J., Schmitt-Ulms G., Meyer H. E., Mandelkow E. M., Mandelkow E. Microtubule-associated protein/microtubule affinity-regulating kinase (p110mark). A novel protein kinase that regulates tau-microtubule interactions and dynamic instability by phosphorylation at the Alzheimer-specific site serine 262. J Biol Chem. 1995 Mar 31;270(13):7679–7688. doi: 10.1074/jbc.270.13.7679. [DOI] [PubMed] [Google Scholar]
- Drubin D. G., Kirschner M. W. Tau protein function in living cells. J Cell Biol. 1986 Dec;103(6 Pt 2):2739–2746. doi: 10.1083/jcb.103.6.2739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujiwara K. Techniques for localizing contractile proteins with fluorescent antibodies. Curr Top Dev Biol. 1980;14(Pt 2):271–296. doi: 10.1016/s0070-2153(08)60198-2. [DOI] [PubMed] [Google Scholar]
- Gallo J. M., Hanger D. P., Twist E. C., Kosik K. S., Anderton B. H. Expression and phosphorylation of a three-repeat isoform of tau in transfected non-neuronal cells. Biochem J. 1992 Sep 1;286(Pt 2):399–404. doi: 10.1042/bj2860399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goedert M., Jakes R., Crowther R. A., Cohen P., Vanmechelen E., Vandermeeren M., Cras P. Epitope mapping of monoclonal antibodies to the paired helical filaments of Alzheimer's disease: identification of phosphorylation sites in tau protein. Biochem J. 1994 Aug 1;301(Pt 3):871–877. doi: 10.1042/bj3010871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goedert M., Jakes R., Crowther R. A., Six J., Lübke U., Vandermeeren M., Cras P., Trojanowski J. Q., Lee V. M. The abnormal phosphorylation of tau protein at Ser-202 in Alzheimer disease recapitulates phosphorylation during development. Proc Natl Acad Sci U S A. 1993 Jun 1;90(11):5066–5070. doi: 10.1073/pnas.90.11.5066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goedert M., Spillantini M. G., Jakes R., Rutherford D., Crowther R. A. Multiple isoforms of human microtubule-associated protein tau: sequences and localization in neurofibrillary tangles of Alzheimer's disease. Neuron. 1989 Oct;3(4):519–526. doi: 10.1016/0896-6273(89)90210-9. [DOI] [PubMed] [Google Scholar]
- Gotoh Y., Nishida E., Matsuda S., Shiina N., Kosako H., Shiokawa K., Akiyama T., Ohta K., Sakai H. In vitro effects on microtubule dynamics of purified Xenopus M phase-activated MAP kinase. Nature. 1991 Jan 17;349(6306):251–254. doi: 10.1038/349251a0. [DOI] [PubMed] [Google Scholar]
- Greenberg S. G., Davies P. A preparation of Alzheimer paired helical filaments that displays distinct tau proteins by polyacrylamide gel electrophoresis. Proc Natl Acad Sci U S A. 1990 Aug;87(15):5827–5831. doi: 10.1073/pnas.87.15.5827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenberg S. M., Koo E. H., Selkoe D. J., Qiu W. Q., Kosik K. S. Secreted beta-amyloid precursor protein stimulates mitogen-activated protein kinase and enhances tau phosphorylation. Proc Natl Acad Sci U S A. 1994 Jul 19;91(15):7104–7108. doi: 10.1073/pnas.91.15.7104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grundke-Iqbal I., Iqbal K., Tung Y. C., Quinlan M., Wisniewski H. M., Binder L. I. Abnormal phosphorylation of the microtubule-associated protein tau (tau) in Alzheimer cytoskeletal pathology. Proc Natl Acad Sci U S A. 1986 Jul;83(13):4913–4917. doi: 10.1073/pnas.83.13.4913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gustke N., Steiner B., Mandelkow E. M., Biernat J., Meyer H. E., Goedert M., Mandelkow E. The Alzheimer-like phosphorylation of tau protein reduces microtubule binding and involves Ser-Pro and Thr-Pro motifs. FEBS Lett. 1992 Jul 28;307(2):199–205. doi: 10.1016/0014-5793(92)80767-b. [DOI] [PubMed] [Google Scholar]
- Gustke N., Trinczek B., Biernat J., Mandelkow E. M., Mandelkow E. Domains of tau protein and interactions with microtubules. Biochemistry. 1994 Aug 16;33(32):9511–9522. doi: 10.1021/bi00198a017. [DOI] [PubMed] [Google Scholar]
- Hagestedt T., Lichtenberg B., Wille H., Mandelkow E. M., Mandelkow E. Tau protein becomes long and stiff upon phosphorylation: correlation between paracrystalline structure and degree of phosphorylation. J Cell Biol. 1989 Oct;109(4 Pt 1):1643–1651. doi: 10.1083/jcb.109.4.1643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanger D. P., Hughes K., Woodgett J. R., Brion J. P., Anderton B. H. Glycogen synthase kinase-3 induces Alzheimer's disease-like phosphorylation of tau: generation of paired helical filament epitopes and neuronal localisation of the kinase. Neurosci Lett. 1992 Nov 23;147(1):58–62. doi: 10.1016/0304-3940(92)90774-2. [DOI] [PubMed] [Google Scholar]
- Hasegawa M., Morishima-Kawashima M., Takio K., Suzuki M., Titani K., Ihara Y. Protein sequence and mass spectrometric analyses of tau in the Alzheimer's disease brain. J Biol Chem. 1992 Aug 25;267(24):17047–17054. [PubMed] [Google Scholar]
- Himmler A., Drechsel D., Kirschner M. W., Martin D. W., Jr Tau consists of a set of proteins with repeated C-terminal microtubule-binding domains and variable N-terminal domains. Mol Cell Biol. 1989 Apr;9(4):1381–1388. doi: 10.1128/mcb.9.4.1381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirokawa N. Microtubule organization and dynamics dependent on microtubule-associated proteins. Curr Opin Cell Biol. 1994 Feb;6(1):74–81. doi: 10.1016/0955-0674(94)90119-8. [DOI] [PubMed] [Google Scholar]
- Ishiguro K., Omori A., Sato K., Tomizawa K., Imahori K., Uchida T. A serine/threonine proline kinase activity is included in the tau protein kinase fraction forming a paired helical filament epitope. Neurosci Lett. 1991 Jul 22;128(2):195–198. doi: 10.1016/0304-3940(91)90259-v. [DOI] [PubMed] [Google Scholar]
- Kanai Y., Takemura R., Oshima T., Mori H., Ihara Y., Yanagisawa M., Masaki T., Hirokawa N. Expression of multiple tau isoforms and microtubule bundle formation in fibroblasts transfected with a single tau cDNA. J Cell Biol. 1989 Sep;109(3):1173–1184. doi: 10.1083/jcb.109.3.1173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanemaru K., Takio K., Miura R., Titani K., Ihara Y. Fetal-type phosphorylation of the tau in paired helical filaments. J Neurochem. 1992 May;58(5):1667–1675. doi: 10.1111/j.1471-4159.1992.tb10039.x. [DOI] [PubMed] [Google Scholar]
- Kilmartin J. V., Wright B., Milstein C. Rat monoclonal antitubulin antibodies derived by using a new nonsecreting rat cell line. J Cell Biol. 1982 Jun;93(3):576–582. doi: 10.1083/jcb.93.3.576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirby B. A., Merril C. R., Ghanbari H., Wallace W. C. Heat shock proteins protect against stress-related phosphorylation of tau in neuronal PC12 cells that have acquired thermotolerance. J Neurosci. 1994 Sep;14(9):5687–5693. doi: 10.1523/JNEUROSCI.14-09-05687.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kosik K. S., Orecchio L. D., Binder L., Trojanowski J. Q., Lee V. M., Lee G. Epitopes that span the tau molecule are shared with paired helical filaments. Neuron. 1988 Nov;1(9):817–825. doi: 10.1016/0896-6273(88)90129-8. [DOI] [PubMed] [Google Scholar]
- Lang E., Szendrei G. I., Lee V. M., Otvos L., Jr Immunological and conformation characterization of a phosphorylated immunodominant epitope on the paired helical filaments found in Alzheimer's disease. Biochem Biophys Res Commun. 1992 Sep 16;187(2):783–790. doi: 10.1016/0006-291x(92)91264-q. [DOI] [PubMed] [Google Scholar]
- Ledesma M. D., Correas I., Avila J., Díaz-Nido J. Implication of brain cdc2 and MAP2 kinases in the phosphorylation of tau protein in Alzheimer's disease. FEBS Lett. 1992 Aug 17;308(2):218–224. doi: 10.1016/0014-5793(92)81278-t. [DOI] [PubMed] [Google Scholar]
- Lee G., Cowan N., Kirschner M. The primary structure and heterogeneity of tau protein from mouse brain. Science. 1988 Jan 15;239(4837):285–288. doi: 10.1126/science.3122323. [DOI] [PubMed] [Google Scholar]
- Lee G. Non-motor microtubule-associated proteins. Curr Opin Cell Biol. 1993 Feb;5(1):88–94. doi: 10.1016/s0955-0674(05)80013-4. [DOI] [PubMed] [Google Scholar]
- Lee V. M., Balin B. J., Otvos L., Jr, Trojanowski J. Q. A68: a major subunit of paired helical filaments and derivatized forms of normal Tau. Science. 1991 Feb 8;251(4994):675–678. doi: 10.1126/science.1899488. [DOI] [PubMed] [Google Scholar]
- Lichtenberg-Kraag B., Mandelkow E. M., Biernat J., Steiner B., Schröter C., Gustke N., Meyer H. E., Mandelkow E. Phosphorylation-dependent epitopes of neurofilament antibodies on tau protein and relationship with Alzheimer tau. Proc Natl Acad Sci U S A. 1992 Jun 15;89(12):5384–5388. doi: 10.1073/pnas.89.12.5384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lieuvin A., Labbé J. C., Dorée M., Job D. Intrinsic microtubule stability in interphase cells. J Cell Biol. 1994 Mar;124(6):985–996. doi: 10.1083/jcb.124.6.985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lo M. M., Fieles A. W., Norris T. E., Dargis P. G., Caputo C. B., Scott C. W., Lee V. M., Goedert M. Human tau isoforms confer distinct morphological and functional properties to stably transfected fibroblasts. Brain Res Mol Brain Res. 1993 Nov;20(3):209–220. doi: 10.1016/0169-328x(93)90043-o. [DOI] [PubMed] [Google Scholar]
- Mandelkow E. M., Drewes G., Biernat J., Gustke N., Van Lint J., Vandenheede J. R., Mandelkow E. Glycogen synthase kinase-3 and the Alzheimer-like state of microtubule-associated protein tau. FEBS Lett. 1992 Dec 21;314(3):315–321. doi: 10.1016/0014-5793(92)81496-9. [DOI] [PubMed] [Google Scholar]
- Mandelkow E. M., Mandelkow E. Tau as a marker for Alzheimer's disease. Trends Biochem Sci. 1993 Dec;18(12):480–483. doi: 10.1016/0968-0004(93)90011-b. [DOI] [PubMed] [Google Scholar]
- Matsuo E. S., Shin R. W., Billingsley M. L., Van deVoorde A., O'Connor M., Trojanowski J. Q., Lee V. M. Biopsy-derived adult human brain tau is phosphorylated at many of the same sites as Alzheimer's disease paired helical filament tau. Neuron. 1994 Oct;13(4):989–1002. doi: 10.1016/0896-6273(94)90264-x. [DOI] [PubMed] [Google Scholar]
- McIntosh J. R., Hering G. E. Spindle fiber action and chromosome movement. Annu Rev Cell Biol. 1991;7:403–426. doi: 10.1146/annurev.cb.07.110191.002155. [DOI] [PubMed] [Google Scholar]
- Melan M. A., Sluder G. Redistribution and differential extraction of soluble proteins in permeabilized cultured cells. Implications for immunofluorescence microscopy. J Cell Sci. 1992 Apr;101(Pt 4):731–743. doi: 10.1242/jcs.101.4.731. [DOI] [PubMed] [Google Scholar]
- Mercken M., Vandermeeren M., Lübke U., Six J., Boons J., Van de Voorde A., Martin J. J., Gheuens J. Monoclonal antibodies with selective specificity for Alzheimer Tau are directed against phosphatase-sensitive epitopes. Acta Neuropathol. 1992;84(3):265–272. doi: 10.1007/BF00227819. [DOI] [PubMed] [Google Scholar]
- Morishima-Kawashima M., Hasegawa M., Takio K., Suzuki M., Yoshida H., Titani K., Ihara Y. Proline-directed and non-proline-directed phosphorylation of PHF-tau. J Biol Chem. 1995 Jan 13;270(2):823–829. doi: 10.1074/jbc.270.2.823. [DOI] [PubMed] [Google Scholar]
- Nigg E. A. Cellular substrates of p34(cdc2) and its companion cyclin-dependent kinases. Trends Cell Biol. 1993 Sep;3(9):296–301. doi: 10.1016/0962-8924(93)90011-o. [DOI] [PubMed] [Google Scholar]
- Otvos L., Jr, Feiner L., Lang E., Szendrei G. I., Goedert M., Lee V. M. Monoclonal antibody PHF-1 recognizes tau protein phosphorylated at serine residues 396 and 404. J Neurosci Res. 1994 Dec 15;39(6):669–673. doi: 10.1002/jnr.490390607. [DOI] [PubMed] [Google Scholar]
- Paudel H. K., Lew J., Ali Z., Wang J. H. Brain proline-directed protein kinase phosphorylates tau on sites that are abnormally phosphorylated in tau associated with Alzheimer's paired helical filaments. J Biol Chem. 1993 Nov 5;268(31):23512–23518. [PubMed] [Google Scholar]
- Pope W. B., Lambert M. P., Leypold B., Seupaul R., Sletten L., Krafft G., Klein W. L. Microtubule-associated protein tau is hyperphosphorylated during mitosis in the human neuroblastoma cell line SH-SY5Y. Exp Neurol. 1994 Apr;126(2):185–194. doi: 10.1006/exnr.1994.1057. [DOI] [PubMed] [Google Scholar]
- Schliwa M., Euteneuer U., Bulinski J. C., Izant J. G. Calcium lability of cytoplasmic microtubules and its modulation by microtubule-associated proteins. Proc Natl Acad Sci U S A. 1981 Feb;78(2):1037–1041. doi: 10.1073/pnas.78.2.1037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schweers O., Schönbrunn-Hanebeck E., Marx A., Mandelkow E. Structural studies of tau protein and Alzheimer paired helical filaments show no evidence for beta-structure. J Biol Chem. 1994 Sep 30;269(39):24290–24297. [PubMed] [Google Scholar]
- Shiina N., Moriguchi T., Ohta K., Gotoh Y., Nishida E. Regulation of a major microtubule-associated protein by MPF and MAP kinase. EMBO J. 1992 Nov;11(11):3977–3984. doi: 10.1002/j.1460-2075.1992.tb05491.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Studier F. W., Rosenberg A. H., Dunn J. J., Dubendorff J. W. Use of T7 RNA polymerase to direct expression of cloned genes. Methods Enzymol. 1990;185:60–89. doi: 10.1016/0076-6879(90)85008-c. [DOI] [PubMed] [Google Scholar]
- Sygowski L. A., Fieles A. W., Lo M. M., Scott C. W., Caputo C. B. Phosphorylation of tau protein in tau-transfected 3T3 cells. Brain Res Mol Brain Res. 1993 Nov;20(3):221–228. doi: 10.1016/0169-328x(93)90044-p. [DOI] [PubMed] [Google Scholar]
- Szendrei G. I., Lee V. M., Otvos L., Jr Recognition of the minimal epitope of monoclonal antibody Tau-1 depends upon the presence of a phosphate group but not its location. J Neurosci Res. 1993 Feb 1;34(2):243–249. doi: 10.1002/jnr.490340212. [DOI] [PubMed] [Google Scholar]
- Trojanowski J. Q., Lee V. M. Paired helical filament tau in Alzheimer's disease. The kinase connection. Am J Pathol. 1994 Mar;144(3):449–453. [PMC free article] [PubMed] [Google Scholar]
- Vandré D. D., Centonze V. E., Peloquin J., Tombes R. M., Borisy G. G. Proteins of the mammalian mitotic spindle: phosphorylation/dephosphorylation of MAP-4 during mitosis. J Cell Sci. 1991 Apr;98(Pt 4):577–588. doi: 10.1242/jcs.98.4.577. [DOI] [PubMed] [Google Scholar]
- Vulliet R., Halloran S. M., Braun R. K., Smith A. J., Lee G. Proline-directed phosphorylation of human Tau protein. J Biol Chem. 1992 Nov 5;267(31):22570–22574. [PubMed] [Google Scholar]
- Watanabe A., Hasegawa M., Suzuki M., Takio K., Morishima-Kawashima M., Titani K., Arai T., Kosik K. S., Ihara Y. In vivo phosphorylation sites in fetal and adult rat tau. J Biol Chem. 1993 Dec 5;268(34):25712–25717. [PubMed] [Google Scholar]
- Weisshaar B., Doll T., Matus A. Reorganisation of the microtubular cytoskeleton by embryonic microtubule-associated protein 2 (MAP2c). Development. 1992 Dec;116(4):1151–1161. doi: 10.1242/dev.116.4.1151. [DOI] [PubMed] [Google Scholar]
- West R. R., Tenbarge K. M., Olmsted J. B. A model for microtubule-associated protein 4 structure. Domains defined by comparisons of human, mouse, and bovine sequences. J Biol Chem. 1991 Nov 15;266(32):21886–21896. [PubMed] [Google Scholar]
- Wille H., Drewes G., Biernat J., Mandelkow E. M., Mandelkow E. Alzheimer-like paired helical filaments and antiparallel dimers formed from microtubule-associated protein tau in vitro. J Cell Biol. 1992 Aug;118(3):573–584. doi: 10.1083/jcb.118.3.573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida H., Ihara Y. Tau in paired helical filaments is functionally distinct from fetal tau: assembly incompetence of paired helical filament-tau. J Neurochem. 1993 Sep;61(3):1183–1186. doi: 10.1111/j.1471-4159.1993.tb03642.x. [DOI] [PubMed] [Google Scholar]