Abstract
The tumor suppressor p53, a 393-amino acid transcription factor, induces cell cycle arrest and apoptosis in response to genotoxic stress. Its inactivation via the mutation of its gene is a key step in tumor progression, and tetramer formation is critical for p53 post-translational modification and its ability to activate or repress the transcription of target genes vital in inhibiting tumor growth. About 50% of human tumors have TP53 gene mutations; most are missense ones that presumably lower the tumor suppressor activity of p53. In this study, we explored the effects of known tumor-derived missense mutations on the stability and oligomeric structure of p53; our comprehensive, quantitative analyses encompassed the tetramerization domain peptides representing 49 such substitutions in humans. Their effects on tetrameric structure were broad, and the stability of the mutant peptides varied widely (ΔTm = 4.8 ∼ −46.8 °C). Because formation of a tetrameric structure is critical for protein-protein interactions, DNA binding, and the post-translational modification of p53, a small destabilization of the tetrameric structure could result in dysfunction of tumor suppressor activity. We suggest that the threshold for loss of tumor suppressor activity in terms of the disruption of the tetrameric structure of p53 could be extremely low. However, other properties of the tetramerization domain, such as electrostatic surface potential and its ability to bind partner proteins, also may be important.
Keywords: Mutant, p53, Protein Stability, Thermodynamics, Tumor Suppressor, Protein Oligomerization, Thermal Denaturation
Introduction
Genome instability and DNA breakage are the hallmarks of cancer cells that arise in response to the activation of oncogenes through point mutations, gene amplifications, or gene translocations (1, 2). Counterbalancing the effects of oncoproteins are tumor suppressor proteins, the most important of which is p53, a transcription factor that modulates cell cycle arrest, senescence, apoptosis, and DNA repair largely via the direct or indirect induction or repression of hundreds of genes (3).
The p53 tumor suppressor monomer is a 393-amino acid protein with five domains: an N-terminal transactivation domain (amino acids 1–42); a proline-rich domain (amino acids 61–92); a central site-specific DNA-binding domain (amino acids 101–300); a tetramerization domain (TD,2 amino acids 326–356); and a C-terminal basic domain (amino acids 364–393). Several stressors, including DNA damage, activate p53 partly through multiple post-translational modifications modulating its activity and stability (4). However, wild-type p53 acts as a transcription factor only when it binds site-specific DNA response elements as a tetramer (5). Furthermore, a number of the post-translational modifications that are believed to be important regulators of p53 activity depend on its quaternary structure (6–11). The p53 protein also exhibits transcription-independent apoptogenesis, possibly contributing to its role in tumor suppression, which is mediated through its interaction with BCL2 family members, including Bak. The efficient targeting to and oligomerization of Bak in the mitochondrial membrane reportedly depends on p53 oligomerization (12). Thus, tetramer formation by p53 is crucial to its tumor suppressive activity.
About half of human tumors carry inactivating mutations in the TP53 gene (13, 14). Unlike other tumor suppressor genes, such as RB1, APC, BRCA1, and CDKN2A, which are inactivated primarily by deletion or nonsense mutations, 74% of TP53 tumor-derived mutations are point mutations that change a single amino acid. More than 95% of these missense mutations occur in the DNA-binding domain; they fall into two main categories, commonly termed DNA contact and conformational mutations. In contrast, ∼17% of germ line p53 mutations in people with Li-Fraumeni syndrome and Li-Fraumeni-like syndromes affect amino acids in the TD, even though it consists only of a short amino acid segment (≈30 amino acids), whereas ∼80% of germ line mutations affect DNA-binding domain residues, viz., six times as long as the TD (14). This finding implies that germ line mutations exist at similar frequencies in the tetramerization and DNA-binding domains, and both are essential for p53-mediated tumor suppressor activity.
The p53TD consists of a β-strand (Glu326–Arg333), a tight turn (Gly334), and an α-helix (Arg335–Gly356) (15). The structure of the TD was determined by NMR spectroscopy (16) and x-ray crystallography (17). Two monomers form a dimer through their antiparallel β-sheets and α-helices, and two dimers become a tetramer through the formation of an unusual four-helix bundle. Ala-scanning of p53TD revealed that 9 hydrophobic residues constitute critical determinants of its stability and oligomerization status (18). An earlier study of tumor-derived mutants R337H, R337C, or L344P from patients with Li-Fraumeni-like syndrome revealed a propensity for dramatic destabilization; the presence of the R337H mutation entailed pH-dependent instability of the mutant p53 tetramer (19, 20). Leu344 occurs in the α-helix, and after introducing a helix-breaking proline (L344P), p53 could not form tetramers. R337C forms dimers and tetramers at low temperature; however, even though its tetrameric structure is destabilized significantly at physiological temperatures, it is only partially inactivated in several functional assays (21, 22). The p53 proteins with these mutations, as with other p53TD mutations (e.g. L330H, R337L, R342P, E349D, and G334V), exhibit an overall decrease in DNA binding and transactivation activity (23, 24).
Because the p53 tetramer is in equilibrium with the monomer, the protein concentration of p53 will affect its oligomeric status (18, 25). In unstressed normal cells, p53 is maintained at low levels by continuous ubiquitylation and subsequent degradation by the 26 S proteasome (26). DNA damage-induced phosphorylation of N-terminal residues of p53, and of Mdm2, a ubiquitin protein ligase, inhibits its binding to the latter and enables p53 stabilization and accumulation (4). A high concentration of p53 shifts the monomer-tetramer equilibrium toward the tetramer state, thereby promoting increased DNA binding, interactions with proteins important for p53 activation and function, and heightening post-translational modifications that activate p53. Past research used only semiquantitative analyses to assess the effects of mutations on the oligomeric structure and transcriptional activity of p53 (27–29). Although this research determined the oligomeric status of the mutant p53 protein by cross-linking (28) or by fluorescence intensity distribution analysis (29), the abundance of p53 protein was not controlled; thus, a destabilized mutant might show wild-type stability under high concentrations of mutant p53.
Because of the difficulty of constructing numerous cells lines with TD mutant p53 in which the p53 concentration can be carefully regulated, in this study, we quantitatively analyzed the oligomeric structure and stability of TD peptides from the reported cancer-associated TD mutants of p53. Surprisingly, the abilities of these mutants to form tetramers spanned a broad, almost continuous distribution. Although mutants that changed the domain core drastically prevented tetramer formation and/or folding as reported previously, the effects of many mutants were much more subtle. Nevertheless, even for mutants that slightly destabilized tetramer formation, at an endogenous concentration of p53, the fraction of tetramer decreased significantly. Our data further suggested that additional studies of the biochemical and biophysical properties of the TD may be required to explain why some p53 TD mutations are cancer-associated.
EXPERIMENTAL PROCEDURES
Peptide Synthesis and Purification
WT and mutant p53TD peptides, comprising residues 319–358 of the extended TD, were synthesized as described previously (30). We measured peptide concentrations spectrophotometrically using an extinction coefficient for mutant p53TD peptides, ϵ280 = 1280 m−1 cm−1, corresponding to a single tyrosine; for G334W and G356W, ϵ280 = 6800 m−1 cm−1, corresponding to a single tyrosine and a tryptophan. Because the peptides Y327D, Y327H, and Y327S have no Tyr or Trp, peptide concentrations were determined by the BCA method (Thermo Fisher Scientific) using a WT peptide as the standard.
Gel Filtration Chromatography
We resolved the WT and mutant p53TD peptides using a Superdex 75 PC 3.2/30 (GE Healthcare) with a Precision Column Holder (GE Healthcare) in 50 mm phosphate buffer, pH 7.5, 100 mm NaCl (30). Peptide concentrations were 100 μm. The flow rate was 0.1 ml/min at 15 °C, and we monitored the effluent at 280 nm. Each peak was quantified by calculating the peak area using IGOR software (Wavemetrics).
Thermal Denaturation by CD Spectroscopy
For our CD measurements, we employed a Jasco-805 spectropolarimeter using a 1-mm path length quartz cell. CD spectra were recorded in 50 mm sodium phosphate buffer containing 100 mm NaCl, pH 7.5. For our thermal denaturation studies, spectra were recorded at discrete temperatures from 4 to 96 °C with a scan rate of 1 °C/min; ellipticity was measured at 222 nm for the p53TD solutions (10 μm monomer in 50 mm phosphate buffer, pH 7.5). We fitted the unfolding process of the p53TD peptide to a two-state transition model wherein the native tetramer directly converts to an unfolded monomer, as described previously (18, 25). The thermodynamic parameters of the peptides were determined by calculation with the functions described by Mateu and Fersht (18). We determined the Tm and ΔHuTm by fitting the fraction of monomer; we estimated the Kd value of the tetramer-monomer transition from Kd = ((1 − Ku)/2)−1/3 (31, 32). For dimer mutants, we used Kd = Ku−1. The oligomeric states at 37 °C against the peptide concentration were assessed via the Kd value.
Structural Modeling of p53TD Mutants
The three-dimensional coordinates of p53TD wild-type (Protein Data Bank code 3SAK) were used as a template. Homology modeling of mutants was performed with Modeler software (33).
RESULTS
Oligomerization State of Mutant p53 Tetramerization Domains
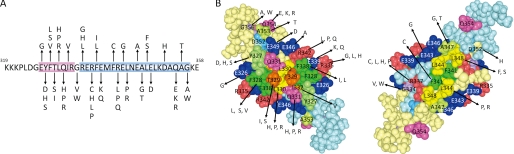
Forty-nine distinct mutations in human cancers occur in 24 of the 31 residues that comprise the p53 core TD (amino acids 326–356) (Fig. 1). We synthesized WT and mutant p53TD peptides corresponding to residues 319–358 and analyzed their oligomeric state and thermodynamic stability; we quantified this state from the peak areas corresponding to a monomer and tetramer during gel-filtration chromatography (Table 1 and supplemental Fig. S1). WT and most mutant peptides eluted as tetramers, but five mutants, L330P, L330R, R337P, L342P, and L344P, eluted as a single peak contemporaneously with the monomer mutant L330A. Interestingly, three mutants (F341C, L344R, and A347T) eluted between the tetramer and monomer fractions. Accordingly, five mutants, L330R/P, R337P, L342P, and L344P, exist as monomers, three mutants, F341C, L344R, and A347T as dimers, and the others as tetramers under our conditions. Moreover, some mutants, such as L330H, R337C, and L348S, contained a lower tetramer fraction (45.1, 54.8, and 40.5%, respectively), and part of these peptides were chromatographed as monomers, implying destabilization of their tetrameric structure, thus favoring the monomer side of the monomer-tetramer equilibrium.
FIGURE 1.
Amino acid sequences for the WT and mutant p53TD peptides. A, amino acid sequences and the positions of the missense mutations in the TD of p53; β-strand residues are highlighted in red, α-helical residues are highlighted in blue. B, space-filling model of p53TD (Protein Data Bank code 3SAK) prepared with MolFeat (version 4.0, FiatLux Corp.) The amino acid residues of the mutation site in the p53TD and the location of these residues in the tetrameric structure are shown. The primary dimers are depicted, and the other dimer is removed to give a direct view of the interior of the protein. The right dimer was obtained by rotating the structure in the left picture by 180° around the horizontal axis.
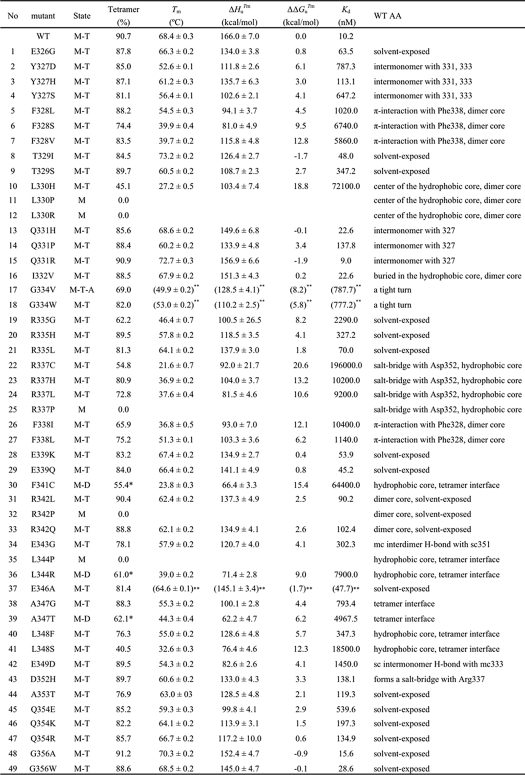
TABLE 1.
Thermodynamic parameters for the mutant peptides
The fraction of the tetramer was determined by gel filtration chromatography; asterisks indicate the fraction of dimer. M-T (monomer-tetramer); M-D (monomer-dimer); M (monomer); Tm (transition temperature); ΔHuTm (variation in the enthalpy of unfolding at Tm); ΔΔGuTm (the difference in ΔG between WT and mutant peptides at the Tm of the WT peptide); sc (side chain); mc (main chain). The standard errors of fittings are indicated. The dissociation constant at 37°C is calculated by Kd = ((1 − Ku)/2)−1/3 (31). For dimer mutants, we used Kd = Ku−1. **, three mutants (G334V, G334W, and E346A) showed some irreversibility under the condition of thermal denaturation employed here.
Secondary Structure of Mutant p53 Tetramerization Domains
We deduced the secondary structures of all mutant peptides from their CD spectra (supplemental Fig. S2). Five monomeric mutants (L330P, L330R, R337P, R342P, and L344P) showed a negative minimum near 200 nm, characteristic of a random coil, even under a high (10 μm) peptide concentration and low temperature of 4 °C. Presumably, substitutions by Pro catastrophically affect tetramer formation. Five mutants (L330H, Q331P, R337C, F338I, and L348S) showed weaker negative CD spectra between ∼210 and 240 nm compared with the WT, pointing to destabilized WT-like tetrameric structures. The three dimer mutants (F341C, L344R, and A347T) displayed the same spectra as the other tetrameric mutants, indicating that their α-helical segment and β-strand are structurally similar to those of the WT tetramer. Still other mutant peptides formed WT-like tetrameric structures under these same conditions. After heating and subsequent cooling, WT and mutants except for G334V, G334W, and E346A showed the same spectra as those before heating. These three mutants showed a different spectrum, which was shifted to a β-dominant spectrum after heating and subsequent cooling.
Thermal Stability of Mutant p53 Tetramerization Domains
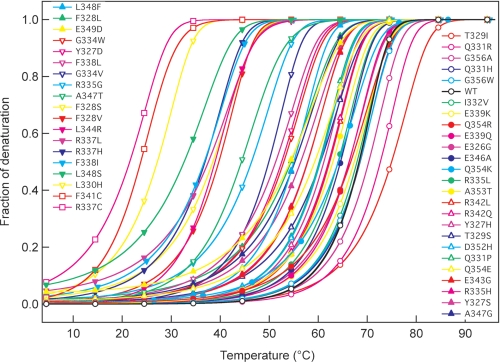
We analyzed the effects of temperature on the conformation of the WT and mutant peptides by calculating the thermal denaturation curves for each p53 peptide from changes in CD ellipticity at 222 nm, using a two-state transition mode. Fig. 2 and Table 1 show that the effects of amino acid changes on the tetramer stability elicited alterations in the ΔTm values of the mutant peptides from 4.8 to −46.8 °C. Changes to the TD hydrophobic core residues (Phe328, Leu330, Arg337, Phe338, Phe341, Leu344, and Leu348), except for I332V, dramatically lowered stability; modifications to solvent-exposed residues had less profound effects. The introduction of proline into the α-helix (R337P, R342P, and L344P) devastated tetramer formation; these peptides existed substantially as monomers only. No cancer-associated mutation has been reported in the codon for the hydrophobic core residue Met340. Four cancer-associated mutants had amino acids changed (T329I, Q331H, Q331R, or G356A; Table 1 and Fig. 2) such that tetramer stability actually increased. We noted a good correlation (r2 = 0.64) between the fraction of oligomers analyzed by gel filtration and the Tm value of the mutants obtained by CD (supplemental Fig. S3), indicating that these thermodynamic parameters corresponded to the tetrameric state of the p53 peptides.
FIGURE 2.
Thermal denaturation of WT and mutant p53TD peptides. Thermal denaturation of the peptides was analyzed by measuring the ellipticity at 222 nm for peptide solutions containing 10 μm peptide in 50 mm sodium phosphate, pH 7.5, 100 mm NaCl over the range of 4 to 96 °C, with a scan rate of 1 °C per minute.
Modeling of Mutant p53TDs
Mutations that changed some solvent-exposed p53TD amino acid residues had little or no significant affect on the thermal stability of the tetramers. To elucidate why these mutations occur in human cancers, we modeled the TD of each mutant (supplemental Fig. S4), finding that changes in some solvent-exposed residues altered the calculated electrostatic potential on the surface of the p53TD. This was especially so for E339K, E339Q, E343G, E346A, and Q354K. We suggest that these changes might influence either the interdomain or the intermolecular interactions with binding partners that thereby could account for their selection as cancer mutants.
Correlation between Stability of p53TD Peptides and That of Full-length p53 Protein
We compared the stabilities of the tetrameric structures of the mutant p53TD peptides with the oligomeric state of full-length p53-EGFP fusion proteins carrying TD mutations; we employed fluorescence intensity distribution analysis, which measures the fluorescence intensity of EGFP-tagged p53 in a very small volume (10−13 liter) of cell extract using confocal microscopy and yields a quantitative assessment of the fraction of protein oligomers in vivo at physiologically relevant concentrations (29). The clear correlation (r2 = 0.75) between the Tm measured here and the in vivo oligomerization state (supplemental Fig. S5) strongly suggests that our quantitative data on the tetrameric structure of p53 peptides can be extended to the full p53 protein.
DISCUSSION
Our study represents the first comprehensive, quantitative biophysical analysis of the oligomeric state and thermal stability of the 49 TD mutants identified in human cancers. Most mutant p53TD peptides formed a WT-like tetrameric structure with diminished stability (Fig. 2). However, tetrameric mutants with altered hydrophobic core residues (Phe328, Leu330, Arg337, Phe338, Phe341, Leu344, and Leu348), except I322V, exhibited dramatic reductions in stability and, in some cases, unfolding of the peptide (e.g. L330H/P/R, R337C/P, R342P, and L344P) as determined by CD measurements (supplemental Fig. S2). In particular, mutations that introduced proline in the α-helix devastated tetramer formation; some mutants could not form tetramers and existed as unfolded monomers (L330P/R, R337P, R342P, and L344P), or as folded dimers (F341C, L344R, and A347T). Indeed, our thermal denaturation study predicted that several TD mutants (e.g. L330H, R337C/H/L, F338I, F341C, L344R, and L348S) are thermally unstable at or near body temperature. These results are consistent with an alanine-scanning study of the p53TD that identified 9 key hydrophobic resides important for TD thermal stability and oligomerization (18).
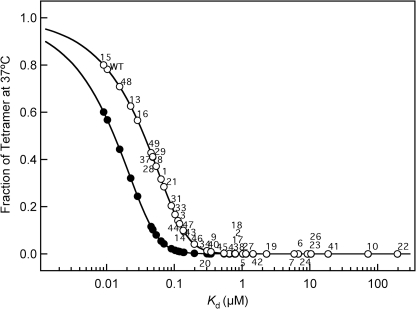
In contrast to mutations that affect hydrophobic core residues, mutations that affect residues accessible to solvent were less destabilizing. The WT p53TD is thermally quite stable (Tm ∼ 70 °C, Table 1) compared with the core DNA-binding domain (15); most mutations that affect TD domain residues, except as noted above, would not be expected to unfold the domain structure. Nevertheless, for most thermally stable TDs, the change in amino acids significantly altered the disassociation constant for tetramer formation (Table 1). Importantly, because tetramers are essential for DNA binding and activating transcription (9), and the p53 tetramer is in equilibrium with the monomer, the intranuclear p53 concentration is a critical factor in determining p53 function. In cultured, undamaged human embryonic skin fibroblasts (WS-1 cells), p53 abundance was 7.8 × 103 molecules/cell, whereas after DNA damage from neocarzinostatin, it rose ∼3-fold to 21.8 × 103 molecules/cell (34). The volume of the nucleus of a human fibroblast is ∼10−12 liters (35). Correspondingly, the p53 concentration in the nucleus of normal, unstressed human cells is ≈13 nm and increases to ≈36 nm after DNA damage. At these concentrations, we can assess the fraction of tetramer for TD mutants at 37 °C (Fig. 3). Thus, we predict that ∼80% of the accumulated WT p53 protein (WT p53TD Kd = 10.2 nm) is in the tetrameric state following DNA damage. These values might be somewhat high as the skin fibroblasts were cultured under normoxic conditions (∼21% oxygen), i.e. much higher that the oxygen concentration in most tissues (∼5–8%). Oxidative stress activates p53, and normoxia causes oxidative stress in cultured cells (36). Nevertheless, the dissociation constant for the formation of the WT p53 tetramer seems tuned to accommodate a much greater change in p53 function than the ∼3-fold change in nuclear concentration. Hence, cancer mutations that affect the p53TD Kd by more than ∼10-fold might not elicit a sufficient concentration of tetramers in cells to induce the transcriptional responses important for tumor suppression (3). Additionally, p53-mediated transcription-independent apoptosis, which reportedly depends on the ability of p53 to form tetramers in the cytoplasm (12), might be diminished.
FIGURE 3.
Fraction of tetramer at 37 °C at concentrations of 13 nm (endogenous p53 level in unstressed cell) and 36 nm (stressed cell) against the value of Kd. Each data point represents the value of a mutant at 13 nm (solid circles) and 36 nm (open circles). The fraction of tetramer at each concentration was calculated from the dissociation constant given in Table 1 assuming a two-state monomer-tetramer model (31). Monomer mutants 11, 12, 25, 32, and 35, and dimer mutants 30, 36, and 39 are not shown.
In addition to the direct effects of mutations on tetramer formation, several indirect effects may further acerbate p53 function. First, the p53TD contains a nuclear export signal (Met340–Lys351) that is exposed in the monomer and dimer but not in the tetramer (37). Except for those mutants in the α-helix between Met340 and Lys351 that affect the interaction of the nuclear export signal with CRM1 (37), TD mutants potentially could exacerbate tetramer formation by increasing the cytoplasmic export of p53 and preventing its nuclear accumulation to normal levels. Second, several post-translational modifications that potentially modulate p53 activity are influenced by its oligomeric state, and the oligomeric state can be modulated by post-translational modifications. The phosphorylation of Ser392 enhances tetramer formation (31). The p53TD peptide in which His replaced Leu330 was thermally destabilized (Table 1), and the phosphorylation of p53 Ser392 by casein kinase 2 was diminished when Leu330 was mutated to His (38). Deletion of the residues 334–354 abolishes the ability of Chk1 to phosphorylate p53 (8); sites that Chk1 can phosphorylate are believed to modulate p53 activity and stability (4). The p300/CBP-associated factor (PCAF) acetyltransferase, which acetylates Lys320, specifically recognizes the tetrameric structure of p53 (6). In addition, Pirh2, an E3 ubiquitin protein-ligase that binds and ubiquitylates p53 protein in vitro and in vivo, only acts on its tetrameric form (39).
More than 50 proteins reportedly interact with the C-terminal region of the p53 protein, and several of them either require or influence tetramer formation. Tetramer formation of p53 is essential for its interaction with human papillomavirus (HPV)-16 E2, c-Abl, and Mdm2 (9, 22). The binding affinity of p53 to MDM2 fell when p53 contained the mutation L344P or R337C found in Li-Fraumeni patients (22). c-Abl binds directly to the C-terminal basic domain of p53, and this interaction requires a tetramer. c-Abl may stabilize the tetrameric conformation, resulting in a more stable p53-DNA complex (40). In contrast, the interaction of caspase recruitment domain (ARC) with the p53TD inhibits tetramer formation and increases nuclear export (41). The binding of S100 family proteins depends on the oligomeric status of p53 and controls the balance between monomer and tetramer (42). Binding of the 14-3-3 protein to p53 enhances sequence-specific DNA binding by inducing p53 to form tetramers at lower concentrations (43).
The p53TD from ∼13 apparently cancer-associated mutants in eight residues, mostly in the α-helical region of the TD, only moderately affected, by ∼5-fold, the Kd of tetramer formation of E326G, T329I, R335L, E339K, E339Q, and E346A, and very slightly affected, by 2-fold, that of Q331H, Q331R, I332V, G356A, and G356W (Table 1). The apparently minimal effect of these changes is particularly surprising for mutations that affect Glu326, Ile332, Glu339, and Glu346, because these are among the 12 most highly evolutionarily conserved residues in the TD (9), and changes to conserved residues often are deleterious to function. Thus, we questioned why mutations causing these changes exist among p53-associated cancer mutants. As Soussi et al. (44) noted, mistakes occur in the literature on p53, possibly due to errors in sequencing or PCR, so caution is needed about accepting mutants that have been reported in cancers only once or a few times; data on germ line mutants should be more reliable. Of the 13 mutants noted above, all but four (Q331H, Q331R, R342Q, and G356W) occur only once in the International Agency for Research on Cancer TP53 mutant database, and none have been reported as germ line mutations. Thus, some of these mutants may be false reports. Of the remaining four mutants (supplemental Fig. S4), two are clearly solvent-exposed residues that either change the surface charge (R342Q) or replace a small residue with one bearing a bulky, hydrophobic side chain at the surface (G356W) (supplemental Table S1). Although mutations affecting solvent exposed residues and altering the electrostatic potential of the surface of p53 were less thermally destabilizing than core mutants were, the change in surface charge potential might well affect intraprotein interactions or the interaction of the TD with one or more of its many binding partners. R342Q represents such a change, and the G356W change might disrupt surface complementarity that could affect protein interactions important for p53 function. Many mutants with greatly changed Kd values also involve surface-exposed alterations in charge that affect the predicted electrostatic potential of the p53TD surface (supplemental Fig. S4). In the crystal structure, E349D, a change that moderately increases the Kd (to 1450 nm) is implicated in crystal contacts and, therefore, probably is important for such interactions (45). Three mutants (G334V, G334W, and E346A) showed a β-dominant spectrum after heating and subsequent cooling (supplemental Fig. S2). We have previously reported that G334V peptide forms amyloid fibrils under physiological conditions of temperature and pH (46). Also, other p53 domains (the transactivation domain and the DNA-binding domain) have been shown to undergo aggregation (47–49). The aggregates of p53 domains might be correlated with cancer.
Residue Gln331 is in the short β-sheet that forms part of the monomer-monomer interface, but Gln331 is not involved in monomer-monomer interactions, and the change to either His, Arg, or even Pro had only minor effects on p53TD thermal stability (Table 1). A recent yeast-based assay for transcriptional activation revealed that almost any amino acid sufficed at this position (50). Thus, biophysical or biochemical measurements do not show why mutations that alter this residue appear among cancer-associated mutations. Nevertheless, we argue that even mild changes to the Kd for tetramer formation or in p53 stability could significantly affect p53 function because of the sensitivity of tetramer formation to the Kd and p53 concentration in the physiological range. Many previous studies involved a ∼20-fold p53 overexpression (supplemental Fig. S6A) that would not reveal the detrimental effects of many p53TD mutations (supplemental Fig. S6B).
Analyses of SNPs in p53 and its pathway support our suggestion of the potential importance of the subtle effects of TD mutants on tetramer formation. The TP53 gene reportedly contains 19 exonic polymorphisms, among which researchers have validated four (R47S, R72P, V217M, and G360A). The P47S and R72P polymorphisms subtly alter expression of p53 transcriptional targets. Although controversial (51), molecular evidence suggests that both polymorphisms alter cancer risk (50–54). Additional evidence comes from SNPs in p53 pathways. Bond et al. (55), working on the most intensively studied T/G SNP at nucleotide 309 in the first intron of the MDM2 gene, demonstrated that the 309G variant is bound more efficiently by the transcription factor SP1, thereby increasing the efficiency of synthesizing MDM2, and consequently, slightly lowering levels of p53. The estrogen receptor also binds the MDM2 promoter in the region of SNP309 and also can increase MDM2 expression in response to the hormone. Several studies associated MDM2 SNP309 polymorphism and increased cancer risk in males and females, although others saw no such connection (51). Thus, although further work is required, these studies support our hypothesis that relatively small changes in p53 concentration or the ability to form tetramers could contribute to cancer risk or progression. Furthermore, the study of cancer-associated mutants in the TD that minimally affect tetramer formation may reveal additional functions for this domain in p53 biology.
Supplementary Material
This work was supported in part by Grants-in-aid for Scientific Research on Priority Areas 16041202 from The Ministry of Education, Culture, Sports, Science and Technology and Scientific Research (B) 21310133 and 18310140 from Japan Society for the Promotion of Science (to K. S.), “Molecular and System Life Science” Promotion of Novel Interdisciplinary Fields Based on Nanotechnology and Materials from The Ministry of Education, Culture, Sports, Science and Technology (to K. S.), Research Fellowships of the Japan Society for the Promotion of Science for Young Scientists 20004981 (to R. K.) and 19001703 (to T. N.) from Japan Society for the Promotion of Science, and Program Development funds from the Brookhaven National Laboratory under contract with the U.S. Department of Energy (to C. W. A.).

The on-line version of this article (available at http://www.jbc.org) contains supplemental “Experimental Procedures,” Table S1, Figs. S1–S6, and an additional reference.
- TD
- tetramerization domain.
REFERENCES
- 1. Hanahan D., Weinberg R. A. (2000) Cell 100, 57–70 [DOI] [PubMed] [Google Scholar]
- 2. Halazonetis T. D., Gorgoulis V. G., Bartek J. (2008) Science 319, 1352–1355 [DOI] [PubMed] [Google Scholar]
- 3. Zilfou J. T., Lowe S. W. (2009) Cold Spring Harb. Perspect. Biol. 1, a001883 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Meek D. W., Anderson C. W. (2009) Cold Spring Harb. Perspect. Biol. 1, a000950 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Halazonetis T. D., Kandil A. N. (1993) EMBO J. 12, 5057–5064 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Sakaguchi K., Herrera J. E., Saito S., Miki T., Bustin M., Vassilev A., Anderson C. W., Appella E. (1998) Genes Dev. 12, 2831–2841 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Maki C. G. (1999) J. Biol. Chem. 274, 16531–16535 [DOI] [PubMed] [Google Scholar]
- 8. Shieh S. Y., Ahn J., Tamai K., Taya Y., Prives C. (2000) Genes Dev. 14, 289–300 [PMC free article] [PubMed] [Google Scholar]
- 9. Chène P. (2001) Oncogene 20, 2611–2617 [DOI] [PubMed] [Google Scholar]
- 10. Warnock L. J., Knox A., Mee T. R., Raines S. A., Milner J. (2008) Cancer Biol. Ther. 7, 1481–1489 [DOI] [PubMed] [Google Scholar]
- 11. Itahana Y., Ke H., Zhang Y. (2009) J. Biol. Chem. 284, 5158–5164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Pietsch E. C., Perchiniak E., Canutescu A. A., Wang G., Dunbrack R. L., Murphy M. E. (2008) J. Biol. Chem. 283, 21294–21304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Hainaut P., Hollstein M. (2000) Adv. Cancer Res. 77, 81–137 [DOI] [PubMed] [Google Scholar]
- 14. Petitjean A., Mathe E., Kato S., Ishioka C., Tavtigian S. V., Hainaut P., Olivier M. (2007) Hum. Mutat. 28, 622–629 [DOI] [PubMed] [Google Scholar]
- 15. Joerger A. C., Fersht A. R. (2010) Cold Spring Harb. Perspect. Biol. 2, a000919 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Clore G. M., Ernst J., Clubb R., Omichinski J. G., Kennedy W. M., Sakaguchi K., Appella E., Gronenborn A. M. (1995) Nat. Struct. Biol. 2, 321–333 [DOI] [PubMed] [Google Scholar]
- 17. Jeffrey P. D., Gorina S., Pavletich N. P. (1995) Science 267, 1498–1502 [DOI] [PubMed] [Google Scholar]
- 18. Mateu M. G., Fersht A. R. (1998) EMBO J. 17, 2748–2758 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. DiGiammarino E. L., Lee A. S., Cadwell C., Zhang W., Bothner B., Ribeiro R. C., Zambetti G., Kriwacki R. W. (2002) Nat. Struct. Biol. 9, 12–16 [DOI] [PubMed] [Google Scholar]
- 20. Zambetti G. P. (2007) J. Cell Physiol. 213, 370–373 [DOI] [PubMed] [Google Scholar]
- 21. Davison T. S., Yin P., Nie E., Kay C., Arrowsmith C. H. (1998) Oncogene 17, 651–656 [DOI] [PubMed] [Google Scholar]
- 22. Lomax M. E., Barnes D. M., Hupp T. R., Picksley S. M., Camplejohn R. S. (1998) Oncogene 17, 643–649 [DOI] [PubMed] [Google Scholar]
- 23. Atz J., Wagner P., Roemer K. (2000) J. Cell Biochem. 76, 572–584 [DOI] [PubMed] [Google Scholar]
- 24. Rollenhagen C., Chène P. (1998) Int. J. Cancer 78, 372–376 [DOI] [PubMed] [Google Scholar]
- 25. Johnson C. R., Morin P. E., Arrowsmith C. H., Freire E. (1995) Biochemistry 34, 5309–5316 [DOI] [PubMed] [Google Scholar]
- 26. Maki C. G., Huibregtse J. M., Howley P. M. (1996) Cancer Res. 56, 2649–2654 [PubMed] [Google Scholar]
- 27. Kato S., Han S. Y., Liu W., Otsuka K., Shibata H., Kanamaru R., Ishioka C. (2003) Proc. Natl. Acad. Sci. U.S.A. 100, 8424–8429 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Kawaguchi T., Kato S., Otsuka K., Watanabe G., Kumabe T., Tominaga T., Yoshimoto T., Ishioka C. (2005) Oncogene 24, 6976–6981 [DOI] [PubMed] [Google Scholar]
- 29. Imagawa T., Terai T., Yamada Y., Kamada R., Sakaguchi K. (2009) Anal. Biochem. 387, 249–256 [DOI] [PubMed] [Google Scholar]
- 30. Nomura T., Kamada R., Ito I., Chuman Y., Shimohigashi Y., Sakaguchi K. (2009) Biopolymers 91, 78–84 [DOI] [PubMed] [Google Scholar]
- 31. Sakaguchi K., Sakamoto H., Lewis M. S., Anderson C. W., Erickson J. W., Appella E., Xie D. (1997) Biochemistry 36, 10117–10124 [DOI] [PubMed] [Google Scholar]
- 32. Poon G. M., Brokx R. D., Sung M., Gariépy J. (2007) J. Mol. Biol. 365, 1217–1231 [DOI] [PubMed] [Google Scholar]
- 33. Šali A., Blundell T. L. (1993) J. Mol. Biol. 234, 779–815 [DOI] [PubMed] [Google Scholar]
- 34. Wang Y. V., Wade M., Wong E., Li Y. C., Rodewald L. W., Wahl G. M. (2007) Proc. Natl. Acad. Sci. U.S.A. 104, 12365–12370 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Swanson J. A., Lee M., Knapp P. E. (1991) J. Cell Biol. 115, 941–948 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Parrinello S., Samper E., Krtolica A., Goldstein J., Melov S., Campisi J. (2003) Nat. Cell Biol. 5, 741–747 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Stommel J. M., Marchenko N. D., Jimenez G. S., Moll U. M., Hope T. J., Wahl G. M. (1999) EMBO J. 18, 1660–1672 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Chène P. (2000) BioTechniques 28, 240–242 [PubMed] [Google Scholar]
- 39. Sheng Y., Laister R. C., Lemak A., Wu B., Tai E., Duan S., Lukin J., Sunnerhagen M., Srisailam S., Karra M., Benchimol S., Arrowsmith C. H. (2008) Nat. Struct. Mol. Biol. 15, 1334–1342 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Nie Y., Li H. H., Bula C. M., Liu X. (2000) Mol. Cell. Biol. 20, 741–748 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Foo R. S., Nam Y. J., Ostreicher M. J., Metzl M. D., Whelan R. S., Peng C. F., Ashton A. W., Fu W., Mani K., Chin S. F., Provenzano E., Ellis I., Figg N., Pinder S., Bennett M. R., Caldas C., Kitsis R. N. (2007) Proc. Natl. Acad. Sci. U.S.A. 104, 20826–20831 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. van Dieck J., Fernandez-Fernandez M. R., Veprintsev D. B., Fersht A. R. (2009) J. Biol. Chem. 284, 13804–13811 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Rajagopalan S., Jaulent A. M., Wells M., Veprintsev D. B., Fersht A. R. (2008) Nucleic Acids Res. 36, 5983–5991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Soussi T., Kato S., Levy P. P., Ishioka C. (2005) Hum. Mutat. 25, 6–17 [DOI] [PubMed] [Google Scholar]
- 45. Miller M., Lubkowski J., Rao J. K., Danishefsky A. T., Omichinski J. G., Sakaguchi K., Sakamoto H., Appella E., Gronenborn A. M., Clore G. M. (1996) FEBS Lett. 399, 166–170 [DOI] [PubMed] [Google Scholar]
- 46. Higashimoto Y., Asanomi Y., Takakusagi S., Lewis M. S., Uosaki K., Durell S. R., Anderson C. W., Appella E., Sakaguchi K. (2006) Biochemistry 45, 1608–1619 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Rigacci S., Bucciantini M., Relini A., Pesce A., Gliozzi A., Berti A., Stefani M. (2008) Biophys. J. 94, 3635–3646 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Ishimaru D., Andrade L. R., Teixeira L. S., Quesado P. A., Maiolino L. M., Lopez P. M., Cordeiro Y., Costa L. T., Heckl W. M., Weissmüller G., Foguel D., Silva J. L. (2003) Biochemistry 42, 9022–9027 [DOI] [PubMed] [Google Scholar]
- 49. Silva J. L., Vieira T. C., Gomes M. P., Bom A. P., Lima L. M., Freitas M. S., Ishimaru D., Cordeiro Y., Foguel D. (2010) Acc. Chem. Res. 43, 271–279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Merritt J., Roberts K. G., Butz J. A., Edwards J. S. (2007) Genomic Med. 1, 113–124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Whibley C., Pharoah P. D., Hollstein M. (2009) Nat. Rev. Cancer 9, 95–107 [DOI] [PubMed] [Google Scholar]
- 52. Feng L., Hollstein M., Xu Y. (2006) Cell Cycle 5, 2812–2819 [DOI] [PubMed] [Google Scholar]
- 53. Mantovani F., Tocco F., Girardini J., Smith P., Gasco M., Lu X., Crook T., Del Sal G. (2007) Nat. Struct. Mol. Biol. 14, 912–920 [DOI] [PubMed] [Google Scholar]
- 54. Bergamaschi D., Samuels Y., Sullivan A., Zvelebil M., Breyssens H., Bisso A., Del Sal G., Syed N., Smith P., Gasco M., Crook T., Lu X. (2006) Nat. Genet. 38, 1133–1141 [DOI] [PubMed] [Google Scholar]
- 55. Bond G. L., Hu W., Bond E. E., Robins H., Lutzker S. G., Arva N. C., Bargonetti J., Bartel F., Taubert H., Wuerl P., Onel K., Yip L., Hwang S. J., Strong L. C., Lozano G., Levine A. J. (2004) Cell 119, 591–602 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.