Abstract
Brown adipose tissue has a central role in thermogenesis to maintain body temperature through energy dissipation in small mammals and has recently been verified to function in adult humans as well. Here, we demonstrate that the heart-type fatty acid-binding protein, FABP3, is essential for cold tolerance and efficient fatty acid oxidation in mouse brown adipose tissue, despite the abundant expression of adipose-type fatty acid-binding protein, FABP4 (also known as aP2). Fabp3−/− mice exhibit extreme cold sensitivity despite induction of uncoupling and oxidative genes and hydrolysis of brown adipose tissue lipid stores. However, using FABP3 gain- and loss-of-function approaches in brown adipocytes, we detected a correlation between FABP3 levels and the utilization of exogenous fatty acids. Thus, Fabp3−/− brown adipocytes fail to oxidize exogenously supplied fatty acids, whereas enhanced Fabp3 expression promotes more efficient oxidation. These results suggest that FABP3 levels are a determinant of fatty acid oxidation efficiency by brown adipose tissue and that FABP3 represents a potential target for modulation of energy dissipation.
Keywords: Adipose Tissue Metabolism, Fatty Acid-binding Protein, Fatty Acid Oxidation, Gene Knockout, Mouse, Brown Adipose Tissue, Cold Tolerance
Introduction
Lipids are crucial metabolic fuels, and their transport, storage, and utilization is governed by numerous mechanisms to maintain homeostasis. The dysregulation of lipid homeostasis contributes to the pathogenesis of common diseases such as obesity, diabetes, and cardiovascular disease. Fatty acids are one of the key lipid types that must be transported, stored, or utilized in tissues with high rates of lipid metabolism, including adipose tissues, liver, heart, and skeletal muscle. Members of the fatty acid-binding protein (FABP)2 family are prominently expressed in these tissues and are thought to act as lipid “chaperones,” trafficking fatty acids to mitochondria and peroxisomes for oxidation, to lipid droplets for storage, to the endoplasmic reticulum for membrane synthesis and signaling, and to the nucleus for regulation of lipid-modulated nuclear transcription factors (1, 2). These activities are accomplished through increasing fatty acid solubility, mobility, and rate of utilization within the cell (2).
The FABP family consists of at least eight members in mammals, which appear to have arisen through duplication of an ancestral gene (2–4). Members of the FABP family have been named according to the tissue where they are expressed most prominently, although there is significant overlap in tissue distribution among some members (2, 4, 5). Multiple FABP family members are expressed in adipose tissue, but their physiological roles in this tissue have not been characterized. The adipose-type FABP4 (also known as aP2) has been considered the major FABP in white and brown adipose tissue, as well as in macrophages (6, 7). FABP4 is generally thought to facilitate fatty acid transport between intracellular compartments for storage or export, as disruption of the Fabp4 gene in mice led to increased intracellular free fatty acids in the cytosol (8, 9). The epidermis-type FABP5 (also known as Mal1) is expressed in epidermal cells and other tissues, including brown adipose tissue (10). Finally, heart-type FABP3, which is most abundantly expressed in heart, skeletal muscle, brain, and other tissues, is induced in brown adipose tissue (BAT) in hibernating squirrel (11, 12) and bat (13). It is also induced by acute cold exposure in rat (14, 15).
The physiological roles of FABP3 have been investigated with knock-out mouse models (reviewed in Ref. 16). Characterization of FABP3-deficient mice has focused largely on the effects of fatty acid metabolism in heart and skeletal muscle, tissues in which this protein is abundantly expressed. In vivo labeling studies revealed reduced palmitic and arachidonic acid uptake in heart of Fabp3−/− mice (17) and reduced palmitic acid uptake and utilization in isolated cardiomyocytes (18, 19). There was a compensatory reliance on glucose oxidation in the heart of Fabp3−/− mice. Fabp3−/− and Fabp3+/− mice also exhibit increased insulin sensitivity, perhaps related to the increased reliance on glucose rather than fatty acid fuels (20, 21). Thus, FABP3 has important roles in fatty acid metabolism in heart and skeletal muscle, with effects on systemic glucose homeostasis.
The induction of FABP3 in BAT during cold exposure described above raised the possibility that this protein may have a role in fatty acid trafficking in adaptive thermogenesis. Adaptive thermogenesis involves shivering to increase energy output in skeletal muscle, as well as nonshivering heat generation in BAT upon stimulation by the sympathetic nervous system (22, 23). Nonshivering thermogenesis is well known as a mechanism for heat generation in rodents and other small mammals, and recently, it has been shown to be metabolically active in adult humans (24–29). The elucidation of mechanisms that modulate BAT thermogenesis is therefore of high significance in understanding the regulation of energy balance in mammals, including humans.
Several factors that are required for adaptive thermogenesis in BAT have been defined in rodents (22, 23). One key factor is the mitochondrial uncoupling protein-1 (UCP1). UCP1 is responsible for enabling the proton leak in mitochondria that dissipates energy resulting from oxidative metabolism. In the mouse, UCP1 is essential for adaptive thermogenesis in response to acute cold exposure (30) but not for gradual adaptation to the cold (31). Furthermore, UCP1 deficiency prevents diet-induced thermogenesis and promotes obesity in mice maintained at thermoneutrality (32), whereas enhanced UCP1 expression in BAT protects against diet-induced obesity, due to increased energy expenditure (33). Beyond UCP1, fatty acids have a key role in thermogenesis as the source of oxidative fuel in the mitochondria (34–38). The process by which fatty acids destined for oxidation during BAT thermogenesis are targeted to the mitochondria is not fully understood but is likely to involve members of the FABP family.
Here, using FABP3-deficient mice, we demonstrate an essential role for FABP3 in whole body thermoregulation and in fatty acid oxidation in BAT, a tissue in which FABP4 and FABP5 have been considered to be the major players. Mice lacking FABP3 displayed severely impaired cold tolerance, whereas FABP4-deficient mice had normal cold tolerance. FAPB3 cold sensitivity occurred even in the presence of a normal response of muscle to cold and normal induction of thermogenic and fatty acid oxidation machinery in BAT. Using isolated BAT, as well as gain- and loss-of-function approaches in cultured brown adipocytes, we detected a direct effect of FABP3 on the rate of exogenous fatty acid oxidation in this tissue. These studies establish a unique role for FABP3 in directing the utilization of exogenously supplied fatty acids for energy expenditure in BAT.
EXPERIMENTAL PROCEDURES
Mice
Mouse embryonic stem cells containing a gene-trap insertion in the Fabp3 gene (cell line XE705) were obtained from the BayGenomics gene-trap consortium (39). Chimeric mice were generated by blastocyst microinjection and then crossed with C57BL/6J mice for at least four generations. The site of the gene-trap insertion within intron 1 of Fabp3 was determined by inverse PCR. Offspring were genotyped by PCR of genomic DNA with primers specific for the wild-type Fabp3 allele and for the mutant allele carrying the gene-trap insertion. FABP4-deficient mice were a generous gift from Dr. Judith Storch (Rutgers University). Mice were housed in standard conditions (12-h/light/dark cycle) and fed Purina 5001 chow diet. Mouse studies were performed under approval from the Veterans Affairs Greater Los Angeles or UCLA IACUC.
Gene Expression Analyses
Total RNA was isolated from mouse tissues by extraction with TRIzol (Invitrogen). cDNA synthesis, RT-PCR, and real time PCR were performed as described previously (40). Many of the primer sequences used in this study have been described previously (40–42). Other primer sequences are as follows: Fabp3 (agtcactggtgacgctggacg; aggcagcatggtgctgagctg); Fabp4 (gaacctggaagcttgtcttcg; accagcttgtcaccatctcg); Fabp5 (cacggctttgaggagtacatg; accgtcttggaaggtgcaga); Myl1 (gcaacaggaggacttcaagg; tctcttcattgctggggttc); Myh7 (acaacccctacgattatgcgt; acgtcaaaggcgctatccgtg); Tnni1 (tccacaacaccagagagatcaagg; gcatggcatcggctgagacacg); Tnni2 (cctgaagagtgtgatgctcca; cccgttccttctcagtgtctt); G6p (acttgttccctggccctgctgc; ctctgcaaatcagccgaggc); Ucp2 (gccacttcacttctgccttc; gaaggcatgaaccccttgta); Ucp3 (atgagttttgcctccattcg; ggcgtatcatggcttgaaat); Cebpα (gaacagcaacgagtaccgggta; gccatggccttgaccaaggag); Serca1a (ctgccgatgatcttcaagctc; cagggcacaagggctggttac); and Serca2a (atctccttgcctgtgatcctc; agtcatgcagagggctggtag).
FABP3 Detection by Immunoassay
Tissue concentrations of FABP3 were measured with a sandwich-type ELISA (HK403, HyCult Biotechnology) according to the manufacturer's protocol. Briefly, tissues were homogenized with a mechanical Ultra-Turrax® in 0.25 m sucrose, 2 mm EDTA, 10 mm Tris-Cl (pH 7.4) supplemented with protease inhibitors (Complete Mini, EDTA-free, Roche Applied Science). Total proteins were quantified with a commercial kit (BCA kit; Pierce) for normalization between samples. Data are reported as the average of determinations from samples of three mice, each measured in duplicate.
Cold Exposure
Prior to cold exposure, mice were provided with food and water ad libitum overnight. The next morning, the mice were housed without food or bedding and placed at 4 °C for 4 h. Body temperature was measured with a BAT-10 digital thermometer with a Ret-3 rectal probe (Physitemp Instruments). BAT surface temperature was measured by placing the temperature probe on the interscapular surface. Three separate cold exposure studies were performed with independent groups of male or female mice. These studies gave very similar results, indicating that this phenotype is not sex-specific. Data presented here are from male mice.
Plasma and Lipid Analysis
Plasma glucose levels were measured with a OneTouch® Ultra® blood glucose monitor (LifeScan). Tissue lipid analyses were conducted as described previously (40). Creatine kinase was determined in 25 μl of plasma (Pointe Scientific, Inc.) with an automated spectrophotometer connected to a water bath at 37 °C. Samples were measured twice and expressed as the average absorbance difference per min, multiplied by the factor 6592 (IU/liter).
Mitochondrial DNA Content
Total genomic DNA from BAT tissue was isolated by phenol/chloroform/isoamyl alcohol extraction. Mitochondrial and nuclear DNA were amplified by real time PCR with 25 ng of DNA and primers in the D-loop region (aatctaccatcctccgtgaaacc; tcagtttagctacccccaagtttaa) and Tert gene (ctagctcatgtgtcaagaccctctt; gccagcacgtttctctcgtt), respectively.
Analysis of Hepatic Glycogen Levels
Glycogen was extracted from frozen tissues (100 mg) as described previously (43). The supernatant fluid was used to measure glucose (Autokit Glucose, Wako Pure Chemicals); values were expressed as nanograms of glycosyl residue per g of tissue.
Histology
For β-galactosidase activity staining, fresh tissues were directly embedded in optimum cutting temperature compound (Sakura Finetek), frozen, and sectioned. Sections were fixed with 2% formaldehyde and 0.2% glutaraldehyde in PBS for 5 min and incubated in X-Gal solution at 37 °C overnight (44). For immunochemical detection of β-galactosidase protein, cryosections were quenched for endogenous peroxidase activity with 0.6% H2O2 in methanol for 20 min. Sections were fixed with cold acetone for 10 min, blocked with 10% rabbit serum in PBS, 0.1% Tween 20 for 3 h, and incubated overnight with primary anti-β-galactosidase antibody (1:250 dilution, ab9361, Abcam). Following a 1-h incubation with secondary HRP-conjugated anti-chicken antibody (1:1000 dilution, ab6753, Abcam), staining was revealed with diaminobenzidine (D4293, Sigma).
Indirect Calorimetry
Oxygen consumption was determined with an Oxymax single cage system and recorded with Oxymax version 3.2 software (Columbus Instruments). Oxygen consumption and CO2 production were recorded every 6 min throughout a 24-h period. The respiratory quotient represents the ratio of O2 over CO2 values. Values were averaged over light (7 a.m. to 6 p.m.) and dark (6 p.m. to 7 a.m.) periods.
Brown Adipocyte Cell Culture
For the culture of primary brown adipocytes, interscapular brown adipose tissue was isolated, minced, and digested with 20 mg of collagenase type II (clostridiopeptidase A, Sigma C-6885) in 10 ml of DMEM (without serum) with 2% BSA and 25 mm HEPES at 37 °C for 30 min. The homogenate was filtered through a 70-μm filter and decanted for 20 min on ice to remove mature adipocytes. Precursor cells were centrifuged at 800 × g for 5 min and resuspended in differentiation medium (DMEM supplemented with 4 nm insulin and 25 μg/ml sodium ascorbate, 10% serum). Cells were allowed to differentiate into mature brown adipocytes for 7 days. Some studies were conducted in an established mouse brown adipocyte cell line obtained from Dr. Bruce Spiegelman (38). Cells were cultured and differentiated into mature brown adipocytes as described previously (38).
In Vitro Fatty Acid Oxidation Experiments
For labeled fatty acid oxidation experiments, 3H2O production was measured as the products of exogenous fatty acid oxidation from [3H]palmitate as described previously (45, 46). Primary brown adipocytes were preincubated with 0.5 μm norepinephrine, and cells were collected, and protein levels were determined for normalization.
Intracellular lipid labeling was determined by TLC. Cells were collected with 200 μl of 5% TCA, and an aliquot was saved for protein determination. Lipids were extracted with chloroform/MeOH (2:1) and resolved on TLC plates with hexane/ether/acetic acid (80:20:1) solvent. Using standards for each lipid species, the bands corresponding to FFA and triglyceride were scraped from the plate and measured by liquid scintillation counting. Experiments were performed twice with triplicate wells.
Cellular Oxygen Consumption and Extracellular Acidification Rates
Cellular metabolic rates were measured in primary tissue pieces or in cultured cells using an XF24 extracellular flux analyzer (Seahorse Bioscience). For measurements of metabolism in primary brown fat, tissues were collected and finely minced in PBS. Pieces of tissue of equal size were rinsed with 20 ml of PBS, placed in the bottom of a Seahorse Islet Capture Microplate, and kept in place with the screen provided with the kit. The pieces of tissue were washed with unbuffered Krebs-Henseleit buffer containing 1 μm norepinephrine and incubated 1 h before starting the experiment. Palmitate oxidation was measured as described using a final palmitate concentration of 200 μm (47). Mixing, waiting, and measurement times were 4, 2, and 2 min, respectively. Data were normalized by total genomic DNA from each tissue sample.
A brown adipocyte cell line (38) was transfected with Fabp3 expression vector or Fabp3 RNAi and then plated into Seahorse Bioscience V28 plates. For Fabp3 overexpression, mouse Fabp3 cDNA was cloned in pcDNA3.1/V5-His vector (Invitrogen) and transfected with BioT (Bioland). For RNA interference, cells were reverse-transfected with ON-TARGETplus SMARTpool siRNA (Dharmacon) and siPORT amine reagent (Ambion). 48 h prior to analysis, differentiated cells were transfected and seeded at 60,000 cells per well in V28 plates (Seahorse Bioscience). Cells were preincubated with 0.5 μm norepinephrine for 3 h prior to start of the experiment. Immediately before the measurement, cells were washed with unbuffered DMEM or DMEM buffered with Krebs-Henseleit Buffer as described previously (47). Plates were placed into the XF24 instrument for measurement of oxygen consumption and extracellular acidification rates. Palmitate oxidation was measured as described using a final palmitate concentration of 200 μm (47). Mixing, waiting, and measurement times were 5, 2, and 1.5 min, respectively. Data were normalized to total cellular protein.
Statistical Analyses
Differences in mean values between groups were assessed using a two-tailed Student's t test. Data are presented as mean ± S.D., with a statistically significant difference between samples defined as p < 0.05. Significance levels are indicated in figure legends.
RESULTS
Fabp3 Is Expressed in BAT and Induced after Cold Exposure
FABP4 has been shown to be highly abundant in white and brown adipose tissues (48), but FABP3 has also been shown to be expressed in BAT of bats and rodents under conditions such as hibernation and cold exposure (11–13, 15). To begin to tease apart the specific roles of these two fatty acid-binding proteins in BAT, we examined the relative tissue distributions and response to cold exposure in mouse. Analysis of Fabp3 mRNA levels in tissues from C57BL/6J mice revealed highest expression in heart, followed by skeletal muscle (Fig. 1A). Under standard laboratory conditions, Fabp3 was also expressed at substantial levels in BAT, at ∼50% the level in skeletal muscle. By contrast, the adipocyte-type Fabp4 was expressed at highest levels in BAT, followed by gonadal and inguinal white adipose tissue, with lower levels in heart and muscle (Fig. 1A). FABP3 protein levels mirrored the mRNA levels, with highest levels in heart and lower levels in skeletal muscle and BAT (Fig. 1B). Thus, FABP3 mRNA and protein are present in BAT of C57BL/6J mice maintained at room temperature.
FIGURE 1.
FABP3 is expressed in brown fat and induced by cold exposure. A, Fabp3 and Fabp4 mRNA levels in mouse tissues determined by real time PCR. cDNA was prepared from C57BL/6J mice, fasted overnight (n = 3). Values shown are in arbitrary units, normalized to housekeeping genes. B, FABP3 protein concentrations in the same samples as in A were determined by immunoassay. Values are expressed as nanograms of FABP3 per μg of total cellular protein. C, Fabp3 and Fabp4 mRNA levels in BAT, heart, skeletal muscle (quadriceps), and white adipose tissue at room temperature (RT) and after 4 h at 4 °C (n = 3). D, Fabp5 mRNA levels in BAT, heart, and skeletal muscle (quadriceps) at room temperature and after 4 h at 4 °C (n = 3). E, FABP3 protein concentration in BAT, heart, and skeletal muscle at room temperature and after 4 h at 4 °C. Protein extracts were obtained from the same mice as in C. In all panels, values represent the mean ± S.D. Sk, skeletal. *, p < 0.05 versus room temperature values.
Because BAT plays a key role in the maintenance of body temperature in rodents, we determined whether mouse Fabp3 gene expression in BAT is induced by acute cold exposure. C57BL/6J mice were maintained at 4 °C for 8 h. After 8 h, Fabp3 mRNA levels were induced significantly in BAT, heart, and skeletal muscle but not in white adipose tissue (Fig. 1C). Fabp4 (Fig. 1C) and Fabp5 mRNA levels (Fig. 1D) were not affected by cold exposure. In parallel with changes in mRNA levels, FABP3 protein was significantly increased after cold exposure in BAT, skeletal muscle, and heart (Fig. 1E). These results establish that Fabp3, but not Fabp4 or Fabp5 expression, increases in BAT, heart, and skeletal muscle in response to cold exposure.
Generation of Fabp3−/− Mice by Gene-trap Mutation
To investigate the physiological role of FABP3 in adaptive thermogenesis, we generated FABP3-deficient mice with an embryonic stem cell line carrying a gene-trap insertional mutation in the Fabp3 gene. The gene-trap insertion occurred in intron 1, generating a fusion protein containing the first 24 amino acids of FABP3 joined in-frame to a βgeo reporter (Fig. 2A). Mice homozygous for the mutant allele are deficient in FABP3, confirmed by the failure to detect Fabp3 mRNA by RT-PCR (Fig. 2B). β-Galactosidase expression in tissues of Fabp3−/− mice was detected at the sites where Fabp3 is normally expressed in wild-type mice. We observed intense staining of cardiomyocytes and specific fibers in skeletal muscle (Fig. 2C). Robust staining was also present in interscapular BAT (Fig. 2C). Fabp3 expression within brown adipocytes was confirmed by immunocytochemical detection of β-galactosidase in lipid droplet-containing cells (Fig. 2D).
FIGURE 2.
Generation of FABP3-deficient mice. A, insertional mutation in Fabp3. The gene-trap vector inserted into intron 1 of the Fabp3 gene, resulting in the production of a fusion protein consisting of the 24 amino-terminal amino acids from FABP3 joined in-frame to βgeo. B, absence of Fabp3 mRNA in the heart of Fabp3−/− mice determined by RT-PCR. PCR results are shown for three mice of each genotype using PCR primers located downstream of the gene-trap vector insertion site (see “Experimental Procedures”). The housekeeping gene hypoxanthine phosphoribosyltransferase (Hprt) was expressed at similar levels in wild-type and Fabp3−/− heart. C, detection of sites of Fabp3 gene expression determined by β-galactosidase staining of heart, skeletal muscle, and BAT sections from Fabp3−/− gene-trap mice. Tissues were cryosectioned at 10 μm (heart and skeletal muscle) or 40 μm (BAT). Pictures show cross-section (cross) or longitudinal sections, as indicated. Magnification is ×100 for heart and is ×400 for skeletal muscle and BAT. D, immunocytochemical detection of β-galactosidase in BAT. Tissue was cryosectioned at 10 μm. An adjacent section was stained with control IgY antibody. Magnification is ×630.
Impaired Temperature Regulation in Fabp3−/− Mice after Cold Exposure or Fasting
To determine the effect of FABP3 deficiency on thermoregulation during acute cold exposure, mice were placed at 4 °C, and body temperature and BAT surface temperature were monitored (Fig. 3A). Both body and BAT surface temperatures in Fabp3−/− mice were significantly lower than in wild-type mice after just 2 h. After 4 h, the difference in body temperature between wild-type and Fabp3−/− mice was more than 13 °C (34.4 ± 0.9 and 21.1 ± 2.1 °C, respectively), and the difference in BAT surface temperature was more than 11 °C (34.0 ± 1.0 and 22.7 ± 2.5 °C, respectively). By contrast, FABP4-deficient mice (9) demonstrated a cold response comparable with their wild-type littermates (Fig. 3B). The cold sensitivity in Fabp3−/− mice could not be explained by reduced body weight or white adipose tissue stores (Fig. 3C) nor by increased muscle shivering, as indicated by circulating creatine kinase levels similar to wild-type mice at both room temperature and 4 °C (Fig. 3D). Furthermore, markers of skeletal muscle oxidative and glycolytic metabolism were similarly affected by cold exposure in wild-type and Fabp3−/− mice. Acute cold exposure slightly induced genes expressed in fast twitch/glycolytic muscle fibers (myosin light chain 1, Myl1, and troponin I type 2, Tnni2) and repressed genes expressed in slow twitch/oxidative muscle fibers (myosin heavy chain 7, Myh7, and troponin I type 1, Tnni1) (Fig. 3E). Together, these results indicate that FABP3, but not FABP4, is required for cold tolerance and suggest that this may be independent of the role of FABP3 in skeletal muscle. We therefore investigated the potential role of BAT FABP3 in body temperature maintenance.
FIGURE 3.
FABP3-deficient mice are cold-intolerant. A, rectal and BAT surface temperature was measured at indicated times during cold exposure in Fabp3−/− mice. Data represent the mean ± S.D. (n = 4). B, rectal temperature during cold exposure in Fabp4−/− mice. Data represent the mean ± S.D. (n = 4). C, body weight and percent white adipose tissue mass in 6-month-old mice fed chow diet and maintained at room temperature (RT). Data represent the mean ± S.D. (n = 8–10). lng. fat, inguinal fat; Gon. fat, gonadal fat. D, creatine kinase activity in the plasma of mice at room temperature or after 4 h at 4 °C. All mice were fasted for 4 h before blood collection. Data represent the mean ± S.D. (n = 4). E, mRNA levels in skeletal muscle (quadriceps) of wild-type and Fabp3−/− mice at room temperature and after 4 h at 4 °C. Myl1, myosin light chain 1; Tnni2, troponin 1, skeletal, fast 2; Myh7, myosin heavy chain 7; Tnni, troponin 1, skeletal, slow 1. Data represent the mean ± S.D. (n = 4). *, p < 0.05 versus control group; **, p < 0.01 versus control group.
Key processes in the activation of BAT thermogenesis include mitochondrial biogenesis and induction of genes that lead to uncoupling of fatty acid oxidation from ATP synthesis. We investigated whether FABP3 deficiency leads to impairment in these processes. In Fabp3−/− mice, mitochondrial DNA content in BAT was increased nearly 2-fold compared with wild-type littermates but was not altered in skeletal muscle (Fig. 4A). Fabp3−/− mice had increased oxygen consumption compared with wild-type mice during the dark period of the circadian cycle, suggesting higher basal energy expenditure (Fig. 4B). Fabp3−/− mice also had a higher respiratory quotient, reflecting an increased proportional use of carbohydrate metabolic substrates over fatty acids (Fig. 4B). These results indicate that neither reduced mitochondrial number nor impaired whole body respiration are responsible for the impaired thermogenesis in Fabp3−/− mice. The BAT-specific increase in mitochondria may represent an attempt to compensate for a deficiency in energy metabolism specifically in this tissue.
FIGURE 4.
Normal induction of mitochondriogenesis and thermogenic gene expression in Fabp3−/− mice. A, relative mitochondrial DNA content in BAT of wild-type and Fabp3−/− mice at room temperature (RT) and after 4 h at 4 °C. Mitochondrial DNA content was measured by real time PCR with primers in the D-loop region and normalized to nuclear DNA (Tert gene). #, p < 0.05 versus wild type. B, oxygen consumption (ml/h) and respiratory quotient (RQ) determined by indirect calorimetry. n = 6. White and black bars represent wild-type and Fabp3−/−, respectively. C, mRNA levels in brown fat of wild-type and Fabp3−/− mice at room temperature and after 4 h at 4 °C. Data represent the mean ± S.D. (n = 4). D, mRNA levels in skeletal muscle (quadriceps) of wild-type and Fabp3−/− mice at room temperature and after 4 h at 4 °C. Data represent the mean ± S.D. (n = 4). *, p < 0.05; **, p < 0.01; ***, p < 0.001 versus values at room temperature.
Fabp3−/− Mice Exhibit Normal Induction of Thermogenic Gene Expression in BAT
Expression levels of the key thermogenic genes encoding peroxisome proliferator-activated receptor γ coactivator-1α (Pgc1a) and uncoupling protein-1 (Ucp1) were similar in wild-type and Fabp3−/− mice at room temperature and were induced to the same extent after 4 h of cold exposure (Fig. 4C). Gene expression of uncoupling protein-2 and -3 (Ucp2 and Ucp3) in Fabp3−/− mice also matched or exceeded levels in wild-type mice. Expression of genes involved in fatty acid oxidation, such as carnitine palmitoyltransferase-1b (Cpt1b) and acyl-CoA oxidase-1 (Acox1), were increased to the same extent by cold exposure in wild-type and Fabp3−/− mice. The transcription factor CCAAT/enhancer-binding protein α (Cebpa) is normally repressed during cold exposure (49), and this response was observed in both wild-type and Fabp3−/− mice. The absence of FABP3 did not lead to increased Fabp4 gene expression in BAT (Fig. 4C).
Fabp3−/− mice also exhibited normal cold-induced gene expression in skeletal muscle. Ucp2 and Ucp3 genes were expressed at similar basal levels in wild-type and Fabp3−/− mice, and both were induced to the same degree by cold exposure (Fig. 4D). Muscle expression of Cpt1b and Acox1 was not altered by the cold nor by FABP3 deficiency. Members of the sarco/endoplasmic reticulum calcium-ATPase family have the potential to contribute to heat production (50, 51), but we did not uncover any differences in expression levels in BAT or muscle of wild-type and Fabp3−/− mice (Fig. 4, C and D). Thus, aside from increased Ucp2 mRNA levels in BAT of the Fabp3−/− mice, expression levels of key thermogenic and fatty acid oxidation genes were similar in BAT and skeletal muscle in wild-type and Fabp3−/− mice.
Fabp3−/− Mice Have Increased Reliance on Glucose in the Cold
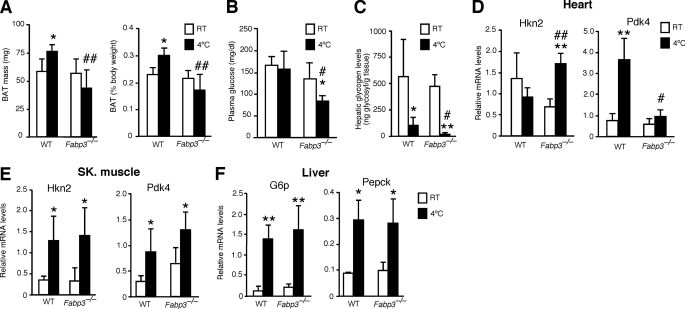
We investigated the effect of cold exposure on BAT composition and lipid mobilization in wild-type and Fabp3−/− mice. At room temperature, BAT depots weighed the same in both groups of mice, when expressed as absolute weight or as percent body weight (Fig. 5A). After 4 h in the cold, BAT mass was significantly lower in Fabp3−/− mice than in wild-type mice. The cold-induced reduction in BAT mass was associated with 65% lower triglyceride (TG) levels and 25% lower FFA levels (Table 1). In contrast, the lipid content of BAT of wild-type mice was not altered by cold exposure. In heart, FFA content was increased in response to cold in wild-type mice but decreased in Fabp3−/− mice (Table 1). In skeletal muscle, neither TG nor FFA levels changed, either in the wild-type or Fabp3−/− mice. Cold exposure led to higher FFA levels in the liver and circulation of both wild-type and Fabp3−/− mice, but the response was more prominent in the knock-out mice (Table 1). The elevated circulating FFA levels suggest that FFA release from white adipose tissue occurs normally in the FABP3-deficient mice and that the defect in thermogenesis may involve inadequate utilization of these FFAs as fuel in BAT.
FIGURE 5.
Lipid and glucose metabolism during cold exposure in wild-type (WT) and Fabp3−/− mice. A, BAT mass at room temperature and after 4 h at 4 °C, expressed as milligrams of body weight and as percent of body weight. B, plasma glucose levels. C, glycogen levels in liver. Glycogen was extracted from frozen tissues and hydrolyzed. Levels correspond to glycosyl molecules per g of tissue. D, induction of glycolytic gene expression in heart of Fabp3−/− mice after cold exposure. Hkn2, hexokinase II; Pdk4, pyruvate dehydrogenase kinase 4. E, glycolytic gene expression in skeletal muscle. F, induction of glucose synthesis gene expression in liver after cold exposure. G6p, glucose-6-phosphatase; Pepck, phosphoenolpyruvate carboxykinase. Open and black bars represent room temperature (RT) and 4 h at 4 °C, respectively. *, p < 0.05, and **, p < 0.01, compared with room temperature. #, p < 0.05, and ##, p < 0.01, compared with wild-type mice at the same temperature. Data represent the mean ± S.D. (n = 4).
TABLE 1.
TG and FFA levels in plasma (mg/dl) and tissues
TG are given as mg/mg protein; FFA are given as nmol/mg protein. RT, room temperature.
| TG, RT | TG, cold | FFA, RT | FFA, cold | |
|---|---|---|---|---|
| Plasma | ||||
| Wild type | 23.5 ± 3.5 | 43.0 ± 4.0a | 16.0 ± 2.8 | 28.3 ± 0.6b |
| Fabp3−/− | 24.5 ± 9.2 | 51.3 ± 5.5a | 22.0 ± 5.7 | 43.0 ± 4.4a,c |
| Heart | ||||
| Wild type | 0.04 ± 0.01 | 0.07 ± 0.01a | 37.6 ± 6.3 | 47.2 ± 7.1 |
| Fabp3−/− | 0.07 ± 0.06c | 0.06 ± 0.01 | 56.5 ± 9.0c | 40.6 ± 4.5a |
| Muscle | ||||
| Wild type | 0.06 ± 0.02 | 0.03 ± 0.01a | 18.4 ± 4.5 | 16.1 ± 1.4 |
| Fabp3−/− | 0.05 ± 0.02 | 0.03 ± 0.01 | 19.6 ± 3.1 | 15.2 ± 2.4 |
| BAT | ||||
| Wild type | 0.96 ± 0.36 | 0.88 ± 0.19 | 426 ± 42 | 460 ± 62 |
| Fabp3−/− | 1.67 ± 0.40c | 0.59 ± 0.18b,c | 430 ± 120 | 332 ± 102c |
| Liver | ||||
| Wild type | 0.63 ± 0.30 | 1.21 ± 0.18a | 130.6 ± 37.6 | 188.8 ± 21.5a |
| Fabp3−/− | 1.02 ± 0.26 | 1.47 ± 0.39 | 188.8 ± 30.2c | 220.0 ± 21.4c |
a p < 0.05.
b p < 0.01 within a genotype for room temperature versus cold.
c p < 0.05 for wild-type versus Fabp3−/− mice. n = 4 for each genotype at each temperature.
Whereas circulating FFA levels were elevated in cold-exposed Fabp3−/− mice, glucose levels were substantially reduced (Fig. 5B and Table 1). Hepatic glycogen levels after cold exposure were reduced 80% in wild-type mice and nearly completely exhausted in Fabp3−/− mice (Fig. 5C). Enhanced glucose metabolism in Fabp3−/− mice was associated with characteristic gene expression changes. Hexokinase (Hkn2) gene expression in the heart was not significantly altered by cold exposure in wild-type mice, but it increased 2-fold in Fabp3−/− mice (Fig. 5D). Pyruvate dehydrogenase kinase 4 (Pdk4), a negative regulator of glucose oxidation, is known to be induced by cold exposure in squirrel (52, 53). Pdk4 expression levels were similar in wild-type and Fabp3−/− mice at room temperature, and induced in both upon cold exposure. However, the induction was 7-fold in wild-type and only 2-fold in Fabp3−/− mice (Fig. 5D). Together, these data suggest that glycolysis is more active in heart of FABP3-deficient mice than wild-type mice after cold exposure.
In skeletal muscle, Hkn2 and Pdk4 expression levels were induced to similar levels by cold in wild-type and Fabp3−/− mice, in contrast to what was observed in heart (Fig. 5, D and E). In liver, gluconeogenic gene expression was induced by cold exposure, with an 8-fold increase in glucose-6-phosphatase (G6p) and a 3-fold induction of phosphoenolpyruvate carboxykinase (Pepck) (Fig. 5F). However, no differences were observed between wild-type and mutant mice. This suggests that there is no compensatory increase in hepatic glucose production in cold-exposed Fabp3−/− mice above that occurring in wild-type mice.
FABP3 Level in Brown Adipocytes Is a Determinant of Exogenous Fatty Acid Oxidation Rate
As described above, we demonstrated that the cold sensitivity of Fabp3−/− mice cannot be explained by a defect in the induction of thermogenic genes in BAT. However, the simultaneous increase of circulating FFA and decrease in circulating glucose suggested that Fabp3−/− mice have a defect in oxidation of exogenously supplied fatty acids that are derived from lipolysis in white adipose tissue. To test this possibility, we quantitated fatty acid oxidation rate directly in BAT from wild-type and Fabp3−/− mice. BAT was isolated from mice maintained at room temperature or after exposure to 4 °C for 4 h, and oxygen consumption rate (OCR) in response to fatty acid administration was measured using an XF24 extracellular flux analyzer. BAT from wild-type mice maintained at room temperature responded to palmitate administration with a high level of oxygen consumption, which was enhanced further in BAT isolated after cold exposure (Fig. 6A). The OCR was substantially blunted in Fabp3−/− mice, reaching only 30–35% of wild-type values. Thus, in the absence of muscle, heart, white adipose tissue, or other tissues that might affect whole body energy metabolism, FABP3 deficiency leads to a reduction in oxidation of extrinsically supplied fatty acids in BAT.
FIGURE 6.
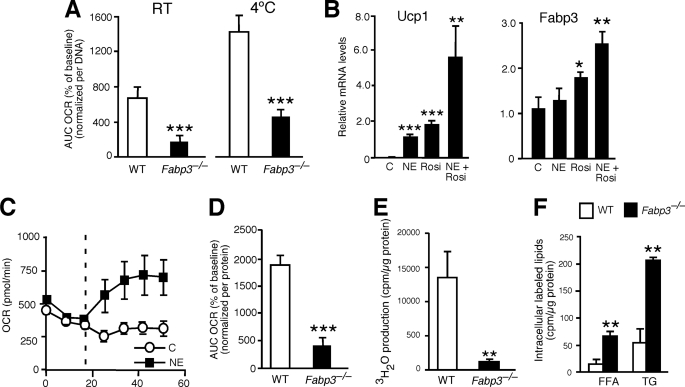
Impaired fatty acid oxidation in brown adipose tissue and primary brown adipocytes from Fabp3−/− mice. A, fatty acid oxidation in primary BAT from wild-type and Fabp3−/− mice was determined using the XF24 extracellular flux analyzer. Percent increase in OCR was measured after 200 μm palmitate injection. Results are expressed as the area under the curve (AUC). Values represent mean ± S.E. (n = 10). B, mRNA levels for Ucp1 and Fabp3 were quantitated by real time RT-PCR in differentiated wild-type primary brown adipocytes. Cells were treated overnight with 1 μm norepinephrine (NE), 2 μm rosiglitazone (Rosi), or a combination of the two. C, control. Values represent mean ± S.D. (n = 3). C, differentiated primary brown adipocytes with and without overnight treatment with 1 μm norepinephrine were analyzed for oxygen consumption rate using the XF24 extracellular flux analyzer. The vertical dotted line indicates the addition of 200 μm palmitate. Values represent mean ± S.E. (n = 10). D, fatty acid oxidation in differentiated primary brown adipocytes from wild-type and Fabp3−/− mice. Cells were pretreated with 1 μm norepinephrine for 3 h before fatty acid oxidation rates were measured with the XF24 extracellular flux analyzer. Values represent the area under the curve (AUC) of the OCR in response to 200 μm palmitate. Mean ± S.E. (n = 10). E, fatty acid oxidation in differentiated primary brown adipocytes of wild-type and Fabp3−/− mice determined using a radiometric method. Cells were incubated with 0.5 μm norepinephrine and [3H]palmitate for 4 h, and the oxidation rate was determined by measuring the concentration of 3H2O in the culture medium. Values represent mean ± S.D. (n = 5). F, intracellular lipid labeling after 4 h of [3H]palmitate incubation. Cells were labeled and lipids extracted as described under “Experimental Procedures.” Labeled FFA and TG were isolated, quantitated, and normalized to cellular protein. *, p < 0.05; **, p < 0.01; ***, p < 0.001 versus controls.
We confirmed the effects of FABP3 on brown adipocyte fatty acid oxidation using primary brown adipocytes. Primary brown adipocytes from wild-type mice responded to norepinephrine, rosiglitazone, or a combination of the two with substantial up-regulation of Ucp1 gene expression (Fig. 6B). Fabp3 expression was also induced by the peroxisome proliferator-activated receptor γ agonist rosiglitazone alone or in combination with norepinephrine. As expected, norepinephrine treatment increased OCR in wild-type brown adipocytes (Fig. 6C), and this effect was reduced in Fabp3−/− brown adipocytes (Fig. 6D). The impairment in fatty acid oxidation in Fabp3−/− brown adipocytes was the same when quantitated using a standard radiometric assay employing radiolabeled palmitate (Fig. 6E). Furthermore, in the radiometric assay we detected an accumulation of labeled palmitate within Fabp3−/− adipocytes, both as free fatty acid and incorporated into triglycerides, consistent with reduced disposal via oxidation (Fig. 6F).
We further demonstrated that FABP3 levels are a determinant of fatty acid oxidation using gain- and loss-of function approaches in a brown adipocyte cell line (38). As with the primary brown adipocytes, upon differentiation this cell line induces Ucp1 expression in response to norepinephrine or rosiglitazone (Fig. 7A), and it induces Fabp3 expression in response to the peroxisome proliferator-activated receptor γ agonist. In addition, norepinephrine increases OCR in the brown adipocyte cell line in a manner similar to primary brown adipocytes (compare Fig. 7B with 6C). To investigate the effects of modulating FABP3 levels, we assessed OCR in differentiated brown adipocytes in which Fabp3 expression was increased by transfection or decreased by siRNA knockdown (Fig. 7C). OCR was increased more than 5-fold by Fabp3 overexpression and reduced by 75% as a result of Fabp3 knockdown (Fig. 7D). Together with the studies from Fabp3−/− BAT and brown adipocytes, these results demonstrate that the levels of FABP3 are a determinant of exogenous fatty acid oxidation rate.
FIGURE 7.
FABP3 levels are a determinant of fatty acid oxidation in a brown adipocyte cell line. A, mRNA levels for Ucp1 and Fabp3 in differentiated brown adipocytes were determined by real time RT-PCR. Cells were treated overnight with 1 μm norepinephrine (NE), 2 μm rosiglitazone (Rosi), or a combination of the two. Values represent mean ± S.D. (n = 3). B, OCR in differentiated brown adipocytes with and without overnight treatment with 1 μm norepinephrine was measured with the XF24 extracellular flux analyzer. The vertical dashed line indicates the addition of 200 μm palmitate. Values represent mean ± S.E. (n = 10). C, modulation of Fabp3 expression levels in differentiated brown adipocytes after transfection with an overexpression vector (left) or administration of RNAi (right). Values represent mean ± S.E. (n = 3). D, oxygen consumption rate in differentiated brown adipocytes after Fabp3 overexpression or siRNA knockdown. Cells were pretreated with 1 μm norepinephrine for 3 h before fatty acid oxidation rates were measured with the XF24 extracellular flux analyzer. Values represent mean ± S.E. (n = 10). *, p < 0.05; **, p < 0.01; ***, p < 0.001 versus controls.
DISCUSSION
The current studies establish a unique role for the heart-type fatty acid-binding protein, FABP3, in BAT fatty acid oxidation and thermogenesis. Cold intolerance has been documented in several mouse models with genetic modifications that impair brown adipose tissue development, mitochondrial biosynthesis and function, uncoupling, and fatty acid oxidation enzyme reactions (30, 34–38, 49). The mechanism of cold sensitivity in FABP3-deficient mouse is distinct from most of these models, as Fabp3−/− mice exhibit normal cold induction of genes involved in mitochondrial biogenesis, uncoupling, and fatty acid oxidation in BAT and skeletal muscle. Furthermore, machinery for lipolysis in peripheral tissues and fatty acid oxidation in brown adipocytes is functional in Fabp3−/− mice, as evidenced by a doubling of BAT mitochondrial number, depletion of TG stored in BAT, and increased circulating FFA. The defect lies in the inability of brown adipocytes to utilize exogenous fatty acids as fuel, highlighting a critical role for the supply of exogenous fatty acids in BAT during acute cold exposure. Of the known cold-intolerant mouse models, the FABP3-deficient phenotype is most similar to that of mice lacking acyl-CoA synthetase-1 (ACSL1) in adipose tissue, which leads to cold intolerance associated with reduced BAT fatty acid oxidation and increased glucose utilization, despite normal thermogenic gene induction in BAT (34). ACSL1 has a critical role in directing fatty acids stored in lipid droplets within brown adipocytes toward β-oxidation. Our studies suggest that FABP3 may play a parallel role, directing exogenously supplied fatty acids that are released from white adipose tissue stores to mitochondria for β-oxidation.
FABP3 and FABP4 are closely related members of the FABP family (63% identity), are both expressed in BAT, and have similar biochemical properties in vitro (6). However, only FABP3 is critical during thermogenesis, as FABP4 is not induced in response to cold and FABP4-deficient mice are cold-tolerant. Our results clearly indicate that FABP3 and FABP4 have distinct roles in fatty acid transport/utilization in brown adipocytes. FABP3-deficient cells can store TG and utilize endogenous TG stores for oxidation, suggesting that fatty acid transport in those processes may be mediated by FABP4. However, FABP3 is required to utilize exogenous fatty acids derived from white adipose tissue hydrolysis in vivo or supplied in the medium of cultured adipocytes in vitro. This suggests a specific role for FABP3 within adipocytes in the transport and delivery of exogenous fatty acids to the mitochondria for oxidation. As predicted by this model, FABP3 deficiency leads to impaired thermogenesis due to a limited supply of fatty acid fuel to the mitochondria. This model and our in vitro data in brown adipocytes with FABP3 gain- and loss-of-function raise the interesting possibility that enhanced FABP3 action can direct fatty acids to the mitochondria for increased rates of oxidation. Our studies are consistent with and extend the findings of Yamashita et al. (15), who observed that UCP1-deficient mice exhibit increased FABP3 expression in BAT and higher rates of FFA clearance from the plasma.
At this point it is unclear how the distinct actions of FABP3 and FABP4 are mediated within the brown adipocyte. Differential cellular compartmentalization or specific binding affinities could underlie the distinct roles of FABP3 and FABP4 in brown adipose tissue. For example, it may be that the two proteins interact differently with specific fatty acid species or with specific proteins on acceptor membranes. However, in vitro studies with the two FABPs have not shown differences in their affinities for acceptor membranes. Both FABP3 and FABP4 exchange FFA with membrane structures via collisional transfer, and no distinction has been observed in the transfer of fatty acids from FABP3 and FABP4 to acceptor membranes (54, 55). Future studies with intact cells isolated from FABP3-deficient and FABP4-deficient mice may shed light on this mechanism.
Although previously controversial, it is becoming widely accepted that metabolically active BAT is present in depots extending from the neck to thorax in healthy adult humans and may contribute to energy expenditure (26, 29). Biopsies have demonstrated that human BAT exhibits the expected morphology and expression of UCP1 and is also induced upon cold exposure (24, 28, 29). If BAT activity contributes to energy expenditure in humans, then it is reasonable to expect that correlations may exist between variations in BAT activity and body weight and fat. Although still controversial, some data exist demonstrating that variations in BAT mass are inversely correlated with several measures of adiposity, such as body mass index, body fat, or visceral fat (26, 29), and it has been proposed that the human propensity to develop obesity in middle age may be related to a condition of diminished brown adipose tissue activity (56). Our findings suggest that genetic variations affecting FABP3 levels could influence energy expenditure and related characteristics such as body fat accumulation and insulin resistance. A genetic association has been detected between an insertion/deletion polymorphism in the human FABP3 gene and type 2 diabetes risk and waist/hip ratio (57). Multiple studies in pigs and cattle have also detected associations between FABP3 genotypes and adiposity traits (58–61). Thus, FABP3 may be a viable candidate to explain some genetic variation in energy balance traits, and moreover, modulation of FABP3 levels may represent a novel therapeutic target to modulate energy expenditure.
Acknowledgments
We thank Dr. Judith Storch for providing FABP4-deficient mice and Dr. Bruce Spiegelman for the kind gift of the mouse brown adipocyte cell line.
This work was supported, in whole or in part, by National Institutes of Health Grants P01 HL90553 (to K. R. and S. G. Y.) and U01 HL66621 (to S. G. Y.). This work was also supported by American Heart Association Grant 09BGIA2080363 (to L. V.).
- FABP
- fatty acid-binding protein
- BAT
- brown adipose tissue
- FFA
- free fatty acid
- TG
- triglyceride
- OCR
- oxygen consumption rate.
REFERENCES
- 1. Furuhashi M., Hotamisligil G. S. (2008) Nat. Rev. Drug Discov. 7, 489–503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Storch J., Corsico B. (2008) Annu. Rev. Nutr. 28, 73–95 [DOI] [PubMed] [Google Scholar]
- 3. Chmurzyńska A. (2006) J. Appl. Genet. 47, 39–48 [DOI] [PubMed] [Google Scholar]
- 4. Haunerland N. H., Spener F. (2004) Prog. Lipid Res. 43, 328–349 [DOI] [PubMed] [Google Scholar]
- 5. Zimmerman A. W., Veerkamp J. H. (2002) Cell. Mol. Life Sci. 59, 1096–1116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Coe N. R., Bernlohr D. A. (1998) Biochim. Biophys. Acta 1391, 287–306 [DOI] [PubMed] [Google Scholar]
- 7. Makowski L., Hotamisligil G. S. (2005) Curr. Opin. Lipidol. 16, 543–548 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Coe N. R., Simpson M. A., Bernlohr D. A. (1999) J. Lipid. Res. 40, 967–972 [PubMed] [Google Scholar]
- 9. Hotamisligil G. S., Johnson R. S., Distel R. J., Ellis R., Papaioannou V. E., Spiegelman B. M. (1996) Science 274, 1377–1379 [DOI] [PubMed] [Google Scholar]
- 10. Maeda K., Uysal K. T., Makowski L., Görgün C. Z., Atsumi G., Parker R. A., Brüning J., Hertzel A. V., Bernlohr D. A., Hotamisligil G. S. (2003) Diabetes 52, 300–307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Hittel D., Storey K. B. (2001) Biochim. Biophys. Acta 1522, 238–243 [DOI] [PubMed] [Google Scholar]
- 12. Hittel D., Storey K. B. (2002) Arch. Biochem. Biophys. 401, 244–254 [DOI] [PubMed] [Google Scholar]
- 13. Eddy S. F., Storey K. B. (2004) Biochim. Biophys. Acta 1676, 63–70 [DOI] [PubMed] [Google Scholar]
- 14. Daikoku T., Shinohara Y., Shima A., Yamazaki N., Terada H. (1997) FEBS Lett. 410, 383–386 [DOI] [PubMed] [Google Scholar]
- 15. Yamashita H., Wang Z., Wang Y., Segawa M., Kusudo T., Kontani Y. (2008) Biochem. Biophys. Res. Commun. 377, 632–635 [DOI] [PubMed] [Google Scholar]
- 16. Binas B., Erol E. (2007) Mol. Cell. Biochem. 299, 75–84 [DOI] [PubMed] [Google Scholar]
- 17. Murphy E. J., Barcelo-Coblijn G., Binas B., Glatz J. F. (2004) J. Biol. Chem. 279, 34481–34488 [DOI] [PubMed] [Google Scholar]
- 18. Binas B., Danneberg H., McWhir J., Mullins L., Clark A. J. (1999) FASEB J. 13, 805–812 [DOI] [PubMed] [Google Scholar]
- 19. Schaap F. G., Binas B., Danneberg H., van der Vusse G. J., Glatz J. F. (1999) Circ. Res. 85, 329–337 [DOI] [PubMed] [Google Scholar]
- 20. Erol E., Cline G. W., Kim J. K., Taegtmeyer H., Binas B. (2004) Am. J. Physiol. Endocrinol. Metab. 287, E977–E982 [DOI] [PubMed] [Google Scholar]
- 21. Shearer J., Fueger P. T., Bracy D. P., Wasserman D. H., Rottman J. N. (2005) Diabetes 54, 3133–3139 [DOI] [PubMed] [Google Scholar]
- 22. Cannon B., Nedergaard J. (2004) Physiol. Rev. 84, 277–359 [DOI] [PubMed] [Google Scholar]
- 23. Lowell B. B., Spiegelman B. M. (2000) Nature 404, 652–660 [DOI] [PubMed] [Google Scholar]
- 24. Cypess A. M., Lehman S., Williams G., Tal I., Rodman D., Goldfine A. B., Kuo F. C., Palmer E. L., Tseng Y. H., Doria A., Kolodny G. M., Kahn C. R. (2009) N. Engl. J. Med. 360, 1509–1517 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Nedergaard J., Bengtsson T., Cannon B. (2007) Am. J. Physiol. Endocrinol. Metab. 293, E444–E452 [DOI] [PubMed] [Google Scholar]
- 26. Saito M., Okamatsu-Ogura Y., Matsushita M., Watanabe K., Yoneshiro T., Nio-Kobayashi J., Iwanaga T., Miyagawa M., Kameya T., Nakada K., Kawai Y., Tsujisaki M. (2009) Diabetes 50, 1526–1531 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. van Marken Lichtenbelt W. D., Vanhommerig J. W., Smulders N. M., Drossaerts J. M., Kemerink G. J., Bouvy N. D., Schrauwen P., Teule G. J. (2009) N. Engl. J. Med. 360, 1500–1508 [DOI] [PubMed] [Google Scholar]
- 28. Virtanen K. A., Lidell M. E., Orava J., Heglind M., Westergren R., Niemi T., Taittonen M., Laine J., Savisto N. J., Enerbäck S., Nuutila P. (2009) N. Engl. J. Med. 360, 1518–1525 [DOI] [PubMed] [Google Scholar]
- 29. Zingaretti M. C., Crosta F., Vitali A., Guerrieri M., Frontini A., Cannon B., Nedergaard J., Cinti S. (2009) FASEB J. 23, 3113–3120 [DOI] [PubMed] [Google Scholar]
- 30. Enerbäck S., Jacobsson A., Simpson E. M., Guerra C., Yamashita H., Harper M. E., Kozak L. P. (1997) Nature 387, 90–94 [DOI] [PubMed] [Google Scholar]
- 31. Shabalina I. G., Hoeks J., Kramarova T. V., Schrauwen P., Cannon B., Nedergaard J. (2010) Biochim. Biophys. Acta 1797, 968–980 [DOI] [PubMed] [Google Scholar]
- 32. Feldmann H. M., Golozoubova V., Cannon B., Nedergaard J. (2009) Cell Metab. 9, 203–209 [DOI] [PubMed] [Google Scholar]
- 33. Kopecky J., Clarke G., Enerbäck S., Spiegelman B., Kozak L. P. (1995) J. Clin. Invest. 96, 2914–2923 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Ellis J. M., Li L. O., Wu P. C., Koves T. R., Ilkayeva O., Stevens R. D., Watkins S. M., Muoio D. M., Coleman R. A. (2010) Cell Metab. 12, 53–64 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Guerra C., Koza R. A., Walsh K., Kurtz D. M., Wood P. A., Kozak L. P. (1998) J. Clin. Invest. 102, 1724–1731 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Lee S. H., Dobrzyn A., Dobrzyn P., Rahman S. M., Miyazaki M., Ntambi J. M. (2004) J. Lipid Res. 45, 1674–1682 [DOI] [PubMed] [Google Scholar]
- 37. Leone T. C., Lehman J. J., Finck B. N., Schaeffer P. J., Wende A. R., Boudina S., Courtois M., Wozniak D. F., Sambandam N., Bernal-Mizrachi C., Chen Z., Holloszy J. O., Medeiros D. M., Schmidt R. E., Saffitz J. E., Abel E. D., Semenkovich C. F., Kelly D. P. (2005) PLoS Biol. 3, e101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Lin J., Wu P. H., Tarr P. T., Lindenberg K. S., St-Pierre J., Zhang C. Y., Mootha V. K., Jäger S., Vianna C. R., Reznick R. M., Cui L., Manieri M., Donovan M. X., Wu Z., Cooper M. P., Fan M. C., Rohas L. M., Zavacki A. M., Cinti S., Shulman G. I., Lowell B. B., Krainc D., Spiegelman B. M. (2004) Cell 119, 121–135 [DOI] [PubMed] [Google Scholar]
- 39. Stryke D., Kawamoto M., Huang C. C., Johns S. J., King L. A., Harper C. A., Meng E. C., Lee R. E., Yee A., L'Italien L., Chuang P. T., Young S. G., Skarnes W. C., Babbitt P. C., Ferrin T. E. (2003) Nucleic Acids Res. 31, 278–281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Vergnes L., Beigneux A. P., Davis R., Watkins S. M., Young S. G., Reue K. (2006) J. Lipid Res. 47, 745–754 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Phan J., Reue K. (2005) Cell Metab. 1, 73–83 [DOI] [PubMed] [Google Scholar]
- 42. Xu J., Lee W. N., Phan J., Saad M. F., Reue K., Kurland I. J. (2006) Diabetes 55, 3429–3438 [DOI] [PubMed] [Google Scholar]
- 43. Crosson S. M., Khan A., Printen J., Pessin J. E., Saltiel A. R. (2003) J. Clin. Invest. 111, 1423–1432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Beigneux A. P., Kosinski C., Gavino B., Horton J. D., Skarnes W. C., Young S. G. (2004) J. Biol. Chem. 279, 9557–9564 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Cabrero A., Alegret M., Sánchez R. M., Adzet T., Laguna J. C., Vázquez M. (2001) Diabetes 50, 1883–1890 [DOI] [PubMed] [Google Scholar]
- 46. Saddik M., Lopaschuk G. D. (1991) J. Biol. Chem. 266, 8162–8170 [PubMed] [Google Scholar]
- 47. Wu M., Neilson A., Swift A. L., Moran R., Tamagnine J., Parslow D., Armistead S., Lemire K., Orrell J., Teich J., Chomicz S., Ferrick D. A. (2007) Am. J. Physiol. Cell Physiol. 292, C125–C136 [DOI] [PubMed] [Google Scholar]
- 48. Ross S. R., Choy L., Graves R. A., Fox N., Solevjeva V., Klaus S., Ricquier D., Spiegelman B. M. (1992) Proc. Natl. Acad. Sci. U.S.A. 89, 7561–7565 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Carmona M. C., Hondares E., Rodríguez de la Concepción M. L., Rodríguez-Sureda V., Peinado-Onsurbe J., Poli V., Iglesias R., Villarroya F., Giralt M. (2005) Biochem. J. 389, 47–56 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. de Meis L., Arruda A. P., Carvalho D. P. (2005) Biosci. Rep. 25, 181–190 [DOI] [PubMed] [Google Scholar]
- 51. de Meis L., Arruda A. P., da Costa R. M., Benchimol M. (2006) J. Biol. Chem. 281, 16384–16390 [DOI] [PubMed] [Google Scholar]
- 52. Brauch K. M., Dhruv N. D., Hanse E. A., Andrews M. T. (2005) Physiol. Genomics 23, 227–234 [DOI] [PubMed] [Google Scholar]
- 53. Buck M. J., Squire T. L., Andrews M. T. (2002) Physiol. Genomics 8, 5–13 [DOI] [PubMed] [Google Scholar]
- 54. Storch J., Veerkamp J. H., Hsu K. T. (2002) Mol. Cell. Biochem. 239, 25–33 [PubMed] [Google Scholar]
- 55. Wootan M. G., Storch J. (1994) J. Biol. Chem. 269, 10517–10523 [PubMed] [Google Scholar]
- 56. Nedergaard J., Cannon B. (2010) Cell Metab. 11, 268–272 [DOI] [PubMed] [Google Scholar]
- 57. Shin H. D., Kim L. H., Park B. L., Jung H. S., Cho Y. M., Moon M. K., Lee H. K., Park K. S. (2003) Hum. Mutat. 22, 180 [DOI] [PubMed] [Google Scholar]
- 58. Arnyasi M., Grindflek E., Jávor A., Lien S. (2006) J. Anim. Breed. Genet. 123, 198–203 [DOI] [PubMed] [Google Scholar]
- 59. Chmurzynska A., Szydlowski M., Stachowiak M., Stankiewicz M., Switonski M. (2007) Anim. Biotechnol. 18, 37–44 [DOI] [PubMed] [Google Scholar]
- 60. Cho S., Park T. S., Yoon D. H., Cheong H. S., Namgoong S., Park B. L., Lee H. W., Han C. S., Kim E. M., Cheong I. C., Kim H., Shin H. D. (2008) BMB Rep. 41, 29–34 [DOI] [PubMed] [Google Scholar]
- 61. Ueno T., Soma M., Tabara Y., Tokunaga K., Tahira K., Fukuda N., Matsumoto K., Nakayama T., Katsuya T., Ogihara T., Makita Y., Hata A., Yamada M., Takahashi N., Hirawa N., Umemura S., Miki T. (2008) Am. J. Hypertens. 21, 691–695 [DOI] [PubMed] [Google Scholar]