Abstract
A large conductance (∼300 picosiemens) channel (LCC) of unknown molecular identity, activated by Ca2+ release from the sarcoplasmic reticulum, particularly when augmented by caffeine, has been described previously in isolated cardiac myocytes. A potential candidate for this channel is pannexin 1 (Panx1), which has been shown to form large ion channels when expressed in Xenopus oocytes and mammalian cells. Panx1 function is implicated in ATP-mediated auto-/paracrine signaling, and a crucial role in several cell death pathways has been suggested. Here, we demonstrate that after culturing for 4 days LCC activity is no longer detected in myocytes but can be rescued by adenoviral gene transfer of Panx1. Endogenous LCCs and those related to expression of Panx1 share key pharmacological properties previously used for identifying and characterizing Panx1 channels. These data demonstrate that Panx1 constitutes the LCC of cardiac myocytes. Sporadic openings of single Panx1 channels in the absence of Ca2+ release can trigger action potentials, suggesting that Panx1 channels potentially promote arrhythmogenic activities.
Keywords: Adenoviruses, ATP, Cell-Cell Interaction, Connexin, Gap Junctions, Cardiac Myocytes, Hemichannel, Pannexin
Introduction
The pannexin gene family (Panx1–3) is homologous to the invertebrate gap junction-forming proteins, the innexins (1). Pannexin proteins and innexins share considerable structural similarities but no significant sequence homology to the prototypical gap junction proteins, the connexins (Cx)3 (2). Important structural similarities between the three protein families led to the initial hypothesis that pannexins form intercellular gap junctions. However, this concept has received little experimental support (3–5). Recent studies of trafficking, cell surface localization, and dynamics, as well as interplay with the cytoskeleton, demonstrate that pannexins are distinct from connexins (6). Panx1 as yet represents the best studied isoform. It is ubiquitously expressed (7–9) and has been demonstrated to form large conductance channels in neurons, glial cells, and erythrocytes (10–12). Opening of Panx1 channels has been induced by various experimental conditions, including activation of purinergic receptors, stretch, high intracellular calcium, and membrane depolarization (13–15). Panx1 channels lack sensitivity to extracellular Ca2+, an important property, as Panx1 channels are not excluded from opening under physiological ionic conditions, unlike most Cx hemichannels. In neurons and possibly astrocytes, Panx1 may contribute to novel forms of synaptic and nonsynaptic communication and Ca2+ wave propagation (16). In the mammalian skin, Panx1 plays a key role in keratinocyte differentiation. Furthermore, Panx1 activation is implicated in ischemic, excitotoxic, and ATP-dependent cell death, whereas Panx1 coupling with purinergic receptors triggers the inflammasome (18–20). The latter data suggest a crucial role for Panx1 in several cell death pathways (16, 21).
Several studies have shown that cardiac myocytes from different species express a large conductance channel (LCC) with unknown physiological function (22–25). This channel responds to spontaneous or caffeine-induced Ca2+ release from internal stores and has the paradoxical feature that unitary current fluctuations can be resolved in the whole cell mode of the patch clamp technique, suggesting a very low density of functional channels. Previous attempts to identify the molecular identity of LCC, primarily based on pharmacological tools with limited specificity, were not conclusive. Interpretations as to its molecular nature included Cx hemichannels (22), ryanodine receptors (23), or polycystin-2 (24).
LCC activities investigated in the quoted studies share some common features beyond their large conductance as follows: they are nonselective cation channels, they can be activated by Ca2+ release from the sarcoplasmic reticulum, particularly when synchronized by caffeine; and they appear to have a low density in the plasma membrane or a low open probability. Here, we demonstrate that the LCC, activated by Ca2+ release from the sarcoplasmic reticulum in cardiac myocytes, shares key pharmacological properties previously used for identifying and characterizing Panx1 channels. These data are in support of the notion that Panx1 constitutes this LCC. Beyond the pharmacological analogies, we can demonstrate that Panx1 protein and LCC are lost within 4–5 days in vitro but can be rescued by adenoviral gene transfer of Panx1. Because sporadic openings of single Panx1 channels in the absence of caffeine can trigger action potentials, we suggest that Panx1 channels potentially promote arrhythmogenic activities.
EXPERIMENTAL PROCEDURES
Isolation and Culture of Adult Rat Atrial Myocytes
Experiments were performed in accordance with local ethics committee approval. Wistar-Kyoto rats of either sex (weight around 200 g) were anesthetized by intravenous injection of urethane (1g/kg). The chest was opened, and the heart was removed and mounted on the cannula of a Langendorff perfusion system for coronary perfusion at a constant flow. The method of enzymatic isolation of atrial myocytes has been described in detail previously (26). Cells were plated at a low density (several hundred cells per dish) and cultured in fetal calf serum-free medium (M199, Invitrogen) containing 25 μg/ml gentamycin (Sigma) and 25 μg/ml kanamycin (Sigma). Medium was changed every 2nd day. Myocytes were used experimentally from day 1 until day 6 after isolation.
Adenovirus Constructs and Gene Transfer in Atrial Myocytes
The pAd-Easy-1 plasmid encoding for the adenovirus type 5 and pAd-Track-CMV were kindly provided by Dr. B. Vogelstein (The Johns Hopkins University, Baltimore). A recombinant adenovirus was generated by subcloning the full-length mouse Panx1 coding sequence (amino acids 1–426; GI:86262134) into the pAd-Track-CMV shuttle vector to yield pAd-Panx1. The construct was sequence-verified. Generation of recombinant adenovirus encoding either for Panx1 and GFP or GFP only (mock control) was performed as described in detail previously (27). Briefly, adenoviral recombinant plasmids were generated by homologous recombination between pAd-Panx1 and pAd-Easy-1 in Escherichia coli. Recombinant viruses (Ad-Panx1) were propagated in HEK 293 cells and recovered after several freezing-thawing cycles. Virus titers were determined by serial dilution and infection of myocyte cultures. For infection, cells were incubated with 1 ml of culture medium containing 105 infectious particles (gene transforming units).
Real Time PCR
Total RNA was extracted, reverse-transcribed, and used in real time PCR as described previously (8). The primer pairs were as follows: Panx1 sense (5′-tgttctggcgctttgcggcggc-3′), Panx1 antisense (5′-ggtccaggtccatctctcagg-3′); 18 S sense (5′-catggtgaccacgggtgac-3′) and 18 S antisense (5′-ttccttggatgtggtagccg-3′) designed for rat Panx1 and 18 S rRNA amplification using VectorNTI software (Invitrogen). Amplification reactions were performed in triplicate using SYBR Green I reaction conditions as recommended by the manufacturer (Clontech) using the DNA Engine Opticon 2 real time PCR detection system (Bio-Rad). The Ct values for the reference gene (18 S rRNA) were used to normalize mRNA levels of the samples. The changes of Panx1 mRNA expression levels were calculated as ratios relative to the Panx1 mRNA levels found in isolated newborn rat hippocampal neurons cultivated for 7 days (set to 1). All experiments represented three independent sets of samples analyzed in triplicate. Statistical analysis was performed using the relative expression software tool (REST) software (28).
Western Blot Analysis
For Western blot analysis, total protein extracts from tissues of adult rats were isolated by direct homogenization in denaturing Laemmli buffer. Proteins were separated by 10% SDS-PAGE, transferred to 0.2-μm nitrocellulose membrane (Protran BA83, Schleicher & Schüll), and processed as described previously (3). Primary antibodies were diluted 1:1,000 (chi-Panx1, gift by Dr. G. Dahl, Miami, FL (12)) and 1:15,000 (anti-GAPDH; Sigma). Secondary antibodies were IRDye680- (1:15,000) and IRDye800-coupled (1:20,000), and Western blot detection was performed using the Odyssey® infrared imaging system (LI-COR Biosciences).
Immunohistochemistry and Immunocytochemistry
Sections (10 μm) from adult heart tissue of transcardially perfused rats (4% paraformaldehyde) were fixed with 3% paraformaldehyde for 15 min, permeabilized with 1% Triton X-100 for 10 min, and blocked for 1 h with 3% normal horse serum, 1% BSA in PBS, pH 7.4. Isolated adult rat myocytes cultured on 35-mm μ-dishes (ibidi) were fixed with 3% paraformaldehyde for 15 min, permeabilized with 1% Triton X-100 for 10 min, and blocked for 1 h with 3% normal horse serum, 1% BSA in PBS, pH 7.4. The chicken anti-Panx1 antibody was diluted 1:100. Secondary antibodies were Alexa 488 nm or Alexa 563 nm coupled (1:3,000). Specimen were finally stained with Hoechst 33342 (Invitrogen) and mounted with Prolong Antifade Gold (Invitrogen). Confocal image analysis was performed using the LSM 510 meta system (Carl Zeiss MicroImaging GmbH,), equipped with argon and HeNe lasers, 63× (NA 1.4) oil objectives, and the LSM 510 META software as described previously (3).
Solutions and Chemicals
For the patch clamp measurements, an extracellular solution of the following composition was used (in mm): 132 NaCl, 7 CsCl, 1 MgCl2, 10 HEPES, 2 CaCl2, pH 7.4. The solution for filling the patch clamp pipettes contained (in mm) 65 Cs3citrate, 10 CsCl, 1 MgCl2, 10 HEPES, 4 Na2ATP, 200 μm EGTA, pH 7.3. For investigating mechanosensitivity of single channel currents, cardiac myocytes were immersed in isotonic solution (composed of (in mm) the following: 100 NaCl, 30 mannitol, 1 MgCl2, 5 CaCl2, 7 CsCl, 10 HEPES, pH 7.4, 300 mosmol/kg) and then in a hypotonic solution of the same composition, except that mannitol was omitted (∼270 mosmol/kg).
For current clamp experiments the following solutions were used (in mm) were as follows: 132 NaCl, 5.4 KCl, 1 MgCl2, 10 HEPES, 2 CaCl2, pH 7.4. The patch pipette contained 100 potassium aspartate, 40 KCl, 5 NaCl, 2 MgCl2, 2 EGTA, 20 HEPES, 0.025 Na2GTP, 5 Na2ATP, pH 7.3.
Standard chemicals were from Merck. EGTA, HEPES, Na2ATP, 2-methylthioadenosine-5′-triphosphate (2Mes-ATP), Na2GTP, probenecid, carbenoxolone (CBX), and caffeine were from Sigma.
Electrophysiology
Membrane currents were recorded using whole cell patch clamp. The DC resistance of the filled pipettes ranged from 4 to 6 megohms. Current measurements were performed with a patch clamp amplifier (List LM/EPC 7). Signals were analog-filtered (1 kHz), digitally sampled, and stored on a computer equipped with a hardware/software package (Iso2, MFK) for voltage control, data acquisition, and analysis. Experiments were performed at ambient temperature (22–24 °C). Membrane holding potential was −60 mV. Rapid superfusion of the cells for application and withdrawal of different solutions was performed by a custom-made solenoid-operated flow system that permitted switching between different solutions within less than 100 ms. Recordings of membrane potential were performed in the current clamp mode. Channel activity was expressed as nPo (i.e. number of channels multiplied by the individual channel open probability) from recordings that were at least 10 s in duration (during caffeine application) or 40 s in duration for with spontaneous LCC activity.
Statistics
For single channel current measurements, the paired t test was used to compare the means of LCC activity before and during drug application. Independent t test was used for evaluating the means of two different groups (e.g. nPo of LCCs in atrial cells measured on days 2 and 5). Values of p < 0.05 were considered statistically significant.
RESULTS
Ca2+ Release and LCC Activity
In isolated rat myocytes, experimental conditions using citrate as a low affinity Ca2+ buffer favor spontaneous cyclic Ca2+ release, which activates an inward current carried by electrogenic Na+/Ca2+ exchange (NCX) (29). The NCX current was superimposed by single channel current fluctuations with an amplitude of ∼15 pA at holding potential (Fig. 1A). Rapid exposure to caffeine, a common tool to discharge Ca2+ from the sarcoplasmic reticulum by opening of the ryanodine-receptor release channels (30), caused an increased and prolonged channel activity. In the recording shown, which was from a myocyte in culture for about 20 h, upon exposure to caffeine a maximum of four simultaneous openings was identified. In general, channel activity slowly decayed in the continuous presence of caffeine; as a rule, channel openings could be recorded for up to 10 min. Because experiments were facilitated by firm attachment of the myocytes, most measurements were performed after at least 24 h in vitro. In line with previous studies, LCC was not induced by short term culture but could be recorded also in freshly isolated atrial and ventricular myocytes, as shown in supplemental Fig. S1, A and B. Inhibition of caffeine-induced Ca2+ release using the local anesthetic tetracaine (1 mm), a common tool to block the ryanodine receptor release channel, abolished both the NCX current and LCC activity, as illustrated in supplemental Fig. S2. This clearly supports the notion that LCC is activated by caffeine-induced Ca2+ release rather than by caffeine itself (25). In line with previous studies, the unitary conductance of the LCC was determined as 298 ± 14 picosiemens (Fig. 1). As shown in Fig. 1, B and D, activity of the channel was almost completely lost after 5 days in vitro, whereas spontaneous Ca2+ cycling and NCX current were not different from short term (48 h) cultured atrial myocytes.
FIGURE 1.
A, membrane currents related to spontaneous and caffeine-induced Ca2+ release. Aa, representative recording of Na+-Ca2+ exchange currents and LCC activity in a rat atrial myocyte (2 days in vitro) caused by spontaneous and caffeine-induced Ca2+ release, Ab and Ac represent expanded sections from A. C, O1, and O2 indicate closed state and two open levels. B, representative recording from a myocyte cultured for 5 days. C, current/voltage relation of LCC unitary current (mean values from 11 different cells). D, nPo of caffeine-induced LCC activity and density of whole cell NCX current calculated for myocytes of 2 and 5 days in culture. nPo periods of 10 s in duration, starting at the peak of NCX current, were used.
Panx1 Is Expressed in Isolated Rat Cardiac Myocytes
Expression of Panx1 has been previously demonstrated in the mouse heart and in neonatal rat cardiac myocytes on the transcriptional level (8, 38). Quantitative mRNA PCR analysis demonstrated that isolated rat atrial myocytes express Panx1 mRNA at levels comparable with isolated newborn hippocampal neurons or adult brain but at higher levels than in heart tissue (Fig. 2A). Western blots confirmed the presence of the differentially glycosylated Panx1 (GLY0–2; see Refs. 31–33) in heart and brain (Fig. 2B). In sections of ventricular tissue, Panx1 immunoreactivity is localized to myocytes (Fig. 2C). Confocal imaging of isolated atrial and ventricular myocytes reveals a preferential localization to the surface membrane (Fig. 2D). In accordance with previous publications (26, 29), adult atrial myocytes but not ventricular myocytes undergo a morphological change from spindle-shaped to spherical within several hours after isolation (supplemental Fig. S1C). Most importantly, in cultured cardiac myocytes Panx1 protein expression recapitulates the pattern observed for LCC activity. Panx1 is abundantly expressed on day 2 and lost by day 5 in vitro (Fig. 2E).
FIGURE 2.
Panx1 expression in cell isolates and native tissues. A, Panx1 mRNA expression quantified by real time PCR and normalized to levels detected in isolated rat hippocampal neurons cultured for 7 days. Panx1 expression levels in atrial myocytes (day 2 in culture) and whole brain tissue were similar to neurons, whereas the expression in rat heart tissue was significantly lower (−2.8 + 0.9-fold; p < 0.001). B, Western blot analysis of total protein extracts (30 μg/lane) from adult rat heart and brain demonstrating Panx1 immunoreactivity in brain and heart. Note that Panx1 levels in lung are below the detection limit. Neuro2A cells expressing transiently transfected mouse Panx1 (mPanx1) were used as positive control. Unglycosylated (GLY0) and glycosylated isoforms (GLY1 and GLY2) are indicated. C, Panx1 immunoreactivity (in green) in adult ventricular tissue showing localization in myocytes; nuclei were DAPI-stained (in blue). Scale bar, 20 μm. D, plasma membrane localization of Panx1 in isolated rat atrial (a, upper panel) and ventricular (v, lower panel) myocytes (about 24 h after isolation). Scale bar, 10 μm. E, detection of Panx1 in atrial myocytes on day 2 in vitro but not on day 5 in vitro. In the image labeled day 2 peptide control, the antibody was blocked by preincubation with excess peptide. Scale bar, 50 μm.
Loss of LCC Activity Is Restored by Adenovirus Vector-driven Expression of Panx1
Expression and localization of Panx1 in the heart and the parallel loss in expression of Panx1 (Fig. 2D) and LCC activity in isolated myocytes (Fig. 1) led us hypothesize that the large unitary current events were carried by Panx1. To challenge this hypothesis, myocytes were infected with Ad-Panx1 about 16 h after plating. Caffeine-induced channel activity was measured on day 5 in vitro and quantified as nPo for periods of 10 s starting at the peak of the NCX current. As illustrated in Fig. 3, in contrast to time-matched native or mock-infected myocytes, LCC activity in terms of nPo was not significantly different from short term (48 h) cultured myocytes, i.e. LCC activity was rescued by expression of Panx1 (compare Figs. 1D and 3B), demonstrating a direct link between Panx1 expression and LCC activity.
FIGURE 3.
Infection with Ad-Panx1 rescues LCC activity in vitro. A, representative recordings of caffeine-induced NCX currents and superimposed LCC in Ad-Panx1-infected and mock (empty virus)-infected rat atrial myocytes cultured for 5 days. B, nPo of caffeine-induced LCC activity in mock-infected cells (n = 25) and time-matched Ad-Panx1-infected cells (n = 29). Pharmacological characterization of LCC is in Ad-Panx1-infected cells. nPo of caffeine-induced LCC activity is in the absence and presence of 20 μm carbenoxolone (C, n = 11) or 200 μm probenecid (D, n = 11).
Pharmacological Properties of LCCs Are Identical to Panx1
Although to date highly selective inhibitors of Panx1 channels are not available, two compounds are suitable to distinguish Panx1-related currents from other charge-carrying mechanisms such as Cx hemichannels. Panx1 channels are sensitive to carbenoxolone, a naturally occurring 11β-hydroxysteroid dehydrogenase inhibitor, commonly used as gap junction inhibitor (34). As illustrated in Figs. 3C and 4, A and C, 20 μm CBX, a concentration that does not significantly affect Cx-related current (35), reduced caffeine-induced channel activity in Ad-Panx1-infected and native atrial myocytes. Cyclic Ca2+ release and the resulting NCX current were not affected by this compound.
FIGURE 4.
Sensitivity of endogenous LCC to carbenoxolone and probenecid. A, representative current recordings from rat atrial myocytes (day 2 in culture) showing caffeine-induced LCC openings in the absence and presence of 20 μm carbenoxolone (A) or 200 μm probenecid (B). C and D, summarized data (C, n = 9; D, n = 10).
Probenecid, a drug for the treatment of gout by virtue of its inhibitory effect on organic anion transporters (36), inhibits Panx1 without affecting Cx channels at concentrations up to 1 mm (37). Application of 200 μm probenecid on Ad-Panx1-infected atrial myocytes substantially reduced nPo of the caffeine-induced LCC activity, consistent with the effective concentration for inhibition of Panx1 channels expressed in Xenopus oocytes (Fig. 3C) (37). An analogous pharmacological profile was observed for LCCs in native atrial myocytes cultured for 2 days. Under these conditions, caffeine-induced LCCs were inhibited by 200 μm probenecid (Fig. 4, B and D) by about 50% on average. Thus, both exogenously expressed channels related to Panx1 and endogenous LCC activity display analogous pharmacological properties.
LCC Activity Is Sensitive to ATP
Evidence has been provided that Panx1 is involved in the release of ATP and UTP from cardiac myocytes under conditions of pressure overload (38). More recently, a complex of Panx1 and P2X7 receptors has been suggested to be responsible for the release of cardioprotectants induced by ischemic pre- and postconditioning (39). Evidence for a role of Panx1 as ATP-release channel, which is modulated by ATP via P2X and P2Y receptors, has been provided in different cellular systems (10, 11, 14, 40).
Furthermore, a direct inhibition of Panx1 channels by extracellular ATP at concentrations in the millimolar range (up to 10 mm) has been demonstrated (41, 42), suggesting a self-regulatory feedback mechanism of channel activity. Therefore, we hypothesized that extracellular ATP might affect gating of LCC in cardiac myocytes via purinergic P2 receptors. This notion is supported by two facts as follows: (i) purinergic P2X and P2Y receptors are expressed in various regions of the rat heart, including atria and sinoatrial node (43), and (ii) extracellular ATP is known to promote (patho)physiological functional changes in cardiac cells by stimulation of P2 purinoceptors, including induction of arrhythmic activity through activation of depolarizing membrane currents (44).
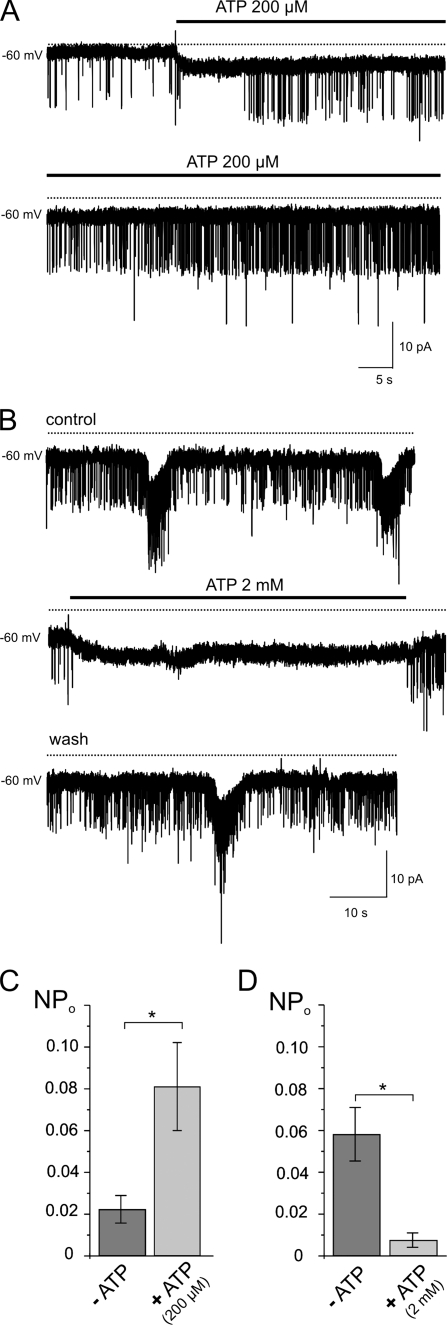
Sporadic LCC openings with identical conductance as caffeine-induced LCC in native atrial myocytes could be identified in the absence of caffeine or cyclic Ca2+ release with a very low open probability (Fig. 5A). Extracellular application of 200 μm ATP gradually increased LCC activity (Fig. 5, A and C). Channel activity remained elevated in the presence of ATP and decreased within 1–3 min after removal of the purinergic agonist. The increase in LCC by ATP was sensitive to probenecid, as shown in supplemental Fig. S3. Modulation of LCC activity might reflect ATP-induced activation of P2Y receptors and subsequent inositol 1,4,5-trisphosphate-induced increase in [Ca2+]i. In line with this hypothesis, exposure to ATP caused a small but consistent inward shift of holding current. Presumably this reflects NCX. A clear identification of the charge-carrying mechanism, however, was impeded because of the low density of this current. Activation of LCC by ATP could be mimicked by 2Mes-ATP (10 μm), a P2X/P2Y1 agonist (Fig. 6). Consistent with a feedback inhibition of Panx1 channels suggested previously (37), ATP at higher concentrations (2 mm) suppressed spontaneous LCC activity in atrial myocytes (Fig. 5, B and D).
FIGURE 5.
Effects of extracellular ATP on spontaneous LCC activity in native atrial myocytes (day 2 in culture). A and C, low basal LCC activity is increased by 200 μm ATP (n = 6). B and D, reduction of LCC activity by 2 mm ATP (n = 6).
FIGURE 6.
Increase of LCC activity through activation of purinergic receptors by 10 μm 2Mes-ATP. A, recording of spontaneous LCC activity in atrial myocyte (2 days in culture) without caffeine. Application of the P2X and P2Y1 agonist 2Mes-ATP reversibly increased LCC openings. Channel activity increased after a short delay of about 30 s and slowly decreased after removal of the agonist within 60 s. B, summarized nPo (n = 7).
LCC Activity and Panx1-related Channels Are Mechanosensitive
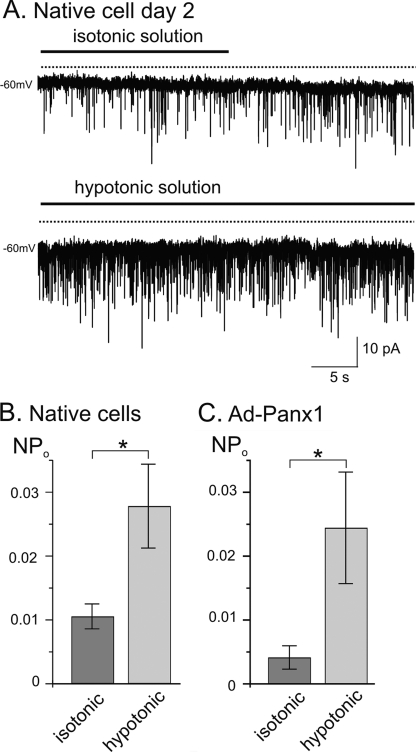
The open state probability of Panx1 channels expressed in Xenopus oocytes has been shown to be sensitive to stretch (15). Here, we induced mechanical strain by exposure of atrial cells to hypotonic solution causing cell swelling. Spontaneous LCC activity was recorded in an isotonic bath solution (300 mosmol/kg) (Fig. 7A), before switching to a hypotonic solution (270 mosmol/kg). Cells were allowed to stabilize in the hypotonic solution before channel activity was recorded again. Under these conditions, single channel activity gradually increased during the adaptation time and remained elevated in the presence of the hypotonic solution (Fig. 7B). Analogously, channels in Ad-Panx1-infected atrial cells (day 5) responded to hypotonic cell swelling with increased opening activity (Fig. 7C).
FIGURE 7.
Mechanosensitivity of LCC. A, representative current recording from a native myocyte (2 days in culture). Spontaneous LCC activity in isotonic bath solution (300 mosmol/kg) followed by hypotonic solution, which had the same ionic composition except that mannitol was omitted (270 mosmol/kg). B, nPo of LCC in native cells (n = 6) and Ad-Panx1-infected cells (day 5 in culture, n = 6).
LCC Elicits Action Potentials
Inward current through single LCCs in an intact cell gives rise to a depolarization, which might reach threshold for eliciting an action potential, as shown previously (25). In a fraction of about 70% of myocytes studied using a physiological K+ gradient ([K+]o = 5.4 mm) in whole cell recordings at high gain, we observed LCC openings without caffeine or a purinergic agonist with very low probability. Representative examples are illustrated in Fig. 8. Spontaneous irregular action potentials or action potential-like electrical activity were detected under this condition in both native (A–C) and Ad-panx1-infected myocytes (D and E). As shown on the expanded time scale (Fig. 8C), these action potentials were preceded by a slight depolarization that activated voltage-gated sodium channels resulting in a fast action potential upstroke. As one would expect, inhibition of spontaneous LCC activity by probenecid resulted in electrical quiescence under current clamp conditions (Fig. 8, D and E).
FIGURE 8.
Aberrant electrical activity induced by spontaneous openings of LCC. A, sample recording of current from a myocyte (2 days) that showed brief spontaneous channel openings. B, spontaneous action potentials recorded in current clamp mode. C, single action potential on expanded time scale. D, sample recording of current from a myocyte (Ad-Panx1-infected, 5d) that showed spontaneous LCC bursts. These and corresponding action potential-like depolarizations in current clamp (E) were inhibited by probenecid (200 μm).
DISCUSSION
This study provides substantial evidence that Panx1 represents the molecular equivalent of the large conductance cation channel first described more than 20 years ago (29). The major finding is the full reconstitution of spontaneous and/or caffeine-induced LCC activity in 5-day cultured atrial myocytes by adenovirus-driven expression of Panx1. In contrast to Ad-Panx1-infected myocytes, time-matched native or empty virus-infected cells revealed negligible channel activity. The loss in LCC activity was paralleled by a loss in Panx1 immunoreactivity. Previous studies related cardiac LCC to polycystin-2-like channels (24), ryanodine receptors (23), or Cx hemichannels (22). The inconsistent interpretations of the molecular nature of LCCs were solely based upon the pharmacological profile of channel inhibition, e.g. by tetracaine, ruthenium red, amiloride, or octanol. Although the channel specificity of CBX is rather low, it inhibits Panx1 channels at concentrations 5–20-fold less than required for inhibition of connexin hemichannels (37). Thus, 20 μm CBX used in this study acts on Panx1 rather than gap junctional channels.
Probenecid inhibits current carried Panx1 channels expressed in Xenopus oocytes with an IC50 of 150 μm (37). In identical experimental conditions, this compound at a concentration of 5 mm did not affect currents carried by different connexin channels. In our experiments 200 μm probenecid caused substantial inhibition of LCC activity in native and Ad-Panx1-infected myocytes, which excludes connexins as conductance pathways of LCC currents. The spontaneous openings of LCCs at physiological extracellular Ca2+ concentrations further argue against connexin channels being the molecular LCC correlate, because connexons should be closed at physiological Ca2+ concentrations of 1–2 mm. The bidirectional modulation of LCCs by extracellular ATP, activation by submillimolar concentrations, and inhibition by millimolar concentrations appears to be another characteristic feature of Panx1 channels (41, 42), supporting our interpretation of Panx1 protein forming the cardiac LC channel.
The modulation of LCC activity by extracellular ATP and membrane stretch and its sporadic spontaneous activity imply a considerable significance for cardiac (patho)physiology. Locally released ATP may affect cardiac excitability via activation of metabotropic purinergic receptors and subsequent openings of Panx1 channels. This notion is supported by the observation that in the mouse heart stretch-induced ATP release through Panx1 activates P2Y6 receptors in an auto-/paracrine way. This stimulates the heterotrimeric G12 family protein Gα12/13, which is suggested to trigger fibrosis via Rho activation under conditions of pressure overload (38). The same study demonstrated that stretch-induced ATP release from neonatal cardiac myocytes was substantially reduced by siRNA targeting Panx1, confirming Panx1 channels as a major ATP-release pathway.
Likewise, Panx1 was described to mediate the release of cardioprotectants induced by ischemic pre- and post-conditioning (39). Brief cycles of ischemia/reperfusion are supposed to induce the formation of Panx1-P2X7 complexes with concomitant release of ATP.
Openings of LCC in rat cardiac myocytes are accompanied by Ca2+ influx and membrane depolarizations, which can trigger action potentials (25). In the intact tissue, depolarizations resulting from brief spontaneous openings are likely to be dampened by the three-dimensional cable properties of the myocardial electrical syncytium.
However, spontaneous LCC openings mediated by Panx1 might be potentially arrhythmogenic under certain pathological conditions that cause an increased opening activity or Panx1 expression (38) analogous to the involvement of Panx1 channels in the generation of aberrant bursting in the hippocampus, as described recently (17).
Supplementary Material
Acknowledgments
We thank Drs. E. Petrasch-Parwez and N. Prochnow for helpful discussions and A. Galhoff, B. Liu, S. Peuckert, and C. Zoidl for technical assistance.

The on-line version of this article (available at http://www.jbc.org) contains supplemental Figs. S1–S3.
- Cx
- connexin
- LCC
- large conductance channel
- Panx
- pannexin
- NCX
- Na+/Ca2+-exchange
- CBX
- carbenoxolone.
REFERENCES
- 1. Bruzzone R., Hormuzdi S. G., Barbe M. T., Herb A., Monyer H. (2003) Proc. Natl. Acad. Sci. U.S.A. 100, 13644–13649 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Baranova A., Ivanov D., Petrash N., Pestova A., Skoblov M., Kelmanson I., Shagin D., Nazarenko S., Geraymovych E., Litvin O., Tiunova A., Born T. L., Usman N., Staroverov D., Lukyanov S., Panchin Y. (2004) Genomics 83, 706–716 [DOI] [PubMed] [Google Scholar]
- 3. Zoidl G., Petrasch-Parwez E., Ray A., Meier C., Bunse S., Habbes H. W., Dahl G., Dermietzel R. (2007) Neuroscience 146, 9–16 [DOI] [PubMed] [Google Scholar]
- 4. Prochnow N., Hoffmann S., Vroman R., Klooster J., Bunse S., Kamermans M., Dermietzel R., Zoidl G. (2009) Neuroscience 162, 1039–1054 [DOI] [PubMed] [Google Scholar]
- 5. Dando R., Roper S. D. (2009) J. Physiol. 587, 5899–5906 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Bhalla-Gehi R., Penuela S., Churko J. M., Shao Q., Laird D. W. (2010) J. Biol. Chem. 285, 9147–9160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Celetti S. J., Cowan K. N., Penuela S., Shao Q., Churko J., Laird D. W. (2010) J. Cell Sci. 123, 1363–1372 [DOI] [PubMed] [Google Scholar]
- 8. Ray A., Zoidl G., Weickert S., Wahle P., Dermietzel R. (2005) Eur. J. Neurosci. 21, 3277–3290 [DOI] [PubMed] [Google Scholar]
- 9. Vogt A., Hormuzdi S. G., Monyer H. (2005) Brain Res. Mol. Brain Res. 141, 113–120 [DOI] [PubMed] [Google Scholar]
- 10. Iglesias R., Dahl G., Qiu F., Spray D. C., Scemes E. (2009) J. Neurosci. 29, 7092–7097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Locovei S., Bao L., Dahl G. (2006) Proc. Natl. Acad. Sci. U.S.A. 103, 7655–7659 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Thompson R. J., Zhou N., MacVicar B. A. (2006) Science 312, 924–927 [DOI] [PubMed] [Google Scholar]
- 13. Pelegrin P., Surprenant A. (2006) EMBO J. 25, 5071–5082 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Locovei S., Wang J., Dahl G. (2006) FEBS Lett. 580, 239–244 [DOI] [PubMed] [Google Scholar]
- 15. Bao L., Locovei S., Dahl G. (2004) FEBS Lett. 572, 65–68 [DOI] [PubMed] [Google Scholar]
- 16. MacVicar B. A., Thompson R. J. (2010) Trends Neurosci. 33, 93–102 [DOI] [PubMed] [Google Scholar]
- 17. Thompson R. J., Jackson M. F., Olah M. E., Rungta R. L., Hines D. J., Beazely M. A., MacDonald J. F., MacVicar B. A. (2008) Science 322, 1555–1559 [DOI] [PubMed] [Google Scholar]
- 18. Silverman W. R., de Rivero Vaccari J. P., Locovei S., Qiu F., Carlsson S. K., Scemes E., Keane R. W., Dahl G. (2009) J. Biol. Chem. 284, 18143–18151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Pelegrin P., Barroso-Gutierrez C., Surprenant A. (2008) J. Immunol. 180, 7147–7157 [DOI] [PubMed] [Google Scholar]
- 20. Kanneganti T. D., Lamkanfi M., Kim Y. G., Chen G., Park J. H., Franchi L., Vandenabeele P., Núñez G. (2007) Immunity 26, 433–443 [DOI] [PubMed] [Google Scholar]
- 21. Bargiotas P., Monyer H., Schwaninger M. (2009) Curr. Mol. Med. 9, 186–194 [DOI] [PubMed] [Google Scholar]
- 22. Pott L., Mechmann S. (1990) J. Membr. Biol. 117, 189–199 [DOI] [PubMed] [Google Scholar]
- 23. Kondo R. P., Weiss J. N., Goldhaber J. I. (2000) Pflugers Arch. 440, 125–131 [DOI] [PubMed] [Google Scholar]
- 24. Volk T., Schwoerer A. P., Thiessen S., Schultz J. H., Ehmke H. (2003) Cardiovasc. Res. 58, 76–88 [DOI] [PubMed] [Google Scholar]
- 25. Zhang Y. A., Tuft R. A., Lifshitz L. M., Fogarty K. E., Singer J. J., Zou H. (2007) Am. J. Physiol. Heart Circ. Physiol. 293, H2448–H2461 [DOI] [PubMed] [Google Scholar]
- 26. Bechem M., Pott L., Rennebaum H. (1983) Eur. J. Cell Biol. 31, 366–36929 [PubMed] [Google Scholar]
- 27. He T. C., Zhou S., da Costa L. T., Yu J., Kinzler K. W., Vogelstein B. (1998) Proc. Natl. Acad. Sci. U.S.A. 95, 2509–2514 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Pfaffl M. W., Horgan G. W., Dempfle L. (2002) Nucleic Acids Res. 30, e36 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Mechmann S., Pott L. (1986) Nature 319, 597–599 [DOI] [PubMed] [Google Scholar]
- 30. Fabiato A., Fabiato F. (1975) J. Physiol. 249, 497–517 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Boassa D., Ambrosi C., Qiu F., Dahl G., Gaietta G., Sosinsky G. (2007) J. Biol. Chem. 282, 31733–31743 [DOI] [PubMed] [Google Scholar]
- 32. Penuela S., Bhalla R., Gong X. Q., Cowan K. N., Celetti S. J., Cowan B. J., Bai D., Shao Q., Laird D. W. (2007) J. Cell Sci. 120, 3772–3783 [DOI] [PubMed] [Google Scholar]
- 33. Penuela S., Bhalla R., Nag K., Laird D. W. (2009) Mol. Biol. Cell 20, 4313–4323 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Goldberg G. S., Moreno A. P., Bechberger J. F., Hearn S. S., Shivers R. R., MacPhee D. J., Zhang Y. C., Naus C. C. (1996) Exp. Cell Res. 222, 48–53 [DOI] [PubMed] [Google Scholar]
- 35. Bruzzone R., Barbe M. T., Jakob N. J., Monyer H. (2005) J. Neurochem. 92, 1033–1043 [DOI] [PubMed] [Google Scholar]
- 36. Shitara Y., Sato H., Sugiyama Y. (2005) Annu. Rev. Pharmacol. Toxicol. 45, 689–723 [DOI] [PubMed] [Google Scholar]
- 37. Silverman W., Locovei S., Dahl G. (2008) Am. J. Physiol. Cell Physiol. 295, C761–C767 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Nishida M., Sato Y., Uemura A., Narita Y., Tozaki-Saitoh H., Nakaya M., Ide T., Suzuki K., Inoue K., Nagao T., Kurose H. (2008) EMBO J. 27, 3104–3115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Vessey D. A., Li L., Kelley M. (2010) J. Cardiovasc. Pharmacol. Ther. 15, 190–195 [DOI] [PubMed] [Google Scholar]
- 40. Buvinic S., Almarza G., Bustamante M., Casas M., López J., Riquelme M., Sáez J. C., Huidobro-Toro J. P., Jaimovich E. (2009) J. Biol. Chem. 284, 34490–34505 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Ma W., Hui H., Pelegrin P., Surprenant A. (2009) J. Pharmacol. Exp. Ther. 328, 409–418 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Qiu F., Dahl G. (2009) Am. J. Physiol. Cell Physiol. 296, C250–C255 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Musa H., Tellez J. O., Chandler N. J., Greener I. D., Maczewski M., Mackiewicz U., Beresewicz A., Molenaar P., Boyett M. R., Dobrzynski H. (2009) Naunyn Schmiedebergs Arch. Pharmacol. 379, 541–549 [DOI] [PubMed] [Google Scholar]
- 44. Gurung I. S., Kalin A., Grace A. A., Huang C. L. (2009) J. Mol. Cell. Cardiol. 47, 622–633 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.