Abstract
Thrombomodulin (TM) is a cofactor for thrombin-mediated activation of protein C and thrombin-activatable fibrinolysis inhibitor (TAFI) and thereby helps coordinate coagulation, anticoagulation, fibrinolysis, and inflammation. Platelet factor 4 (PF4), a platelet α-granule protein and a soluble cofactor for TM-dependent protein C activation, stimulates protein C activation in vitro and in vivo. In contrast to stimulation of protein C activation, PF4 is shown here to inhibit activation of TAFI by thrombin-TM. Consequences of inhibition of TAFI activation by PF4 included loss of TM-dependent prolongation of clot lysis times in hemophilia A plasma and loss of TM-stimulated conversion of bradykinin (BK) to des-Arg9-BK by TAFIa in normal plasma. Thus, PF4 modulates the substrate specificity of the thrombin-TM complex by selectively enhancing protein C activation while inhibiting TAFI activation, thereby preventing the generation of the antifibrinolytic and anti-inflammatory activities of TAFIa. To block the inhibitory effects of PF4 on TAFI activation, heparin derivatives were tested for their ability to retain high affinity binding to PF4 despite having greatly diminished anticoagulant activity. N-acetylated heparin (NAc-Hep) lacked detectable anticoagulant activity in activated partial thromboplastin time clotting assays but retained high affinity binding to PF4 and effectively reversed PF4 binding to immobilized TM. NAc-Hep permitted BK conversion to des-Arg9-BK by TAFIa in the presence of PF4. In a clot lysis assay on TM-expressing cells using hemophilia A plasma, NAc-Hep prevented PF4-mediated inhibition of TAFI activation and the antifibrinolytic functions of TAFIa. Accordingly, NAc-Hep or similar heparin derivatives might provide therapeutic benefits by diminishing bleeding complications in hemophilia A via restoration of TAFIa-mediated protection of clots against premature lysis.
Keywords: Carboxypeptidase, Hemostasis, Heparin, Thrombolysis, Thrombomodulin, Hemophilia A, Platelet Factor 4, Thrombin-activatable Fibrinolysis Inhibitor
Introduction
A well coordinated balance between coagulation, anticoagulation, fibrinolysis, and inflammation is essential to maintain normal hemostasis, as evident from the pathological manifestations associated with disproportional activation of either system (1, 2). The endothelial cell receptor thrombomodulin (TM;2 CD141) controls key regulatory steps for these systems (3–5). The regulatory functions of TM originate from its ability to bind and alter the substrate specificity of thrombin. Binding of thrombin to TM shields the procoagulant exosite I of thrombin and unveils its anticoagulant and antifibrinolytic properties (6). TM is an essential cofactor for thrombin-mediated activation of protein C and the generation of activated protein C (APC), an enzyme with anticoagulant, antithrombotic, and multiple cytoprotective activities (7). Moreover, TM greatly stimulates thrombin-mediated activation of thrombin-activatable fibrinolysis inhibitor (TAFI), resulting in attenuation of fibrinolysis, increased clot stability, and anti-inflammatory effects (8). Potential strategies for therapeutic intervention have been proposed based on these properties of TM. Stimulation of endogenous APC generation in patients with severe sepsis might prove to augment the beneficial effects of APC, as pharmacologic applications of APC in these patients increased survival (9, 10), and endogenous cytoprotective APC actions prevented LPS-induced and traumatic shock-induced death in mice (11, 12).
Platelet Factor 4 (PF4) or derivatives thereof are promising candidates to stimulate endogenous generation of APC as PF4 can act as a soluble cofactor for protein C activation in the presence of TM (13–15). Infusion of PF4, a platelet α-granule protein that is normally released from platelets upon activation, in cynomolgus monkeys resulted in a strong anticoagulant response and increased circulating APC levels indicating that the PF4 cofactor function is preserved in a biologically relevant in vivo systems (16). Furthermore, a murine deficiency of PF4 aggravated LPS-induced lethality, which was suggestive of diminished protein C activation as transgenic overexpression of human PF4 in protein C+/− heterozygote mice corrected the LPS-susceptible phenotype associated with expression of low protein C levels in these mice (17). These results suggest that the application of PF4 to boost the deteriorating protein C pathway in inflammatory disease and sepsis seems ultimately feasible, especially as PF4 has been used successfully in humans to reverse therapeutic heparinization (18).
Stimulation of TM-dependent TAFI activation has been proposed to correct the premature lysis of clots associated with a defect in the intrinsic pathway and might be an attractive approach to diminishing bleeding complications in hemophilia A patients that have developed inhibitory antibodies (19–22). Secondary thrombin formation via thrombin-mediated activation of factor XI and subsequent amplification by the intrinsic pathway that is required for TAFI activation in the absence of TM is defective in hemophilia A patients (23, 24). Consequently, TAFI activation by thrombin is severely compromised, and clot lysis times are decreased in hemophilia A plasma. Hemophilia A patients are thus dependent on the presence of TM for efficient TAFI activation and adequate clot protection against fibrinolysis (19, 25). Effects of PF4 on thrombin-TM-mediated activation of TAFI have not been studied before.
Protein C and TAFI are two major substrates for the thrombin-TM complex, which controls key regulatory steps for coagulation, anticoagulation, fibrinolysis, and inflammation. Our understanding of the interactions and effects of PF4 on TM are incomplete. Although PF4 stimulates TM-dependent activation of protein C, the following question arises: what effect will PF4 have on TM-dependent TAFI activation? The results of this study indicate that PF4 specifically stimulates the TM-dependent activation of protein C, whereas PF4 inhibits the activation of TAFI by the thrombin-TM complex. Thus, PF4 regulates the substrate specificity of the thrombin-TM complex and modulates the biologic thrombin-TM complex activities in a ligand-specific manner. In addition, we identified a nonanticoagulant derivative of heparin that blocks the ability of PF4 to inhibit TM-dependent TAFI activation.
EXPERIMENTAL PROCEDURES
Materials
Normal and factor VIII-deficient plasmas were from George King (Overland Park, KS). Rabbit lung thrombomodulin (rl-TM) and rabbit anti-PF4 IgG were from American Diagnostica (Stamford, CT). Hirudin, lisinopril, fluorescamine, heparin, heparin-biotin, and heparin derivatives N-acetyl heparin, N-acetyl-de-O-sulfated heparin and de-N-sulfated heparin (all from porcine intestinal mucosa) were from Sigma. Chromogenic substrate S-2366 was obtained from Chromogenix (Franklin, OH). Thrombin (specific activity, 3425 units/mg) and corn trypsin inhibitor were from Enzyme Research Laboratories (South Bend, IL). Tissue-type plasminogen activator (tPA) was from Aniara (Mason, OH). Bradykinin (BK) and des-Arg9-BK were from Phoenix Pharmaceuticals (Burlingame, CA). Synthetic phospholipids 1,2-dioleoyl-sn-glycero-3-phosphatidylcholine (PC), 1,2-dioleoyl-sn-glycero-3-phosphatidylserine (PS), and 1,2-dioleoyl-sn-glycero-3-phosphatidylethanolamine (PE) were from Avanti (Alabaster, AL). Tissue factor (Innovin) was from Dade Behring (Newark, DE). H-d-Phe-Pro-Arg-chloromethylketone was obtained from Bachem (Torrance, CA). Carboxypeptidase inhibitor (CPI) from potato tubers and Plummer's inhibitor (d,l-2-mercaptomethyl-3-guanidinoethylthiopropanoic acid) were from Calbiochem. A blocking antibody against TM (clone RTM96) was from Cell Sciences (Canton, MA). Peroxidase-labeled antirabbit IgG was from DACO (Carpinteria, CA).
Purified TAFI
Outdated human plasma was used to purify TAFI (also known as procarboxypeptidase U, plasma procarboxypeptidase B, or procarboxypeptidase R) as described using affinity and anion exchange chromatography (26).
Purified Protein C
Plasma-derived protein C was purified from factor concentrate Prothromplex-T TIM4 (Baxter, Vienna, Austria) by affinity chromatography on anti-protein C (HPC4)-coupled Sepharose and anion exchange chromatography (see supplemental Methods for details).
Purified PF4
PF4 was extracted from frozen outdated platelet packs by a 1:1 dilution with buffer A (20 mm Tris, 250 mm NaCl, pH 7.7) containing 5 mm benzamidine and 1% Triton X-100. PF4 extracts were cleared by centrifugation (1000 × g for 30 min at 4 °C), and the supernatant was set aside, whereas the pellet was subjected to another round of PF4 extraction after which PF4-containing supernatants were applied to heparin-Sepharose (Sigma). Following washing with buffer A containing 1 m NaCl, PF4 was recovered by elution with buffer A containing 1.5 m NaCl. The NaCl concentration in the PF4 elution was lowered by a 1:5 dilution with 50 mm MES, pH 6.0, and applied on a mono-S-Sepharose column (GE Healthcare) equilibrated in 50 mm MES, pH 6.0 containing 250 mm NaCl. Purified PF4 was recovered in a 250–1000 mm NaCl gradient in 50 mm MES, pH 6.0 as described (27).
All purified proteins were >95% homogeneous as determined by SDS-PAGE. Protein concentrations were determined by BCA (Pierce) or by absorbance using extinction coefficients (280 nm, 1%, 1 cm) 14.5 for protein C and 2.6 for PF4 (28, 29).
Platelets
Washed platelets preparations were prepared as described (30).
TM-bearing Cells
TM was cloned from EA.hy926 endothelial cells (see supplemental Methods for details) and transfected into human kidney 293 (K293) cells for stable expression (TM-K293). Full-length TM with a C-terminal His-tag (TM-His) was generated as described (see supplemental Methods for details). Stable TM-K293 and TM-His-K293 cells were maintained in Dulbecco's modified Eagle's medium:Ham's F-12 (Invitrogen) supplemented with penicillin-streptomycin-glutamine (Invitrogen), 10% fetal bovine serum (Omega Scientific, Tarzana, CA), and 0.6 mg/ml G418.
Purified Human TM
TM-His was purified from TM-His-K293 cell lysates (Hepes-buffered saline buffer (20 mm Hepes, pH 7.4, 147 mm NaCl, 3 mm KCl) supplemented with 250 mm sucrose, 1% Nonidet, 5 mm benzamidine, 0.02% NaN3, and 1/100 diluted protease inhibitor mixture for His-tagged proteins (Sigma)). Briefly, 50 ml of cell lysate (derived from 1000 cm2 of confluent cells) was applied to NTA-Sepharose (Pharmacia) equilibrated in HBS containing 0.5 m NaCl and 10 mm imidazole. After extensive washing with the same buffer, TM-His was eluted in HBS with 100 mm EDTA, followed by extensive dialysis against HBS buffer.
TAFI Activation Assays
Activation of TAFI and determination of TAFIa activity were described previously (26). Briefly, purified TAFI (500 nm) was activated by thrombin and rl-TM (both 5 nm) and PF4 in HBS (20 mm Hepes, 147 mm NaCl, 3 mm KCl, pH 7.4) containing 1 mm CaCl2 at room temperature for 10 min after which the reaction was terminated by addition of the thrombin inhibitor H-d-Phe-Pro-Arg-chloromethylketone. Samples of activated TAFI (80 μl) were supplemented with 20 μl of TAFIa substrate hipuryl-Arg (20 mm). Substrate conversion was allowed to take place at room temperature and was stopped after 10 min by the addition of 20 μl of 1 m HCl. Subsequently, generation of hippuric acid was determined using cyanuric chloride as described (26). Alternatively, washed platelets were used as the source of PF4 and TAFI activation was induced by thrombin (2 nm) and rl-TM (5 nm) as described above. TAFI activation in normal plasma was performed as described (26).
Protein C Activation Assays
Activation of purified protein C (500 nm) by thrombin and rl-TM (both 5 nm) and PF4 in HBS containing 1 mm CaCl2 was determined at room temperature for 10 min after which the reaction was terminated by addition of hirudin. Generation of APC was quantified using the chromogenic substrate S-2366.
Coagulation Assays
APTT clotting assays were performed as described using kaolin/cephalin as the APTT reagent (Stago, Parsippany, NJ) (31). Clot lysis was studied in a plasma system of thrombin-induced clot formation and tPA-mediated fibrinolysis (see supplemental Methods for details).
Inactivation of Bradykinin
Inactivation of BK in plasma was analyzed using a combination of plasma filtration and HPLC as described (32). Various concentrations of PF4 and rl-TM in HBS/0.1% BSA were mixed with 17 mm CaCl2, 10 μm phospholipid vesicles (PC/PS/PE, 40/20/40), 4 pm tissue factor, 100 μm bradykinin, and 50% (v/v) normal pooled plasma supplemented with 0.5 mm lisinopril and 1.48 μm corn trypsin inhibitor. At 30 min or the indicated times, reactions were halted by addition of 1/8 (v/v) 160 mm EDTA, 10 mm benzamidine, and 800 μm Plummer's inhibitor, clots were removed, and the remaining supernatant (200 μl) was filtered (Microcon Ultracel YM-10; Millipore). Analysis of BK and des-Arg9-BK was performed as described (32).
Determination of Relative Affinities of PF4 for Heparin Derivatives and TM
Relative affinities of PF4 for heparin derivatives were determined by their ability to compete for PF4 binding to immobilized heparin or TM using solid phase binding assays. For heparin competition assays, microtiter plates (Maxisorp, Nunc) were coated with avidin (10 μg/ml in 15 mm NaCO3/35 mm NaHCO3, pH 9.5), blocked in Tris-buffered saline (TBS, 50 mm Tris, pH 7.4, 150 mm NaCl) containing 3% BSA and incubated with heparin-biotin (0.10 μg/ml) in TBS/0.1% BSA for 1 h. For TM competition, Maxisorp microtiter plates were coated with purified human recombinant TM-His (10 μg/ml) and blocked in TBS/3% BSA. After washing of the plates (TBS/0.1% Tween), preincubated mixtures (45 min) of PF4 (1 μg/ml) and heparin or heparin derivative, were added, and PF4 binding to immobilized heparin or TM was allowed to reach equilibrium for 1 h. After washing, bound PF4 was detected using a rabbit anti-PF4 antibody (2 μg/ml) and peroxidase-labeled swine anti-rabbit antibodies (1:1000). Peroxidase activity was detected with 0.4 mg/ml orthophenylene diamine (Sigma) in phosphate citrate buffer (100 mm Na2HPO4, 50 mm citric acid, pH 5.0) with 0.035% H2O2. PF4 labeling with fluorescamine was performed as described (27). Briefly, PF4 (90 μg/ml) in Dulbecco's phosphate-buffered saline was incubated in black 96-well plates (FluoroNunc) with heparin or heparin derivatives and mixed with fluorescamine (150 μg/ml). Fluorescence was measured after 5 min (SpectraMax Gemini EM, Molecular Devices) using excitation/emissions of 390/475 nm.
RESULTS
Inhibition of TAFI Activation by PF4
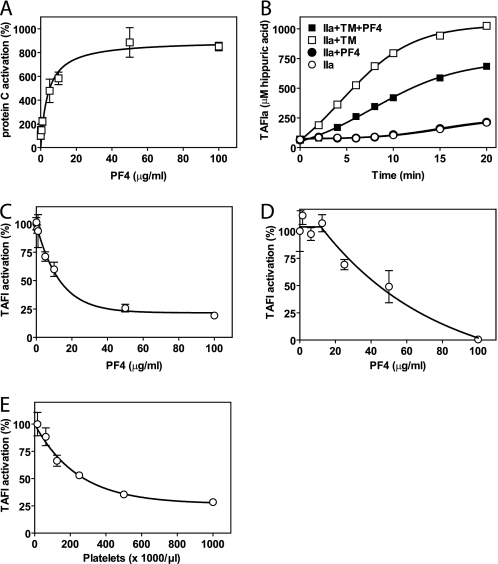
PF4 was purified to homogeneity from outdated platelet concentrates (supplemental Fig. 1). Purified PF4 enhanced protein C activation by the thrombin-TM complex (Fig. 1A) as reported (13, 15). Similar to the stimulation of protein C activation by thrombin-TM, thrombin-mediated activation of TAFI was enhanced by the presence of TM as expected (25, 33). This confirmed PF4 and other reagents have previously reported activities. Under conditions where PF4 enhanced thrombin-TM-dependent activation of protein C, PF4 decreased thrombin-TM-dependent activation of TAFI (Fig. 1B). PF4 did not inhibit the activation of TAFI in the absence of TM by thrombin alone. However, in the presence of TM, PF4 showed a dose-dependent inhibition of TAFI activation in a system comprised of purified components (Fig. 1C) as well as in normal plasma (Fig. 1D). When washed platelets were used as the source of PF4, TM-dependent TAFI activation was inhibited at increasing platelet concentrations (Fig. 1E), similar to that observed in the presence of purified PF4. Thus, inhibition of thrombin/TM-dependent TAFI activation by PF4 can occur within the normal range of platelet counts. These results suggest that PF4 altered the substrate specificity of the thrombin-TM complex from stimulation of both protein C and TAFI activation in the absence of PF4 to selectively favoring protein C activation over TAFI activation in the presence of PF4.
FIGURE 1.
PF4 modulation of protein C and TAFI activation by the thrombin-TM complex. A, stimulation of TM-dependent protein C activation by PF4. Protein C activation in the absence of PF4 was set to 100%. B, time course of thrombin-mediated activation of TAFI in the presence and absence of TM (5 nm) or PF4 (10 μg/ml). C, inhibition of TM-dependent TAFI activation by PF4. TAFI activation in the absence of PF4 was set to 100%. D, PF4-mediated inhibition of TAFI activation in normal plasma initiated by thrombin (20 nm) in the presence of TM (5 nm). TAFI activation in the absence of PF4 was set to 100%. E, inhibition of TM-dependent TAFI activation by washed platelets as the source of PF4. TAFI (500 nm) activation was induced by thrombin (2 nm) and TM (5 nm). TAFI activation in the absence of platelets was set to 100%. Each point represents the mean ± S.E. from at least three independent experiments. IIa denotes thrombin.
Consequences of Inhibition of TAFI Activation by PF4 for Clot Stability in Hemophilia A Plasma
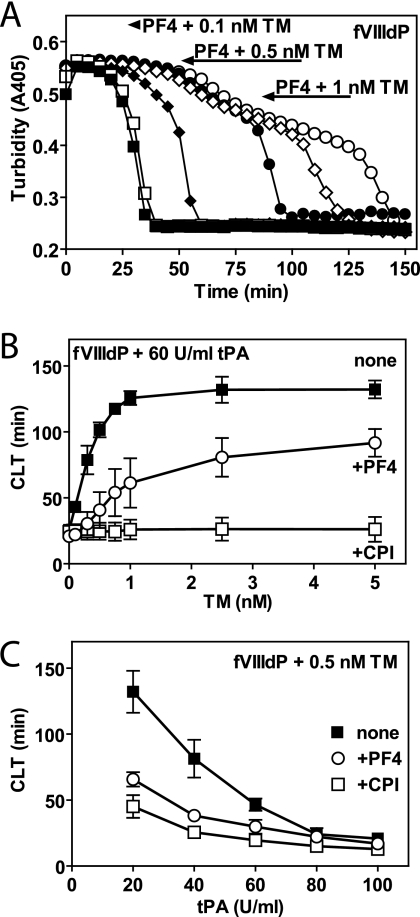
In normal plasma, in the absence of TM, TAFI is activated by relatively high concentrations of thrombin that are generated after the initial clot has been formed by thrombin-mediated feedback activation of factor XI and subsequent amplification by the intrinsic pathway (8, 34). Activation of TAFI is impaired in plasma from hemophilia A patients due to ineffective feedback amplification of thrombin generation via the tenase complex. In the absence of sufficient thrombin generation in hemophilia A plasma, TAFI activation, and TAFIa-mediated clot protection are thus dependent on the presence of TM (20). To determine whether PF4 inhibited TM-mediated TAFI activation in plasma, clot lysis was determined in normal and hemophilia A plasmas in the presence of PF4 and TM. Addition of PF4 in the absence of TM did not affect clot lysis in normal pooled plasma nor did it affect clot lysis in the presence of CPI (supplemental Fig. 2A), indicating that PF4 did not significantly interfere with thrombin-mediated clot formation or with tPA-mediated fibrinolysis. In the absence of TM, hemophilia A plasma showed rapid lysis of the clot similar to that in the presence of the TAFIa inhibitor CPI, confirming defective thrombin-mediated TAFI activation, which is restored in the presence of TM (supplemental Fig. 2B). As anticipated, low concentrations of TM (≤1 nm) prolonged clot lysis time in hemophilia A plasma by stimulation of TAFI activation. PF4 largely reversed this TM-dependent prolongation of clot lysis times (68% at 0.5 nm TM and 32% at 1 nm TM; Fig. 2A). Inhibition of TM-dependent prolongation of clot lysis time by PF4 was dependent on both the TM and tPA concentration used, consistent with the proposed threshold mechanism for inhibition of fibrinolysis by TAFIa (35, 36). Stimulation of TM-dependent prolongation of clot lysis time reached saturation at ∼1 nm TM in the absence of PF4, whereas in the presence of PF4, higher TM concentrations were required to inhibit fibrinolysis (Fig. 2B). TAFIa-dependent prolongation of clot lysis was more pronounced at lower tPA concentrations, and consequently, inhibition of TM-dependent prolongation of clot lysis time by PF4 was most effective at low tPA concentrations (Fig. 2C). Thus, PF4 inhibited TM-dependent TAFI activation in plasma similar to the inhibition of TM-dependent TAFI activation by PF4 observed in the purified system.
FIGURE 2.
Inhibition of TM-dependent TAFI activation by PF4 and loss of TAFIa-mediated clot protection in hemophilia A plasma. A, clot lysis initiated in factor VIII-deficient plasma (fVIIIdP) by thrombin (10 nm) and tPA (60 units (U)/ml) in the absence (open symbols) and presence (closed symbols) of PF4 (100 μg/ml) and various concentrations of TM (□ and ■, 0.1 nm TM; ○ and ●, 0.5 nm TM; and ♢ and ♦, 1 nm TM). B, clot lysis time (CLT) in factor VIII-deficient plasma initiated by thrombin (10 nm), tPA (60 units/ml), and TM in the absence (■, none) or presence of CPI (□, CPI 20 μg/ml) or PF4 (○, PF4 100 μg/ml). C, clot lysis time in factor VIII-deficient plasma initiated by thrombin (10 nm), TM (0.5 nm), and tPA in the absence (■, none) or presence of CPI (□, CPI 20 μg/ml) or PF4 (○, PF4 100 μg/ml). Each point represents the mean ± S.E. from at least three independent experiments.
Inhibition of TM-mediated TAFI Activation by PF4 in Normal Plasma
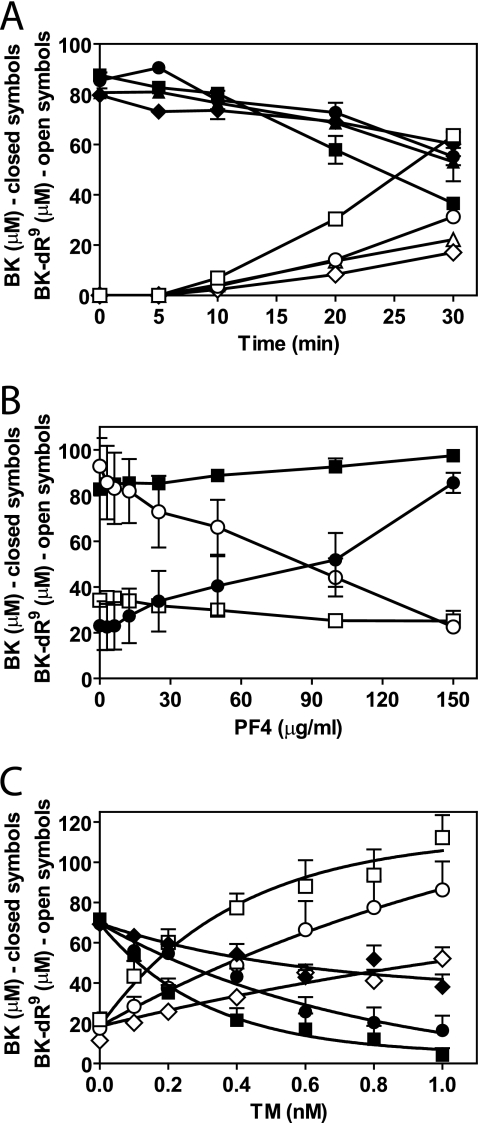
In addition to inhibition of fibrinolysis, TAFIa also exhibits anti-inflammatory activities via the inactivation of BK and the C3a and C5a anaphylatoxins by removal of C-terminal arginine residues. To determine the effect of PF4 on TM-dependent TAFI activation in normal plasma, inactivation of BK in plasma by TAFIa was analyzed using an HPLC-based quantitative analysis of BK and its conversion to des-Arg9-BK (32). In this system, lisinopril was used to inhibit angiotensin-converting enzyme, which, in the absence of lisinopril, is responsible for an 11% decrease in BK without generation of des-Arg9-BK under the conditions employed (32). Using tissue factor-induced coagulation, analysis of the BK inactivation time course and concomitant generation of des-Arg9-BK showed that BK was decreased after 30 min by ∼25%. Thrombin-activated TAFIa and constitutively active carboxypeptidase N accounted each for about equal parts of the BK inactivation under these conditions (32). Presence of TM accelerated BK inactivation and generation of des-Arg9-BK (Fig. 3A), consistent with TM-mediated stimulation of TAFI activation and subsequent increased BK conversion to des-Arg9-BK by TAFIa. PF4 largely neutralized the TM-enhanced BK inactivation and reversed BK and des-Arg9-BK levels to that observed in the absence of TM (Fig. 3A). PF4 in the absence of TM did not notably alter the conversion of BK into des-Arg9-BK (Fig. 3A). Inhibition of TAFIa-dependent BK conversion to des-Arg9-BK by PF4 was PF4 concentration-dependent and specific for TM-mediated TAFI activation, as PF4 in the absence of TM had no effect on BK inactivation by TAFIa that was generated via thrombin-mediated activation (Fig. 3B). The activation of TAFI was dependent on the concentration of TM and resulted in a TM dose-dependent conversion of BK to des-Arg9-BK. Increasing the TM concentration could eventually overcome inhibition of des-Arg9-BK generation by PF4 (Fig. 3C). Thus, PF4 modulates the substrate specificity of the thrombin-TM complex in normal plasma by enhancing activation of protein C by the thrombin-TM complex and by inhibition of TM-dependent TAFI activation.
FIGURE 3.
Inhibition of TM-dependent TAFI activation by PF4 and loss of TAFIa-mediated BK inactivation in normal plasma. A, time course of BK (closed symbols) conversion to des-Arg9-BK (BK-dR9, open symbols) during tissue factor-induced coagulation in normal pooled plasma (▴ and △, no TM and no PF4; ♦ and ♢, 50 μg/ml PF4; □ and ■, 0.3 nm TM; and ○ and ●, 0.3 nm TM + 50 μg/ml PF4). B, PF4 effect on BK (closed symbols) conversion to des-Arg9-BK (open symbols) during tissue factor-induced coagulation in normal pooled plasma (□ and ■, no TM; and ○ and ●, 0.3 nm TM). C, TM effect on BK (closed symbols) conversion to des-Arg9-BK (open symbols) during tissue factor-induced coagulation in normal pooled plasma (□ and ■, no PF4; ○ and ●, 50 μg/ml PF4; and ♦ and ♢, 100 μg/ml PF4). Each point represents the mean ± S.E. from at least three independent experiments.
Affinity of Non-anticoagulant Heparins for PF4
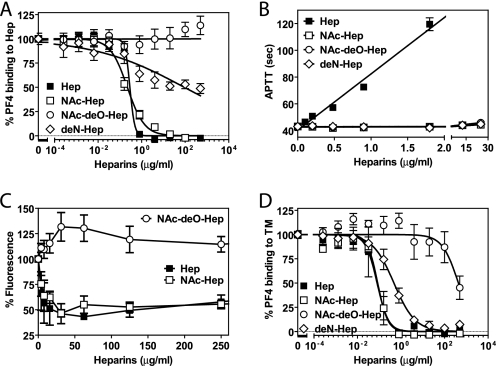
Previously, heparin was demonstrated to effectively abrogate stimulation of protein C activation by PF4 due to its exceptional high affinity for PF4 (13). Because the potent anticoagulant effects of heparin prohibit its use as a PF4 reversal reagent in biological systems, several heparin derivatives were tested for their ability to retain high affinity binding to PF4 while having greatly diminished anticoagulant activity (supplemental Fig. 3). The high affinity of PF4 for heparin is derived from two binding sites on the tetramer of PF4, one on each PF4 dimer, that can by occupied by either two hexa-decasaccharides or by a single polysaccharide of ≥34 units that wraps around the positively charged groove on the PF4 tetramer (37, 38). Analysis of the multiple interactions between the oxygen atoms of heparin and basic residues in PF4 revealed that the majority of interaction involved O-linked sulfate group oxygens (>55%) with minor contributions of sulfylamine group oxygens (20%), suggesting that a heparin derivative with modified N-sulfate groups might retain significant affinity for PF4 (37). Heparin N-desulfation and acetylation of the free amine group yields NAc-Hep (supplemental Fig. 3) with greatly diminished anticoagulant activity as it lacks the essential sulfamine group in the internal residue of the unique pentasaccharide that comprises the antithrombin recognition sequence (39, 40). N-Acetylation of heparin came at a relatively minor cost with respect to affinity for PF4. Compared with unmodified heparin, NAc-Hep showed similar IC50 values (0.22 μg/ml NAc-Hep versus 0.29 μg/ml Hep) for competition with PF4 binding to heparin-biotin complexed to immobilized avidin (Fig. 4A and supplemental Table 1). APTT clotting assays confirmed the absence of detectable anticoagulant activity for NAc-Hep, whereas Hep induced a pronounced anticoagulant response (Fig. 4B). Additional O-desulfation of NAc-Hep (supplemental Fig. 3) resulted in complete abrogation of anticoagulant activity in APTT clotting assays (Fig. 4B) but also resulted in a complete loss of affinity for PF4 (Fig. 4A). Alternatively, heparin N-desulfation without N-acetylation, leaving the free amine group intact (deN-Hep; supplemental Fig. 3), did not show an additional decrease in anticoagulant activity compared with NAc-Hep (Fig. 4B), whereas the positively charged amine groups of deN-Hep were incompatible with high affinity binding to PF4 (Fig. 4A).
FIGURE 4.
Relative affinity of non-anticoagulant heparin-derivatives for PF4. A, competition of heparin derivatives for PF4 binding to immobilized heparin. B, anticoagulant activity of heparin derivatives determined in an APTT clotting assay. C, effect of heparin derivatives on fluorescence induced by fluorescamine labeling of PF4. D, competition of heparin derivatives for PF4 binding to immobilized human recombinant TM-His. ■, Hep; □, NAc-Hep; ○, N-acetyl-de-O-sulfated heparin (NAc-deO-Hep); and ♢, deN-Hep. Each point represents the mean ± S.E. from at least three independent experiments.
Nonfluorescent fluorescamine acquires an intense fluorescent signal upon reacting with primary amines (mostly derived from Lys residues) in PF4. Binding of Hep in the positively charged groove of the PF4 prevented the interaction of fluorescamine with several of the Lys residues located in the heparin binding groove as indicated by the deceased fluorescent signal in the presence of increasing Hep concentrations. Similar diminished fluorescamine-induced fluorescence was observed for NAc-Hep but not for N-acetyl-de-O-sulfated heparin (Fig. 4C). This suggests that NAc-Hep retained its ability to wrap around the PF4 tetramer.
Both NAc-Hep and Hep effectively reversed PF4 binding to immobilized TM (IC50, 0.084 μg/ml NAc-Hep and 0.079 μg/ml Hep), whereas deN-Hep required a 6-fold higher concentration to achieve half-maximal inhibition of PF4 binding to TM (Fig. 4D and supplemental Table 1). These concentrations were 2.6-fold (NAc-Hep), 3.6-fold (Hep), and 194-fold (deN-Hep) lower compared with half-maximal inhibition of PF4 binding to heparin, consistent with the ∼10-fold lower affinity of PF4 for immobilized TM compared with PF4 binding to heparin (human full-length TM, Kd, apparent 11.7 ± 0.020 nm; rabbit lung TM, Kd, apparent 12.7 ± 5.0 nm; heparin Kd, apparent 1.0 ± 0.020 nm).
Reversal of PF4-mediated Inhibition of TM-dependent TAFI Activation by Non-anticoagulant Heparins
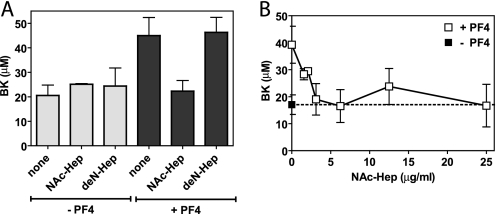
To determine whether a non-anticoagulant heparin derivative that has retained high affinity for PF4 could restore TM-dependent TAFI activation in the presence of PF4, TAFIa-mediated BK conversion to des-Arg9-BK was determined in the presence of TM, PF4, and NAc-Hep in normal plasma during tissue factor-induced coagulation. Presence of PF4 induced a marked increase of BK levels, indicative of decreased BK conversion to des-Arg9-BK by TAFIa due to the inhibition of TAFI activation by PF4. NAc-Hep effectively restored BK conversion to des-Arg9-BK by TAFIa, similar to BK conversion in the absence of PF4, whereas NAc-Hep in the absence of PF4 did not significantly affect BK levels (Fig. 5A). Inhibition of PF4-mediated decreased BK conversion by NAc-Hep was dose-dependent (Fig. 5B). Thus, NAc-Hep reversed PF4-mediated inhibition of TAFI activation. In contrast, deN-Hep, with significantly lower affinity for PF4 compared with NAc-Hep, did not restore BK conversion to des-Arg9-BK by TAFIa under the conditions of the assays (Fig. 5A).
FIGURE 5.
Reversal of PF4-induced inhibition of TM-dependent TAFI activation by non-anticoagulant heparin derivatives. A, effect of heparin derivatives (6.25 μg/ml) on PF4 (100 μg/ml)-induced inhibition of TAFI activation and consequent TAFIa-mediated inactivation of BK. Activation of TAFI in normal pooled plasma was induced by 4 pm tissue factor in the presence of 0.3 nm TM. B, dose-dependent NAc-Hep reversal of PF4-induced inhibition of TAFI activation and consequently TAFIa-mediated inactivation of BK. Activation of TAFI in normal pooled plasma was induced by 4 pm tissue factor in the presence of 0.6 nm TM in the absence (■) and presence (□) of PF4 (50 μg/ml). Each point represents the mean ± S.E. from at least three independent experiments.
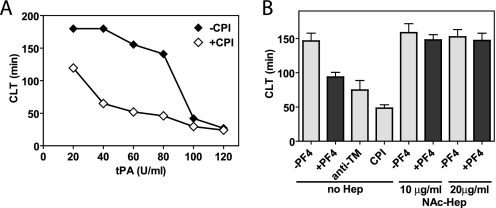
The ability of NAc-Hep to reverse PF4-mediated inhibition of TAFI activation, and thereby the antifibrinolytic functions of TAFIa, was tested in a clot lysis assays using factor VIII-deficient plasma and cellular TM (TM-K293 monolayer) as a source of TM. Under the conditions employed, the typical ∼3-fold TAFIa-dependent prolongation of the clot lysis time was observed at tPA concentration ranging from 40–80 units/ml (Fig. 6A). The prolongation of the clot lysis was TAFIa-dependent, and the activation of TAFI required TM (Fig. 6B). PF4 decreased clot lysis times in the absence of NAc-Hep but failed to affect clot lysis times in the presence of NAc-Hep (Fig. 6B). Therefore, NAc-Hep effectively reversed PF4-mediated inhibition of TM-dependent TAFI activation and restored clot protection in hemophilia A plasma by TAFIa.
FIGURE 6.
Reversal of PF4-induced inhibition of TAFIa-mediated clot protection in hemophilia A plasma on TM-bearing cells by non-anticoagulant heparin derivatives. A, clot lysis initiated in factor VIII-deficient plasma by thrombin (10 nm) and tPA on TM-expressing K293 cells in the absence (♦) and presence (♢) of CPI 20 μg/ml. A representative experiment is shown. B, clot lysis initiated in factor VIII-deficient plasma by thrombin (10 nm) and tPA (60 units/ml) on TM-expressing K293 cells in the absence (light bars) and presence (dark bars) of 50 μg/ml PF4. Blocking antibodies against TM (20 μg/ml) preincubated with cells 10 min before the start of the experiment, CPI (20 μg/ml) and NAc-Hep were included as indicated. Each point represents the mean ± S.E. from at least three independent experiments.
DISCUSSION
PF4 is released upon platelet activation and was shown previously to enhance protein C activation in vitro and in vivo (13–15). The results of this study show that PF4, either purified or derived from washed platelets, inhibited the activation of TAFI by the thrombin-thrombomodulin complex in systems of purified proteins and in plasma (Fig. 1). PF4 did not interfere with thrombin-mediated TAFI activation in the absence of thrombomodulin. This suggests that PF4 alters the substrate specificity of the thrombin-TM complex from stimulation of both protein C and TAFI activation in the absence of PF4 to selectively favor protein C activation over TAFI activation in the presence of PF4.
In contrast to the synergy between the anti-inflammatory effects of APC and TAFI are the differential effects of APC and TAFIa on fibrinolysis. TAFIa stabilizes the clot by protecting it from degradation by plasmin, whereas APC promotes fibrinolysis by decreasing the generation of thrombin that is required for the activation of TAFIa in the absence of TM (41, 42). Shifting the substrate specificity of the thrombin-TM complex will therefore resonate in a change in up or down-regulation of fibrinolysis. Because protein C and TAFI are both substrates for thrombin that require TM for efficient activation, this raises the question: “How is activation of protein C versus TAFI by the thrombin-TM complex regulated?”
Different regions on TM are required for protein C activation versus TAFI activation. The minimal TM structure to support protein C activation is comprised of EGF 4–6 and the interdomain peptide connecting EGF 3–4, whereas TAFI activation requires additional residues comprising the C-loop of EGF 3 (22, 43). Nevertheless, the kinetic constants for activation of protein C and TAFI by the thrombin-TM complex are similar, and their plasma concentrations are approximately an order of magnitude below their respective Km values for activation, indicating that reciprocal competitive inhibition is unlikely under physiological conditions (3, 25).
Previously, we found that the concentration of TM is an important factor in the regulation of TAFI activation and fibrinolysis (44). Consistent with this observation that relatively high concentration of TM promote fibrinolysis and that TAFIa-mediated anti-fibrinolytic properties are favored at low TM concentration, inhibition of TAFI activation by PF4 was most notable at low TM concentration (Figs. 2A and 3C). Thus, PF4 enhances the profibrinolytic effects of TM by modulating the substrate specificity of the thrombin-TM complex in favor of protein C activation over TAFI activation. In addition, by inhibiting TM-dependent TAFI activation, PF4 induces a shift in the pathways responsible for TAFI activation from TM-dependent toward thrombin-dependent mechanisms. Thrombin-dependent TAFI activation requires thrombin-mediated activation of factor XI and amplification of secondary thrombin formation via the intrinsic pathway that is sensitive to down-regulation by APC (11, 12). This implies that by emphasizing a thrombin-dependent activation mechanism for TAFI, PF4 also increases susceptibility of TAFI activation to down-regulation by APC, consistent with the enhancing effects of PF4 on protein C activation.
In hemophilia A plasma, where the thrombin generating amplification potential of the intrinsic pathway is impaired due to the deficiency of factor VIII, TM is required to generate adequate amounts of TAFIa for TAFIa-mediated clot protection. Inhibition of TM-dependent TAFI activation in hemophilia A plasma by PF4 induced pronounced profibrinolytic effects as thrombin-mediated TAFI activation was unable to substitute the loss of TM-dependent TAFI activation in this plasma (Fig. 2). In contrast, the effects on fibrinolysis of the PF4-induced shift in the mechanisms underlying the activation of TAFI in normal plasma were uneventful (supplemental Fig. 2A), due to the threshold-dependent mechanism by which TAFIa attenuates fibrinolysis (35, 36). Nevertheless, processing of BK into to des-Arg9-BK by TAFIa in normal plasma, which is not threshold-dependent, was decreased markedly at increasing PF4 concentrations, indicating that PF4 did inhibit TM-dependent TAFI activation in normal plasma (Fig. 3).
Instability of the initial hemostatic plug is typical for hemophilia A and these results suggest that PF4 released during the formation of the platelet plug could further hamper the already defective TAFIa-mediated protection of the forming clot and exacerbate the risk of bleeding. Blunting the PF4-induced substrate specificity shift for the thrombin-TM complex could therefore potentially contribute to improved clot stability in hemophilia A. Based on the exceptional high affinity of PF4 for heparin, a non-anticoagulant heparin derivative was sought to reverse PF4 inhibition of TM-dependent TAFI activation. NAc-Hep, with greatly diminished anticoagulant activity, retained the ability to bind PF4 with high affinity (Fig. 4).
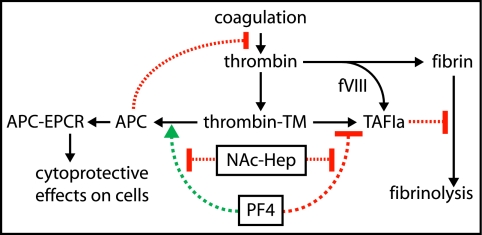
Proof-of-principle for reversal of PF4 inhibition of TM-dependent TAFI activation by NAc-Hep was obtained in normal and hemophilia A plasma. In normal plasma, NAc-Hep restored TM-dependent processing of BK into to des-Arg9-BK by TAFIa that was inhibited by PF4 (Fig. 5). In hemophilia A plasma, NAc-Hep increased clot protection against fibrinolysis in the presence of PF4 but not in the absence of PF4 by restoring TM-dependent activation of TAFI (Fig. 6). This suggests that NAc-Hep or similar other non-anticoagulant heparin derivatives that have retained high affinity for PF4 might provide therapeutic benefits in hemophilia A patients by diminishing bleeding complications via restoration of TM-dependent TAFIa-mediated protection of clots against premature lysis (Fig. 7).
FIGURE 7.
Schematic model for PF4 regulation of substrate specificity of the thrombin-TM complex. PF4 modulates the substrate specificity of the thrombin-TM complex by selectively enhancing protein C activation (green arrow), while inhibiting TAFI activation (red bar). PF4 thereby stimulates the anticoagulant of APC and cytoprotective activities and prevents the generation of the antifibrinolytic and anti-inflammatory activities of TAFIa. Non-anticoagulant heparin derivatives such as NAc-Hep have negligible anticoagulant activity but retain high affinity binding to PF4 and thereby can block the effects of PF4 on the substrate specificity of the thrombin-TM complex. Normal thrombin-mediated TAFI activation is impaired in hemophilia A patients due to the inability of the intrinsic pathway to amplify secondary thrombin formation required for TAFI activation sufficiently in the absence of factor VIII. Consequently, TAFIa-mediated clot protection in hemophilia A patients requires TM to generate adequate amounts of TAFIa. Release of PF4 from activated platelets, e.g. during formation of the initial platelet plug upon injury, contributes further to a diminishment of TM-dependent activation of TAFI, resulting in increased susceptibility of the clot to fibrinolytic attack and associated bleeding complications. Accordingly, NAc-Hep might provide therapeutic benefits by diminishing bleeding complications in hemophilia A patients via restoration of TM-dependent TAFIa-mediated protection of clots against premature lysis and via blunting anticoagulant APC generation that is enhanced by PF4. Green arrows indicate stimulation; red arrows indicate inhibition; and black arrows indicate cause and effect relations.
Impaired TAFIa-dependent protection of the fibrin clot in hemophilia A would predict hemostatic plug instability and increased risk of bleeding. Although the bleeding manifestations in hemophilia A that occur typically hours after injury are consistent with inadequate protection of the hemostatic plug against premature degradation, the contribution of impaired TAFIa-dependent clot protection to the phenomena of delayed bleeding in hemophilia A has yet to be determined. Nevertheless, high TAFI levels corrected the premature lysis of factor VIII deficient clots in vitro and were associated with milder clinical symptoms of hemophilia A patients (21, 45), supporting an anticipated role of defective TAFI activation in the bleeding diathesis of hemophilia A.
Could PF4 release during platelet activation contribute to the bleeding diathesis in hemophilia A patients? Elevated markers for platelet activation are found in hemophilia A patients, suggesting sustained in vivo platelet activation and excess circulating PF4 in these patients (46). Based on the concentration of PF4 in serum (7–20 μg/ml), it is likely that physiological achievable PF4 concentrations at sites of local platelet activation can reach levels required to stimulate APC generation and/or to inhibit TAFI activation. Observations in literature support the anticipated contributions of APC to increased bleeding risk. Designer inhibitors with increased specificity for APC improved thrombin generation in hemophilia A plasma (47, 48). Cynomolgus monkeys showed a marked APC-mediated anticoagulant response at PF4 concentrations sustainable in vivo after platelet activation (16).
Neutralization of PF4 with non-anticoagulant heparin derivatives, such as NAc-Hep, would not only diminish PF4 stimulated APC generation but also promote endogenous TM-dependent TAFI activation. Although its affinity for PF4 has not yet been tested, fucoidan, a sulfated polysaccharide with low anticoagulant activity tested for its ability to inhibit tissue factor pathway inhibitor, was found to partially correct excessive bleeding in hemophilia A mice (49). Identification of novel beneficial effects of NAc-Hep and other non-anticoagulant heparins for cancer treatment and ischemia/reperfusion injury identified several sulfated polysaccharide and heparin derivates with potential applications in hemophilia A (40, 50). Especially in hemophilia A patients with inhibitory antibodies where factor replacement is very challenging and therapeutic alternatives (such as recombinant factor VII) are limited and expensive, NAc-Hep or other similar heparin derivates might provide a novel therapeutic strategy to decrease bleeding risks.
Supplementary Material
This work was supported, in whole or in part, by National Institutes of Health, NHLBI Grant HL087618.

The on-line version of this article (available at http://www.jbc.org) contains supplemental Methods, Table 1, Figs. 1–3, and additional references.
- TM
- thrombomodulin
- APC
- activated protein C
- APTT
- activated partial thromboplastin time
- BK
- bradykinin
- CPI
- carboxypeptidase inhibitor
- NAc-Hep
- N-acetylheparin
- deN-Hep
- de-N-sulfated heparin
- PF4
- platelet factor 4
- PC
- 1,2-dioleoyl-sn-glycero-3-phosphatidylcholine
- PS
- 1,2-dioleoyl-sn-glycero-3-phosphatidylserine
- PE
- 1,2-dioleoyl-sn-glycero-3-phosphatidylethanolamine
- TAFI
- thrombin-activatable fibrinolysis inhibitor
- TAFIa
- activated TAFI
- rl-TM
- TM from rabbit lung
- tPA
- tissue-type plasminogen activator
- MES
- 4-morpholineethanesulfonic acid.
REFERENCES
- 1. Levi M., de Jonge E., van der Poll T., ten Cate H. (2001) Semin. Thromb. Hemost. 27, 569–575 [DOI] [PubMed] [Google Scholar]
- 2. Chan M. Y., Andreotti F., Becker R. C. (2008) Circulation 118, 2286–2297 [DOI] [PubMed] [Google Scholar]
- 3. Esmon C. T., Owen W. G. (1981) Proc. Natl. Acad. Sci. U.S.A. 78, 2249–2252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Van de Wouwer M., Collen D., Conway E. M. (2004) Arterioscler. Thromb. Vasc. Biol. 24, 1374–1383 [DOI] [PubMed] [Google Scholar]
- 5. Nesheim M., Wang W., Boffa M., Nagashima M., Morser J., Bajzar L. (1997) Thromb. Haemost. 78, 386–391 [PubMed] [Google Scholar]
- 6. Esmon C. T. (1995) FASEB J. 9, 946–955 [DOI] [PubMed] [Google Scholar]
- 7. Mosnier L. O., Zlokovic B. V., Griffin J. H. (2007) Blood 109, 3161–3172 [DOI] [PubMed] [Google Scholar]
- 8. Mosnier L. O., Bouma B. N. (2006) Arterioscler. Thromb. Vasc. Biol. 26, 2445–2453 [DOI] [PubMed] [Google Scholar]
- 9. Isermann B., Vinnikov I. A., Madhusudhan T., Herzog S., Kashif M., Blautzik J., Corat M. A., Zeier M., Blessing E., Oh J., Gerlitz B., Berg D. T., Grinnell B. W., Chavakis T., Esmon C. T., Weiler H., Bierhaus A., Nawroth P. P. (2007) Nat. Med. 13, 1349–1358 [DOI] [PubMed] [Google Scholar]
- 10. Bernard G. R., Vincent J. L., Laterre P. F., LaRosa S. P., Dhainaut J. F., Lopez-Rodriguez A., Steingrub J. S., Garber G. E., Helterbrand J. D., Ely E. W., Fisher C. J., Jr. (2001) N. Engl. J. Med. 344, 699–709 [DOI] [PubMed] [Google Scholar]
- 11. Chesebro B. B., Rahn P., Carles M., Esmon C. T., Xu J., Brohi K., Frith D., Pittet J. F., Cohen M. J. (2009) Shock 32, 659–665 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Xu J., Ji Y., Zhang X., Drake M., Esmon C. T. (2009) J. Thromb. Haemost. 7, 851–856 [DOI] [PubMed] [Google Scholar]
- 13. Slungaard A., Key N. S. (1994) J. Biol. Chem. 269, 25549–25556 [PubMed] [Google Scholar]
- 14. Slungaard A. (2004) Crit. Care Med. 32, S331–335 [DOI] [PubMed] [Google Scholar]
- 15. Dudek A. Z., Pennell C. A., Decker T. D., Young T. A., Key N. S., Slungaard A. (1997) J. Biol. Chem. 272, 31785–31792 [DOI] [PubMed] [Google Scholar]
- 16. Slungaard A., Fernandez J. A., Griffin J. H., Key N. S., Long J. R., Piegors D. J., Lentz S. R. (2003) Blood 102, 146–151 [DOI] [PubMed] [Google Scholar]
- 17. Kowalska M. A., Mahmud S. A., Lambert M. P., Poncz M., Slungaard A. (2007) Blood 110, 1903–1905 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Dehmer G. J., Lange R. A., Tate D. A., Pirwitz M., Daniel W., Fisher M., Bonnem E. M. (1996) Circulation 94, II347–352 [PubMed] [Google Scholar]
- 19. Broze G. J., Jr., Higuchi D. A. (1996) Blood 88, 3815–3823 [PubMed] [Google Scholar]
- 20. Foley J. H., Nesheim M. E. (2009) J. Thromb. Haemost. 7, 453–459 [DOI] [PubMed] [Google Scholar]
- 21. Mosnier L. O., Lisman T., van den Berg H. M., Nieuwenhuis H. K., Meijers J. C., Bouma B. N. (2001) Thromb. Haemost. 86, 1035–1039 [PubMed] [Google Scholar]
- 22. Wang W., Nagashima M., Schneider M., Morser J., Nesheim M. E. (2000) J. Biol. Chem. 275, 22942–22947 [DOI] [PubMed] [Google Scholar]
- 23. von dem Borne P. A., Meijers J. C., Bouma B. N. (1995) Blood 86, 3035–3042 [PubMed] [Google Scholar]
- 24. Von dem Borne P. A., Bajzar L., Meijers J. C., Nesheim M. E., Bouma B. N. (1997) J. Clin. Invest. 99, 2323–2327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Bajzar L., Morser J., Nesheim M. E. (1996) J. Biol. Chem. 271, 16603–16608 [DOI] [PubMed] [Google Scholar]
- 26. Mosnier L. O., von dem Borne P. A., Meijers J. C., Bouma B. N. (1998) Thromb. Haemost. 80, 829–835 [PubMed] [Google Scholar]
- 27. Medici I., Di Martino A., Cella G., Callegaro L., Prosdocimi M. (1989) Thromb. Res. 54, 277–287 [DOI] [PubMed] [Google Scholar]
- 28. Mosnier L. O., Griffin J. H. (2006) Front. Biosci. 11, 2381–2399 [DOI] [PubMed] [Google Scholar]
- 29. Kisiel W. (1979) J. Clin. Invest. 64, 761–769 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Cazenave J. P., Ohlmann P., Cassel D., Eckly A., Hechler B., Gachet C. (2004) Methods Mol. Biol. 272, 13–28 [DOI] [PubMed] [Google Scholar]
- 31. Mosnier L. O., Gale A. J., Yegneswaran S., Griffin J. H. (2004) Blood 104, 1740–1744 [DOI] [PubMed] [Google Scholar]
- 32. Mosnier L. O., Yang X. V., Griffin J. H. (2007) J. Biol. Chem. 282, 33022–33033 [DOI] [PubMed] [Google Scholar]
- 33. Bajzar L., Nesheim M., Morser J., Tracy P. B. (1998) J. Biol. Chem. 273, 2792–2798 [DOI] [PubMed] [Google Scholar]
- 34. Bouma B. N., Mosnier L. O., Meijers J. C., Griffin J. H. (1999) Thromb. Haemost. 82, 1703–1708 [PubMed] [Google Scholar]
- 35. Leurs J., Nerme V., Sim Y., Hendriks D. F. (2004) J. Thromb. Haemost. 2, 416–423 [DOI] [PubMed] [Google Scholar]
- 36. Walker J. B., Bajzar L. (2004) J. Biol. Chem. 279, 27896–27904 [DOI] [PubMed] [Google Scholar]
- 37. Stuckey J. A., St., Charles R., Edwards B. F. (1992) Proteins 14, 277–287 [DOI] [PubMed] [Google Scholar]
- 38. Bock P. E., Luscombe M., Marshall S. E., Pepper D. S., Holbrook J. J. (1980) Biochem. J. 191, 769–776 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Casu B., Lindahl U. (2001) Adv. Carbohydr. Chem. Biochem. 57, 159–206 [DOI] [PubMed] [Google Scholar]
- 40. Casu B., Vlodavsky I., Sanderson R. D. (2008) Pathophysiol. Haemost. Thromb. 36, 195–203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Bajzar L., Nesheim M. E., Tracy P. B. (1996) Blood 88, 2093–2100 [PubMed] [Google Scholar]
- 42. Bajzar L., Nesheim M. E. (1993) J. Biol. Chem. 268, 8608–8616 [PubMed] [Google Scholar]
- 43. Kokame K., Zheng X., Sadler J. E. (1998) J. Biol. Chem. 273, 12135–12139 [DOI] [PubMed] [Google Scholar]
- 44. Mosnier L. O., Meijers J. C., Bouma B. N. (2001) Thromb. Haemost. 85, 5–11 [PubMed] [Google Scholar]
- 45. Shetty S., Vora S., Kulkarni B., Mota L., Vijapurkar M., Quadros L., Ghosh K. (2007) Br. J. Haematol. 138, 541–544 [DOI] [PubMed] [Google Scholar]
- 46. Al-Mondhiry H. (1987) Thromb. Haemost. 57, 294–297 [PubMed] [Google Scholar]
- 47. De Nanteuil G., Gloanec P., Béguin S., Giesen P. L., Hemker H. C., Mennecier P., Rupin A., Verbeuren T. J. (2006) J. Med. Chem. 49, 5047–5050 [DOI] [PubMed] [Google Scholar]
- 48. Butenas S., Orfeo T., Kalafatis M., Mann K. G. (2006) J. Thromb. Haemost. 4, 2411–2416 [DOI] [PubMed] [Google Scholar]
- 49. Liu T., Scallan C. D., Broze G. J., Jr., Patarroyo-White S., Pierce G. F., Johnson K. W. (2006) Thromb. Haemost. 95, 68–76 [PubMed] [Google Scholar]
- 50. Friedrichs G. S., Kilgore K. S., Manley P. J., Gralinski M. R., Lucchesi B. R. (1994) Circ. Res. 75, 701–710 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.