Abstract
Neuropilin-1 (NRP-1) is present on the cell surface of endothelial cells, or as a soluble truncated variant. Membrane NRP-1 is proposed to enhance angiogenesis by promoting the formation of a signaling complex between vascular endothelial growth factor-A165 (VEGF-A165), VEGF receptor-2 (VEGFR-2) and heparan sulfate, whereas the soluble NRP-1 is thought to act as an antagonist of signaling complex formation. We have analyzed the angiogenic potential of a chimera comprising the entire extracellular NRP-1 region dimerized through an Fc IgG domain and a monomeric truncated NRP-1 variant. Both NRP-1 proteins stimulated tubular morphogenesis and cell migration in HDMECs and HUVECs. Fc rNRP-1 was able to induce VEGFR-2 phosphorylation and expression of the VEGFR-2 specific target, regulator of calcineurin-1 (RCAN1.4). siRNA mediated gene silencing of VEGFR-2 revealed that VEGFR-2 was required for Fc rNRP-1 mediated activation of the intracellular signaling proteins PLC-γ, AKT, and MAPK and tubular morphogenesis. The stimulatory activity was independent of VEGF-A165. This was evidenced by depleting the cell culture of exogenous VEGF-A165, and using instead for routine culture VEGF-A121, which does not interact with NRP-1, and by the inability of VEGF-A sequestering antibodies to inhibit the angiogenic activity of the NRP proteins. Analysis of angiogenesis over a period of 6 days in an in vitro fibroblast/endothelial co-culture model revealed that Fc rNRP-1 could induce endothelial cell tubular morphogenesis. Thus, we conclude that soluble Fc rNRP-1 is a VEGF-A165-independent agonist of VEGFR-2 and stimulates angiogenesis in endothelial cells.
Keywords: Differentiation, Endothelium, Growth Factors, Protein Phosphorylation, Receptor Tyrosine Kinase, Signal Transduction, Tyrosine Protein Kinase (Tyrosine Kinase), Endothelial Cell, Angiogenesis, Neuropilin
Introduction
Neuropilin-1 (NRP-1)4 is a protein known for playing important functions in neural and vascular systems (reviewed in Refs. 1, 2). Initially, it was described as a regulator of axon collapse and enhancer of angiogenesis. Subsequently, new functions of NRP-1 were characterized and the expanded contemporary view of NRP-1 includes among its functions antigen recognition (3), adhesion via interaction with β integrins (4, 5), activation of latent forms of cytokines (6), control of stem cell differentiation (7–9), and viral infection (10).
The majority of studies analyzing NRP-1 function in endothelial cells have focused on the role of the native transmembrane protein. NRP-1 was shown to be abundantly expressed in human, mouse, and chick with highest expression in the vascular endothelium, heart and placenta (11–13). Moreover, it was shown that a homozygous deletion of the Nrp1 gene in mice causes embryonic lethality, because of defects in the vessels and general vascularization (14), while exogenously overexpressed NRP-1 led to formation of excess capillaries and hemorrhages (11).
Overexpression of NRP-1 has been observed in the tumor microenvironment, where apart from endothelial cells, the tumor cells themselves were shown to express NRP-1 (15, 16). Current knowledge of NRP-1 places it among the key drivers of angiogenesis (17); however, it must be emphasized that the exact mechanism of its action is not clear. It has been proposed that NRP-1 forms signaling complexes, where, as a co-receptor with no intrinsic kinase activity, it associates with other tyrosine kinase receptors, their ligands and heparan sulfate moieties of heparan sulfate proteoglycans (1). The formation of such complexes is regulated by the availability of NRP-1 in the cell membrane, dependent on its down-regulation by ligand-mediated internalization. Recent data have shown that VEGF-A165 binding to both VEGFR-2 and NRP-1 facilitates the activation of p38 MAPK indicating that NRP-1 plays an active role in VEGFR-2 signaling (18).
Several studies have shown that molecules interacting with NRP-1 cause its disappearance from the cell surface and this mechanism together with ligand binding preference might provide a mechanism for NRP-1 signaling selectivity (5, 19–22). The hypothesis that the internalization process might be a means of selecting signaling pathways is supported by observations that VEGF-A165 induces NRP-1 internalization at a much higher level than SEMA-3A, whereas VEGF-A121, which does not bind NRP-1, fails to affect the internalization of NRP-1 (19). Another mechanism controlling the angiogenic activity of NRP-1 is the secretion of soluble truncated isoforms of the receptors, which bind the same ligands as membrane NRP-1. For example, in the presence of soluble NRP-1 species, which sequester VEGF-A165, membrane NRP-1 cannot enhance VEGF signaling nor be internalized, which may lead to an increased probability of NRP-1 interacting with the antagonizing SEMA-3A (19).
Because of its crucial role in angiogenesis, NRP-1 is currently the target of various prospective anticancer therapies. The most common approaches aim to inhibit NRP-1 function, and, consequently, block such phenotypes as pathological angiogenesis, and consequently tumor growth (23). Among these are antagonistic soluble NRP-1 (24, 25), VEGF-A165-derived blocking peptides (25–27), siRNA against NRP-1 (25), antibodies to NRP-1 (28) and recently developed synthetic small molecule inhibitors (29). Other approaches use NRP-1 to allow drug delivery inside the cells (30–33), thus providing a route for selective drug delivery into the cells expressing NRP-1.
In this study we hypothesized that dimeric NRP-1, a proxy for oligomerized membrane NRP-1, could be a potential proangiogenic agent mimicking in trans an intercellular activity of NRP-1 (34). Consequently, we have examined the molecular components required for NRP-1 to exert an angiogenic effect in human dermal microvascular endothelial cells (HDMECs) and human umbilical vein endothelial cells (HUVECs). We used a recombinant dimeric rat NRP-1 (Fc rNRP-1), as a proxy for native oligomerized NRP-1 species embedded on the cell surface and a soluble human NRP-1 isoform, comprising the a and b, but not the c domain. Fc rNRP-1 contains all the main extracellular domains from a to c that are considered essential for ligand/receptor interactions and also for NRP-1 oligomerization (1). Surprisingly, our data demonstrate that both forms of NRP-1 can cause tube formation independently of VEGF ligand in a collagen-based angiogenesis assay of both cell lines. The mechanism of Fc rNRP-1 action is VEGFR-2 dependent, as shown by the stimulation of VEGFR-2 phosphorylation, RCAN-1.4 induction and blockage by a VEGFR-2 knock-down and a VEGFR-2 kinase inhibitor. The soluble human NRP-1 isoform was similarly shown to cause tube formation, though less effectively. Thus, NRP-1 behaves as a VEGFR-2 agonist and does not require partner growth factors to exert its angiogenic activity.
EXPERIMENTAL PROCEDURES
Materials
Recombinant rat NRP-1 chimera (Fc rNRP-1), soluble human truncated variant (shNRP-1) and human Fc were purchased from R&D Systems (Abingdon, Oxon, UK), VEGF-A121 was purchased from PeproTech EC Ltd (London, UK). VEGF-A165 was kindly provided by NCI-Frederick (Frederick, MD). The tissue culture reagents were purchased from Promocell GmbH (Heidelberg, Germany) and Lonza (Wokingham, Berks, UK). Antibodies against phospho-VEGFR-2 clone 19A10 (Y1175), VEGFR-2 clone 55B11, phospho-PLCγ (Y783), phospho-ERK-1/2 (Y202,204/Y185,187), phospho-AKT (S473) and AKT were purchased from Cell Signaling Technology (NEB, Hitchin, Herts, UK); against RCAN-1.4 (DSCR-1/ADAPT78/Calcipressin-1) from Sigma-Aldrich; against actin from Santa Cruz Biotechnology (Insight Biotechnology, Wembley, Middx, UK); anti-mouse-HRP and anti-rabbit-HRP antibodies were purchased from GE Healthcare (Amersham Biosciences, Bucks, UK); sequestering anti-VEGF antibody was from R&D Systems. VEGFR-2 kinase inhibitor (ZM323881) (35) was purchased from Tocris Bioscience (Bristol, Avon, UK).
Tissue Culture
HDMECs and HUVECs were purchased from Promocell and were cultured with the endothelial cell growth medium MV2 kit (Promocell) consisting of the endothelial cell basal medium with appropriate supplements provided by manufacturer (5% v/v fetal calf serum, 5 ng/ml human epidermal growth factor, 10 ng/ml FGF-2, 20 ng/ml insulin-like growth factor, 0.5 ng/ml VEGF-A165, 1 μg/ml ascorbic acid, 0.2 μg/ml hydrocortisone). Alternatively, the cells were grown with 0.5 ng/ml VEGF-A121 instead of VEGF-A165 where indicated. Cells were used until passage 10. For routine cell maintenance, cells were grown on 1% (w/v) gelatin-coated plates at 37 °C and 5% (v/v) CO2. 24 h prior to the experiments, the cells were serum-starved by replacing the fully supplemented medium with 1% (v/v) fetal calf serum endothelial cell basal medium. For regular maintenance cells were detached from the plates using solution of 0.05% (w/v) trypsin in 0.53 mm EDTA (Invitrogen). For the experiments the cells were detached using Accutase solution (Promocell GmbH) to preserve the extracellular membrane proteins.
Transfection of siRNA
Two validated short interfering (siRNA) duplexes directed against the target sequence 5′-AACGCTGACATGTACGGTCTA-3′ for Hs_KDR_5 (denoted KDR siRNA1) and 5′-AAGGCTAATACAACTCTTCAA-3′ for Hs_KDR_6 (denoted KDR siRNA2), were used to silence kdr, together with a non-silencing (N.S) control siRNA (Qiagen, Crawley, West Sussex, UK). The procedure of transfection was performed as described previously (36). Briefly, HDMECs were seeded at 1 × 105 per well, on gelatin-coated six well dishes in 2 ml of endothelial cell growth medium MV2 containing 1% (v/v) FCS and incubated for 24 h. Transfection was carried out in 2.5 ml per well of Opti-MEM® medium (Invitrogen, Paisley, UK) containing 10 nm siRNA and 0.1% (v/v) LipofectamineTM RNAiMAX (Invitrogen). The cells were incubated with the trasfection mix for 4 h and washed in dPBS containing Ca2+/Mg2+. Next, the cells were cultured for 24 h in endothelial cell growth medium MV2 containing 1% (v/v) FCS and subsequently serum-starved overnight in preparation for the tube formation and signaling assays.
Tube Formation Assay
The tube formation assay was performed as described previously (37). Collagen type I (Vitrogen, Cohesion Technologies, Palo Alto, CA) was mixed at a ratio 8:1:1 with 0.1 m NaOH and 10× concentrated Ham's F-12 medium (Promocell). The solution was supplemented to contain 0.02 m Hepes, 0.1% (w/v) sodium bicarbonate and 2 mm Glutamax-I (Invitrogen), and kept on ice until placed into the wells. The assay was prepared in 24-well plate format, and the bottom layer of collagen was formed by adding 300 μl of the solution per well and allowing subsequent gelation at 37 °C overnight. Following serum starvation, cells were plated at 8–9 × 104 in 500 μl per well in 1% (v/v) fetal calf serum endothelial cell basal medium. After 120 min, the medium was aspirated and a second layer of collagen (200 μl per well) was prepared according to the same protocol and gently placed on top of the cell layer. After 90 min of incubation at 37 °C, the medium was returned to the wells. The photos were taken after 17–20 h incubation at 37 °C from approximately the same coordinates in each well within the experimental plate.
The final volume of the assay was 1 ml, comprising of 0.5 ml of the collagen layers and 0.5 ml of medium. Depending on the ligand type, various orders of addition were employed. The higher molecular weight ligands, such as Fc, shNRP-1 and Fc rNRP-1 were added to the cells at the adhesion step (final concentration 5 nm, unless indicated otherwise), while smaller molecular weight ligands, such as VEGF-A165 (final concentration 10 ng/ml) and VEGFR-2 inhibitor ZM323881 (final concentration 3 μm) were added to the medium after formation of the top layer of collagen. In experiments using sequestering VEGF antibody, the antibody (20 μg/ml) was incubated with VEGF-A165 (10 ng/ml) for 60 min at room temperature and added after formation of the top layer of collagen. For variants with the Fc rNRP-1/shNRP-1 the antibody was added directly to the medium.
Signaling Assay
Serum-starved cells were plated at 1.9 × 105 cells in 500 μl per well of 12-well plates either on collagen or a layer of 1% (w/v) gelatin in 1% (v/v) fetal calf serum endothelial cell basal medium. 2 h later, the medium with appropriate ligands was added to the wells, and the plate was returned to the incubator. After an appropriate time of incubation (10 and 180 min), plates were placed on ice and the cells were lysed by 3 min of incubation with RIPA buffer (20 mm Tris, pH 7.5, 150 mm NaCl, 2.5 mm EDTA, 10% (w/v) glycerol, 1% (w/v) Triton X-100, 1 mm sodium vanadate, 10 μg/ml aprotinin, 10 μg/ml leupeptin, 1 mm PMSF, 0.1% (w/v) SDS, 0.5% (w/v) sodium deoxycholate). The lysates were collected, clarified by centrifugation at 11,000 rpm at 4 °C for 20 min, boiled with NuPAGE LDS Sample Buffer and analyzed by SDS-PAGE.
Gel Electrophoresis and Western Blot
For gel electrophoresis the NuPAGE Bis-Tris system was used (Invitrogen). The gradient 4–12% (w/v bis-acrylamide) gels were resolved at 50 mA constant current and transferred onto nitrocellulose membranes (GE Healthcare) using XCell IITM Blot Module for 120 min at 140 mA (Invitrogen). The membranes were blocked in 5% (w/v) BSA, 0.1% (v/v) Tween-20 in Tris-buffered saline (TBS) for 60 min at room temperature and subsequently incubated overnight in the cold room with the appropriate antibody solution prepared in 2% (w/v) BSA, 0.1% (v/v) Tween-20 in TBS. After washing, the membranes were incubated with the appropriate secondary antibodies in 2% (w/v) BSA 0.1% (v/v) Tween-20 in TBS for 60 min in the cold room. The membranes were developed using Super Signal West Dura Extended Duration Substrate kit (Pierce, Perbio Science). Silver staining of recombinant proteins was performed as described previously (38).
Scratch Wound Migration Assay
The assay was performed as described previously (39). Briefly, HDMECs and HUVECs were seeded in a 24-well plate coated with gelatin at 3 × 104 and 2 × 104 cells per well, respectively. After 24–36 h the cells were subjected to a serum-starvation step for 24 h for HDMECs and 48 h for HUVECs. Subsequently, a scratch was introduced to the cell monolayer using a sterile 200-μl pipette tip. At that stage the cells were stimulated with the agonists and after ∼18 h of incubation fixed with 2% (w/v) paraformaldehyde, stained with 2.3% (w/v) crystal violet (Sigma-Aldrich) and photographed. Each variant was performed in triplicate, and three representative photographs were used for the calculations.
Fibroblast-HDMEC Co-culture
The assay was performed as described previously (39). Briefly, human dermal fibroblasts were seeded at 2 × 104 cells per well in a 24-well gelatinized plate and grown until confluent for 3–4 days in fibroblast growth medium (Promocell). After reaching the confluence, HDMECs were seeded on top of the fibroblast monolayer at 4 × 104 cells per well in endothelial cell growth medium MV2 containing 1% (v/v) FCS (Promocell). After 24 h, the medium was replaced with the fresh batch and the agonists were added at indicated concentrations. After 72 h, the medium was aspirated and replaced with fresh medium containing agonists, and the cells were further incubated for 72 h. To visualize tube formation the co-cultures were fixed with 70% (v/v) ethanol, and then stained for the endothelial specific marker CD31 (Dako UK, Ely, Cambs, UK).
Data Analysis
Photographs of HDMECs and HUVECs in the tube formation three-dimensional collagen assays were analyzed with Adobe® Photoshop® CS3 Extended version 10.0 (San Jose, CA). For each experimental condition three squares of 400 × 400 pixels were selected in the photograph and on each the area covered by tubes was calculated. The output values were used for the Student's t test evaluation of the significant difference between the experiments. To allow comparison between experiments, the mean value of the tube surface in the control containing no agonist was set as a reference value 1, and used to normalize all other experimental values.
Tube formation in the co-culture experiment was analyzed by photographing three randomly selected fields of view (10 × objective), in triplicate wells per condition. Total tube length was quantified using AngioQuant software (40).
The band intensities in analyzed Western blots were estimated using Adobe® Photoshop® CS3 Extended 10.0 and presented as relative values compared with the control. An additional step of normalization versus the intensity of the actin band was performed to account for the variations in sample loading.
In migration scratch assays the gap size after allowed migration was compared with an average size of a starting scratch line. The obtained values were normalized against the respective controls. The unpaired Student's t test was used for evaluation of the significant difference between variants and their respective controls.
RESULTS
Characterization of Recombinant Soluble NRP-1 Proteins
The NRP-1 proteins used in this study are commercially available recombinant proteins (supplemental Fig. S1). They differ from the membrane-localized NRP-1 (supplemental Fig. S1A) in several aspects. Recombinant rat NRP-1 chimera (Fc rNRP-1) has the rat sequence of NRP-1, which shows high similarity (∼93% identity within the overlapping regions) to the human sequence (supplemental Fig. S1A). It covers all extracellular domains (a1, a2, b1, b2, and c) of the membrane NRP-1 and also fragments of the linker region that follows the c domain (where amino acids 811–828 are substituted by arginine residues, but subsequent sequence 829–854 is present). This sequence is fused to the Fc part of human IgG1 and a histidine tag. Consequently, the protein is expressed as a dimer due to the formation of disulfide bridges between the Fc domains of the two identical recombinant constructs. Upon SDS-PAGE in reducing conditions it migrates as a monomer around 150 kDa (supplemental Fig. S1D). Soluble truncated human NRP-1 (shNRP-1) encodes the human amino acid sequence of the native soluble isoform fused to a histidine tag (supplemental Fig. S1C). It differs from Fc rNRP-1 by the absence of the c domain and minor sequence variation due to alternative splicing (1) (supplemental Fig S2B). shNRP-1 migrates around 90 kDa upon SDS-PAGE in reducing conditions, and both proteins were confirmed to be pure as judged by silver staining (supplemental Fig. S1D). When analyzed under native conditions, Fc rNRP-1 migrates as a large aggregate, as expected from previous gel-filtration analyses with the same protein (41), while the migration pattern of shNRP-1 is not much affected (supplemental Fig. S1E).
Induction of Endothelial Tube Formation by Fc rNRP-1 and shNRP-1 via VEGFR-2 Activation
We utilized cultures of two different primary human endothelial cells to characterize recombinant NRP-1s' effect in vitro. First, the most commonly applied in endothelial research HUVECs; second, HDMECs, which are of similar characteristics to HUVECs (42), however, in contrast to the latter, they are of micro-, not macrovascular origin. This makes them a more appropriate subject of angiogenic studies, as angiogenesis is their direct physiological function (43, 44). The batches of HDMECs and HUVECs used in this study were confirmed to be CD31(+ve), VEGFR-2(+ve), and PROX-1(-ve), which supports their blood endothelial origin with minimal lymphatic endothelial contamination (data not shown).
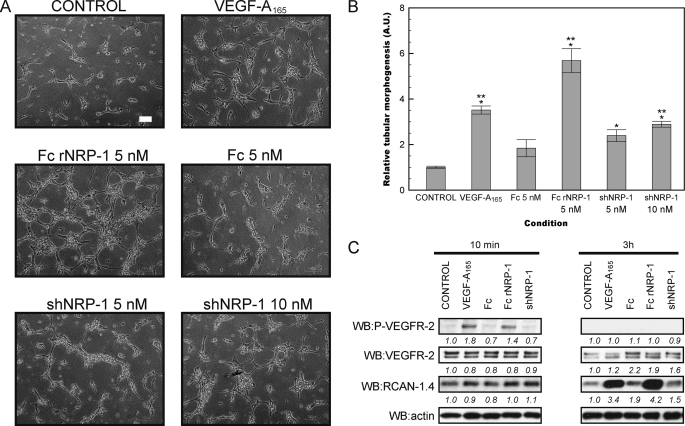
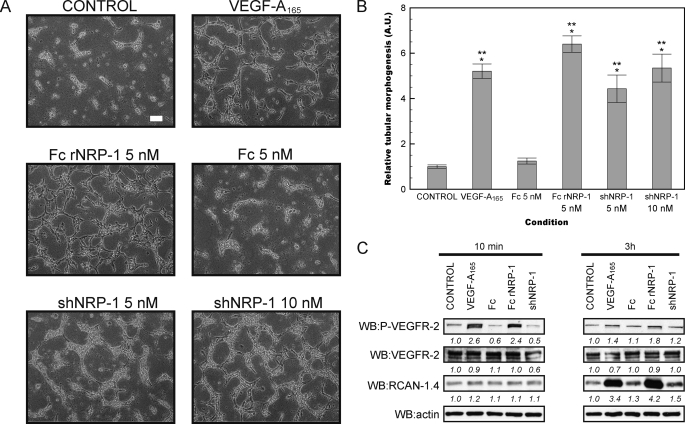
The tubular morphogenesis assay measures the ability of cells to form capillary-like structures in a three-dimensional collagen type I gel in response to growth factors and represents an in vitro angiogenesis assay (37, 45). This assay confirmed the ability of VEGF-A165 to induce angiogenesis in vitro in both HDMECs and HUVECs. It also demonstrated that both recombinant NRP-1 proteins induced tube formation (Figs. 1, A and B and 2, A and B). Fc rNRP-1 (5 nm) had the highest proangiogenic activity and at the applied conditions it exerted more potent stimulation of angiogenesis than VEGF-A165 (10 ng/ml; 224 pm). The Fc region (5 nm) of Fc rNRP-1 had little significant stimulatory effect on tube formation (p = 0.02 HDMEC, Fig. 1B and p = 0.03, HUVEC, Fig. 2B), demonstrating that the effects observed with the Fc rNRP-1 chimeric protein were likely to be due to the NRP-1 part. The monomeric shNRP-1 (5 nm) induced tube formation to a lesser extent than Fc rNRP-1. However, as the NRP-1 proteins were added at equimolar concentrations, the dimeric form of Fc rNRP-1 had double the content of NRP-1 moieties. Therefore, shNRP-1 was also tested at 10 nm, but this did not increase its ability to induce the formation of tubes in either of the cell lines (Figs. 1, A and B and 2, A and B), indicating that the dimerization of Fc rNRP-1 and/or the presence of the c domain is the most likely reason for the increased potency of the latter in this assay.
FIGURE 1.
Recombinant NRP-1 proteins stimulate tube formation in HDMEC and activate VEGFR-2 signaling. A and B, influence of addition of VEGF-A165 (10 ng/ml), Fc and Fc rNRP-1 (5 nm), and shNRP-1 (5 nm and 10 nm) on HDMEC tube formation on collagen substratum (white bar indicates 100 μm). The tubes were photographed and analyzed as described under “Experimental Procedures.” Significant differences between the tested agonists and the control were determined by Student's t test. Single asterisk (*) indicates that the p value is <0.001 for comparison to control; double asterisk (**) indicates that the p value is <0.01 for comparison to Fc. C, Western blot for phosphorylated VEGFR-2 and for RCAN-1.4 protein of cells from a parallel experiment conducted on a layer of collagen. Samples were collected for analysis 10 min and 3 h after addition of VEGF-A165 (10 ng/ml), Fc, shNRP-1, and Fc rNRP-1 (10 nm). The band intensities were analyzed, as described under “Experimental Procedures.”
FIGURE 2.
Recombinant NRP-1 proteins stimulate tube formation in HUVEC and activate VEGFR-2 signaling. A and B, influence of addition of VEGF-A165 (10 ng/ml), Fc and Fc rNRP-1 (5 nm), and shNRP-1 (5 nm and 10 nm) on HDMEC tube formation on collagen substratum (white bar indicates 100 μm). The tubes were photographed and analyzed as described under “Experimental Procedures.” Significant differences between the tested agonists and the control were determined by Student's t test. Single asterisk (*) indicates that the p value is <0.001 for comparison to control, double asterisk (**) indicates that the p value is <0.001 for comparison to Fc. C, Western blot for phosphorylated VEGFR-2 and RCAN-1.4 of cells from a parallel experiment conducted on a layer of collagen. Samples were collected for analysis 10 min and 3 h after addition of VEGF-A165 (10 ng/ml), Fc, shNRP-1, and Fc rNRP-1 (10 nm). The band intensities were analyzed as described under “Experimental Procedures.”
To identify the potential molecular partners of NRP-1 in the stimulation of in vitro angiogenesis, the activation of VEGFR-2 and induction of a downstream VEGF-responsive protein, RCAN-1.4/DSCR-1 (39, 46) (Uniprot P53805–2) (47) were analyzed by Western blotting. Surprisingly, Fc rNRP-1 in the absence of exogenous VEGF-A165 was able to stimulate the phosphorylation of VEGFR-2 in the cells plated on collagen (Figs. 1C and 2C). Moreover, after 3 h, the level of RCAN-1.4 was higher, supporting a VEGFR-2-dependent mechanism of activation of its synthesis (48). Importantly, in view of the small stimulatory effect of Fc seen in Figs. 1B and 2B, Fc alone had no detectable effect on the phosphorylation of VEGFR-2 or on the levels of RCAN-1.4. This, therefore, demonstrates that any effect of Fc is likely to be mediated through a different mechanism and unrelated to the effects of NRP-1 proteins. The same molar amount of shNRP-1 did not have any detectable effect on the signaling proteins tested, which is in accordance with its weaker angiogenic activity, and explained by the similar, but much weaker response it can induce. Indeed, when the amount of shNRP-1 is doubled a very weak activation of VEGFR-2 and ERK-1/2 is observed (Fig. 4, D and E), which supports the notion that the effects of shNRP-1 are the same, but less potent than those of Fc rNRP-1. Consequently, these data indicate that exogenously added recombinant Fc rNRP-1 and shNRP-1 are likely to elicit their proangiogenic response in a VEGFR-2 dependent manner both in HDMECs and HUVECs.
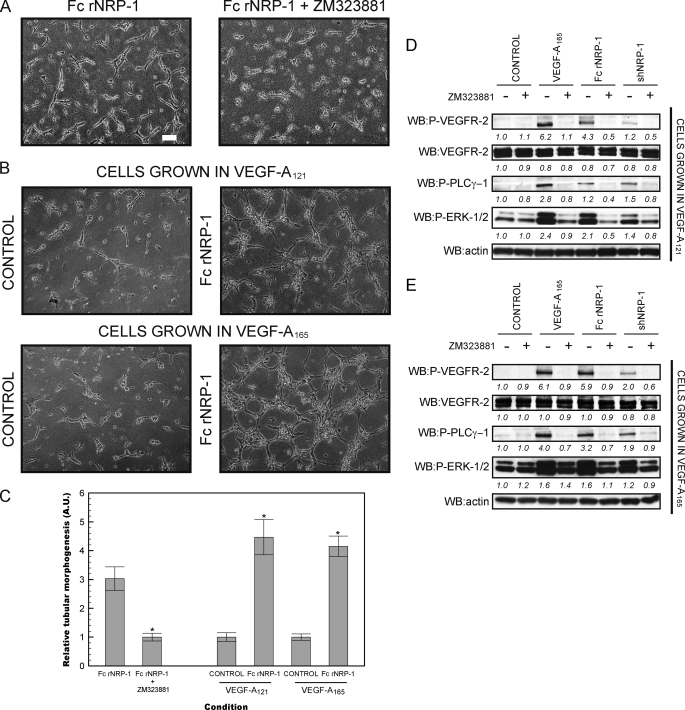
FIGURE 4.
The stimulation of tube formation by Fc rNRP-1 depends on VEGFR-2 kinase activity, but not on VEGF-A in HDMEC. A, HDMECs were placed in a collagen gel and stimulated with Fc rNRP-1 (5 nm) in the presence and absence of the inhibitor of the VEGFR-2 tyrosine kinase, ZM323881. B, angiogenic effect of Fc rNRP-1 (5 nm) on HDMEC cells grown 7 days prior to serum starvation in medium supplemented with VEGF-A165 or VEGF-A121 (white bar indicates 100 μm). C, tubes from A and B were photographed and analyzed as described under “Experimental Procedures.” Significance of the effect of addition of the ZM323881 inhibitor to the Fc rNRP-1 variant and the effect of the Fc rNRP-1 in the two tested pools of cells versus their respective controls were determined by Student's t test. Single asterisk (*) indicates that the p value is <0.001. D, Western blot of cells from a parallel experiment to B conducted on a collagen layer, where the top panel presents effect of stimulation with VEGF-A165 (10 ng/ml) and recombinant NRP-1 proteins (10 nm Fc rNRP-1 and 20 nm shNRP-1) on cells grown 7 days prior to serum starvation in medium supplemented with VEGF-A121, and panel E presents an experiment with cells grown in standard conditions in the medium supplemented with VEGF-A165. The band intensities were analyzed as described under “Experimental Procedures,” with the exception, that all band intensities were normalized to their respective actin bands.
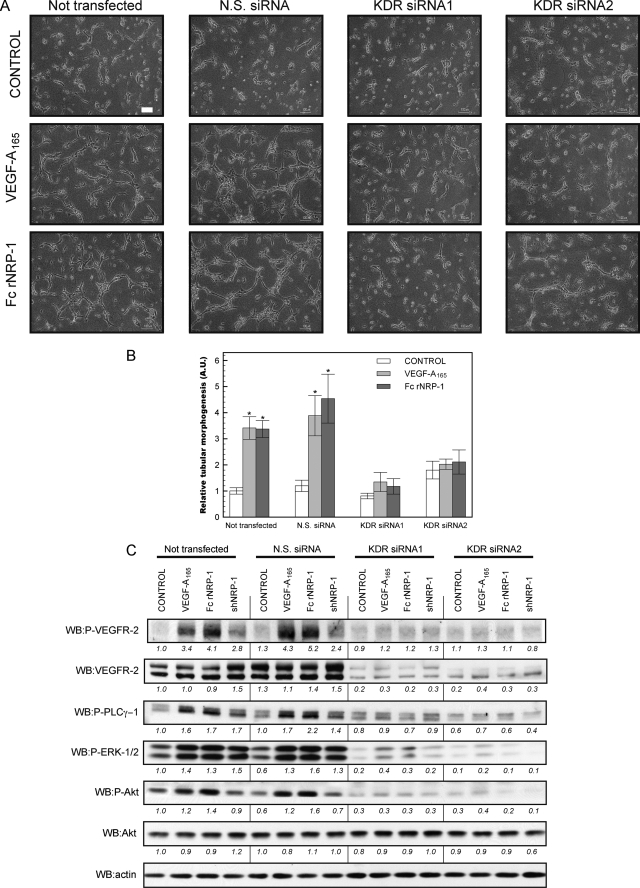
Knock-down of VEGFR-2 and Its Inhibition by a VEGFR-2 Specific Inhibitor ZM323881 Impede Fc rNRP-1 Response
As the previous data clearly indicated that VEGFR-2 was involved in the signaling cascade arising after Fc rNRP-1 administration, the next step we undertook was to verify the dependence of the effect of NRP-1 on the observed phosphorylation of VEGFR-2. To do so the HDMECs were transfected with two distinct siRNAs against human vegfr2 (kdr) and the effect on intracellular signaling of Fc rNRP-1 was analyzed. The result confirmed the crucial function of VEGFR-2 in mediating the downstream effect of NRP-1. First of all, after a knock-down of VEGFR-2 expression, no tubes were formed either in response to VEGF-A165 or Fc rNRP-1 (Fig. 3, A and B). Secondly, knock-down of VEGFR-2 expression substantially reduced the ability of VEGF-A165 and Fc rNRP-1 to stimulate VEGFR-2 phosphorylation and the concomitant activation of the downstream signaling molecules PLC-γ, AKT and ERK1/2 (Fig. 3C), which are critical components of the VEGFR-2 signaling pathway in endothelial cells (48) (Fig. 3C). This observation supports the earlier conclusion that NRP-1 elicits its proangiogenic effects through VEGFR-2.
FIGURE 3.
Silencing of the kdr gene impedes NRP-1 driven tube formation and related signaling in HDMEC. A and B, influence of addition of VEGF-A165 (10 ng/ml) and Fc rNRP-1 (5 nm) on HDMEC tube formation on collagen substratum (white bar indicates 100 μm) on four pools of cells, where one comprised of untrasfected cells, second of the cells transfected with non-silencing siRNA (N.S. siRNA) and third and fourth of cells transfected by two different siRNAs against kdr gene. The tubes were photographed and analyzed as described under “Experimental Procedures.” The relative values depicting tubular morphogenesis were normalized versus control variant of the untransfected cells. Significant differences between the tested agonists and their respective controls were determined by Student's t test. Single asterisk (*) indicates that the p value is <0.01. C, Western blot of each pool of cells from a parallel experiment conducted on a layer of collagen. Samples were collected for analysis 10 min after addition of VEGF-A165 (10 ng/ml), shNRP-1, and Fc rNRP-1 (10 nm). The band intensities were analyzed as described under “Experimental Procedures” and normalized versus the untransfected cell control.
To confirm the results obtained with siRNAs, a pharmacological approach directed against VEGFR-2 was then used to block the VEGFR-2 kinase. The cells were treated with ZM323881, a potent cell-permeable inhibitor of the VEGFR-2 tyrosine kinase (35) (Fig. 4). This inhibitor has the benefit of a very high affinity for the VEGFR-2 kinase (<2 nm), and at the same time much lower affinity for other related receptor tyrosine kinases, including VEGFR-1, FGFR-1, EGFR, ErbB2, and PDGFR-β (>50 μm), which makes it a highly specific VEGFR-2 inhibitor. Under these conditions, Fc rNRP-1 stimulation of tube formation in HDMECs was fully blocked (Fig. 4A). The efficiency of the inhibitor was confirmed by Western blot, where it was able to abolish detectable phosphorylation of VEGFR-2 and downstream responses induced either by VEGF-A165 or the recombinant NRP-1 proteins on collagen substrata (Fig. 4, D and E). These data demonstrate that NRP-1 stimulates angiogenesis by stimulating VEGFR-2 phosphorylation and activation of signaling cascades downstream of the receptor.
Fc rNRP-1-driven Tube Formation Occurs in VEGF-A165-depleted Cells
The recombinant NRP-1 proteins were shown to induce angiogenesis in an exclusively VEGFR-2-dependent manner and an inhibitor of the VEGFR-2 tyrosine kinase could block this response. However, most of the published data have been interpreted to suggest that NRP-1 exerts its angiogenic effects by potentiating the effects of VEGF-A165, as generally these experiments were performed in the presence of VEGF-A165 (34, 49, 50). In contrast, the experimental design of the present study implied lack of involvement of endogenous VEGF-A165, because of the 24 h incubation of the HDMECs in low-serum medium in the absence of VEGF-A165, prior to the addition of NRP-1 proteins (51–54). To rigorously exclude the presence of small amounts of VEGF-A165 carried over from the culture medium or bound to extracellular matrix, the cells were grown for at least 7 days in medium containing 0.5 ng/ml VEGF-A121 isoform, instead of VEGF-A165. Cells grown in such conditions are most unlikely to contain any residual VEGF-A165 and the response observed is expected to be VEGF-A165 independent. In parallel, another batch of cells was grown in the standard conditions containing VEGF-A165 for the purpose of comparison. Cells grown in both conditions appeared to be similarly sensitive to Fc rNRP-1 (Fig. 4, B and C). Additionally, they showed exactly the same profile of signaling activation, whereby VEGF-A165, Fc rNRP-1 and to a lesser extent shNRP-1 (whose amount was doubled to 20 nm, whereas Fc rNRP-1 was at 10 nm), caused immediate phosphorylation of VEGFR-2 and activation of PLCγ-1 and ERK-1/2 on collagen (Fig. 4, D and E). This shows that cells devoid of VEGF-A165 by extended medium depletion are also induced by the NRP-1 proteins to undergo a proangiogenic response.
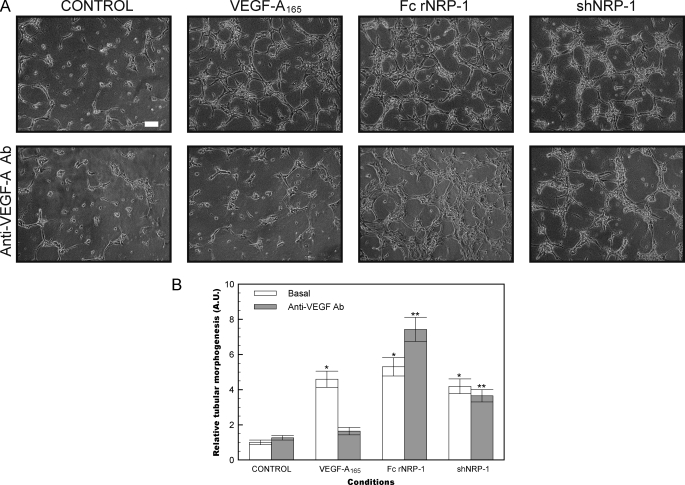
To further eliminate the possibility of VEGF-A165 contributing to the angiogenic activity of the NRP-1 proteins, a sequestering antibody that targets the N-terminal region of human VEGF-A was introduced into the tube formation assay. The antibody successfully blocked the tube formation of exogenously administered VEGF-A165, however, the stimulation of the formation of tubes by the recombinant NRP-1 proteins was completely unaffected by the VEGF-A sequestering antibody (Fig. 5).
FIGURE 5.
Effect of a sequestering antibody to VEGF-A on tube formation by HDMEC. A, top panels present controls (VEGF-A165 at 10 ng/ml and Fc rNRP-1 and shNRP-1 at 5 nm), while the bottom panels present the same conditions, but with the addition of VEGF-A sequestering antibody (white bar indicates 100 μm). B, tubes were photographed and analyzed as described under “Experimental Procedures.” The relative values depicting tubular morphogenesis were normalized versus basal variant of the control cells. Significant differences between the tested agonists and their respective controls were determined by Student's t test. Single asterisk (*) indicates that the p value is <0.001 versus basal control, double asterisk (**) indicates that the p value is <0.001 for comparison to the control variant containing antibody.
Fc rNRP-1 Enhances Migration of HDMECs and HUVECs
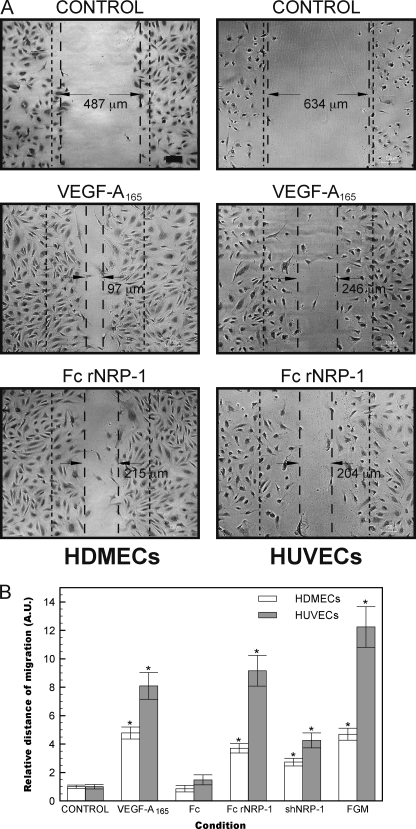
VEGF is known to stimulate the migration of endothelial cells. To further investigate the physiological effects of Fc rNRP-1 we conducted a series of migration assays of HDMECs and HUVECs. The scratch wound assay revealed that both NRP-1s could also drive a significant pro-migratory response (Fig. 6). This further reinforced our findings and supports the hypothesis of Fc rNRP-1 stimulating angiogenesis.
FIGURE 6.
Fc rNRP-1, similarly to VEGF-A165, induces HDMEC and HUVEC migration in a scratch wound assay. A, left panels present the migratory effect after addition of VEGF-A165 (10 ng/ml) and Fc rNRP-1 (5 nm) in HDMEC, while the right panels in HUVEC. The dashed lines present the arbitrary scope of migration, the dense dashed lines indicate the approximate width of the initial scratch (black bar indicates 100 μm). The photos presented are representatives of three independent measurements, absolute values of the measurements are indicated. B, migration distance was calculated as described under “Experimental Procedures.” Additional variants are included in the graph, where 5 nm Fc, 10 nm shNRP-1, and full-growth medium (FGM) migration is presented. Significant differences between the tested agonists and their respective controls were determined by Student's t test. Single asterisk (*) indicates that the p value is <0.001.
Fc rNRP-1 Drives Tube Formation in a Long-term Fibroblast-HDMEC Co-culture
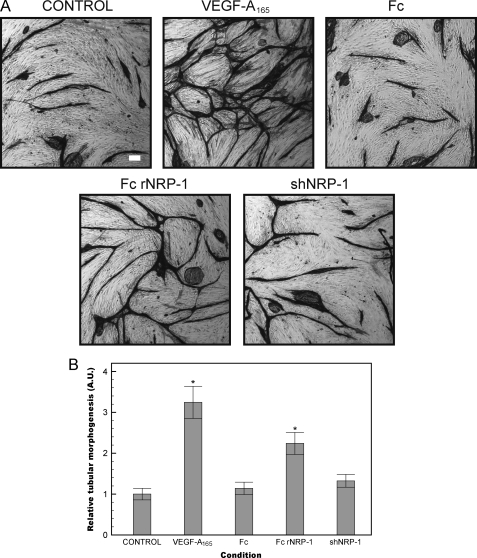
Angiogenesis involves the co-ordinated migration, proliferation, and eventual differentiation of endothelial cells into a lumen-containing vessel (45, 55). We utilized an endothelial/fibroblast co-culture assay to investigate the role of the recombinant NRPs in an assay representative of in vivo angiogenesis (56, 57). In this assay HDMECs are plated on a confluent monolayer of fibroblasts and stimulated with agonist for a period of 6 days and the extent of tubular network formation is revealed by staining for the endothelial-specific marker CD31. This assay confirmed that Fc rNRP-1 was able to evoke an angiogenic response (Fig. 7). In contrast, the shNRP-1 was not able to evoke significant angiogenesis in this assay.
FIGURE 7.
Fc rNRP-1 drives angiogenesis in a fibroblast-HDMEC co-culture. A, HDMEC cells were grown for 6 days on a monolayer of confluent human dermal fibroblasts in the presence of VEGF-A165 (30 ng/ml), Fc and Fc rNRP-1 (10 nm), and shNRP-1 (20 nm) (white bar indicates 100 μm). B, tube-like structures were photographed after staining for CD31 and analyzed as described under “Experimental Procedures.” The relative values depicting tubular morphogenesis were normalized versus the control cells. Significant differences between the tested agonists and the control were determined by Student's t test. Single asterisk (*) indicates that the p value is <0.01 for comparison to control.
DISCUSSION
The addition of Fc rNRP-1/shNRP-1 triggered tubular morphogenesis in two distinct endothelial cell types, HUVECs and HDMECs. This early in vitro angiogenic response was a consequence of the activation of the VEGFR-2 receptor and was inhibited by siRNA directed to kdr and a specific inhibitor of this receptor's kinase. At the same time, cells depleted of VEGF-A165 or incubated with a sequestering antibody to VEGF-A could still be induced by the NRP-1 proteins to form tubes. Therefore, we conclude that Fc rNRP-1, and to a lesser extent shNRP-1, can behave as a VEGFR-2 agonist, possessing a weaker (shNRP-1) or comparable/stronger (Fc rNRP-1) pro-angiogenesis activity compared with the VEGF-A165 ligand in two different types of endothelial cell, HDMECs and HUVECs. Thus, the present results extend considerably the regulatory potential of NRP-1.
Fc rNRP-1 was able to stimulate phosphorylation of VEGFR-2 and evoke a robust signaling response confirmed by activation of PLC-γ, AKT, and ERK1/2, which are critical downstream mediators of VEGFR-2 activation regulating proliferation and survival of endothelial cells (48). The small activating effect of the Fc observed on Figs. 1 and 2 can be explained by the presence of the Fc receptors on the endothelial cells (58) and their downstream signaling, since no detectable VEGFR-2 activation was observed and no long-term morphological changes could be evoked by Fc. Therefore, we conclude that this activity is unrelated to the proangiogenic activity of NRP-1 proteins observed in this study.
The few studies that have focused on the angiogenic activity of recombinant NRP-1, as opposed to its activity in the presence of VEGF-A165, have provided contradictory views. A study employing monomeric full-length NRP-1 suggested that it inhibited angiogenesis and caused necrosis in chloroma solid tumor in SCID mice. Moreover, localized expression of this dimeric form was able to block tumor progression (49). However, the inhibitory effect of NRP-1 in these experiments may be due to the NRP-1 sequestering VEGF-A165 from the immediate environment of the endothelial cells. A similar effect was observed in another study, conducted in explants of para-aortic splanchnopleural mesoderm cells, where monomeric NRP-1 was found to have an inhibitory effect on vasculogenesis (50). Further studies of dimeric NRP-1 provide more contradictory data, perhaps related to different experimental systems. According to one study, a chimeric construct similar to the one used here was shown to bind to endothelial cells expressing VEGFR-2 only in the presence of VEGF-A165 (34), whereas another study showed that the dimeric form of the protein induced angiogenesis in the presence of VEGF-A165 and alone, however even 50 μg/ml of the dimeric NRP-1 alone had no comparable activity to VEGF-A165 (50). In the presented results the pro-angiogenic activity of Fc rNRP-1 was stronger than in the case of the shNRP-1 protein, which may be explained by the dimerization of the protein by the Fc fusion (49, 59). Alternatively the c domain, which is known to cause dimerization of native NRP-1 (60, 61) and may have other functions may be critical for NRP-1 angiogenic activity.
Overall, the present findings suggest that the dimerization of NRP-1 by the Fc domain and/or the presence of the c domain results in a strong VEGFR-2 agonist. Thus, Fc NRP-1 may be a good candidate for therapeutic angiogenesis in conditions requiring revascularization such as ischemia (62). It elicits a strong proangiogenic response and could be an additional/complementary contribution in the well-studied VEGF-A165-based therapies (63). To become clinically relevant, Fc rNRP-1 would need to undergo two major adaptations: humanization and reduction of Fc function (64), which are both currently attainable and allow reduction of undesirable immunological response without affecting the benefits of the fusion nature (64). The practical significance is that the half-life of VEGF-A165, does not exceed 1 h (65), limiting the amount of administered agonist. Importantly, the modified Fc rNRP-1 may have a considerably longer half-life, as has been found with analogous fusion proteins, which have been already shown successful in preclinical and clinical studies (66), such as etanercept (Fc-p75 TNF receptor) (67), alefacept (Fc-LFA-3) (68), and abatacept (Fc-CTLA-4) (69), Fc-endostatin (70), and VEGF-TRAP (selected VEGFR-1/2 domains trapping VEGF-A independently and Fc) (71). Thus the discovery that Fc rNRP-1 is a potent VEGFR-2 agonist opens new possibilities for the development of proangiogenic therapies using a powerful approach that so far has been scarcely employed in proangiogenic field (72).
Supplementary Material
This work was supported by the European Commission (Marie Curie Early Stage Training Fellowship, to K. A. U.), the North West Cancer Research Fund, and the Cancer and Polio Research Fund.

The on-line version of this article (available at http://www.jbc.org) contains supplemental Figs. S1 and S2.
- NRP-1
- neuropilin-1
- DSCR-1
- Down Syndrome Critical Region-1
- HDMECs
- human dermal microvascular endothelial cells
- HUVECs
- human umbilical vein endothelial cells
- KDR
- kinase insert domain receptor
- Fc rNRP-1
- Fc linked rat neuropilin-1
- shNRP-1
- soluble human neuropilin-1
- PLCγ
- phospholipase C-γ
- RCAN
- regulator of calcineurin
- VEGFR
- vascular endothelial growth factor receptor.
REFERENCES
- 1. Uniewicz K. A., Fernig D. G. (2008) Front. Biosci. 13, 4339–4360 [DOI] [PubMed] [Google Scholar]
- 2. Zachary I. C., Frankel P., Evans I. M., Pellet-Many C. (2009) Biochem. Soc. Trans. 37, 1171–1178 [DOI] [PubMed] [Google Scholar]
- 3. Sarris M., Andersen K. G., Randow F., Mayr L., Betz A. G. (2008) Immunity 28, 402–413 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Fukasawa M., Matsushita A., Korc M. (2007) Cancer Biol. Ther. 6, 1173–1180 [DOI] [PubMed] [Google Scholar]
- 5. Valdembri D., Caswell P. T., Anderson K. I., Schwarz J. P., König I., Astanina E., Caccavari F., Norman J. C., Humphries M. J., Bussolino F., Serini G. (2009) PLoS Biol. 7, e25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Glinka Y., Prud'homme G. J. (2008) J. Leukoc. Biol. 84, 302–310 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Gualandris A., Noghero A., Geuna M., Arese M., Valdembri D., Serini G., Bussolino F. (2009) FASEB J. 23, 68–78 [DOI] [PubMed] [Google Scholar]
- 8. Chitteti B. R., Cheng Y. H., Poteat B., Rodriguez-Rodriguez S., Goebel W. S., Carlesso N., Kacena M. A., Srour E. F. (2010) Blood 115, 3239–3248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Ball S. G., Bayley C., Shuttleworth C. A., Kielty C. M. (2010) Biochem. J. 427, 29–40 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Lambert S., Bouttier M., Vassy R., Seigneuret M., Petrow-Sadowski C., Janvier S., Heveker N., Ruscetti F. W., Perret G., Jones K. S., Pique C. (2009) Blood 113, 5176–5185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Kitsukawa T., Shimono A., Kawakami A., Kondoh H., Fujisawa H. (1995) Development 121, 4309–4318 [DOI] [PubMed] [Google Scholar]
- 12. Herzog Y., Kalcheim C., Kahane N., Reshef R., Neufeld G. (2001) Mech. Dev. 109, 115–119 [DOI] [PubMed] [Google Scholar]
- 13. Soker S., Takashima S., Miao H. Q., Neufeld G., Klagsbrun M. (1998) Cell 92, 735–745 [DOI] [PubMed] [Google Scholar]
- 14. Kitsukawa T., Shimizu M., Sanbo M., Hirata T., Taniguchi M., Bekku Y., Yagi T., Fujisawa H. (1997) Neuron 19, 995–1005 [DOI] [PubMed] [Google Scholar]
- 15. Bielenberg D. R., Pettaway C. A., Takashima S., Klagsbrun M. (2006) Exp. Cell Res. 312, 584–593 [DOI] [PubMed] [Google Scholar]
- 16. Kawakami T., Tokunaga T., Hatanaka H., Kijima H., Yamazaki H., Abe Y., Osamura Y., Inoue H., Ueyama Y., Nakamura M. (2002) Cancer 95, 2196–2201 [DOI] [PubMed] [Google Scholar]
- 17. Staton C. A., Kumar I., Reed M. W., Brown N. J. (2007) J. Pathol. 212, 237–248 [DOI] [PubMed] [Google Scholar]
- 18. Kawamura H., Li X., Goishi K., van Meeteren L. A., Jakobsson L., Cébe-Suarez S., Shimizu A., Edholm D., Ballmer-Hofer K., Kjellén L., Klagsbrun M., Claesson-Welsh L. (2008) Blood 112, 3638–3649 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Narazaki M., Tosato G. (2006) Blood 107, 3892–3901 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Narazaki M., Segarra M., Tosato G. (2008) Blood 111, 4126–4136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Matsushita A., Götze T., Korc M. (2007) Cancer Res. 67, 10309–10316 [DOI] [PubMed] [Google Scholar]
- 22. Castellani V., Falk J., Rougon G. (2004) Mol. Cell. Neurosci. 26, 89–100 [DOI] [PubMed] [Google Scholar]
- 23. Geretti E., Klagsbrun M. (2007) Cell Adh. Migr. 1, 56–61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Bartsch G., Jr., Eggert K., Soker S., Bokemeyer C., Hautmann R., Schuch G. (2008) J. Urol. 179, 326–332 [DOI] [PubMed] [Google Scholar]
- 25. Hong T. M., Chen Y. L., Wu Y. Y., Yuan A., Chao Y. C., Chung Y. C., Wu M. H., Yang S. C., Pan S. H., Shih J. Y., Chan W. K., Yang P. C. (2007) Clin. Cancer Res. 13, 4759–4768 [DOI] [PubMed] [Google Scholar]
- 26. Barr M. P., Byrne A. M., Duffy A. M., Condron C. M., Devocelle M., Harriott P., Bouchier-Hayes D. J., Harmey J. H. (2005) Br. J. Cancer 92, 328–333 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Starzec A., Ladam P., Vassy R., Badache S., Bouchemal N., Navaza A., du Penhoat C. H., Perret G. Y. (2007) Peptides 28, 2397–2402 [DOI] [PubMed] [Google Scholar]
- 28. Liang W. C., Dennis M. S., Stawicki S., Chanthery Y., Pan Q., Chen Y., Eigenbrot C., Yin J., Koch A. W., Wu X., Ferrara N., Bagri A., Tessier-Lavigne M., Watts R. J., Wu Y. (2007) J. Mol. Biol. 366, 815–829 [DOI] [PubMed] [Google Scholar]
- 29. Jarvis A., Allerston C. K., Jia H., Herzog B., Garza-Garcia A., Winfield N., Ellard K., Aqil R., Lynch R., Chapman C., Hartzoulakis B., Nally J., Stewart M., Cheng L., Menon M., Tickner M., Djordjevic S., Driscoll P. C., Zachary I., Selwood D. L. (2010) J. Med. Chem. 53, 2215–2226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Sugahara K. N., Teesalu T., Karmali P. P., Kotamraju V. R., Agemy L., Girard O. M., Hanahan D., Mattrey R. F., Ruoslahti E. (2009) Cancer Cell 16, 510–520 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Bechet D., Tirand L., Faivre B., Plénat F., Bonnet C., Bastogne T., Frochot C., Guillemin F., Barberi-Heyob M. (2010) Pharm. Res. 27, 468–479 [DOI] [PubMed] [Google Scholar]
- 32. Teesalu T., Sugahara K. N., Kotamraju V. R., Ruoslahti E. (2009) Proc. Natl. Acad. Sci. U.S.A. 106, 16157–16162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Slimani H., Guenin E., Briane D., Coudert R., Charnaux N., Starzec A., Vassy R., Lecouvey M., Perret Y. G., Cao A. (2006) J. Drug Target. 14, 694–706 [DOI] [PubMed] [Google Scholar]
- 34. Soker S., Miao H. Q., Nomi M., Takashima S., Klagsbrun M. (2002) J. Cell. Biochem. 85, 357–368 [DOI] [PubMed] [Google Scholar]
- 35. Whittles C. E., Pocock T. M., Wedge S. R., Kendrew J., Hennequin L. F., Harper S. J., Bates D. O. (2002) Microcirculation 9, 513–522 [DOI] [PubMed] [Google Scholar]
- 36. Roberts O. L., Holmes K., Müller J., Cross D. A., Cross M. J. (2010) J. Cell Sci. 123, 3189–3200 [DOI] [PubMed] [Google Scholar]
- 37. Mellberg S., Dimberg A., Bahram F., Hayashi M., Rennel E., Ameur A., Westholm J. O., Larsson E., Lindahl P., Cross M. J., Claesson-Welsh L. (2009) FASEB J. 23, 1490–1502 [DOI] [PubMed] [Google Scholar]
- 38. Heukeshoven J., Dernick R. (1988) Electrophoresis 9, 28–32 [DOI] [PubMed] [Google Scholar]
- 39. Holmes K., Chapman E., See V., Cross M. J. (2010) PLoS ONE 5, e11435 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Niemistö A., Dunmire V., Yli-Harja O., Zhang W., Shmulevich I. (2005) IEEE Trans. Med. Imaging 24, 549–553 [DOI] [PubMed] [Google Scholar]
- 41. West D. C., Rees C. G., Duchesne L., Patey S. J., Terry C. J., Turnbull J. E., Delehedde M., Heegaard C. W., Allain F., Vanpouille C., Ron D., Fernig D. G. (2005) J. Biol. Chem. 280, 13457–13464 [DOI] [PubMed] [Google Scholar]
- 42. Unger R. E., Krump-Konvalinkova V., Peters K., Kirkpatrick C. J. (2002) Microvasc. Res. 64, 384–397 [DOI] [PubMed] [Google Scholar]
- 43. Harper S. J., Bates D. O. (2008) Nat. Rev. Cancer 8, 880–887 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Peirce S. M. (2008) Microcirculation 15, 739–751 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Montesano R., Orci L., Vassalli P. (1983) J. Cell Biol. 97, 1648–1652 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Hesser B. A., Liang X. H., Camenisch G., Yang S., Lewin D. A., Scheller R., Ferrara N., Gerber H. P. (2004) Blood 104, 149–158 [DOI] [PubMed] [Google Scholar]
- 47. Fuentes J. J., Pritchard M. A., Estivill X. (1997) Genomics 44, 358–361 [DOI] [PubMed] [Google Scholar]
- 48. Holmes K., Roberts O. L., Thomas A. M., Cross M. J. (2007) Cell. Signal. 19, 2003–2012 [DOI] [PubMed] [Google Scholar]
- 49. Schuch G., Machluf M., Bartsch G., Jr., Nomi M., Richard H., Atala A., Soker S. (2002) Blood 100, 4622–4628 [DOI] [PubMed] [Google Scholar]
- 50. Yamada Y., Takakura N., Yasue H., Ogawa H., Fujisawa H., Suda T. (2001) Blood 97, 1671–1678 [DOI] [PubMed] [Google Scholar]
- 51. Gupta K., Kshirsagar S., Li W., Gui L., Ramakrishnan S., Gupta P., Law P. Y., Hebbel R. P. (1999) Exp. Cell Res. 247, 495–504 [DOI] [PubMed] [Google Scholar]
- 52. Abcouwer S. F., Marjon P. L., Loper R. K., Vander Jagt D. L. (2002) Invest. Ophthalmol. Vis. Sci. 43, 2791–2798 [PubMed] [Google Scholar]
- 53. Bartoli M., Platt D., Lemtalsi T., Gu X., Brooks S. E., Marrero M. B., Caldwell R. B. (2003) FASEB J. 17, 1562–1564 [DOI] [PubMed] [Google Scholar]
- 54. Uchida K., Uchida S., Nitta K., Yumura W., Marumo F., Nihei H. (1994) Am. J. Physiol. 266, F81–88 [DOI] [PubMed] [Google Scholar]
- 55. Koike N., Fukumura D., Gralla O., Au P., Schechner J. S., Jain R. K. (2004) Nature 428, 138–139 [DOI] [PubMed] [Google Scholar]
- 56. Donovan D., Brown N. J., Bishop E. T., Lewis C. E. (2001) Angiogenesis 4, 113–121 [DOI] [PubMed] [Google Scholar]
- 57. Sorrell J. M., Baber M. A., Caplan A. I. (2007) Cells Tissues Organs 186, 157–168 [DOI] [PubMed] [Google Scholar]
- 58. Sedmak D. D., Davis D. H., Singh U., van de Winkel J. G., Anderson C. L. (1991) Am. J. Pathol. 138, 175–181 [PMC free article] [PubMed] [Google Scholar]
- 59. Schneider P. (2000) Methods Enzymol. 322, 325–345 [DOI] [PubMed] [Google Scholar]
- 60. Nakamura F., Tanaka M., Takahashi T., Kalb R. G., Strittmatter S. M. (1998) Neuron 21, 1093–1100 [DOI] [PubMed] [Google Scholar]
- 61. Giger R. J., Urquhart E. R., Gillespie S. K., Levengood D. V., Ginty D. D., Kolodkin A. L. (1998) Neuron 21, 1079–1092 [DOI] [PubMed] [Google Scholar]
- 62. Cristofaro B., Emanueli C. (2009) Curr. Opin. Pharmacol. 9, 102–108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Ferrara N. (1999) Kidney Int. 56, 794–814 [DOI] [PubMed] [Google Scholar]
- 64. An Z., Forrest G., Moore R., Cukan M., Haytko P., Huang L., Vitelli S., Zhao J. Z., Lu P., Hua J., Gibson C. R., Harvey B. R., Montgomery D., Zaller D., Wang F., Strohl W. (2009) mAbs 1, 572–579 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Eppler S. M., Combs D. L., Henry T. D., Lopez J. J., Ellis S. G., Yi J. H., Annex B. H., McCluskey E. R., Zioncheck T. F. (2002) Clin. Pharmacol. Ther. 72, 20–32 [DOI] [PubMed] [Google Scholar]
- 66. Huang C. (2009) Curr. Opin. Biotechnol. 20, 692–699 [DOI] [PubMed] [Google Scholar]
- 67. Scallon B., Cai A., Solowski N., Rosenberg A., Song X. Y., Shealy D., Wagner C. (2002) J. Pharmacol. Exp. Ther. 301, 418–426 [DOI] [PubMed] [Google Scholar]
- 68. da Silva A. J., Brickelmaier M., Majeau G. R., Li Z., Su L., Hsu Y. M., Hochman P. S. (2002) J. Immunol. 168, 4462–4471 [DOI] [PubMed] [Google Scholar]
- 69. Fiocco U., Sfriso P., Oliviero F., Pagnin E., Scagliori E., Campana C., Dainese S., Cozzi L., Punzi L. (2008) Autoimmun. Rev. 8, 76–82 [DOI] [PubMed] [Google Scholar]
- 70. Lee T. Y., Tjin Tham Sjin R. M., Movahedi S., Ahmed B., Pravda E. A., Lo K. M., Gillies S. D., Folkman J., Javaherian K. (2008) Clin. Cancer Res. 14, 1487–1493 [DOI] [PubMed] [Google Scholar]
- 71. Holash J., Davis S., Papadopoulos N., Croll S. D., Ho L., Russell M., Boland P., Leidich R., Hylton D., Burova E., Ioffe E., Huang T., Radziejewski C., Bailey K., Fandl J. P., Daly T., Wiegand S. J., Yancopoulos G. D., Rudge J. S. (2002) Proc. Natl. Acad. Sci. U.S.A. 99, 11393–11398 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Foubert P., Silvestre J. S., Souttou B., Barateau V., Martin C., Ebrahimian T. G., Leré-Déan C., Contreres J. O., Sulpice E., Levy B. I., Plouët J., Tobelem G., Le Ricousse-Roussanne S. (2007) J. Clin. Invest. 117, 1527–1537 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.