Abstract
Phagocytosis of foreign pathogens by cells of the immune system is a vitally important function of innate immunity. The phagocytic response is initiated when ligands on the surface of invading microorganisms come in contact with receptors on the surface of phagocytic cells such as neutrophils, monocytes/macrophages, and dendritic cells. The complement receptor CR3 (CD11b/CD18, Mac-1) mediates the phagocytosis of complement protein (C3bi)-coated particles. Fcγ receptors (FcγRs) bind IgG-opsonized particles and provide a mechanism for immune clearance and phagocytosis of IgG-coated particles. We have observed that stimulation of FcγRs modulates CR3-mediated phagocytosis and that FcγRIIA and FcγRI exert opposite (stimulatory and inhibitory) effects. We have also determined that an intact FcγR immunoreceptor tyrosine-based activation motif is required for these effects, and we have investigated the involvement of downstream effectors. The ability to up-regulate or down-regulate CR3 signaling has important implications for therapeutics in disorders involving the host defense system.
Keywords: Complement, Immunology, Macrophage, Phagocytosis, Signal Transduction, Fcγ Receptor, Complement Receptor 3
Introduction
Both complement receptor 3 (CR3)3 and Fcγ receptors (FcγRs) play essential roles in the immune response to infection and in inflammatory processes (1–8). CR3 (CD11b/CD18, Mac-1), a member of the β2 integrin family, is a heterodimeric receptor composed of a unique α-chain (CD11b) and a β-chain (CD18) common to other β2 integrin family members (1–5). CR3 is expressed primarily on monocytes/macrophages and neutrophils in humans and mice and is activated during the initial immune response to infection. C3b, a cleavage product of the CR3 ligand C3, coats invading microbes and is further cleaved to form C3bi, which is recognized and bound by CR3, thus initiating a phagocytic response.
FcγRs provide a mechanism for the clearance and phagocytosis of IgG-coated particles (6–10). The FcγR response to infection becomes prominent later in host defense when significant IgG production occurs as part of the adaptive immune response. FcγRI and FcγRIIIA consist of an α-chain, which contains the extracellular ligand-binding site, and an associated γ-chain, required for signaling (9–13). The γ-chain contains within its cytoplasmic domain the immunoreceptor tyrosine-based activation motif (ITAM), required for many Ig gene family signaling events (14, 15). FcγRI and FcγRIIIA are expressed on human and murine macrophages (9, 10). FcγRIIA is unique to humans and is expressed on monocytes, macrophages, neutrophils, dendritic cells, and platelets (9, 10). FcγRIIA contains an ITAM sequence within its own cytoplasmic domain and does not require the γ-chain for signaling (9, 10, 16). Upon engagement of these FcγRs, the ITAM tyrosines in both the FcR γ-chain and FcγRIIA are rapidly phosphorylated (17). The tandem Src homology 2 domains of the tyrosine kinase Syk bind the diphosphorylated ITAM, leading to the formation of a signaling complex at the cell membrane. Within the complex, Syk-mediated phosphorylation of several adaptor proteins activates downstream signaling pathways (9, 10, 17). FcγRIIB is also expressed on monocytes and macrophages in humans and mice (18–20). This receptor, whose ligand-binding extracellular domain is highly homologous to FcγRIIA (18), contains an immunoreceptor tyrosine-based inhibition motif within its cytoplasmic domain, and, in contrast to the other FcγRs, FcγRIIB does not mediate phagocytosis. Rather, stimulation of this receptor results in the down-regulation of many FcγR signaling events (20).
There is evidence that signaling through CR3 can affect signaling by FcγRs (21–26). Early studies reported that the presence of C3 dramatically reduces the amount of bound IgG required to induce FcγR-mediated particle ingestion by monocytes (21, 22). A later study indicated that blocking CR3 function with anti-CR3 antibodies reduces FcγR-mediated phagocytosis without impairing binding of FcγR to IgG (23). There is also evidence that FcγR stimulation can impact the function of CR3. The binding of IgG to FcγR in a mouse macrophage cell line (RAW264.7) induces the lateral movement of CR3 to FcγR-containing phagocytic cups, stimulates macrophage adhesion to surfaces coated with CR3 ligands, and enhances C3bi binding to CR3 (27). The exact mechanism(s) by which each receptor affects the function of the interacting receptor, however, remains unclear.
We have now observed that CR3-mediated signaling for phagocytosis is altered when FcγRs and CR3 are co-stimulated and that individual FcγRs play different roles in modulating CR3-mediated phagocytosis. Co-stimulation of CR3 with FcγRI inhibits CR3-mediated phagocytosis whereas co-stimulation of CR3 with FcγRIIA enhances CR3-mediated phagocytosis. We show that these effects of FcγRs on CR3 are dependent upon the availability of an intact FcγR ITAM. These observations are important in that they present a potential for selective modulation of the immune response.
EXPERIMENTAL PROCEDURES
Animals, Antibodies, and Reagents
Six- to 10-week-old male and female C57/BL6 mice were used throughout this study. FcγRIIA transgenic (IIA-TG) mice were kindly provided by Dr. Steven E. McKenzie (Thomas Jefferson University, Philadelphia, PA). γ-KO mice were obtained from Taconic Laboratories (Germantown, NY). Mice expressing FcγRIIA and lacking the γ-chain (IIA-TG × γ-KO) were produced by crossing FcγRIIA-TG mice with γ-chain KO mice. All protocols were performed in accordance with National Institutes of Health guidelines and with the approval of the University of Pennsylvania Animal Use Committee. Anti-human FcγRII mAb IV.3 and IV.3 F(ab′)2 fractions were purified and prepared in our laboratory using established methods. Anti-mouse FcγRIIB/FcγRIIIA mAb 2.4G2 was obtained from BD Bioscience (San Jose, CA). Anti-Vav antibody (C-14) and anti-actin antibody (I-19) were obtained from Santa Cruz Biotechnology (Santa Cruz, CA), and anti-phosphotyrosine antibody 4G10 from Upstate Laboratories (Charlottesville, VA).
Cell Culture and Transfection
COS cells were cultured and maintained in Dulbecco's modified Eagle's medium containing glucose (4.5 mg/ml), glutamine (2 mm), streptomycin (100 units/ml), penicillin (100 μg/ml), and 10% heat-inactivated fetal bovine serum (FBS). COS cells stably expressing WT FcγRIIA (COS IIA) and COS cells stably expressing a mutant FcγRIIA whose ITAM tyrosines are replaced with phenylalanine (COS IIA-Y288F/Y304F) were prepared by calcium phosphate transfection as described previously (28). FcγRI-γ-γ and FcγRI-γ-γ Y65F/Y76F cDNAs were constructed as described previously (29) and transfected into COS IIA cells using the FuGENE 6 transfection reagent (Roche Diagnostics). In FcγRI-γ-γ Y65F/Y76F, the γ-chain ITAM tyrosines (positions 65 and 76) were replaced with phenylalanine. THP-1 cells were maintained in RPMI 1640 (Invitrogen) containing 10% heat-inactivated FBS, glutamine (2 mm), streptomycin (100 units/ml), and penicillin (100 μg/ml).
Preparation of Murine Peritoneal Macrophages and Human Peripheral Blood Monocytes
Resident macrophages were flushed from the peritoneal cavity of mice using 10 ml of PBS. After centrifugation and hypotonic lysis to remove red blood cells, the cells were resuspended in RPMI 1640 medium containing 10% FCS and adhered to tissue culture wells.
Peripheral blood mononuclear cells from healthy individuals were isolated as described previously (29). Briefly, heparinized blood was centrifuged on Ficoll-Hypaque (Lymphocyte Separation medium; Organon Teknika, Durham, NC), and mononuclear (interface) cells after brief wash were resuspended in complete RPMI 1640 medium containing 10% heat-inactivated FCS. Cells were allowed to adhere at 37 °C onto flasks precoated with FCS. After 45–60 min, nonadherent cells were removed by extensive washing in PBS and were harvested by vigorous agitation. Isolated monocytes were maintained in RPMI 1640 medium at 37 °C in 5% CO2.
Flow Cytometry
Cell samples (∼1 × 106 cells) were incubated with the appropriate primary mAb for 30 min at 4 °C. The cells were then washed and incubated with fluorescein isothiocyanate-conjugated F(ab′)2 goat anti-mouse IgG (Tago, Inc., Burlingame, CA) for 30 min at 4 °C, washed again, and fixed with 2% paraformaldehyde. Isotype control mAbs were used for all reactions, and fluorescence was measured on a Becton Dickinson FACScan (Mansfield, MA). For all samples, 10,000 events were recorded on a logarithmic fluorescence scale.
Preparation of Monomeric and Heat-aggregated Human IgG
Monomeric human IgG (mon-IgG) was prepared by ultracentrifugation of human IgG (8 mg/800 μl IgG) (MP Biomedicals, Cleveland, OH) at 80,000 rpm for 15 min (Beckman TL-100 ultracentrifuge). Heat-aggregated human IgG (HA-IgG) was prepared by heating human IgG (10 mg/ml) in PBS (without calcium and magnesium) at 62 °C for 20 min, followed by centrifugation at 12,000 rpm for 10 min to remove insoluble aggregates (30). The supernatant was used at a 1:100 dilution (100 μg/ml) to induce FcγR cross-linking.
Preparation of C3bi-opsonized Sheep Red Blood Cells (SRBCs; EC3b)
SRBCs (∼2 × 108 cells) (Rockland Immunochemicals, Inc., Gilbertsville, PA) and rabbit anti-SRBC IgM (1:30 dilution) (Accurate Chemical and Scientific Corp., Westbury, NY) in 600 μl of PBS, were incubated at room temperature for 1 h. Unbound IgM was removed by washing with PBS. IgM-coated SRBCs were then incubated with C5-deficient human serum (1:3 dilution) (Sigma-Aldrich) at 37 °C for 20 min followed by extensive washing with PBS. The C3bi-opsonized SRBCs were resuspended in 500 μl of PBS.
Phagocytosis Assays
Cells grown in 6-well plates were overlaid with EC3bi (50 μl in 1 ml PBS) and incubated at 37 °C for 20 min. In co-stimulation studies, HA-IgG (100 μg/ml final concentration) was added to cells simultaneous with EC3bi. Unbound EC3bi were removed by washing with PBS. Externally bound EC3bi were removed by a short (40-s) hypotonic wash. The cells were stained with Wright-Giemsa, and the number of cells with more than one internalized EC3bi was determined in a blinded manner by light microscopy. The contribution of specific FcγRs to CR3-mediated phagocytosis was determined by blocking input from individual FcγRs. Input from FcγRI was blocked by incubating cells with monomeric IgG (10 mg/ml, equivalent to the physiological levels of IgG found in the human circulation) for 20 min before addition of HA-IgG and EC3bi. Input from FcγRIIA was blocked by incubating cells with F(ab′)2 mAb IV.3 (25 μg/ml), and input from mouse FcγRIIB and FcγRIIIA was blocked by incubating mouse macrophages with mAb 2.4G2 (25 μg/ml). For studies involving the FcγRIIA ITAM, COS cell clones expressing similar levels of WT FcγRIIA or the FcγRIIA ITAM mutant Y288F/Y304F were used. Approximately 300 cells were counted for each determination. The phagocytic index (PI) is the number of EC3bi internalized/100 cells.
γ-Chain Silencing Using Small Interfering RNA (siRNA)
Pooled human γ-chain siRNA was prepared using established protocols provided by Applied Biosytems/Ambien (Austin, TX). The following primers were used to produce cDNA templates: forward, 5′-TAATACGACTCACTATAGGGAAGATGATTCCAGCAGTGGTC-3′ and reverse, 5′-TAATACGACTCACTATAGGGCTGTGGTGGTTTCTCATGCTT-3′. Purified human γ-chain siRNA (3 μg) was transfected into THP-1 cells (2 × 106 cells) using AMAXA technology (Cell Line Nucleofector Kit V; AMAXA Biosystems, Gaithersburg, MD). After 48 h at 37 °C, cells were evaluated for CR3-mediated phagocytosis in the presence or absence of FcγR co-stimulation and for efficiency of γ-chain silencing (by RT-PCR and/or Western blotting).
Vav: Immunoprecipitation and Western Blotting
Human peripheral monocytes were incubated with reagents at 37 °C for 20 min as described for the phagocytosis assays and then lysed directly on culture plates with 500 μl of Brij 96 lysis buffer (1% Brij 96, 5 mm Hepes-KOH, pH 7.4) containing the protease and phosphatase inhibitors, 5 mm EGTA, 3 mm sodium orthovanadate, 2 mm phenylmethylsulfonyl fluoride, and 1 μg/liter aprotinin and leupeptin (Sigma-Aldrich). Lysates were immunoprecipitated with 1 μg of anti-Vav antibody (Santa Cruz Biotechnology) bound to protein G/A plus agarose and washed three times in lysis buffer. Adsorbed proteins were eluted into reducing sample buffer and resolved on NuPAGE 4–12% BisTris gel (Invitrogen). Following electrophoretic transfer to nitrocellulose, proteins were immunoblotted with anti-phosphotyrosine (4G10) or anti-Vav (C-14) blotting antibodies followed by goat anti-mouse-IgG-HRP or goat anti-rabbit-IgG-HRP secondary antibody (Santa Cruz Biotechnology). Proteins were visualized by chemiluminescence (ECL Western blotting detection kit; Amersham Biosciences).
RESULTS
Modulation of CR3-mediated Phagocytosis by FcγRs: Human Monocytes
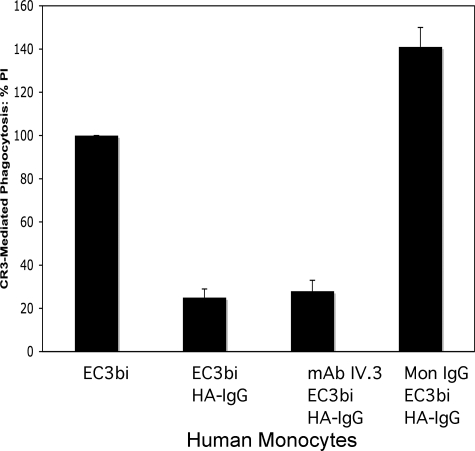
There is accumulating evidence for interaction(s) between CR3 and FcγRs (21–27). To investigate functional consequences and mechanisms involved in the interaction of FcγRs and CR3, we examined the effect of FcγR stimulation on CR3-mediated phagocytosis by human peripheral blood monocytes (PBMs). Although efficient phagocytosis of C3bi-opsonized erythrocytes (EC3bi) by phagocytes often requires a preactivation step (31–33), phagocytosis of EC3bi by freshly isolated human PBMs was robust in the absence of any co-stimulating ligand (Fig. 1). Addition of HA-IgG (which binds to and stimulates FcγRs expressed on the surface of PBMs) at the same time as the addition of EC3bi diminished CR3-mediated phagocytosis by 75% (p < 0.001), suggesting that co-stimulation of FcγRs with CR3 can modulate CR3 phagocytic function.
FIGURE 1.
CR3-mediated phagocytosis of C3bi-opsonized SRBCs (EC3bi) in human PBMs: Effect of FcγRs. Percent phagocytosis of EC3bi was determined for the following conditions: 1) no co-stimulation (no HA-IgG); 2) co-stimulation of all FcγRs with HA-IgG; 3) blocking input from FcγRII with mAb IV.3 before co-stimulation of HA-IgG; and 4) blocking input from FcγRI with mon-IgG before co-stimulation with HA-IgG. Phagocytosis is analyzed as the PI, the number of red blood cells ingested/100 cells. The figure presents the percent change of PI from untreated (basal) levels for each treatment group. In three independent experiments, basal levels of phagocytosis were 158, 118, and 121 (average 132 ± 22). Although the PIs varied, in each experiment the percent change from basal levels was similar for each condition.
Human PBMs express three FcγRs: FcγRI, FcγRIIA, and FcγRIIB (9, 10). To examine the role of individual FcγRs in the CR3 phagocytic response, we blocked the IgG-binding sites of individual FcγRs prior to the addition of EC3bi and HA-IgG. The IgG-binding site of FcγRI was blocked by pretreating cells with mon-IgG. Under these conditions, only class II Fcγ receptors (FcγRIIA and FcγRIIB) are available for stimulation by HA-IgG. Specific stimulation of class II FcγRs resulted in an increase of 41% (p < 0.01) in CR3-mediated phagocytosis compared with cells not exposed to HA-IgG and a 5-fold increase over cells in which all FcγRs were stimulated (Fig. 1). These observations indicate that blocking FcγRI not only negates the inhibitory effect observed when all FcγRs are stimulated, but suggests that FcγRII stimulation has an enhancing effect on phagocytosis by CR3.
To block the class II FcγRs specifically, we used F(ab′)2 mAb IV.3, which binds and blocks the IgG-binding sites of FcγRIIA and FcγRIIB, leaving only FcγRI available for stimulation by HA-IgG. In PBMs pretreated with F(ab′)2 mAb IV.3, co-stimulation with HA-IgG (for FcγRI) and EC3bi (for CR3) reduced CR3-mediated phagocytosis by 72% compared with cells not exposed to HA-IgG (p < 0.002) (Fig. 1). This observation confirms that FcγRI is responsible for the inhibitory effect observed following co-stimulation of CR3 and FcγRs. Fcγ receptor stimulation does not affect the expression of CR3 on the cell surface of human PBMs, nor does CR3 expression change following preblocking of the individual FcγRs with antibody or monomeric IgG (data not shown). In addition, blocking FcγRI with monomeric IgG does not interfere with the ability of FcγRII to bind HA-IgG (data not shown).
To distinguish the contribution of the individual class II Fcγ receptors FcγRIIA and FcγRIIB to CR3-mediated phagocytosis, we utilized peritoneal macrophages from FcγRIIA transgenic mice (IIA-TG). These macrophages have been used as a model system for studies of human macrophage function (34) because they express human FcγRIIA as well as endogenous mouse FcγRI, FcγRIIB, and FcγRIIIA. Importantly, mAb 2.4G2 can be used to block input from mouse FcγRIIB and FcγRIIIA (35), providing a method for distinguishing between the effect of human FcγRIIA and FcγRIIB in these macrophages.
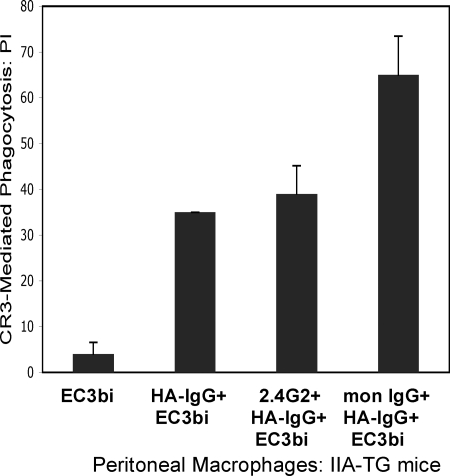
Phagocytosis of EC3bi in IIA-TG macrophages and in WT mouse macrophages is negligible without prepriming or co-stimulation; however, phagocytosis is evident in IIA-TG macrophages co-stimulated with EC3bi and HA-IgG (PI = 35) (Fig. 2). Pretreatment of IIA-TG macrophages with mAb 2.4G2 before co-stimulation with HA-IgG and EC3bi (Fig. 2) did not significantly affect phagocytosis of EC3bi (PI = 39 for cells pretreated with 2.4G2 versus PI = 35 for nonpretreated cells, p = 0.3), strongly suggesting that input from mouse FcγRIIB and FcγRIIIA does not contribute to the FcγR effect on CR3 phagocytosis. Pretreatment with monomeric IgG to block FcγRI engagement significantly increased the phagocytic index for IIA-TG macrophages (PI = 65 versus PI 35, p < 0.01) (Fig. 2). These results parallel our observations in human monocytes; i.e. specific stimulation of FcγRIIA simultaneous with treatment of CR3 with EC3bi produces an enhancing effect on CR3-mediated phagocytosis whereas inclusion of FcγRI in FcγR stimulation diminishes CR3-mediated phagocytosis.
FIGURE 2.
CR3-mediated phagocytosis of EC3bi by peritoneal macrophages from IIA-TG mice: Effect of stimulating specific FcγRs. The PI was determined for 1) cells treated with EC3bi only; 2) cells treated with EC3bi and HA-IgG (CR3 and all FcγRs stimulated); 3) cells preincubated with mAb 2.4G2 to block FcγRIIB and FcγRIIIA before treatment with HA-IgG and EC3bi (CR3, FcγRI and FcγRIIA stimulated); 4) cells preincubated with mon-IgG (10 mg/ml) before treatment with HA-IgG and EC3bi (CR3, FcγRIIA, FcγRIIIA, and FcγRIIB stimulated). Data represent at least three independent experiments.
Requirement for the FcγR γ-Chain in FcγRI Modulation of CR3-mediated Phagocytosis
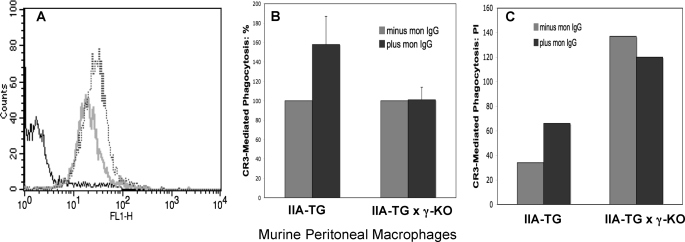
Signaling through FcγRI generally, but not always, requires association with the ITAM-containing FcR γ-chain (9–11, 36–38). We used macrophages from our IIA-TG mice crossed with γ-KO mice (IIA-TG × γ-KO) to examine whether the γ-chain is required for the effect of FcγRI on CR3-mediated phagocytosis. Because in some systems, cell surface expression of FcγRI is severely diminished in the absence of the γ-chain (36), we first examined the cell surface expression of FcγRI on these macrophages. As indicated by fluorescence cytometry, there is somewhat diminished but still robust cell surface expression of FcγRI on these mouse macrophages lacking the γ-chain (Fig. 3A).
FIGURE 3.
FcγR γ-chain requirement for FcγRI-mediated inhibition of CR3 phagocytosis. A, analysis by flow cytometry of FcγRI cell surface expression on peritoneal mouse macrophages (PMMs) from WT mice and γ-chain KO mice. FcγRI on macrophages was stained with fluorescently tagged mon-IgG. The dashed tracing indicates the fluorescence of FcγRI on macrophages from WT mice. The gray tracing represents the fluorescence of macrophages from γ-chain KO mice. The black tracing indicates the fluorescence of macrophages stained with control IgG. B, phagocytosis of EC3bi by PMMs. PMMs from IIA-TG and IIA-TG × γ-chain KO mice were pretreated or not with mon-IgG prior to co-stimulation of cells with HA-IgG and EC3bi. Treatment with mon-IgG blocks the ligand-binding site on FcγRI. For each strain, results are normalized to the PI of macrophages not treated with mon-IgG (100%). Data represent at least three independent experiments. C, representative experiment illustrating that IIA-TG × γ-KO PMMs consistently have a higher PI for EC3bi than have IIA-TG PMMs treated in the same manner.
Our studies have demonstrated that when macrophages from IIA-TG mice are treated with mon-IgG before addition of HA-IgG and EC3bi, there is an increase in CR3 phagocytosis (Fig. 2). That blocking the stimulation of FcγRI increases CR3 phagocytosis indicates that FcγRI exerts a negative effect on CR3 phagocytosis. We hypothesized that if the γ-chain is needed for this negative effect, blocking input from FcγRI in mouse macrophages lacking γ-chain expression (IIA-TG × γ-KO mice) before addition of HA-IgG and EC3bi will not produce an increase in CR3 phagocytosis. The verification of this thesis is demonstrated in Fig. 3B in which the CR3 phagocytic efficiencies of IIA-TG cells and IIA-TG × γ-KO cells pretreated with mon-IgG are compared. Pretreatment of IIA-TG × γ-KO macrophages with mon-IgG did not produce a significant increase in CR3 phagocytosis (p = 0.2, Fig. 3B), whereas, as before, pretreatment of IIA-TG macrophages with mon-IgG significantly increased CR3 phagocytosis (p < 0.01). Further, within each experiment, the PI values for CR3 in IIA-TG × γ-KO macrophages were consistently higher than those observed for IIA-TG macrophages treated in the same manner. For example, in the representative experiment shown in Fig. 3C, the CR3 phagocytic index for nonpretreated cells was 137 for IIA-TG × γ-chain KO cells and 35 for IIA-TG cells.
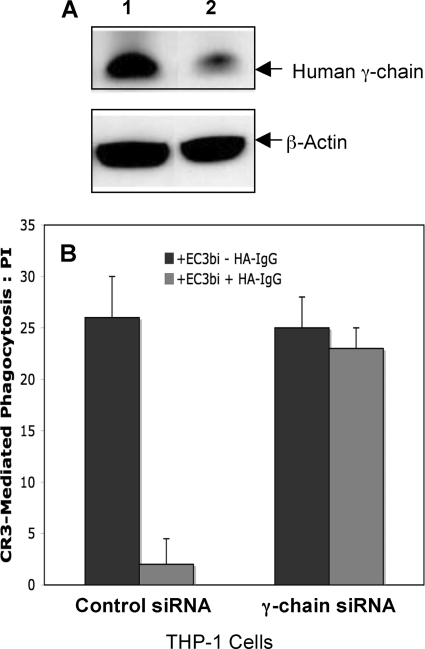
We also examined the requirement for the γ-chain in FcγRI inhibition of CR3-mediated phagocytosis in human cells (THP-1) in which γ-chain expression was knocked down using siRNA. THP-1 cells, like human monocytes, express FcγRI, the γ-chain, FcγRIIA, and FcγRIIB. Treatment of THP-1 cells with γ-chain siRNA decreased γ-chain protein by >60% (Western blot analysis; Fig. 4A). In addition, RT-PCR analysis indicated a 90% decrease in γ-chain mRNA (data not shown). Analysis by flow cytometry indicated little or no difference between surface expression of FcγRI in cells treated with γ-chain siRNA or control siRNA (data not shown).
FIGURE 4.
Effect of γ-chain siRNA on γ-chain protein levels and CR3-mediated phagocytosis in THP-1 cells. THP-1 cells were treated with control siRNA or γ-chain siRNA 48 h before co-stimulation of FcγRI and CR3. A, Western blot of cells treated with control siRNA (lane 1) or γ-chain siRNA (lane 2) is shown. B, CR3-mediated phagocytosis was evaluated for THP-1 cells treated with control siRNA or γ-chain siRNA. Data represent at least three independent experiments.
As expected, in THP-1 cells treated with control siRNA, stimulation of cells with EC3bi and HA-IgG inhibited phagocytosis of EC3bi (PI = 2 versus non-co-stimulated cells PI = 26, p < 0.001, Fig. 4B), reflecting the inhibitory effect of FcγRI/γ. In contrast, phagocytosis of EC3bi was not significantly diminished when cells treated with γ-chain siRNA were co-stimulated with EC3bi and HA-IgG (PI = 23 versus non co-stimulated cells PI = 25, p = 0.2). Together, these studies in mouse macrophages and human monocytic cells provide evidence that signaling through the γ-chain is required for the inhibitory effect of FcγRI on CR3-mediated phagocytosis.
Role of the γ-Chain ITAM
Having demonstrated that the γ-chain is required for the FcγRI effect on CR3-mediated phagocytosis, we next examined whether signaling through the γ-chain ITAM is required for modulation of CR3 phagocytosis by FcγRI (Fig. 5). For these experiments, we used COS cells stably expressing human FcγRIIA (COS IIA) because the baseline for CR3 phagocytosis in COS cells expressing CD11b and CD18 but lacking FcγRIIA is a very low. COS IIA cells were transfected to express CR3 (CD11b and CD18) and RI-γ-γ, a chimera that contains the extracellular domain of FcγRI and the transmembrane and cytoplasmic domains of the γ-chain. Other COS IIA cells were transfected to express CR3 (CD11b and CD18) and a mutant RI-γ-γ chimera, in which the γ-chain ITAM tyrosines were replaced with phenylalanine (Y65F/Y76F). Phosphorylated tyrosines in the ITAM are required for association of the receptor with signaling molecules containing Src homology 2 domains, such as Syk kinase (17, 39), and the Y65F/Y76F ITAM mutation does not support FcγR signaling functions such as phagocytosis (10, 13, 16). The transfection of CR3 plus vector alone provides a control for the effect of FcγRIIA on CR3 phagocytosis.
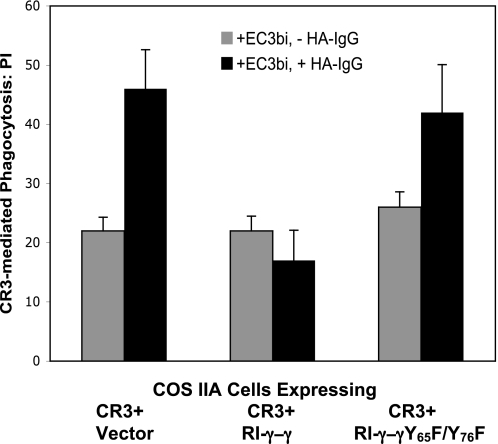
FIGURE 5.
Requirement for an intact γ-chain ITAM in the negative modulation of CR3-mediated phagocytosis by FcγRI/γ. COS cells stably expressing human FcγRIIA (COS IIA) were transfected to express CR3 (CD11b and CD18) and either RI-γ-γ, a chimera that contains the extracellular domain of FcγRI and the transmembrane and cytoplasmic domains of the WT γ-chain, or a mutant RI-γ-γ (Y65F/Y76F) chimera, in which the γ-chain ITAM tyrosines were replaced with phenylalanine. Control cells received CR3 plus vector. Phagocytosis was examined in the absence of co-stimulation with HA-IgG (gray bars) and following co-stimulation with HA-IgG (black bars). Data represent at least three independent experiments.
As expected, CR3-mediated phagocytosis for COS IIA cells expressing CR3 plus vector increased following co-stimulation of cells with HA-IgG and EC3bi (PI = 22 versus PI = 45, p < 0.01, Fig. 5) due to the effect of FcγRIIA, the only FcγR expressed on these cells. The enhancing effect of FcγRIIA was absent in COS IIA cells expressing CR3 plus WT RI-γ-γ (PI = 22 versus PI = 18, p = 0.2) due to inhibitory input from FcγRI/γ. Similar to cells expressing CR3 plus vector, there was enhancement of CR3-mediated phagocytosis for COS IIA cells expressing CR3 and RI-γ-γ (Y65F/Y76F) treated with HA-IgG and EC3bi (PI = 26 versus PI = 44, p < 0.01). This increase in phagocytic efficiency indicates that an intact ITAM is required for the inhibitory effect of the FcγRI γ-chain on CR3 phagocytosis and that FcγRI/γ inhibition of CR3 phagocytosis is mediated through its ITAM signaling pathways.
Role of the FcγRIIA ITAM in Stimulation of CR3 Phagocytosis
Having determined that the γ-chain ITAM is required for inhibition of CR3 phagocytosis by FcγRI, we next examined whether the ITAM of FcγRIIA is essential for enhancement of CR3 phagocytosis. We transfected CD11b and CD18 into COS cell lines stably expressing WT FcγRIIA (COS IIA) or the FcγRIIA-ITAM mutant Y288F/Y304F (COS IIA-Y288F/Y304F). The expression level of FcγRIIA was similar in the COS cell clones expressing the WT ITAM or the ITAM mutant.
In COS IIA cells, co-stimulation with HA-IgG increased CR3-mediated phagocytosis over the basal levels observed in cells not exposed to HA-IgG (p < 0.01, Fig. 6), in accord with our observations in human monocytes and in IIA-TG mouse macrophages (Figs. 1–3). In COS IIA-Y288F/Y304F cells, CR3 phagocytosis remained near basal levels after co-stimulation with HA-IgG (p = 0.2, Fig. 6), an indication that FcγRIIA, like the FcγRI γ-chain, requires an intact ITAM to induce modulation of CR3 phagocytosis.
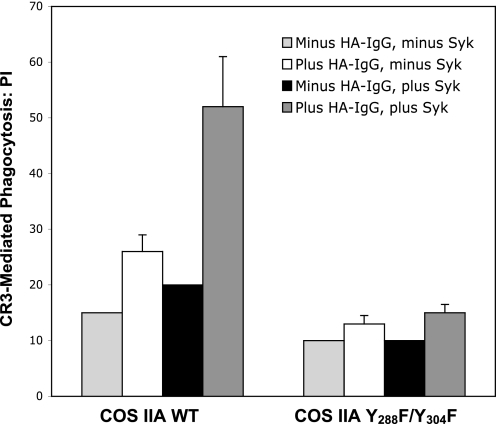
FIGURE 6.
Requirement for an intact ITAM in the positive modulation of CR3-mediated phagocytosis by FcγRIIA. Comparison of phagocytosis of EC3bi in COS cells expressing WT FcγRIIA (COS IIA) or the FcγRIIA mutant Y288F/Y304F lacking ITAM tyrosines (COS IIA-Y288F/Y304F). Cells were transfected to express CR3. The efficiency of CR3 phagocytosis was examined in cells expressing endogenous Syk kinase and in those transfected to overexpress Syk. Data represent at least three independent experiments.
In monocytes and macrophages, Syk kinase is recruited to the tyrosine-phosphorylated FcγRIIA ITAM where it undergoes activation and mediates many FcγRIIA signaling functions (39–42). COS cells express low levels of Syk kinase (28), and we have previously noted that increasing Syk expression by heterologous transfection enhances FcγR signaling responses in these cells (28, 39). Overexpression of Syk increased the enhancement of CR3 phagocytosis in COS IIA (WT ITAM) cells (p < 0.01) but produced no significant increase in CR3 phagocytosis in COS IIA-Y288FY304F cells (p = 0.2) (Fig. 6). Together, these observations indicate that FcγRIIA enhancement of CR3-mediated phagocytosis is diminished in the absence of ITAM tyrosines.
Effect of FcγR Stimulation on Vav Phosphorylation
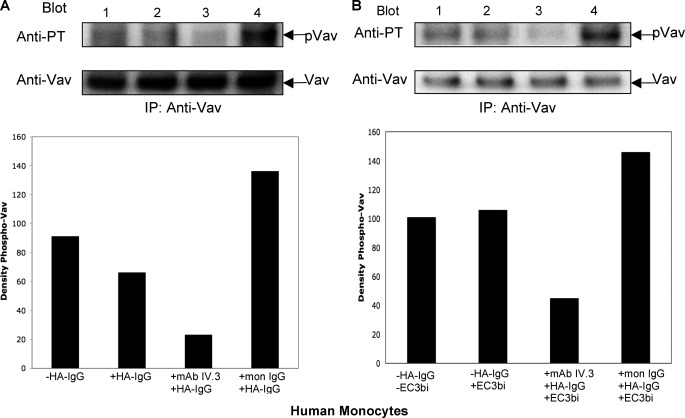
Having demonstrated the requirement for an intact ITAM for modulation of CR3 by both FcγRI/γ and FcγRIIA, we next hypothesized that differential phosphorylation of a downstream effector(s) may determine how these FcγRs affect signaling for CR3 phagocytosis. Guanine exchange factors play important roles in both CR3 and FcγR signaling (43, 44), and it is known that activation of the guanine exchange factor Vav is required for CR3-mediated phagocytosis (44). Although FcγRs do not require Vav for phagocytic signaling, activation of FcγRs does induce tyrosine phosphorylation of Vav (44). We observed that specific engagement of FcγRII (Fig. 7A, lane 4) induces a large increase in Vav tyrosine phosphorylation in human monocytes compared with untreated cells and that specific engagement of FcγRI (lane 3) results in a decrease in Vav tyrosine phosphorylation compared with untreated cells. This pattern of Vav phosphorylation was also observed when CR3 and specific FcγRs were co-stimulated (Fig. 7B). Stimulation of CR3 alone did not produce a discernable change in the basal state of Vav phosphorylation (Fig. 7B). The inhibition and enhancement of Vav phosphorylation by specific FcγRs, evident with and without co-stimulation of CR3, correlate with the pattern of CR3 phagocytosis induced when CR3 and specific FcγRs are co-stimulated.
FIGURE 7.
Tyrosine phosphorylation of Vav. Western blots show tyrosine phosphorylation of Vav in peripheral human monocytes following (A) stimulation of FcγRs with HA-IgG or (B) co-stimulation of FcγRs and CR3 with HA-IgG and EC3bi at 37 °C for 20 min. A, lane 1, no HA-IgG stimulation; lane 2, stimulation of all FcγRs (with HA-IgG); lane 3, cells preincubated with Fab IV.3 mAb before the addition of HA-IgG (stimulation of FcγRI only); and lane 4, cells preincubated with monomeric IgG before the addition of HA-IgG (stimulation of FcγRII only). B, lane 1, no HA-IgG or EC3bi; lane 2, EC3bi but no HA-IgG; lane 3, cells preincubated with Fab IV.3 mAb before the addition of HA-IgG and EC3bi (stimulation of CR3 and only FcγRI; and lane 4, cells preincubated with monomeric IgG before the addition of HA-IgG and EC3bi (stimulation of CR3 and only FcγRII). Quantification of the phospho-Vav bands is shown below each Western blot. To quantitate phospho-Vav, the density of each phospho-Vav band was normalized to the density of the equivalent Vav protein band.
DISCUSSION
Our present studies reveal a new aspect in the interaction between CR3 and FcγRs, two important phagocytic receptors involved in the immune response. We have observed that CR3-mediated phagocytosis is modulated when FcγRs and CR3 are co-stimulated (Figs. 1 and 2). We have also observed that individual FcγRs play different roles in modulating CR3-mediated phagocytosis. Co-stimulation of CR3 and FcγRI inhibits CR3-mediated phagocytosis, and co-stimulation of CR3 and FcγRIIA enhances CR3-mediated phagocytosis. As with most FcγRI signaling events, the γ-chain associated with FcγRI is required for the inhibitory effect of FcγRI (Figs. 3, B and C, and 4), and an intact ITAM is necessary for the effects of both FcγRI and FcγRIIA on CR3 phagocytosis (Figs. 5 and 6). Our studies also indicate that FcγRI, generally considered an activating receptor, can exert a negative effect in signaling pathways. Negative effects on signaling pathways by “activating” ITAM-dependent receptors have also been identified in some myeloid cells (15, 45–47).
Although both FcγRI and FcγRIIA require intact ITAMs for most signaling events, including phagocytosis (9, 10, 15, Figs. 5, 6), they may induce different effects on CR3 signaling for phagocytosis by recruiting/stimulating different downstream effectors and/or by inducing different levels of stimulation (tyrosine phosphorylation) on downstream effectors.
FcγR ITAM domains couple to downstream signaling pathways through the tyrosine kinase Syk. We and others have demonstrated that Syk is essential for FcγR signaling, including phagocytosis (39–41, 48). Syk is also essential for CR3-mediated phagocytosis (48–50). Our observation that overexpression of Syk intensifies the enhancement of CR3 phagocytosis by FcγRIIA (Fig. 6B) suggests that Syk also participates in the modulation of CR3-mediated phagocytosis by FcγRIIA. For experiments with FcγRI/γ, it was necessary to include expression of FcγRIIA because CR3 phagocytosis in the absence of FcγRIIA in COS cells expressing CD11b and CD18 is very low. Participation of Syk in the inhibitory effect of FcγRI could therefore not be evaluated because overexpression of Syk in this system may affect both the FcγRI/γ inhibitory effect and the FcγRIIA-enhancing effect on CR3 phagocytosis.
Studies in macrophage cell lines and other cell lines have suggested that signaling through both CR3 and FcγR requires activation of guanine nucleotide exchange factors, which, in turn, activate members of the Rho GTPase family, implicated in both FcγR- and CR3-mediated phagocytosis (43, 44). Of particular interest is the guanine exchange factor Vav. Vav is required for complement-mediated phagocytosis, and the activity of Vav (a substrate of Syk), is modulated by tyrosine phosphorylation (43). Further, although FcγRs do not require Vav for phagocytic signaling, activation of FcγRs does induce tyrosine phosphorylation of Vav (43, 44). In human monocytes, specific ligation of FcγRII induced an increase in Vav tyrosine phosphorylation compared with cells left untreated, and specific ligation of FcγRI resulted in a decrease in Vav tyrosine phosphorylation, paralleling the stimulatory and inhibitory effects of FcγRs on CR3-mediated phagocytosis (Figs. 1 and 7). The correlation between FcγR-induced Vav phosphorylation and the effects of FcγR stimulation on CR3 phagocytosis is intriguing in that it suggests that the tyrosine phosphorylation status of Vav (and/or other downstream effectors), induced by the specific stimulation of FcγRI or FcγRIIA, may contribute to whether CR3 phagocytosis is enhanced or inhibited.
The differential effects of FcγRs on CR3 phagocytosis may be linked with various pathological conditions. In many autoimmune diseases (e.g. systemic lupus erythematosus, rheumatoid arthritis, glomerulonephritis) C3bi or IgG immune complexes are recognized by inflammatory cells such as macrophages, leading to the release of biologically active products which can lead to tissue damage (51–53). There have been reports that expression of FcγRI is significantly elevated whereas expression of FcγRII is decreased in monocytes from systemic lupus erythematosus patients (54–56). In systemic lupus erythematosus, there are increased levels of circulating immune complexes as well as a reduction in the clearance of apoptotic bodies (56, 57). Increased expression of FcγRI may contribute to defective CR3-mediated clearing of apoptotic cells in lupus patients. Similarly, a decrease in FcγRII expression may minimize an activating contribution to the CR3 response by FcγRIIA. Together, these conditions may interfere with the ability of systemic lupus erythematosus patients to clear circulating immune complexes and apoptotic bodies that arise from the production of autoantibodies, thus contributing to the manifestations of the disease.
That individual FcγRs play different roles in modulating CR3-mediated phagocytosis has important implications for therapeutics, and we anticipate that further investigations of the interactions of CR3 and FcγRs will help identify new approaches for modulating the early stages of the immune response.
Footnotes
- CR3
- complement receptor 3
- BisTris
- bis(2-hydroxyethyl)iminotris(hydroxymethyl)methane
- EC3bi
- sheep erythrocytes coated with C3bi
- FcγR
- Fc receptor for IgG
- HA-IgG
- heat aggregated human IgG
- ITAM
- immunoreceptor tyrosine-based activation motif
- mon-IgG
- monomeric IgG
- PBM
- peripheral blood monocyte
- PI
- phagocytic index
- PMM
- peritoneal mouse macrophage
- SRBC
- sheep red blood cell
- TG
- transgenic.
REFERENCES
- 1. Ehlers M. R. (2000) Microbes Infection 2, 289–294 [DOI] [PubMed] [Google Scholar]
- 2. Gasque P. (2004) Mol. Immunol. 41, 1089–1098 [DOI] [PubMed] [Google Scholar]
- 3. Köhl J. (2006) Adv. Exp. Med. Biol. 586, 71–94 [DOI] [PubMed] [Google Scholar]
- 4. Morgan B. P., Harris C. L. (2003) Mol. Immunol. 40, 159–170 [DOI] [PubMed] [Google Scholar]
- 5. Ross G. D., Vetvicka V., Yan J., Xia Y., Vetvicková J. (1999) Immunopharmacology 42, 61–74 [DOI] [PubMed] [Google Scholar]
- 6. Groves E., Dart A. E., Covarelli V., Caron E. (2008) Cell. Mol. Life Sci. 65, 1957–1976 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Nimmerjahn F., Ravetch J. V. (2008) Nat. Rev. Immunol. 8, 34–47 [DOI] [PubMed] [Google Scholar]
- 8. Greenberg S., Grinstein S. (2002) Curr. Opin. Immunol. 14, 136–145 [DOI] [PubMed] [Google Scholar]
- 9. Ravetch J. V., Bolland S. (2001) Annu. Rev. Immunol. 19, 275–290 [DOI] [PubMed] [Google Scholar]
- 10. Indik Z. K., Park J. G., Hunter S., Schreiber A. D. (1995) Blood 86, 4389–4399 [PubMed] [Google Scholar]
- 11. Indik Z. K., Park J. G., Hunter S., Schreiber A. D. (1995) Semin. Immunol. 7, 45–54 [DOI] [PubMed] [Google Scholar]
- 12. Indik Z. K., Hunter S., Huang M. M., Pan X. Q., Chien P., Kelly C., Levinson A. I., Kimberly R. P., Schreiber A. D. (1994) Exp. Hematol. 22, 599–606 [PubMed] [Google Scholar]
- 13. Park J. G., Murray R. K., Chien P., Darby C., Schreiber A. D. (1993) J. Clin. Invest. 92, 2073–2079 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Isakov N. (1997) J. Leukocyte Biol. 61, 6–16 [DOI] [PubMed] [Google Scholar]
- 15. Underhill D. M., Goodridge H. S. (2007) Trends Immunol. 28, 66–73 [DOI] [PubMed] [Google Scholar]
- 16. Mitchell M. A., Huang M. M., Chien P., Indik Z. K., Pan X. Q., Schreiber A. D. (1994) Blood 84, 1753–1759 [PubMed] [Google Scholar]
- 17. Strzelecka A., Kwiatkowska K., Sobota A. (1997) FEBS Lett. 400, 11–14 [DOI] [PubMed] [Google Scholar]
- 18. Tridandapani S., Siefker K., Teillaud J. L., Carter J. E., Wewers M. D., Anderson C. L. (2002) J. Biol. Chem. 277, 5082–5089 [DOI] [PubMed] [Google Scholar]
- 19. Muta T., Kurosaki T., Misulovin Z., Sanchez M., Nussenzweig M. C., Ravetch J. V. (1994) Nature 368, 70–73 [DOI] [PubMed] [Google Scholar]
- 20. Nimmerjahn F., Ravetch J. V. (2006) Immunity 24, 19–28 [DOI] [PubMed] [Google Scholar]
- 21. Schreiber A. D., Parsons J., McDermott P., Cooper R. A. (1975) J. Clin. Invest. 56, 1189–1197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Ehlenberger A. G., Nussenzweig V. (1977) J. Exp. Med. 145, 357–371 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Brown E. J., Bohnsack J. F., Gresham H. D. (1988) J. Clin. Invest. 81, 365–375 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Kusunoki T., Tsuruta S., Higashi H., Hosoi S., Hata D., Sugie K., Mayumi M., Mikawa H. (1994) J. Leukocyte Biol. 55, 735–742 [DOI] [PubMed] [Google Scholar]
- 25. Tang T., Rosenkranz A., Assmann K. J., Goodman M. J., Gutierrez-Ramos J. C., Carroll M. C., Cotran R. S., Mayadas T. N. (1997) J. Exp. Med. 186, 1853–1863 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. van Spriel A. B., van Ojik H. H., Bakker A., Jansen M. J., van de Winkel J. G. (2003) Blood 101, 253–258 [DOI] [PubMed] [Google Scholar]
- 27. Jongstra-Bilen J., Harrison R., Grinstein S. (2003) J. Biol. Chem. 278, 45720–45729 [DOI] [PubMed] [Google Scholar]
- 28. Huang Z. Y., Hunter S., Kim M. K., Chien P., Worth R. G., Indik Z. K., Schreiber A. D. (2004) J. Leukocyte Biol. 76, 491–499 [DOI] [PubMed] [Google Scholar]
- 29. Kim M. K., Pan X. Q., Huang Z. Y., Hunter S., Hwang P. H., Indik Z. K., Schreiber A. D. (2001) Clin. Immunol. 98, 125–132 [DOI] [PubMed] [Google Scholar]
- 30. Huang Z. Y., Barreda D. R., Worth R. G., Indik Z. K., Kim M. K., Chien P., Schreiber A. D. (2006) J. Leukocyte Biol. 80, 1553–1562 [DOI] [PubMed] [Google Scholar]
- 31. Wiedemann A., Patel J. C., Lim J., Tsun A., van Kooyk Y., Caron E. (2006) J. Cell Biol. 172, 1069–1079 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Caron E., Self A. J., Hall A. (2000) Curr. Biol. 10, 974–978 [DOI] [PubMed] [Google Scholar]
- 33. Wright S. D., Jong M. T. (1986) J. Exp. Med. 164, 1876–1888 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. McKenzie S. E., Schreiber A. D. (1998) Curr. Opin. Hematol. 5, 16–21 [DOI] [PubMed] [Google Scholar]
- 35. Araujo-Jorge T., Rivera M. T., el Bouhdidi A., Daëron M., Carlier Y. (1993) Infect. Immun. 61, 4925–4928 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. van Vugt M. J., Heijnen A. F., Capel P. J., Park S. Y., Ra C., Saito T., Verbeek J. S., van de Winkel J. G. (1996) Blood 87, 3593–3599 [PubMed] [Google Scholar]
- 37. Edberg J. C., Yee A. M., Rakshit D. S., Chang D. J., Gokhale J. A., Indik Z. K., Schreiber A. D., Kimberly R. P. (1999) J. Biol. Chem. 274, 30328–30333 [DOI] [PubMed] [Google Scholar]
- 38. Edberg J. C., Qin H., Gibson A. W., Yee A. M., Redecha P. B., Indik Z. K., Schreiber A. D., Kimberly R. P. (2002) J. Biol. Chem. 277, 41287–41293 [DOI] [PubMed] [Google Scholar]
- 39. Indik Z. K., Park J. G., Pan X. Q., Schreiber A. D. (1995) Blood 85, 1175–1180 [PubMed] [Google Scholar]
- 40. Kiefer F., Brumell J., Al-Alawi N., Latour S., Cheng A., Veillette A., Grinstein S., Pawson T. (1998) Mol. Cell. Biol. 18, 4209–4220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Ghazizadeh S., Bolen J. B., Fleit H. B. (1995) Biochem. J. 305, 669–674 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Chacko G. W., Duchemin A. M., Coggeshall K. M., Osborne J. M., Brandt J. T., Anderson C. L. (1994) J. Biol. Chem. 269, 32435–32440 [PubMed] [Google Scholar]
- 43. Bustelo X. R. (2000) Mol. Cell. Biol. 20, 1461–1477 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Hall A. B., Gakidis M. A., Glogauer M., Wilsbacher J. L., Gao S., Swat W., Brugge J. S. (2006) Immunity 24, 305–316 [DOI] [PubMed] [Google Scholar]
- 45. Boekhoudt G. H., Frazier-Jessen M. R., Feldman G. M. (2007) J. Leukocyte Biol. 81, 1086–1092 [DOI] [PubMed] [Google Scholar]
- 46. Hamerman J. A., Jarjoura J. R., Humphrey M. B., Nakamura M. C., Seaman W. E., Lanier L. L. (2006) J. Immunol. 177, 2051–2055 [DOI] [PubMed] [Google Scholar]
- 47. Pinheiro da Silva F., Aloulou M., Skurnik D., Benhamou M., Andremont A., Velasco I. T., Chiamolera M., Verbeek J. S., Launay P., Monteiro R. C. (2007) Nat. Med. 13, 1368–1374 [DOI] [PubMed] [Google Scholar]
- 48. Turner M., Schweighoffer E., Colucci F., Di Santo J. P., Tybulewicz V. L. (2000) Immunol. Today 21, 148–154 [DOI] [PubMed] [Google Scholar]
- 49. Tohyama Y., Yamamura H. (2006) IUBMB Life 58, 304–308 [DOI] [PubMed] [Google Scholar]
- 50. Shi Y., Tohyama Y., Kadono T., He J., Miah S. M., Hazama R., Tanaka C., Tohyama K., Yamamura H. (2006) Blood 107, 4554–4562 [DOI] [PubMed] [Google Scholar]
- 51. Lourenço E. V., La Cava A. (2009) Curr. Mol. Med. 9, 242–254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Quigg R. J. (2004) Curr. Dir. Autoimmun. 7, 165–180 [DOI] [PubMed] [Google Scholar]
- 53. Feldmann M., Brennan F. M., Maini R. N. (1996) Cell 85, 307–310 [DOI] [PubMed] [Google Scholar]
- 54. Li Y., Lee P. Y., Sobel E. S., Narain S., Satoh M., Segal M. S., Reeves W. H., Richards H. B. (2009) Arthritis Res. Ther. 11, R6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Szücs G., Kávai M., Surányi P., Kiss E., Csipö I., Szegedi G. (1994) Scand. J. Immunol. 40, 481–484 [DOI] [PubMed] [Google Scholar]
- 56. Kavai M., Szegedi G. (2007) Autoimmun. Rev. 6, 497–502 [DOI] [PubMed] [Google Scholar]
- 57. Kruse K., Janko C., Urbonaviciute V., Mierke C. T., Winkler T. H., Voll R. E., Schett G., Muñoz L. E., Herrmann M. (2010) Apoptosis 9, 1098–1113 [DOI] [PubMed] [Google Scholar]